Abstract
Background and objectives
Presymptomatic testing is available for early diagnosis of hereditary autosomal dominant polycystic kidney disease (ADPKD). However, the complex ethical and psychosocial implications can make decision-making challenging and require an understanding of patients’ values, goals and priorities. This study aims to describe patient and caregiver beliefs and expectations regarding presymptomatic testing for ADPKD.
Design, setting and participants
154 participants (120 patients and 34 caregivers) aged 18 years and over from eight centres in Australia, France and Korea participated in 17 focus groups. Transcripts were analysed thematically.
Results
We identified five themes: avoiding financial disadvantage (insecurity in the inability to obtain life insurance, limited work opportunities, financial burden); futility in uncertainty (erratic and diverse manifestations of disease limiting utility, taking preventive actions in vain, daunted by perplexity of results, unaware of risk of inheriting ADPKD); lacking autonomy and support in decisions (overwhelmed by ambiguous information, medicalising family planning, family pressures); seizing control of well-being (gaining confidence in early detection, allowing preparation for the future, reassurance in family resilience); and anticipating impact on quality of life (reassured by lack of symptoms, judging value of life with ADPKD).
Conclusions
For patients with ADPKD, presymptomatic testing provides an opportunity to take ownership of their health through family planning and preventive measures. However, these decisions can be wrought with tensions and uncertainty about prognostic implications, and the psychosocial and financial burden of testing. Healthcare professionals should focus on genetic counselling, mental health and providing education to patients’ families to support informed decision-making. Policymakers should consider the cost burden and risk of discrimination when informing government policies. Finally, patients are recommended to focus on self-care from an early age.
Keywords: genetics, nephrology, medical education & training, mental health, paediatric nephrology
Strengths and limitations of this study.
The focus groups allowed in-depth exploration of patients’ views on presymptomatic testing for autosomal dominant polycystic kidney disease and helped to understand their decision-making process.
The number of participants and the diversity was a strength in this study, including 154 participants across Australia, France and Korea from both stakeholder groups relevant to this study (caregivers and patients).
Research limitations are common to qualitative research methodology in that the data are not generalisable and are restricted to the expressed thoughts of participants.
We acknowledge sensitive topics may be discussed at the focus groups and some views may have been suppressed in the focus group setting.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease and affects about 10% of patients receiving kidney replacement therapy.1 Early phase of ADPKD is often asymptomatic but the development of kidney cysts leads to increased kidney volume, reduced kidney function and eventually follows a relentless course towards end-stage kidney disease.2–8 Clinical management involves pharmacological and lifestyle interventions to control hypertension, slow the progression of cysts, manage complications (kidney and extra-kidney manifestations) and maintain quality of life (QoL).9–11
Diagnosis of ADPKD is usually based on family history, ultrasonography, CT or MRI.12 Testing, however, can facilitate the diagnosis of ADPKD in patients whose kidney phenotypes are atypical or asymptomatic, and in patients with unknown family history. It may also help identify living donors for kidney transplantation.13–15 However, testing has not historically been part of routine care and remains controversial in some countries. Typically, countries used to offer testing when a diagnosis is needed to be confirmed in young patients with unknown family history, for family planning, to determine eligibility for kidney donation, or when the disease presents in childhood or adolescence but testing in adults is overall accepted and encouraged.16 In some countries in Europe and Asia, access to asymptomatic or presymptomatic testing is very restricted or not available.17–20
For the scope of this paper, testing may include any strategy used to identify the presence of ADPKD prior to symptom onset (including genetic tests, blood tests, imaging such as ultrasound, CT, MRI, and so on).13 While testing for ADPKD has the potential to support early intervention, patients can suffer from anxiety and depression from being diagnosed prior to the onset of symptoms.21–24 There are also concerns about potential discrimination with employment and obtaining life insurance, and strains on social and familial relationships.16 The genetic aspect of family planning is emotionally challenging as patients contend with guilt and uncertainty in pursuing parenthood.25 Decision-making about testing is ethically challenging with psychosocial implications, and requires an understanding of the patients’ attitudes, priorities and perspectives of testing. The aim of this study was to describe patient and caregiver perspectives on the value and risks of testing to support the development of strategies and interventions for testing for ADPKD that address their values and needs.
Methods
This focus group study was conducted as part of the Standardized Outcomes in Nephrology-Polycystic Kidney Disease (SONG-PKD) initiative.26 This study is focused on perspectives of patients on testing for themselves and/or their children. We used the Consolidated Criteria for Reporting Qualitative Research to report the study.27
Participant recruitment and selection
Participants were recruited across eight centres in Australia (n=3), France (n=4) and Korea (n=1). Participants were eligible if they spoke English (Australia), French (France) or Korean (Korea), were over 18 years old and diagnosed with ADPKD, or a caregiver. Caregiver refers to family member or support person and not their healthcare professional. We purposively sampled participants to capture a diverse range of demographics (age, gender, employment status) and clinical characteristics (stage of chronic kidney disease, age of diagnosis, current treatment, comorbidities and complications). Recruiting clinicians identified patients with ADPKD who could also invite their caregivers. Participants and researchers had no prior relationship. Participants were given information packages to be able to provide informed consent and received reimbursement (US$25—equivalent in local currency) for travel expenses.
Patient and public involvement
The SONG-PKD initiative26 was developed to ensure outcomes in trials are relevant to patients and other stakeholders. The SONG-PKD Steering Group comprises a multidisciplinary team of healthcare professionals and patients with PKD and was aimed to ultimately develop a core set of outcome domains informed by all stakeholders (including patients) to be reported in all trials in patients with ADPKD.26 Patients on the Steering Group were involved in the initial planning and design of the study. Purposive sampling was done across different centres and patients were able to invite any other patients who would be interested to participate. All participants were invited to be involved in the following step of SONG-PKD which involved completing a Delphi survey.26 Results of this survey will be emailed to all participants. The general public were not involved.
Data collection
The 2-hour focus group discussions were conducted from June to November 2017 until data saturation. Data saturation was achieved when CL, YC and AT agreed that little or no new concepts were arising from subsequent focus groups. We developed the question guide from the literature and with input from the research team (online supplemental material and methods).25 28 29 Focus groups were convened in a venue external to the hospital and facilitated by one investigator (English—AT (researcher), TG (researcher), YC (academic nephrologist); French—BS (academic nephrologist); Korean—YK (academic nephrologist)). Focus groups were designed with the intent to have a broad range of demographic and clinical characteristics (including patients/caregivers, age). We did not consider severity of symptoms a priori. We did not separate patients from a caregiver as they preferred to participate in the same group. A cofacilitator recorded field notes. All discussions were audio recorded and were transcribed.
bmjopen-2020-038005supp001.pdf (20.1KB, pdf)
Data analysis
All transcripts were entered into HyperRESEARCH (V.3.7) for analysis and coded line by line, in the original language and then translated for investigator triangulation, by CL (researcher) (English, French) and HK (academic nephrologist) (Korean) using thematic analysis and drawing on principles from grounded theory to identify concepts related to perspectives on testing for ADPKD.30 From grounded theory, we conducted initial coding (memoing) and line-by-line coding of the data, used constant comparison within and across the transcripts and inductively identified concepts and themes. In accordance with thematic analysis, we identified initial concepts and grouped similar concepts into themes. Codes were grouped by similar concepts into themes and subthemes which were discussed and revised with AT/TG/YC/BS/YK who independently read the translated transcripts. To ensure reliable interpretation of the translated transcripts, CL and HK were available to give more context of the quotes. Investigator triangulation ensured that the analysis captured the full range and breadth of the data.31
Results
In total, 154 participants (120 patients, 33 caregivers) participated in 17 focus groups across Australia, France and Korea. The demographics are shown in table 1. Participants’ age ranged from 19 to 78 years (mean age 54.5 years) and 67 (42%) were men. Most patients were diagnosed between the ages of 21 and 40 years and the majority of patients were predialysis (n=76, 61%), followed by transplant recipients (n=31, 26%) and those on dialysis (n=19, 13%). The majority of caregivers defined themselves as the spouse or partner of the patient (n=24, 71%), but also included child (n=2, 6%), daughter-in-law (n=1, 3%), parent (n=4, 12%) and sibling (n=1, 3%). Reasons for declining to participate included having other commitments and being too unwell to participate.
Table 1.
Participant demographic characteristics
| Characteristic | Australia n=85 (%) | France n=40 (%) | Republic of Korea n=29 (%) | All participants n=154 (%) |
| Participant status | ||||
| Patient | 61 (71) | 36 (90) | 24 (83) | 121 (78) |
| Caregiver | 24 (28) | 4 (10) | 5 (17) | 33 (21) |
| Male | 35 (41) | 17 (43) | 12 (41) | 64 (42) |
| Age (years) | ||||
| 18–39 | 16 (19) | 2 (5) | 3 (10) | 21 (14) |
| 40–59 | 34 (40) | 18 (45) | 20 (69) | 72 (47) |
| 60–79 | 35 (41) | 20 (50) | 6 (21) | 61 (40) |
| Highest level of education* | ||||
| Primary school: grade 6 | 4 (5) | 2 (5) | 1 (3) | 7 (5) |
| Secondary school: grade 10 | 18 (22) | 8 (20) | 2 (7) | 28 (18) |
| Secondary school: grade 12 | 7 (8) | 14 (35) | 5 (17) | 26 (17) |
| Tertiary: certificate/diploma | 25 (30) | 4 (10) | 0 (0) | 29 (19) |
| Tertiary: university degree | 29 (35) | 12 (30) | 21 (72) | 62 (41) |
| Employment | ||||
| Full time | 21 (25) | 12 (30) | 17 (59) | 50 (32) |
| Part time or casual | 17 (20) | 4 (10) | 3 (10) | 24 16) |
| Not employed | 11 (13) | 0 (0) | 4 (14) | 15 (10) |
| Retired | 28 (33) | 19 (48) | 2 (7) | 49 (32) |
| Other (eg, income protection insurance) | 8 (9) | 5 (13) | 3 (10) | 16 (10) |
| Ethnicity | ||||
| White | 72 (85) | 40 (100) | 0 (0) | 112 (73) |
| Asian | 7 (19) | 0 (0) | 29 (100) | 36 (23) |
| Other | 6 (7) | 0 (0) | 0 (0) | 6 (4) |
| CKD stage† | ||||
| Predialysis | 34 (56) | 20 (56) | 20 (83) | 74 (61) |
| Dialysis | 11 (18) | 2 (6) | 3 (13) | 16 (13) |
| Transplantation | 16 (26) | 14 (39) | 1 (4) | 31 (26) |
| Age at diagnosis*† (years) | ||||
| 0–20 | 10 (16) | 6 (17) | 3 (13) | 19 (16) |
| 21–40 | 35 (57) | 21 (58) | 14 (58) | 70 (58) |
| 41–60 | 13 (21) | 7 (19) | 6 (25) | 26 (21) |
| >60 | 3 (5) | 2 (6) | 1 (4) | 6 (5) |
*Missing data from two participants.
†Patient only (n=61; n=31; n=24).
CKD, chronic kidney disease.
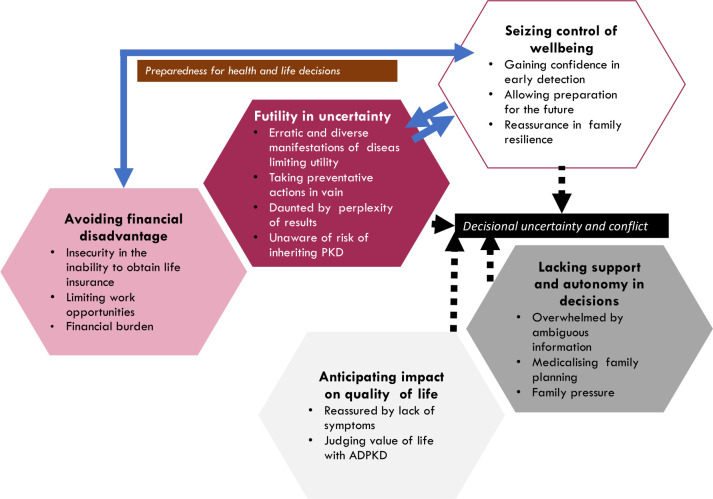
Five themes were identified with both patients and caregivers contributing to the concept unless otherwise stated: avoiding financial disadvantage, futility in uncertainty, lacking autonomy and support in making decisions, seizing control of well-being and anticipating impact on QoL. Subthemes are described in the following section. Illustrative quotations for each theme are provided in table 2. The conceptual links among themes are depicted in figure 1.
Table 2.
Selected illustrative quotations
| Theme | Illustrative quotations |
| Avoiding financial disadvantage | |
| Insecurity in the inability to obtain life insurance | I asked many years ago whether I could have testing done on my children and I was told yes, but it’s not advised, because if it was proven that either of them were likely to get polycystic kidneys, they would never be able to go on a school camp, and they would never get life insurance. (Australia) He’s 21 now and I’m pretty certain he has it and I say to him, ‘Whatever you do don't get it confirmed, just live your life as long as you can without being diagnosed, without getting it there in writing that you’ve got it,’ because superannuation, life insurance, job prospects, all these sorts of things that come up that are going to be detrimental or change his life in some way. (Australia) I actually tried to get some extra life insurance cover through superannuation and they said ‘yeah, polycystic kidneys, nope can’t do it’ so I got [doctor] to write a detailed letter about my renal function, and he reckons I’m going to be good for another 20 years, and they still wouldn’t insure me. (Australia) If you are not insured in health expenses insurance, you can be reimbursed later on but if you are diagnosed with PKD in your teens then you can’t get insured. (Korea) |
| Limited work opportunities | Even applying for jobs now, they ask you about your medical history. If you don’t know, you can’t write it down. (Australia) When I went for jobs, my job provider turned around and said, ‘You have to tell them anything that will affect your job.’ (Australia) My oldest son is in high school and the doctor advised me to organize genetic test for him before he enters army. I think if his test results come back positive and he is unable to attend army that may have a negative impact on his ability to work in future. I worry that it will place him in disadvantaged position. (Korea) |
| Financial burden | My nephew and his wife were pregnant, and she was going to get a test to see whether his daughter had polycystic kidneys. But the cost was huge, so he didn’t do it. (Australia) Genetic testing raises concerns about associated costs. There is added cost when you don’t know about diagnosis. Spending a lot of money in advance is a burden. (Korea) My family decided to undergo genetic testing with government support. It’s quite expensive for a whole family to do. It would be better if these aspects can be improved to reduce the burden on family. (Korea) |
| Futility in uncertainty | |
| Erratic and diverse manifestation of disease limiting utility | There are just so many variables in it, and there are plenty of people that die, and didn’t even know that they had it, they discovered it in autopsy. I just thought, [genetic testing] was a big call to make for something that could never ever actually develop. (Australia) You don’t assume that another person will get those same symptoms… everyone will be different, some similar but not the same. (Australia) Some people can go all through and live to old age and not know. It’s just a slower growing cyst, or a different form of PKD. There are some babies that are born with PKD that’s not conducive to life. (Australia) There are no two people the same in terms of what works or what, why it started or how quickly it declines. (Australia) |
| Taking preventive actions in vain | There’s no benefit to knowing early. There is nothing they can do to change the outcome; it’s going to happen in its own time at this stage anyhow, so why spoil that young person’s life? (Australia) No matter what you’re going to go through the process anyway, if you’ve got it. (Australia) I thought of this [genetic test] very negatively. There is no effective treatment, so why you need to know early. Knowing early without treatment means that you are mentally suffering… you have to live in pain as soon as you know. (Korea) To tell us information when there’s no possibility to make things better, is just giving anxiety for nothing. (France) |
| Daunted by perplexity of results | Everyone is not equal before the disease. To teach a young person that he has a sword of Damocles over his head, that he will be dialyzed, maybe grafted may psychologically damage him. (France) He just didn’t cope [with his possible diagnosis]. In fact, I even wondered if he was going to do something ghastly to himself. (Australia) I would just rather go through life not having to have that cloud over me at any point. So, if I was in my twenties and somebody said, ‘Here you can do genetic testing and it will show you've got this,’ I wouldn't want to. I just don't want to have that limiting my life at that point. (Australia) I have an 18-year-old son, when I broach the subject of him getting tested, what is it going to achieve? It’s only going to cause more stress. (Australia) |
| Unaware of risk of inheriting ADPKD | Fertility and the genetics of PKD really fascinate me and impact me a lot and that’s probably the biggest impact in my life at the moment is whether or not I want to consider passing on the PKD gene, or to adopt, or if I want to terminate if I find out they do have it. (Australia) Knowing at some stage that you've got a possibility of a child having a disease is good because then it can help you with other decisions. (Australia) I'm only 25, I do not have children, and it’s true that this is a question I'm asking myself today. What do I do? Have children? Naturally or do I ask to go into a process of assisted reproduction to try to remove that gene. (France) I cried a lot blaming [my mother in law] why my husband had to suffer from this genetically inherited disease…. My biggest wish is that this does not affect my children. (Korea) |
| Lacking autonomy and support in decisions | |
| Overwhelmed by ambiguous information | He [doctor] didn’t know what to say. Screen or don’t screen. (France) I didn’t have enough information on that, so I tried to search the internet. (France) No one’s ever brought [genetic counseling] up to me, it’s always been, ‘Oh, this is what you’re looking forward to, this is what we have to do to your mother,’ it’s never been on the fertility part of it at all and I actually had to go to a fertility doctor to help me. (Australia) [Genetic testing] is rarely offered to us. (France) |
| Medicalising family planning | I was a young woman, and [the doctor] said when you get to the point of having children, we can certainly test your fetus to see if it has polycystic kidney disease, and then you could terminate if it did. And I didn’t go back to him, ‘cause I didn’t like that. (Australia) I felt like he thought that was my civic duty to try and eliminate this disease, well if your baby’s got polycystic kidney disease, we’ll just terminate it and then you can try for another one, and there’s a 50% chance that it will or won’t, and you could just, terminate any defective ones. (Australia) We cannot detect [ADPKD] before the end date of the abortion authorization. So, this is a debate that leads nowhere, because there is no opportunity to choose. It’s either we do not have children at all or we take the risk. (France) Fetus is also a life. If a genetic test finds PKD, are you going to abort the fetus? No. So, if I find out early with fetus in utero, I will feel guilty and have bad feelings. I just don’t see why this is necessary. (Korea) |
| Family pressure | If they were planning on having children, I’d potentially encourage them to be tested before then just, so they can keep an extra eye on it. (Australia) [PKD is] a family concern, because she’s my sister, she’s a bit concerned. So, she wants to make sure that the boys don’t have it. (Australia) So, you know, my family’s all on my case, oh why don’t you get tested? (Australia) You have to follow up. If someone is found in the family, who is affected, then … I got the report, you have to follow up on the rest of the family too … they are invited to do some research. (France) |
| Seizing control of well-being | |
| Gaining confidence in early detection | If you know about it early, you can do some things to help yourself, to prolong [your kidneys] life. Maybe don’t have a huge steak and have more vegetables and less protein, lots of water and that sort of thing. (Australia) Personally, I think that it must absolutely be done and know if one is sick or not to anticipate and preserve the maximum [kidney function]. (France) I think it may be better to get children tested early. So that parents know, to better look after their health, their diet, care with sport… and then to tell them when they have grown up as adults to allow them to make informed decision. (Korea) If you don’t know the result of genetic test, as a parent it is very difficult to reinforce the importance of dietary health, so as a parent there is definitely an aspect you want to know (through genetic testing). (Korea) |
| Allowing preparation for the future | I might’ve put more away in super rather than running my own business so much, had I known, but that opportunity wasn’t there for me because I didn’t know at that time. (Australia) I went on dialysis a lot quicker because I was working in a job with huge stress. Now, if I would have known I would have got out of that job years before because I didn't realize that my blood pressure was like 180 over something. (Australia) For someone who has gone on dialysis and sold my business and can't travel, if we had had been educated earlier, we would not have worked so hard, we would have had a holiday, all those things would have been done so we had no regrets later. (Australia) |
| Reassurance in family resilience | This is generational, my mother’s father died of it. We’ve been quite used to it, if there’s such a thing as used to it, so our children, I don’t think would have a huge impact on them, they would know what to do. (Australia) She was fine after testing, we discussed it, we had all sorts of chats about; what it’s going to be in the future and look at some of the other things you could have that would be far worse, and look on the bright side, at least you know about it and we can do preventative stuff. (Australia) When we went and saw the genetic doctor the second time he gave us an actual formal letter and we passed it out throughout our kids, and mainly living cousins, so that if they wanted to go they could ring up and get an appointment, see him and get tested or whatever. (Australia) PKD is hereditary in our family, I just think of it as a quirk, it’s just another thing that makes me different and unique so I’ve been very lucky to watch my grandmother and my father go through it and the way that they’ve approached it and dealt with it has given me hopefully a good attitude towards it, it doesn’t affect me yet so I am lucky, at this stage. (Australia) |
| Anticipating impact on quality of life | |
| Reassured by lack of symptoms | If you're getting towards 40, 50, 60 even and it hasn't bothered you until then, you're not going to be worried about it. (Australia) ‘Not until you're older.’ ‘Oh well, I suppose I'll worry about then.’ I wouldn't worry about it until I absolutely have to. (Australia) I had children anyhow, even though I did know there was a risk. I was still healthy. I’ve got four lovely boys, two have got the disease. I do feel a bit of guilt about that for sure, but I wouldn’t give any of them back. (Australia) I don’t think about it as I have not had any major problems related to the disease, but it’s true that I don’t have enough perspective to inform me. (France) |
| Judging the value of life with ADPKD | You want your child to have the best life possible and be healthy and happy and everything like that but I don't see I’ve been ever denied anything or will ever be denied anything in life and if my parents had had the same decision would I exist today? (Australia) I was fairly stubborn that I was never going to have children if I had PKD. I was 100% fixed in my head and nothing was going to change that but I decided I didn’t have PKD so it was all right, so I made that error now because I had the two most beautiful children and I really think you’ve got to be pretty careful in that area because you create beings that are adding quite a bit of value to society. (Australia) My mother said a couple of times if she’d have known; she probably shouldn’t have had children. My take on that is I’ve had a really good 60 years with a bit of intervention, and she had three children without the kidney disease, so I was the only one of four. (Australia) [My mother] said, ‘If I knew like you, you would not be here. Neither you nor your sister.’ I have no family. Because, I made the choice not to have children, but I made the right choice. (France) |
ADPKD, autosomal dominant polycystic kidney disease; PKD, polycystic kidney disease.
Figure 1.
Thematic schema. Participants felt that their ability to take control of their health was influenced by how prepared they were financially and was hindered by the unpredictable nature of their disease symptoms (indicated by the solid lines). Participants often felt that they were conflicted in whether or not they wanted to be tested for polycystic kidney disease (PKD). This decisional uncertainty (indicated by the dotted lines) was prompted by the uncertainty in participant symptoms, whether they felt capable of seizing their health, how they anticipated the impact on PKD on the quality of their life and whether or not they had support and autonomy in their decisions. ADPKD, autosomal dominant polycystic kidney disease.
Avoiding financial disadvantage
Insecurity in the inability to obtain life insurance
Some participants (specifically caregivers) were concerned about patients being labelled as ‘high risk’ when assessed for life insurance and expected they would pay higher premiums, be unable to obtain insurance or be ‘dropped’ by their insurance provider. They suspected they would be unfairly penalised for a disease that may not manifest. For this reason, some did not disclose ADPKD or avoided confirmatory tests—‘Don’t get it confirmed, just live your life as long as you can without being diagnosed’ (caregiver, France). Parents worried that limited insurance would restrict their children from travelling and from attending school camps.
Limiting work opportunities
Some patients feared discrimination from employers who could deny or dismiss them because of a diagnosis. Some worried that the disease would impair their physical ability to perform at work. Parents considered how the risks of early diagnosis through testing may jeopardise work opportunities for their children—‘[my] doctor advised me to organize a genetic test for [my son] … but then I think if his test result comes back positive … this may have a negative impact on his ability to work in future’ (Australia). Some refused tests and avoided disclosing their medical history to protect employment prospects.
Financial burden
Some presymptomatic participants wanted to undergo testing for ADPKD, but the cost was prohibitive, particularly for participants in Korea—‘Genetic testing raises concerns about associated cost…. spending a lot of money in advance is a burden’ (Korea). Some believed that a history of ADPKD warranted reimbursement from the government to improve equity of access to testing.
Futility in uncertainty
Erratic and diverse manifestations of disease limiting utility
The symptoms of ADPKD were regarded as unpredictable, such that a diagnosis would not provide useful information about symptom burden and prognosis. Patients and caregivers believed it was unnecessary to be concerned until symptoms become apparent—‘[confirmatory testing] was a big call to make for something that could never ever actually develop’ (Australia).
Taking preventive actions in vain
Participants who had been diagnosed through screening felt frustrated when attempts to minimise disease progression (eg, with antihypertensive medications or smoking cessation) proved futile. Some felt helpless and perceived that testing prior to experiencing symptoms was useless since they were powerless to change the unpredictable course—‘There’s no benefit to knowing early. There is nothing they can do to change the outcome, it’s going to happen in its own time’ (Australia).
Daunted by perplexity of results
Some parents worried that their child would be overwhelmed in trying to comprehend or interpret the results from testing and that it would create ‘a sword of Damocles over [their] head causing worry, anxiety, depression and even posttraumatic stress disorder’ (France).
Unaware of risk of inheriting PKD
The threat of transmitting the disease to their children caused decisional conflict about testing. Some felt they would be more empowered by knowing the results—‘Knowing that you’ve got a possibility of a child having a disease is good, it can help you with other decisions’ (Australia). Others struggled with the uncertainty of the impact of tests on decisions about family planning—‘probably the biggest impact in my life at the moment is whether or not I want to consider passing on the PKD gene’ (Australia). For parents who were diagnosed after having children, they believed that the diagnosis would not have impacted their decisions and were aware of options such as preimplantation genetic diagnosis—‘Genetically if there was some way of knowing that I was going to pass it on would I take that, or would I just go ahead and have the child? […] I would have the child’ (Australia).
Lacking support and autonomy in decisions
Overwhelmed by ambiguous information
Participants felt ‘completely in the dark’ about testing. They struggled with conflicting opinions, such as what age to get screened, and some felt misled by clinicians—‘I remember the specialist [saying to mum], “girls don’t get polycystic kidney disease so you’re fine having two girls,” so my sister and I lived in oblivion until I was 42’ (Australia). Some thought that clinicians did not provide adequate genetic counselling. In Australia, some were unaware that a genetic test was available and felt they should be informed. They searched for information on the internet and asked other family members with ADPKD but were disappointed by a general lack of information.
Medicalising family planning
Some participants regretted having tests when they were advised against having children—‘[The doctors said] “don’t reproduce, that will stop the disease”’ (Australia). Some diagnosed through screening feared judgement from clinicians and felt pressured against having children. Others appreciated the direct advice in family planning to support their decision—‘[The doctors] told me “don’t do it”. And I made the choice—no kids’ (France). Some resisted prenatal testing to avoid having to confront decisions about termination of pregnancy—‘if genetic tests find PKD, are you going to abort the fetus? No. If I found out early with fetus in utero, I will feel guilty and have bad feelings’ (Korea). Some participants in France thought prenatal testing was useless because abortion was illegal—‘When he was in utero, I wanted to abort. At the time, it was not possible’ (France).
Family pressures
Some thought they should convince their family to get tested—‘From the moment I found that I had it, I wrote to all my relatives, and said, “Get screened”’ (Australia). Some parents expected that testing would motivate behaviour change to maintain health, and were frustrated when their child did not demonstrate effort to protect their kidney health—‘I keep nagging him to see a doctor, see a specialist, and he goes yeah, doctor said my kidneys are alright’ (Australia). For some, tests on children were a collective ‘family concern’ and decision.
Seizing control of well-being
Gaining confidence from early detection
An early diagnosis through testing was thought to provide an opportunity for participants to take control of their health by modifying their diet and taking preventive medications, such as antihypertensive agents. Participants were empowered to monitor their health vigilantly and gained confidence in their ability to preserve their QoL—‘Going to the doctor regularly, just getting your blood pressure checked, because they say that if you can keep your blood pressure under control, they [kidneys] might not fail’ (Australia). For parents with a child with ADPKD, an early diagnosis motivated them to educate and ‘reinforce the importance of dietary health’ (Korea) in their children.
Allowing preparation for the future
An earlier diagnosis through testing enabled patients to mentally prepare for potential symptom burden and make lifestyle changes (including financial and career planning) to protect their QoL and avoid stress—‘Forewarned is forearmed’ (Australia). Some participants (particularly on dialysis) regretted not getting tested as they would have maximised their time while asymptomatic—‘If we had been educated earlier, we would not have worked so hard, we would have had a holiday, all those things would have been done so we had no regrets later’ (Australia).
Reassurance from family resilience
Some observed their parents’ optimism and resilience while on dialysis or with a transplant, and this strengthened confidence in their decision to be tested. Some appreciated that testing was more accessible for their children—‘now any of my family can go and get it done’ (Australia).
Anticipating impact on QoL
Reassured by lack of symptoms
Some participants were not interested in testing because their QoL had not been affected. They questioned ‘well do I actually have it’ and did not worry about their disease or testing—‘I have not had any major problems related to the disease’ (France). Some parents were not concerned with testing or genetic transmission as they believed their child would not suffer a disadvantaged life because they had not.
Judging the value of life with ADPKD
Some parents believed they would have decided against having children if they had been tested because ADPKD had caused their family to suffer—‘If I knew [that I had PKD], you would not be here’ (France). Some participants respected their parents’ decisions to have children but questioned that if they had had been tested ‘would I exist today?’ Some did not see the merit in testing as they valued their lives regardless of ADPKD—‘You’ve got to be pretty careful in that area because you create beings that are adding quite a bit of value to society’ (Australia).
Discussion
For some patients with ADPKD and caregivers, testing provided an opportunity to gain certainty about their health status, foster motivation and confidence for self-management, prepare mentally and financially for the onset of symptomatic disease and seek support from family. However, others perceived testing as futile because they perceived preventive measures had little impact, and the onset and course of ADPKD were unpredictable. They were also concerned about interpreting the results and the implications for their current and future life, which could cause unnecessary worry and anxiety, particularly with regard to family planning. The costs incurred in accessing testing and the potential financial discrimination they expected to endure would impose substantial constraints on their lives and futures.
Overall, the perspectives of patients and caregivers were similar as they felt inadequately equipped and conflicted in making decisions, which was exacerbated by a lack of support and information and perceived pressure from family and healthcare professionals. They were also uncertain about the severity of the symptom burden, and it was difficult to judge the value of life with ADPKD. Patients who witnessed intense suffering in their family members with ADPKD were inclined to refuse testing to avoid becoming anxious about their future and did not expect that the diagnosis would increase their sense of control. Lack of support has been recognised and, through discussions with specialists and patient advocates, this led to the development of a route map for ADPKD (available in three languages) intended to help patients and all stakeholders navigate through the services available to them (including genetic testing, diagnostic, management and treatment options).18
The variability in policies across the countries and parent-child roles may also explain some differences in perspectives. The diagnosis of ADPKD using methods other than genetic testing is routinely offered as the latter is not readily available or accessible in many countries. The cost of testing was of particular concern to patients in Korea, which may reflect the fact that testing is not funded by the government.32
This has led to an increase in direct-to-consumer genetic testing, which has negative ramifications because the public is often unaware of their clinical and social implications.32 Korean patients were particularly concerned about the cost burden of the disease and expressed that they did not understand the added value of paying for a presymptomatic ultrasound test if cysts may develop later in life. A recent study showed that more than 70% of Korean patients believed that genetic testing should be included in Korea’s national health testing programme so these services can be provided at little expense.33 In Australia, access to dialysis and transplantation is provided to all citizens via government-funded Medicare system.34 Transplantation is primarily limited due to insufficient kidney donors available to meet the number of potential recipients on the organ waiting list.34 Dialysis in Korea has also been covered since 1989 and this is similar in France.35 36 In France, genetic testing is not routinely offered to patients, although some could have free access to genetic testing (eg, if they were enrolled in the Genkyst observational cohort study37). In regard to legislative protection, Australia, France and Korea have comprehensive provisions pertaining to consent, autonomy and integrity of the person tested.38 In France, refusal of fetal testing for ADPKD may be due to fear of genetic transmission and the illegality of termination of pregnancy after 12-week conception due to ADPKD.39 No participants mentioned preimplantation genetic diagnosis, which highlights an information gap between countries. Variable perspectives can also be noted depending on the role of the participant regardless of their country of residence. In previous studies, parents have reported largely positive attitudes towards testing for children, while some children became more concerned about their health or the health of their family members.40 41 Younger patients expressed more anxiety around a diagnosis because they feared it could limit their future and were anxious about how quickly their health would decline.
Similar perspectives on testing have also been noted with other later onset progressive conditions including Huntington disease, characterised by a motor and cognitive deterioration with unpredictable prognosis leading to similar decisional uncertainty in views about testing.42–44 For patients with Huntington disease, family members could be perceived to have a supportive role or put pressure on making decisions in terms of being presymptomatically tested. They considered the consequences of sharing or withholding information about the diagnosis.45 Some refused testing to avoid unnecessary anxiety before they experienced symptoms of the disease.45
Our findings are consistent with the concepts of multigenerational transmission process in family system theory, which emphasises that an individual’s behaviour is inextricably connected with the attitudes and behaviours learnt from their family.46 47 The multigenerational transmission process can help explain how decisions about testing can be shaped by observing the extent to which family members (particularly parents) suffered the symptoms of ADPKD.46 Some patients believed that their experience might be different from those of their family members and were uncertain about the chance of genetic transmission in family planning, while others were influenced by the adverse impact that ADPKD had on their family.
Our study spanned three countries and provided in-depth, diverse and novel insights about testing for ADPKD from a relatively large sample of patients and their caregivers purposively selected to include a range of demographic characteristics. We achieved data saturation, coded the data in the language of the focus groups and used investigator triangulation in the analysis to ensure the themes reflected the breadth and depth of the data. However, there are some potential limitations. We are uncertain about the transferability of the findings to other countries with different healthcare policies on testing. We acknowledge that testing can be a sensitive topic and some views may have been suppressed in the focus group setting which may also explain why there was limited variation in the perspectives of caregivers versus patients. We discussed testing for disease presence only in patients diagnosed with ADPKD and 31% were receiving kidney replacement therapy. We acknowledge that the findings may not include views of at-risk persons because of ethical reasons. Other limitations include the relatively low number of caregivers and other subgroups (ie, transplant recipients) and being ethically unable to collect demographic characteristics from patients who declined to participate.
Our findings can inform ways to better inform and communicate with patients and their families. Knowledge of the available tests, prevention and management may support decision-making. Our findings support the value of genetic counsellors and education sessions prior to testing to help address the potential psychological and social consequences of testing to the individual and the family.45 Kidney Disease Improving Global Outcomes guidelines and the European ADPKD Forum suggest that patients with ADPKD should have access to reproductive counselling.29 48 However, as few as 20%–40% of nephrologists may actually inform their patients about the prenatal and preimplantation genetic diagnostic options due to ethical concerns while 68% of patients with ADPKD believe it should be offered.33 49 Clinicians have articulated similar concerns about testing for ADPKD because of the perceived absence of curative treatment options and the perceived minimal burden on QoL. However, perceptions of testing may change with increasing availability and use of vasopressin receptor antagonists to prevent the progression of ADPKD.50 Ongoing clinical trials may provide additional options for treatment to slow progression.51–53 Concerns about discrimination in regard to disclosure of genetic status should also be addressed.54
For some patients with ADPKD, testing could empower them to take charge of their health while for others, receiving a confirmed diagnosis of ADPKD causes unnecessary anxiety over a disease that they limited control over. Testing positive for ADPKD could jeopardise employment opportunities for patients and complicate family planning and dynamics. Providing access to education and genetic counselling in people at risk of ADPKD and their family, and psychosocial support after receiving the test results, are suggested to provide individuals with the capacity to make informed decisions and to empower them for self-management.
Supplementary Material
Acknowledgments
We are grateful to all the patients and their caregivers who generously gave their time to share their insights and perspectives.
Footnotes
Twitter: @pkd_int, @KarineManera, @allisontong1
Contributors: Conceptualisation: GKR, JC, AT, CL, YC. Methodology: JC, ATP, AT, CL, YC. Software: AT. Validation: CL, YC, BS, TG. Formal analysis: CL, YC, BS, ATP, AT, YC, TG. Investigation: CL, YC, BS, GKR, TG, JC, AO, AC, CA, HC, JTWK, RTG, RP, TH, VT, KF, YP, PK, JR, DJ, AV, CG, HK, YK, MH, AJ, KEM, ATP, GP, AT (all authors). Resources: AT. Data curation: YC, BS. Writing—original draft preparation: CL. Writing—review and editing: CL, YC, BS, GKR, TG, JC, AO, AC, CA, HC, JTWK, RTG, RP, TH, VT, KF, YP, PK, JR, DJ, AV, CG, HK, YK, MH, AJ, KEM, ATP, GP, AT (all authors). Visualisation: CL. Supervision: AT, GP, YC, JC. Project administration: TG, MH, AJ, KEM. Funding acquisition: AT, DJ, TG, MH, KEM, YC, AV, BS. All authors have read and agreed to the published version of the manuscript.
Funding: The study was financially supported by a grant from Polycystic Kidney Disease Foundation of Australia and the National Health and Medical Research Council (NHMRC) Better Evidence and Translation in Chronic Kidney Disease (BEAT-CKD) Program (1092957), UMR INSERM 1246-SPHERE Methods in Patient-centered Outcomes and Health Research, and Research of Korea Center for Disease Control and Prevention (2016E3300201). AT is supported by an NHMRC Fellowship (1106716). DJ is supported by an NHMRC Practitioner Fellowship (1117534). TG is supported by an NHMRC Postgraduate Scholarship (1169149). MH is funded as a Post-Doctoral Fellow through the NHMRC BEAT-CKD Program Grant (APP1092957). KEM is supported by an NHMRC Postgraduate Scholarship (1151343). YC is supported by an NHMRC Early Career Fellowship (1126256). AV receives grant support from the NHMRC Medical Postgraduate Scholarship (1114539) and the Royal Australasian College of Physicians (Jacquot NHMRC Award for Excellence).
Competing interests: GKR declares he is site investigator of clinical trials sponsored by Sanofi, Otsuka and Reata, principal investigator of the PREVENT-ADPKD clinical trial (funded in part by the NHMRC, Danone Nutricia—manufacturer of bottled water—PKD Australia, Westmead Medical Research Foundation) and Chair, Scientific Advisory Board, PKD Australia. RP declares he is site investigator of clinical trials sponsored by Sanofi, Kadmon, Reata, Otsuka and the US Department of Defense (TAME PKD), and section editor for Cystic Kidney Disease, UpToDate.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Ethics approval: The Human Research Ethics Committees of the Western Sydney Local Health District (HREC2009/6/4,14), Monash Medical Centre (2010.031), Metro South Health District (17/QPAH/112), France (INSERM/2017) and Republic of Korea (1709-097-886) approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. Data are available upon reasonable request to achieve aims in the approved proposal. Data include deidentified participant data and transcripts from focus groups that underlie the results reported in this article. The study protocol is also available. Data are available from the corresponding author (https://orcid.org/0000-0002-8773-1003).
References
- 1.Lanktree MB, Haghighi A, Guiard E, et al. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol 2018;29:2593–600. 10.1681/ASN.2018050493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med 1993;329:332–42. 10.1056/NEJM199307293290508 [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ. Polycystic kidney disease: neoplasia in disguise. Am J Kidney Dis 1990;15:110–6. 10.1016/S0272-6386(12)80507-5 [DOI] [PubMed] [Google Scholar]
- 4.Higashihara E, Nutahara K, Kojima M, et al. Prevalence and renal prognosis of diagnosed autosomal dominant polycystic kidney disease in Japan. Nephron 1998;80:421–7. 10.1159/000045214 [DOI] [PubMed] [Google Scholar]
- 5.Masoumi A, Reed-Gitomer B, Kelleher C, et al. Developments in the management of autosomal dominant polycystic kidney disease. Ther Clin Risk Manag 2008;4:393–407. 10.2147/tcrm.s1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadnapaphornchai MA, George DM, Masoumi A, et al. Effect of statin therapy on disease progression in pediatric ADPKD: design and baseline characteristics of participants. Contemp Clin Trials 2011;32:437–45. 10.1016/j.cct.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. Clin J Am Soc Nephrol 2008;3:1197–204. 10.2215/CJN.00060108 [DOI] [PubMed] [Google Scholar]
- 8.Ong ACM, Devuyst O, Knebelmann B, et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 2015;385:1993–2002. 10.1016/S0140-6736(15)60907-2 [DOI] [PubMed] [Google Scholar]
- 9.Schrier RW. Optimal care of autosomal dominant polycystic kidney disease patients. Nephrology 2006;11:124–30. 10.1111/j.1440-1797.2006.00535.x [DOI] [PubMed] [Google Scholar]
- 10.Grantham JJ. Clinical practice. autosomal dominant polycystic kidney disease. N Engl J Med 2008;359:1477–85. 10.1056/NEJMcp0804458 [DOI] [PubMed] [Google Scholar]
- 11.Palmer SC, Maggo JK, Campbell KL, et al. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev 2017;4:CD011998. 10.1002/14651858.CD011998.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomsen HS, Levine E, Meilstrup JW, et al. Renal cystic diseases. Eur Radiol 1997;7:1267–75. 10.1007/s003300050288 [DOI] [PubMed] [Google Scholar]
- 13.Huang E, Samaniego-Picota M, McCune T, et al. DNA testing for live kidney donors at risk for autosomal dominant polycystic kidney disease. Transplantation 2009;87:133–7. 10.1097/TP.0b013e318191e729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thong KM, Ong ACM. The natural history of autosomal dominant polycystic kidney disease: 30-year experience from a single centre. QJM 2013;106:639–46. 10.1093/qjmed/hct082 [DOI] [PubMed] [Google Scholar]
- 15.Shaw C, Simms RJ, Pitcher D, et al. Epidemiology of patients in England and Wales with autosomal dominant polycystic kidney disease and end-stage renal failure. Nephrol Dial Transplant 2014;29:1910–8. 10.1093/ndt/gfu087 [DOI] [PubMed] [Google Scholar]
- 16.Tchan M, Savige J, Patel C, et al. KHA-CARI autosomal dominant polycystic kidney disease guideline: genetic testing for diagnosis. Semin Nephrol 2015;35:545–9. 10.1016/j.semnephrol.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 17.Rossler FL. Legislation on genetic testing in different countries. Pediatr Epidemiol 2018;21:30–40. [Google Scholar]
- 18.EAF co-chairs, Harris T, Sandford R, et al. European ADPKD forum multidisciplinary position statement on autosomal dominant polycystic kidney disease care: European ADPKD forum and Multispecialist roundtable participants. Nephrol Dial Transplant 2018;33:563–73. 10.1093/ndt/gfx327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimpel C, Bergmann C, Bockenhauer D, et al. International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat Rev Nephrol 2019;15:713–26. 10.1038/s41581-019-0155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horie S, Mochizuki T, Muto S, et al. Evidence-based clinical practice guidelines for polycystic kidney disease 2014. Clin Exp Nephrol 2016;20:493–509. 10.1007/s10157-015-1219-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miskulin DC, Abebe KZ, Chapman AB, et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1-4: a cross-sectional study. Am J Kidney Dis 2014;63:214–26. 10.1053/j.ajkd.2013.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajwa ZH, Sial KA, Malik AB, et al. Pain patterns in patients with polycystic kidney disease. Kidney Int 2004;66:1561–9. 10.1111/j.1523-1755.2004.00921.x [DOI] [PubMed] [Google Scholar]
- 23.Baker A, King D, Marsh J, et al. Understanding the physical and emotional impact of early-stage ADPKD: experiences and perspectives of patients and physicians. Clin Kidney J 2015;8:531–7. 10.1093/ckj/sfv060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simms RJ, Thong KM, Dworschak GC, et al. Increased psychosocial risk, depression and reduced quality of life living with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2016;31:1130–40. 10.1093/ndt/gfv299 [DOI] [PubMed] [Google Scholar]
- 25.Tong A, Rangan GK, Ruospo M, et al. A painful inheritance-patient perspectives on living with polycystic kidney disease: thematic synthesis of qualitative research. Nephrol Dial Transplant 2015;30:790–800. 10.1093/ndt/gfv010 [DOI] [PubMed] [Google Scholar]
- 26.Cho Y, Sautenet B, Rangan G, et al. Standardised outcomes in Nephrology-Polycystic kidney disease (SONG-PKD): study protocol for establishing a core outcome set in polycystic kidney disease. Trials 2017;18:560. 10.1186/s13063-017-2298-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 28.Tong A, Tunnicliffe DJ, Lopez-Vargas P, et al. Identifying and integrating consumer perspectives in clinical practice guidelines on autosomal-dominant polycystic kidney disease. Nephrology 2016;21:122–32. 10.1111/nep.12579 [DOI] [PubMed] [Google Scholar]
- 29.Chapman AB, Devuyst O, Eckardt K-U, et al. Autosomal-Dominant polycystic kidney disease (ADPKD): Executive summary from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 2015;88:17–27. 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaser BG, Strauss AL, Strutzel E. The discovery of Grounded theory; strategies for qualitative research. Nurs Res 1968;17:364 10.1097/00006199-196807000-00014 [DOI] [Google Scholar]
- 31.Denzin N. Sociological methods. New York: Routledge, 2006. [Google Scholar]
- 32.Kim HJ. Genetic counseling in Korean health care system. J Genet Med 2011;8:89–99. 10.5734/JGM.2011.8.2.89 [DOI] [Google Scholar]
- 33.De Rechter S, Kringen J, Janssens P, et al. Clinicians' attitude towards family planning and timing of diagnosis in autosomal dominant polycystic kidney disease. PLoS One 2017;12:e0185779. 10.1371/journal.pone.0185779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris A. The organization and funding of the treatment of end-stage renal disease in Australia. Int J Health Care Finance Econ 2007;7:113–32. 10.1007/s10754-007-9018-7 [DOI] [PubMed] [Google Scholar]
- 35.Lee HS, Ju Y-S, Song YR, et al. Current treatment status and medical costs for hemodialysis vascular access based on analysis of the Korean health insurance database. Korean J Intern Med 2018;33:1160–8. 10.3904/kjim.2018.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benain J-P, Faller B, Briat C, et al. [Cost of dialysis in France]. Nephrol Ther 2007;3:96–106. 10.1016/j.nephro.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 37.Cornec-Le Gall E, Audrézet M-P, Renaudineau E, et al. PKD2-Related Autosomal Dominant Polycystic Kidney Disease: Prevalence, Clinical Presentation, Mutation Spectrum, and Prognosis. Am J Kidney Dis 2017;70:476–85. 10.1053/j.ajkd.2017.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clayton EW, Evans BJ, Hazel JW, et al. The law of genetic privacy: applications, implications, and limitations. J Law Biosci 2019;6:1–36. 10.1093/jlb/lsz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaumoitre K, Brun M, Cassart M, et al. Differential diagnosis of fetal hyperechogenic cystic kidneys unrelated to renal tract anomalies: a multicenter study. Ultrasound Obstet Gynecol 2006;28:911–7. 10.1002/uog.3856 [DOI] [PubMed] [Google Scholar]
- 40.Lim Q, McGill BC, Quinn VF, et al. Parents' attitudes toward genetic testing of children for health conditions: a systematic review. Clin Genet 2017;92:569–78. 10.1111/cge.12989 [DOI] [PubMed] [Google Scholar]
- 41.Wakefield CE, Hanlon LV, Tucker KM, et al. The psychological impact of genetic information on children: a systematic review. Genet Med 2016;18:755–62. 10.1038/gim.2015.181 [DOI] [PubMed] [Google Scholar]
- 42.Bates GP, Dorsey R, Gusella JF, et al. Huntington disease. Nat Rev Dis Primers 2015;1:15005. 10.1038/nrdp.2015.5 [DOI] [PubMed] [Google Scholar]
- 43.Halvorson CR, Bremmer MS, Jacobs SC. Polycystic kidney disease: inheritance, pathophysiology, prognosis, and treatment. Int J Nephrol Renovasc Dis 2010;3:69–83. 10.2147/ijnrd.s6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagberg A, Bui T-H, Winnberg E. More appreciation of life or regretting the test? experiences of living as a mutation carrier of Huntington's disease. J Genet Couns 2011;20:70–9. 10.1007/s10897-010-9329-6 [DOI] [PubMed] [Google Scholar]
- 45.Sobel SK, Cowan DB. Impact of genetic testing for Huntington disease on the family system. Am J Med Genet 2000;90:49–59. [DOI] [PubMed] [Google Scholar]
- 46.Bowen M. The use of family theory in clinical practice. Compr Psychiatry 1966;7:345–74. 10.1016/S0010-440X(66)80065-2 [DOI] [PubMed] [Google Scholar]
- 47.Young AL, Butow PN, Rhodes P, et al. Talking across generations: family communication about BRCA1 and BRCA2 genetic cancer risk. J Genet Couns 2019;28:516–32. 10.1002/jgc4.1055 [DOI] [PubMed] [Google Scholar]
- 48.Harris T, Sandford R. European ADPKD forum multidisciplinary position statement on autosomal dominant polycystic kidney disease care. European ADPKD forum and Multispecialist roundtable participants. Nephrol Dial Transplant 2017;33:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swift O, Vilar E, Rahman B, et al. Attitudes in patients with autosomal dominant polycystic kidney disease toward prenatal diagnosis and preimplantation genetic diagnosis. Genet Test Mol Biomarkers 2016;20:741–6. 10.1089/gtmb.2016.0050 [DOI] [PubMed] [Google Scholar]
- 50.Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med 2014;371:2267–76. 10.1056/NEJMoa1402686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Study of the efficacy and safety of Tesevatinib in subjects with ADPKD. Available: https://ClinicalTrials.gov/show/NCT03203642
- 52.A medical research study designed to determine if Venglustat can be a future treatment for ADPKD patients. Available: https://ClinicalTrials.gov/show/NCT03523728
- 53.An extended access program for Bardoxolone methyl in patients with CKD (Eagle). Available: https://ClinicalTrials.gov/show/NCT03749447
- 54.Australian Law Reform Commission AHEC Essentially Yours - The Protection of Human Genetic Information in Australia, 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038005supp001.pdf (20.1KB, pdf)