Abstract
Background
There would be over 600 million people living with diabetes by 2040 as predicted by the World Health Organization. Diabetes is characterized by raised blood sugar and insulin resistance. Insulin regulates the influx of glucose into the cell by upregulating the glucose transporter type 4 (GLUT4) expression on the plasma membrane. Besides, PPAR-γ also controls the metabolism of glucose in adipose tissues. Curcuma mangga Val., denoted as C. mangga, is a native Indonesian medicinal plant that has many beneficial effects, including an antidiabetic potential.
Purpose
In this research, we aimed to disclose the hypoglycemic activity of ethanol extract of C. mangga (EECM) in 3T3-L1 fibroblasts-derived adipocyte cells in regulating glucose uptake as confirmed by the GLUT4 and PPAR-γ gene expression.
Methods
The uptake of glucose was determined using radioactive glucose, while the gene expression of GLUT4, PPAR-γ, and β-actin was quantified using mRNA segregation and real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR).
Results
We discovered that EECM interventions (200 and 50 μg/mL) increased glucose uptake in lipid-laden 3T3-L1 cells by 14.75 and 8.86 fold compared to the control non-insulin, respectively (p < 0.05). At the same doses, they also increased GLUT4 mRNA expression by 8.41 and 11.18 fold compared to the control non-insulin, respectively (p < 0.05). In contrast, EECM interventions (200 and 50 μg/mL) showed lower levels of PPAR-γ mRNA expression compared to the control metformin, indicating the anti-adipogenic potentials of EECM.
Conclusion
EECM showed hypoglycemic activity in lipid-laden 3T3-L1 cells by improving glucose ingestion into the cells, which was mediated by increased GLUT4 expression and downregulated PPAR-γ expression.
Keywords: 3T3-L1 adipocytes, Curcuma mangga Val, glucose uptake, GLUT4, PPAR-γ
Introduction
According to the World Health Organization, more than 400 million people were diagnosed with diabetes in 2015, and it was predicted to reach up to 600 million by 2040.1 Diabetes arises as a consequence of inadequate insulin synthesis by the pancreas to regulate the glucose influx into the cells due to the damage of insulin-producing cells, which is mainly categorized as type 1 diabetes. In different cases, however, our body might produce sufficient insulin but unfortunately, our body cannot recognize it and use it properly, which is known as insulin resistance and categorized as type 2 diabetes. As a consequence of both abnormalities, there is a significant increase in the amount of glucose in the bloodstream. The uncurbed glucose level in the bloodstream will damage the blood vessels, nerves, and other organs.2,3
In order to regulate the glucose entrance mechanism into the living cells, insulin activates the glucose transporters (GLUT) in a process called GLUT translocation. There are currently 14 GLUT isoforms and at least one GLUT isoform in each type of cell. However, the type that is mainly regulated by insulin is GLUT4.4,5 Insulin resistance, such as in diabetes, will downregulate the expression of this transporter, causing a reduction in cellular glucose ingestion.5
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) also regulates glucose metabolism.6 This transcription factor is predominantly found within adipocytes, endothelial cells, and vascular smooth muscle cells as it acts as sensors with various compounds. PPAR-γ controls the expression of heterogeneous genes, including those related to cell transformation, lipid deposition, and the glucose transfiguration or, more specifically, the insulin response.1,7
Agricultural products, including fruits and vegetables, as well as herb crops have many potentials for human healths, such as a source of natural antioxidants.8,9 In particular, many members of the Zingiberaceae family, such as Curcuma mangga Val. (C. mangga), have been researched as an alternative treatment for diabetes.10 C. mangga is locally recognized as temu mangga, kunyit putih, or kunir putih. It is an indigenous plant from Java, which has antiallergic, antioxidative, antiproliferative, antitumor, antimicrobial, analgesic, lipid peroxidative, and cytotoxic properties.11,12 According to Pujimulyani et al,13 EECM contains important phytochemical compounds, including gallic acid, catechin, epicatechin, epigallocatechin, epigallocatechin gallate, and gallocatechin gallate of roughly 0.124 × 10−3, 0.134 × 10−3, 0.442 × 10−3, 0.113 × 10−3, 0.037 × 10−3, and 0.159 × 10−3 g/g dried extract, respectively.
The essential oils of C. mangga also have antibacterial potencies toward Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Bacillus cereus, and has antimycotic activities toward Cryptococcus neoformans and Candida albicans.12 However, its antidiabetic potentials are mostly undiscovered. Therefore, in this experiment, we examined whether EECM can improve glucose uptake in lipid-laden 3T3-L1 cells through modulation of GLUT4 and PPAR-γ mRNA expression.
Materials and Methods
White Saffron Rhizomes and Extraction Methods
C. mangga rhizomes were freshly harvested from Bantul, Yogyakarta in October 2017. The rhizomes were harvested after 10 months of growth, as indicated by the natural fall of plants or leaves. At this time, the rhizomes usually have bright yellow flesh and smell like mangoes. The structure of land for planting was clay, where manure was given once in the third month of plant growth. After cleaning, drying, and crushing, the fine powder was extracted by soaking 250 g of fine powder in 1.5 L of 70% distilled ethanol (most of the substantial compounds of C. mangga are soluble in ethanol). Then, the solution was filtered every 24 h until 3 days to gain colorless filtrate. Thereafter, the filtrate was vaporized and stored at −20 °C prior to use.10
Cell Culture, Differentiation, and Treatment
The pre-adipocyte form of 3T3-L1 cell lines (purchased commercially from American Type Culture Collection, Manassas, USA; Catalogue #CL173) was subjected to cell culture in DMEM (Gibco, 11995065) supplemented with 10% of FBS (Gibco, 26140079) and 1% of antibiotic and antimycotic (ABAM) (Gibco, 15240–062) and maintained in a conditioned cell incubator (37 °C, 5% CO2). In order to induce cell differentiation of the pre-adipocyte cells into 3T3-L1 fibroblasts-derived adipocytes, 1 × 106 3T3-L1 cells were seeded in T25 flasks and incubated for 24 h in the conditioned incubator. Then, cells were differentiated using the adipogenesis kit from Merck (ECM950). Every 48 h, the medium was renewed until the cells were fully differentiated on day 8 post-induction (the cells began to differentiate on day 4 post-induction). The differentiated cells were confirmed using Oil Red-O (lipid maculation).14,15 The differentiated cells were treated with EECM (200 and 50 μg/mL), curcumol (Chengdu, 15012104) (200 and 50 μg/mL), metformin (Hexpharm) (100 µM), and insulin (Merck Ecm950FR) (1 µM).10 Cells treated with insulin was set as the positive control, while cells treated with PBS was set as the negative control. Control metformin was set for comparison to EECM and curcumin because metformin is a commonly used drug to treat type-2 diabetes, while curcumol was used because it showed good lipid removal in our previous study.
Glucose Uptake Assay
In order to measure glucose ingestion in 3T3-L1 fibroblasts-derived adipocytes treated with EECM, curcumol, metformin, and insulin, the radioactive 2-deoxyglucose (10 μM 2 and 2,5 μCi 2-deoxy-D-(3H)-glucose) was used (Abcam, ab136955). The primers used in this process were Krebs, Ringer, Phosphate, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). Then, the amount of radioactive glucose entering the cells was measured using Multiskan™ GO Microplate Spectrophotometer at a 412-nm wavelength.15 To quantify the glucose uptake, the 2-deoxyglucose (2-DG) concentration of samples, which is comparable to concentrated 2-DG-6-phosphate (2-DG6P), was calculated using equation (1):
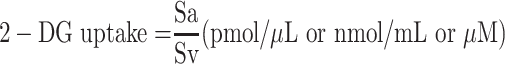 |
(1) |
where Sa is the amount of 2-DG6P (in pmol) in the sample well calculated from the standard curve and Sv is sample volume (in μL) added into the sample well.
mRNA Isolation and Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
mRNAs were segregated from lipid-laden 3T3-L1 cells using the Aurum Total RNA mini kit (Biorad, 7326820). Then, the mRNAs were reversed into cDNAs using iScript reverse transcriptase (Biorad, 1708840). Moreover, RT-qPCR using SsoFast Evergreen Supermix (Biorad, 1725200) was performed according to the manufacturers’ directions, in which the pre-incubation cycle was set at 95 °C for 30 sec followed by 40 cycles of denaturation at 95 °C for 30 sec, annealing at 59 °C for 20 sec, and extension at 72 °C for 10 sec. The sequences of primers used in this study were as follows: 5´GAGCCTGAATGCTAATGGAG3´ (GLUT4 forward), 5´GAGAGAGAGCGTCCAATGTC3´ (GLUT4 reverse), 5´TTATCAAGGGTCCCAGTTTC3´ (PPAR-γ forward), 5´TTATTCATCAGGGAGGCCAG3´ (PPAR-γ reverse), 5´TCTGGCACCACACCTTCTACAATG3´ (β-actin forward), and 5´AGCACAGCCTGGATAGCAACG3´ (β-actin reverse). The interpretation of RT-qPCR was done by the real-time PCR system (Pikoreal 96, Thermo Scientific, TCR0096).15
Statistical Analysis
IBM SPSS Statistics 20 software was used to statistically analyze the data with the interpretation based on One-way Analysis of Variance (ANOVA) and Duncan’s Multiple Range Test (DMRT) with a 95% confidence interval (p < 0.05). Then, the results were displayed as mean ± standard deviation (SD) (n= 3).14,15
Results
Cell Culture and Differentiation
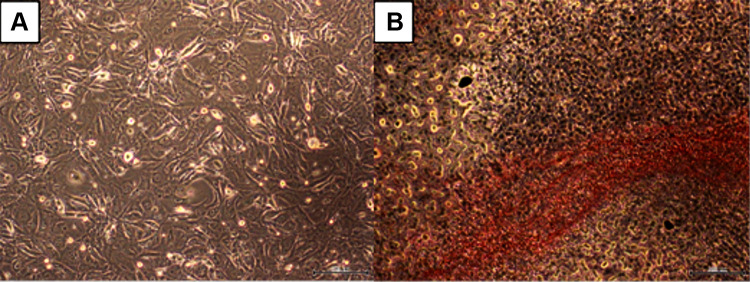
In vitro model of adipocyte-like cells was established from pre-adipocyte cells (3T3-L1) treated with adipogenesis kit (Merck, ECM950). This treatment could trigger cell transformation from fibroblast-like cells into adipocyte-like cells. The pre-adipocyte cells changed their morphology between 4 days and 8 days. The initial morphology of the pre-adipocytes was fibroblast-like cells (Figure 1A), and they changed into adipocyte-like cells within 8 days as confirmed by the intracellular lipid droplet as stained with Oil Red-O (Figure 1B).
Figure 1.
The morphological changes of 3T3-L1 cells. (A) 3T3-L1 pre-adipocytes. (B) 3T3-L1 fibroblasts-derived adipocytes as validated by the Oil Red-O staining. Magnification: 40X, scale bars: 200 µm.
Glucose Uptake Assay
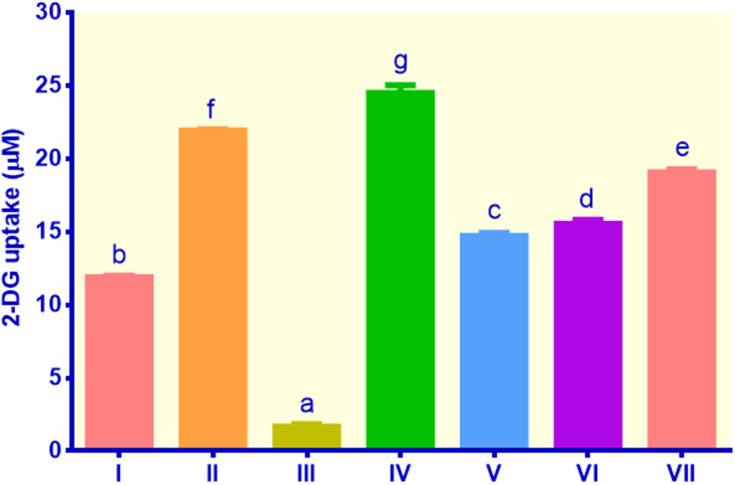
As shown in Figure 2, compared to control non-insulin, all treatments exhibited remarkable increases in glucose uptake (p < 0.05). The highest uptake was found in cells treated with EECM (200 μg/mL), followed by control metformin, curcumol (50 μg/mL), curcumol (200 μg/mL), EECM (50 μg/mL), and control insulin, indicating glucose uptake of 24.49, 21.93, 19.06, 15.54, 14.71, and 11.89 μM, respectively. EECM interventions (200 and 50 μg/mL) could increase glucose uptake in lipid-laden 3T3-L1 cells by 14.75 and 8.86 fold compared to the control non-insulin, respectively (p < 0.05). Moreover, the control metformin as the type-2 diabetes drug showed a relatively high glucose uptake. It could elevate glucose uptake in lipid-laden 3T3-L1 cells by 13.21 fold compared to the control non-insulin. Meanwhile, curcumol interventions (200 and 50 μg/mL) could improve glucose ingestion in lipid-laden 3T3-L1 cells by 9.35 and 11.48 fold, indicating lower glucose uptake than EECM (200 μg/mL), but higher than EECM (50 μg/mL).
Figure 2.
Glucose uptake in lipid-laden 3T3-L1 cells treated with different treatments: (I) control insulin, (II) control metformin, (III) control non-insulin, (IV) EECM 200 μg/mL, (V) EECM 50 μg/mL, (VI) curcumol 200 μg/mL, and (VII) curcumol 50 μg/mL. Data are presented as mean ± SD. Different symbols (a, b, c, d, e, f, and g) display a significant difference (p < 0.05) among treatments. ANOVA and DMRT were used for data interpretation (n= 3).
GLUT4 Gene Expression
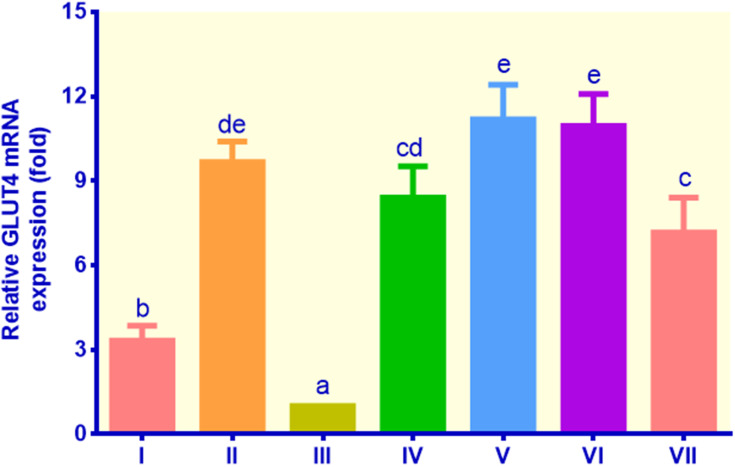
The GLUT4 gene expression of all treatments was significantly higher than that of the control non-insulin (Figure 3). The adipocyte cells treated with insulin, metformin, EECM, and curcumol showed relatively higher GLUT4 mRNA expression ranging from 3.32 to 11.18 fold compared to the control non-insulin, which was only 1.00 fold. EECM interventions (200 and 50 μg/mL) increased the mRNA expression of GLUT4 by 8.41 and 11.18 fold compared to the control non-insulin, respectively (p < 0.05). Moreover, the control metformin indicated a slightly higher GLUT4 gene expression than EECM (200 μg/mL), but lower than EECM (50 μg/mL). Meanwhile, curcumol intervention was not significantly different with EECM at the same dose (200 μg/mL) in improving GLUT4 gene expression in lipid-laden 3T3-L1 cells, but at the lower dose (50 μg/mL), curcumol was less potent than EECM.
Figure 3.
The expression of GLUT4 in lipid-laden 3T3-L1 cells treated with different treatments: (I) control insulin, (II) control metformin, (III) control non-insulin, (IV) EECM 200 μg/mL, (V) EECM 50 μg/mL, (VI) curcumol 200 μg/mL, and (VII) curcumol 50 μg/mL. Data are presented as mean ± SD. Different symbols (a, b, cd, de, and e) display a significant difference (p < 0.05) among treatments. ANOVA and DMRT were used for data interpretation (n= 3).
PPAR-γ Gene Expression
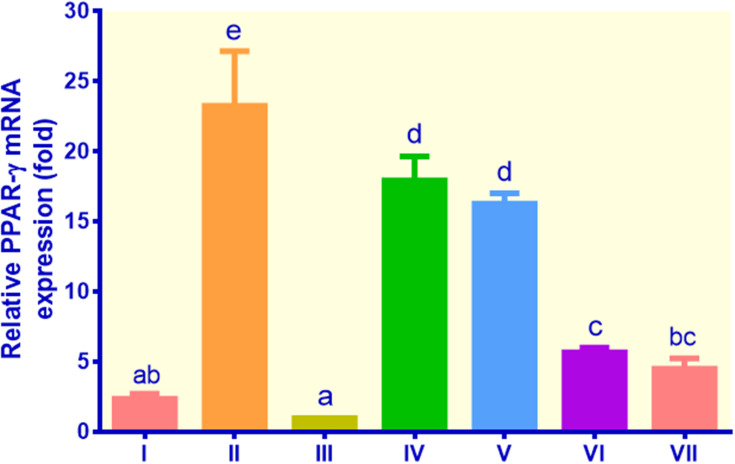
PPAR-γ gene expression was the highest in the control metformin, which was around 23.26 fold, while the figures for EECM (200 μg/mL), EECM (50 μg/mL), curcumol (200 μg/mL), and curcumol (50 μg/mL) were 7.93, 16.27, 5.69, and 4.52 fold, respectively (Figure 4). The PPAR-γ gene expression of the control insulin and non-insulin was slightly different. Although EECM was more potent than metformin in suppressing PPAR-γ gene expression, it was less potent than curcumol. Curcumol could downregulate PPAR-γ mRNA expression by about 4 times compared to the control metformin.
Figure 4.
The expression of PPAR-γ in lipid-laden 3T3-L1 cells treated with different treatments: (I) control insulin, (II) control metformin, (III) control non-insulin, (IV) EECM 200 μg/mL, (V) EECM 50 μg/mL, (VI) curcumol 200 μg/mL, and (VII) curcumol 50 μg/mL. Data are presented as mean ± SD. Different symbols (a, ab, bc, c, d, and e) display a significant difference (p < 0.05) among treatments. ANOVA and DMRT were used for data interpretation (n= 3).
Discussions
Insulin has many functions in the cells, but it has a major function as a glucose uptake regulator in the adipose tissue. This hormone binds to its receptors at the surface of the fat cells and triggers the process called GLUT4 translocation, where the GLUT4 vesicles eject their contents and move GLUT4 to the outermost membrane for protein activation.4,5 It has been proven that, in obese transgenic mice, the overexpression of GLUT4 increases insulin sensitivity, and the lack of GLUT4 expression will cause insulin resistance. In obesity, there is a reduction of the expression of this gene, thus causing insulin resistance. This might be caused by increased oxidative stress and inflammatory cytokines release.16,17 According to Pujimulyani et al,10 C. mangga fractions have nitric oxide and H2O2 scavenging properties. In our previous study, oral administration of pressure-blanched C. mangga in oxidized lipid-treated wistar rats could improve antioxidative properties and lipid profiles in vivo.18 In another research, C. mangga extract was capable of preventing further damages to pancreatic β-cells (insulin-producing cells) caused by alloxan in mice.19 Therefore, in this experiment, we attempted to discover the antidiabetic potentials of C. mangga extract in 3T3-L1 fibroblasts-derived adipocytes in regulating glucose uptake, as confirmed by the expression of GLUT4, which is the glucose transporter that mediates insulin-dependent glucose ingestion and PPAR-γ genes that modulate glucose and lipid metabolism.
Figure 2 shows the profile for glucose uptake in lipid-laden 3T3-L1 cells without and with treatments of EECM, curcumol, and metformin compared to the control groups. In our previous study, EECM showed good biocompatibility because it showed low cytotoxicity in 3T3-L1 adipocytes in all selected concentrations (6.25, 12.5, 25, 50, 100, and 200 μg/mL) with cell viability above 90%. In addition, EECM, curcumol and metformin could reduce the lipid droplet formation in lipid-laden 3T3-L1 cells.20 The biological or pharmacological activity of the studied EECM on cells might be attributed to the multi substance’s active ingredients contained in the extract, including curcumin, gallic acid, catechin, epicatechin, epigallocatechin, epigallocatechin gallate, and gallocatechin gallate.
In the present study, EECM (200 μg/mL) was the most effective treatment among others to improve the glucose uptake in 3T3-L1 fibroblasts-derived adipocytes. This result was linear with a preceding investigation that examined the ability of curcumin, one of the primary compounds in C. mangga, in escalating the glucose ingestion in skeletal muscle as intervened by the increased GLUT4 expression through the PLC-PI3K pathway and in recovering the insulin resistance in muscular tissue through the LKB1-AMPK pathway.21 A previous investigation by Virtanen et al22 revealed that glucose ingestion in adipocytes might increase or remain stable upon metformin intervention. This regulation might be due to the phosphorylation by adenosine monophosphate-activated protein kinase (AMPK) of critical biological catalysts as well as inscription factors that modulate gene expression.23 Consequently, the synthesis of protein, lipid, and glucose was impeded, while glucose ingestion and fatty acid oxidation were induced.24 A previous study found that the translocation of GLUT4 and GLUT8 in the atria cells of healthy mice and type-1 diabetic mice was suppressed by 70% and 90%, respectively.25 However, after insulin stimulation, the expressions of GLUT4 and GLUT8 were upregulated, followed by the increase of glucose uptake.25 Therefore, high GLUT4 expression would increase the uptake of glucose into cells.17,25
In this experiment, we found that treatments with EECM and curcumol could significantly improve the GLUT4 expression compared to the control non-insulin. The result was similar to the control metformin, which acts as a type-2 diabetes drug. However, the control insulin showed lower levels of GLUT4 expression compared to the control metformin, EECM, and curcumol. According to Cheng et al,26 the insulin receptor tyrosine kinase mediates the phosphorylation of insulin receptor substrate 1 (IRS-1), which causes the displacement of the GLUT4 molecules to the outermost layer of insulin-sensitive tissues, such as in smooth muscle cells and adipocytes. Furthermore, curcumin could efficaciously mitigate the IRS-1 phosphorylation on Ser307 and improve the Akt phosphorylation in skeletal muscles.27 Consequently, an increase in glucose ingestion from the blood into these tissues was observed.28 In another study, nevertheless, methanol extract from C. longa root affected the incomplete prohibition of lipid synthesis in 3T3-L1 fibroblasts-derived adipocytes as confirmed by the reduced GLUT4 expression and lipolysis initiation by the hormone-sensitive lipase (HSL) that restricts the lipolysis process.29
As one of the genes regulating glucose and lipid metabolism, PPAR-γ becomes one of the primary targets for antidiabetic drugs. The activation of this receptor will alter its ligand-binding domain, causing the activation of other target genes in glucose and lipid metabolism.30 PPAR-γ has two main isoforms, PPAR-γ1 and PPAR-γ2. The former is generally found in the intestines, macrophages, β-cells, muscles, livers, and adipose tissues, while the latter is found only in adipose tissue. However, lipid deposition will cause obesity appearing in the liver and skeletal muscles.1 Both isoforms are responsible for controlling glucose and lipid metabolism.1,30 Therefore, any defect would lead to insulin resistance. Besides, the PPAR-γ activation by its agonists will ameliorate insulin sensitivity.1,30 In this experiment, we discovered that exposing lipid-laden 3T3-L1 adipocytes to EECM and curcumol led to a decrease of the relative PPAR-γ mRNA expression, compared to the control metformin. Metformin has been observed not to regulate the expression of adenosine triphosphate (ATP) citrate lyases, which are the primary regulator of cholesterol and fatty acid synthesis.31 These results were similar to the investigation by Kim et al,32 demonstrating that lipid-laden 3T3-L1 adipocytes treated with kahweol had a significant reduction in the PPAR-γ mRNA levels and adipogenic marker genes such as adiponectin, mediating lipid efflux. Another study also revealed that dietary supplementation of curcumin could suppress the PPAR-γ and C/EBPa gene expression in vivo.33
Conclusion
In conclusion, we discovered that EECM treatment could improve the GLUT4 mRNA expression and subsequently increase the glucose ingestion in 3T3-L1 fibroblasts-derived adipocytes but downregulated the PPAR-γ mRNA expression. EECM intervention with a dose of 200 μg/mL has a remarkable potential for further in vivo study via oral administration. However, in order to improve its bioavailability, EECM might need to be further prepared through nanoformulation for intravenous injection in mice models. The hypoglycemic activity of EECM through glucose and lipid metabolism might have positive effects on reducing the potency of insulin resistance, which is one of the main factors affecting glucose intolerance and type 2 diabetes.
Acknowledgments
This study was supported by the Grants-in-Aid from Excellence Higher Education Fundamental Research [111/SP2H/LT/DPRM/2019], the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia. We gratefully acknowledge the support from University of Mercu Buana Yogyakarta, Yogyakarta, Indonesia, and Biomolecular and Biomedical Research Center, Bandung, Indonesia.
Disclosure
Hanna Sari Widya Kusuma reports a patent for “white saffron extract and its fractions as antidiabetic agents”, P00201707284, issued. The authors report no other potential conflicts of interest for this work.
References
- 1.Global report on diabetes: executive summary [homepage on the Internet]. Geneva: World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/204874. Accessed April22, 2020. [Google Scholar]
- 2.Salsali A, Nathan M. A review of types 1 and 2 diabetes mellitus and their treatment with insulin. Am J Ther. 2006;13(4):349–361. doi: 10.1097/00045391-200607000-00012 [DOI] [PubMed] [Google Scholar]
- 3.Corrales P, Vidal-Puig A, Medina-Gómez G. PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int J Mol Sci. 2018;19(7):2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokarz VL, MacDonald PE, Klip A. The cell biology of systemic insulin function. J Cell Biol. 2018;217(7):2273–2289. doi: 10.1083/jcb.201802095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayem A, Arya A, Karimian H, Krishnasamy N, Ashok Hasamnis A, Hossain C. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules. 2018;23(2):258. doi: 10.3390/molecules23020258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma AM, Staels B. Peroxisome proliferator-activated receptor γ and adipose tissue–understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92(2):386–395. [DOI] [PubMed] [Google Scholar]
- 7.Adedapo A, Asunloye O, Adeoye B. Peroxisome proliferator-activated receptor-review. Int J Biochem Res Rev. 2018;21:1–19. doi: 10.9734/IJBCRR/2018/40221 [DOI] [Google Scholar]
- 8.Thirugnanasambandham K, Sivakumar V. Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. J Saudi Soc Agric Sci. 2017;16(1):41–48. [Google Scholar]
- 9.Aoussar N, Rhallabi N, Mhand RA, et al. Seasonal variation of antioxidant activity and phenolic content of Pseudevernia furfuracea, Evernia prunastri and Ramalina farinacea from Morocco. J Saudi Soc Agric Sci. 2020;19(1):1–6. [Google Scholar]
- 10.Pujimulyani D, Yulianto WA, Setyowati A, Arumwardana S, Rizal R. Antidiabetic and antioxidant potential of Curcuma mangga Val. extract and fractions. Asian J Agric Biol. 2018;6:162–168. [Google Scholar]
- 11.Rajkumari S, Sanatombi K. Nutritional value, phytochemical composition, and biological activities of edible Curcuma species: a review. Int J Food Prop. 2017;20(3):S2668–S2687. [Google Scholar]
- 12.Dosoky N, Setzer W. Chemical composition and biological activities of essential oils of curcuma species. Nutrients. 2018;10(9):1196. doi: 10.3390/nu10091196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pujimulyani D, Raharjo S, Marsono Y, Santoso U. The phenolic substances and antioxidant activity of white saffron (Curcuma mangga Val.) as affected by blanching methods. World Acad Sci Eng Technol. 2013;7(10):947–950. [Google Scholar]
- 14.Widowati W, Afifah E, Mozef T, et al. Effects of insulin-like growth factor-induced Wharton jelly mesenchymal stem cells toward chondrogenesis in an osteoarthritis model. Iran J Basic Med Sci. 2018;21(7):745–752. doi: 10.22038/IJBMS.2018.28205.6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidayat M, Prahastuti S, Fauziah N, Maesaroh M, Balqis B, Widowati W. Modulation of adipogenesis-related gene expression by ethanol extracts of Detam 1 soybean and Jati belanda leaf in 3T3-L1 cells. Bangladesh J Pharmacol. 2016;11(3):697–702. doi: 10.3329/bjp.v11i3.26471 [DOI] [Google Scholar]
- 16.Leguisamo NM, Lehnen AM, Machado UF, et al. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc Diabetol. 2012;11:100. doi: 10.1186/1475-2840-11-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran V, Saravanan R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum Exp Toxicol. 2015;34(9):884–893. doi: 10.1177/0960327114561663 [DOI] [PubMed] [Google Scholar]
- 18.Pujimulyani D, Santoso U, Luwihana DS, Maruf A. Orally administered pressure-blanched white saffron (Curcuma mangga Val.) improves antioxidative properties and lipid profiles in vivo. Heliyon. 2020;6(6):e04219. doi: 10.1016/j.heliyon.2020.e04219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrikos R, Marusin N, Tjong DH. The effect of mango ginger (Curcuma mangga Val.) rhizome ethanolic extract on the histology of β cell pancreas of alloxan-induce mice. J Biol Univ Andalas. 2014;3(4):317–323. [Google Scholar]
- 20.Pujimulyani D, Yulianto WA, Setyowati A, et al. Curcuma mangga Val. extract as antidiabetic agent in 3T3-L1 adipocyte cells. Mol Cell Biomed Sci. 2020;4(1):45–51. doi: 10.21705/mcbs.v4i1.88 [DOI] [Google Scholar]
- 21.Na L-X, Zhang Y-LY-L, Li Y, et al. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. 2011;21(7):526–533. doi: 10.1016/j.numecd.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 22.Virtanen KA, Hällsten K, Parkkola R, et al. Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects. Diabetes. 2003;52(2):283–290. doi: 10.2337/diabetes.52.2.283 [DOI] [PubMed] [Google Scholar]
- 23.Viollet B, Athea Y, Mounier R, et al. AMPK: lessons from transgenic and knockout animals. Front Biosci (Landmark Ed). 2009;14:19–44. doi: 10.2741/3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012;122(6):253–270. doi: 10.1042/CS20110386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maria Z, Campolo AR, Lacombe VA. Diabetes alters the expression and translocation of the insulin-sensitive glucose Transporters 4 and 8 in the Atria. PLoS One. 2015;10(12):e0146033. doi: 10.1371/journal.pone.0146033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng KK, Iglesias MA, Lam KS, et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9(5):417–427. doi: 10.1016/j.cmet.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 27.Zhang DW, Fu M, Gao SH, Liu JL. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013:636053. doi: 10.1155/2013/636053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maradana MR, Thomas R, O’Sullivan BJ. Targeted delivery of curcumin for treating type 2 diabetes. Mol Nutr Food Res. 2013;57(9):1550–1556. doi: 10.1002/mnfr.201200791 [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Jun W. Methanolic extract of turmeric (Curcuma longa L.) enhanced the lipolysis by up-regulation of lipase mRNA expression in differentiated 3T3-L1 adipocytes. Food Sci Biotechnol. 2009;18(6):1500–1504. [Google Scholar]
- 30.Porskjær Christensen L, Bahij El-Houri R. Development of an in vitro screening platform for the identification of partial PPARγ agonists as a source for antidiabetic lead compounds. Molecules. 2018;23(10):2431. doi: 10.3390/molecules23102431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tebbe C, Chhina J, Dar SA, et al. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget. 2014;5(13):4746–4764. doi: 10.18632/oncotarget.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JS, Lee SG, Kang YJ, Kwon TK, Nam JO. Kahweol inhibits adipogenesis of 3T3-L1 adipocytes through downregulation of PPARγ. Nat Prod Res. 2018;32(10):1216–1219. doi: 10.1080/14786419.2017.1326039 [DOI] [PubMed] [Google Scholar]
- 33.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139(5):919–925. doi: 10.3945/jn.108.100966 [DOI] [PubMed] [Google Scholar]