INTRODUCTION:
Family planning options, including hormonal contraception, are crucial for reproductive-aged women living with HIV or at high-risk for HIV acquisition. Hormonal contraception is highly effective against unintended pregnancy and is particularly important in HIV-infected women to avoid mother-to-child transmission.1 However, concurrent use of antiretroviral (ARV) drugs and hormonal contraceptives has resulted in unexpected drug-drug interactions (DDIs), which may result in decreased efficacy of antiretroviral treatment (ART) or ARV-drug based pre-exposure prophylaxis (PrEP).2 For example, oral contraceptives containing ethinyl estradiol/desogestrel have been found to reduce efavirenz serum concentrations by 18%.3 Additionally, in transgender women, medroxyprogesterone acetate as well as estrogen with or without anti-androgen activity have been associated with reduced tenofovir intracellular and plasma levels, respectively.4–6
Cabotegravir is a novel integrase strand-transfer inhibitor currently in development for use in HIV prevention and treatment. Cabotegravir formulations include a daily oral tablet and a long-acting intramuscular formulation currently being studied as an injection administered every one or two months.7 Long-acting injectable cabotegravir (CAB-LA) is formulated with milled cabotegravir nanocrystals suspended in mannitol, with the rate of drug absorption determined by dissolution of cabotegravir particles into the interstitial fluid.8 Cabotegravir is predominantly excreted in the feces as unchanged drug (58%) and in urine following metabolization by uridine diphosphate glucuronosyltranferase (UGT) 1A1 and UGT1A9 (26.8%).9 Cabotegravir is considered to be low-risk for causing clinically significant DDIs overall, since in vitro and clinical studies have not shown effects on major UGT or cytochrome P450 (CYP) metabolic pathways.10,11 However, the effect of hormonal contraceptives on cabotegravir pharmacokinetics (particularly with the long-acting injectable formulation of cabotegravir) has not been investigated. Given the widespread use of hormonal contraceptive agents, understanding potential DDIs between exogenous sex hormones and ART is critical to understanding the safety and efficacy of novel treatment and prevention regimens in cisgender women and in transgender women receiving gender-affirming hormonal therapy. To address this research gap, we performed a subset analysis of the HIV Prevention Trials Network 077 (HPTN 077) study to evaluate the association between hormonal contraceptive use and CAB-LA pharmacokinetic (PK) parameters.
METHODS:
Study Population and Design
This is a secondary analysis of cisgender women who were enrolled in HPTN 077, a randomized, double-blind, placebo-controlled, phase 2a trial that evaluated safety, tolerability, and pharmacokinetics of CAB-LA at two doses and intervals (clinicaltrials.gov NCT02178800). Detailed methods and results from the trial have been published.7 Briefly, between February 2015 and May 2016, 199 HIV-uninfected, low-risk individuals aged 18-65 years from Malawi, Brazil, South Africa, and the United States were enrolled in two sequential cohorts with different dosing regimens and randomly assigned 3:1 to receive CAB-LA or placebo. Women who were pregnant, breastfeeding, or intending to become pregnant were ineligible, and women of reproductive potential were required to use an effective contraceptive method for the duration of the study; 129 cisgender women were enrolled in the parent study.
All participants received an oral version of their assigned study product (CAB-LA or placebo) for 4 weeks before injections (30 mg oral cabotegravir daily for those randomized to the cabotegravir arm). Cohort 1 then received CAB-LA 800 mg intramuscularly (IM) every 12 weeks for 3 injections, administered as 2 x 2 mL gluteal injections. Cohort 2 received CAB-LA 600 mg IM, administered as a single 3 mL gluteal injection, with the first two injections separated by 4 weeks and the remainder separated by 8 weeks, for a total of 5 injections. Clinical safety, type of hormonal contraceptive used, and laboratory assessments occurred at injection visits and at weeks 6, 9, 13, 18, 23, 30, 35, and 41 (Cohort 1) or 6, 10, 13, 18, 21, 26, 29, 34, 37, and 41 (Cohort 2). Laboratory assessments included HIV testing and plasma cabotegravir levels. The primary study endpoint was at week 41; participants received quarterly follow-up visits for 52-76 weeks after final injection (detailed schedule of study assessments can be found in Landovitz et al.).7 Plasma cabotegravir concentrations were measured using a validated liquid chromatography-mass spectrometric (LC-MS) assay with a lower limit of quantification [LLOQ] of 25 ng/mL. Peak concentration [Cmax], trough concentration [Cτ], and exposure between injections, measured as area under the concentration time curve [AUC0-τ], were estimated for each injection.7 Apparent terminal half-life [T1/2app] and time to LLOQ after the last injection were estimated and included in the analysis.12 All participants provided written informed consent and the institutional review board or ethics committee at each participating site approved the study protocol.
Statistical Analysis
Statistical analysis was limited to cisgender women who were randomized to receive CAB-LA and received at least one injection (n=85). We evaluated the association between self-reported hormonal contraceptive use and CAB LA PK parameters. Self-reported hormonal contraceptive use at each injection visit was categorized by contraceptive type (oral, injectable, implants, other). Of note, as all women who indicated that they were not of reproductive potential reported hormonal contraceptive use (n=12), they were included as a separate variable in our analysis. Generalized estimating equations (GEE) were used to account for correlation of PK parameters and changes in hormonal contraceptive type within individual women across injections and to assess the effect of hormonal contraceptive use on Cmax, Cτ, and AUC0-τ. Linear regression was used to assess the effect of hormonal contraceptive use reported at the last injection visit on T1/2app and time to LLOQ after the last injection. Since body mass index (BMI) was significantly associated with PK parameters,7,12 BMI and CAB-LA dose cohorts as covariates were adjusted in all analyses. All PK parameters were log-transformed before analyses. Geometric mean ratios (GMR), i.e., exp(beta) where beta is the estimate of the effect of hormonal contraceptive use (vs. not on any hormonal contraception) on the PK parameter, 90% confidence intervals, and p-values were reported for all analyses. GMRs with 90% confidence intervals were used based on regulatory guidance for clinical evaluation of DDIs.13 All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS:
Of the 85 cisgender women included in the analysis, median age was 28 years (IQR 23-36), median BMI was 27 (22-35), and 51.8% of women were from Sub-Saharan Africa (Table 1). Hormonal contraceptive use was reported by 79 women; the remaining six women reported no hormonal contraception. Of the women who reported using hormonal contraception, the following types of contraceptives were reported at baseline visit: 35.4% (n=28) injectable, 22.8% (n=18) oral, 12.7% (n=10) implantable, 13.9% (n=11) other; 15.2% (n=12) of the women were not of reproductive potential, but still reported hormonal contraceptive use (hormonal contraception type by injection visit and cohort is shown in Supplementary Figure 1).
Table 1:
Demographics and baseline contraceptive use of cisgender women randomized to receive CAB-LA and received at least one injection in HIV Prevention Trials Network study 077 (N=85)
| Cohort 1 (n=46) | Cohort 2 (n=39) | Overall (n=85) | |
|---|---|---|---|
| Age+ | 26 (23-35) | 31(23-38) | 28 (23-36) |
| BMI+ | 28 (24-35) | 26 (22-35) | 27 (22-35) |
| Race/Ethnicity | |||
| Non-Hispanic black | 24 (52.2%) | 20 (51.3%) | 44 (51.8%) |
| Non-Hispanic white | 12 (26.1%) | 4 (10.3%) | 16 (18.8%) |
| Hispanic/Latino | 9 (19.6%) | 12 (30.8%) | 21 (24.7%) |
| Asian | 0 (0.0%) | 1 (2.6%) | 1 (1.2%) |
| Mixed/Other | 1 (2.2%) | 2 (5.1%) | 3 (3.5%) |
| Region | |||
| United States | 13 (28.3%) | 13 (33.3%) | 26 (30.6%) |
| Sub-Saharan Africa | 26 (56.5%) | 18 (46.2%) | 44 (51.8%) |
| Brazil | 7 (15.2%) | 8 (20.5%) | 15 (17.6%) |
| Contraceptive Type | |||
| None | 2 (4.3%) | 4 (10.3%) | 6 (7.1%) |
| Not of reproductive potential | 5 (10.9%) | 7 (17.9%) | 12 (14.1%) |
| Oral contraceptives | 12 (26.1%) | 6 (15.4%) | 18 (21.2%) |
| Injectable | 12 (26.1%) | 16 (41.0%) | 28 (32.9%) |
| Implants∫ | 6 (13.0%) | 4 (10.3%) | 10 (11.8%) |
| Other∫∫ | 9 (19.6%) | 2 (5.1%) | 11 (12.9%) |
Median(IQR)
Implantable = intradermal implant
Other = vaginal ring, intrauterine device
Oral contraceptive use was associated with lower Cmax when compared to women not on any hormonal contraception (GMR 0.75; 90% CI 0.59-0.93; p=0.033) after adjusting for BMI and cohort. However, oral contraceptive use did not result in significant differences in other CAB-LA PK parameters relative to women not on any hormonal contraception, including Cτ (GMR 0.91; 90% CI 0.72-1.15; p=0.51), AUC0-τ (0.81; 0.65-1.01; p=0.12), T1/2app (0.84; 0.53-1.35; p=0.56), and time to LLOQ (0.96; 0.65-1.40; p=0.85). Compared to women not on hormonal contraception, no statistically significant difference in any cabotegravir PK parameter was observed between women who used injectable, implantable, or other types of hormonal contraceptives. Similarly, women not of reproductive potential had no differences in cabotegravir PK parameters when compared to women not on hormonal contraceptives. When evaluated in aggregate (any use vs. not), self-report of any type of hormonal contraception did not result in significant differences in GMRs for any CAB-LA PK parameter, compared to women not on any hormonal contraceptives (data not shown). Results of GMRs stratified by contraceptive type are shown in Figure 1.
Figure 1: The effect of hormonal contraceptive use on long-acting injectable cabotegravir pharmacokinetic (PK) parameters (Cmax, Cτ, AUC0-τ, T1/2app, LLOQ) compared to women not on hormonal contraception, controlling for body mass index and cohort, among cisgender women in HIV Prevention Trials Network study 077.
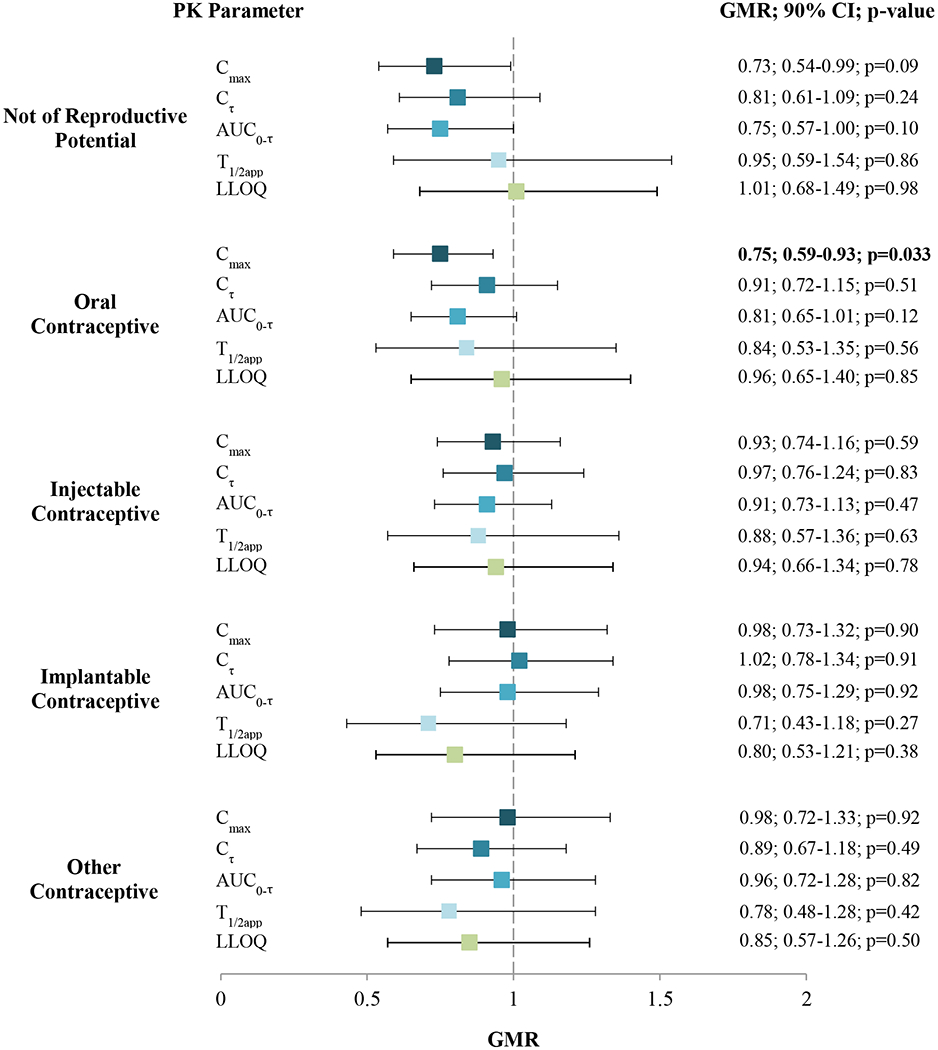
Cmax = peak concentration; Cτ = trough; AUC0-τ = exposure between injections; T1/2app = apparent terminal half-life after last injection; LLOQ = time to lower limit of quantitation; GMR = geometric mean ratio (ref: no hormonal contraception)
Note: bold indicates p ≤ 0.05
DISCUSSION:
In this secondary analysis of HIV-uninfected, cisgender women participating in HPTN 077, use of oral hormonal contraceptives was significantly associated with lower peak CAB concentrations when compared to women not on hormonal contraception. However, oral contraceptive use was not associated with differences in GMRs for other cabotegravir PK parameters (Cτ, AUC0-τ, T1/2app, time to LLOQ). No additional types of hormonal contraceptives (injectable, implantable, or other) were associated with differences in the PK of CAB-LA during serial injections or during the “pharmacokinetic tail”. Given that ARV drugs and hormonal contraceptives are frequently taken concomitantly by women at-risk for or living with HIV, these data highlight the need for further research exploring potential DDIs between CAB-LA and hormonal contraceptives. However, it is important to note that differences in time to LLOQ were not observed with oral contraceptive use, supporting that these differences observed in Cmax are unlikely to be clinically important. Furthermore, significant differences were not observed with oral contraceptive use and Cτ and AUC0-τ, which appear to be the most important PK determinants of virologic control.14
The finding of a decreased Cmax for women taking oral contraceptives was unexpected, as DDIs between ARV drugs and hormonal contraceptives are largely due to alteration in expression of CYP 450 enzymes, suggesting that hormonal contraceptives would not cause significant interactions with cabotegravir.15 In contrast, nevirapine and efavirenz, the two ARV drugs most likely to interact with hormonal contraceptives, are known CYP 450 inducers and may compete with the metabolism of oral contraceptives, potentially altering systemic concentrations of both the ARV drugs and the hormonal contraceptive; the clinical implications of this interaction are unclear, but have led to recommendations for caution in the use of these ARV drugs with hormonal contraception.2,15 Importantly, prior in vitro and clinical studies of cabotegravir demonstrated a low risk profile for clinically significant DDIs, as cabotegravir is metabolized via glucuronidation through UGT1A1 and A9 enzymes.10,11,16 Furthermore, it is unlikely that hormonal contraceptives would alter absorption of CAB-LA nanoparticles, as injection technique, physical activity, body fat distribution, and muscle mass are hypothesized to be the primary determinants of the rate of CAB-LA absorption.8 However, DDIs have been shown to be challenging to predict from in vitro data, highlighting the need for clinical data.
Our study has several strengths. Samples and data were collected as part of a multicenter phase 2a clinical trial that had high rates of retention and included close monitoring of cabotegravir drug levels. However, the study does have some limitations. In HPTN 077, hormonal contraception use was self-reported; it is unknown whether hormonal contraceptives were taken consistently, and the specific hormones taken were not assessed. As the type(s) of hormonal contraceptives used by women who self-reported not being of reproductive potential was unclear, it is also possible that this group was subject to misclassification bias. Given the effect of oral contraceptives observed on CAB-LA Cmax, dedicated PK studies evaluating potential interactions of hormonal contraceptives on cabotegravir are warranted. We did not measure the effect of cabotegravir on hormone levels. However, we do not anticipate that CAB-LA would influence hormonal contraception levels, based on previous study demonstrating that oral cabotegravir did not influence metabolism of oral ethinyl estradiol or levonorgestrel;16 this is a potential area for future investigation. Given the relatively modest sample size, our analysis is not powered for small differences between any hormonal contraceptive use vs. no use. However, HPTN 084, a registrational trial that is currently enrolling 3,200 cisgender women in Sub-Saharan Africa (of whom 1,800 are receiving cabotegravir), will be better powered to address these findings (clinicaltrials.gov NCT03164564). Additionally, as gender-affirming hormonal therapies typically utilize a wide variety of estrogens (e.g., estradiol), anti-androgens, and/or androgens, further study is needed to fully characterize the bidirectional DDI’s between such cross-sex hormonal therapies and CAB-LA.17 Such interactions will need to be fully explored prior to implementation and scale-up in transgender communities.
Supplementary Material
Acknowledgements:
We would like to thank the participants of HPTN 077 and their families. In addition, we acknowledge the study teams and clinical research staff at the study sites.
Conflicts of Interest Source of Funding:
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [UM1AI068619, UM1AI068613, and UM1AI1068617] and National Institute of Mental Health of the National Institutes of Health [T32MH080634]. Study products were provided by ViiV Healthcare. MAM received grant support through the NIH and received grant support through ViiV/GSK on work external to this study. DM and ARR are paid employees of ViiV Healthcare. CWH has research contracts with ViiV/GSK, Gilead Sciences, Merck, Gates Foundation, RTI, inc, and the NIH through Johns Hopkins. RJL has received consulting fees and travel support from Gilead Sciences and Merck, Inc, as well as honoraria from Roche.
Footnotes
Presentation at conference:
Preliminary findings using baseline data were presented at the 2019 Conference on Retroviruses and Opportunistic Infections, March 2019, Seattle, Washington (abstract #473).
REFERENCES
- 1.Robinson JA, Jamshidi R, Burke AE. Contraception for the HIV-positive woman: A review of interactions between hormonal contraception and antiretroviral therapy. Infect Dis Obstet Gynecol. 2012;2012:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanda K, Stuart GS, Robinson J, Gray AL, Tepper NK, Gaffield ME. Drug interactions between hormonal contraceptives and antiretrovirals. AIDS. 2017;31(7):917–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landolt NK, Phanuphak N, Ubolyam S, et al. Efavirenz, in contrast to nevirapine, is associated with unfavorable progesterone and antiretroviral levels when coadministered with combined oral contraceptives. J Acquir Immune Defic Syndr. 2013;62(5):534–539. [DOI] [PubMed] [Google Scholar]
- 4.Shen Z, Rodriguez-Garcia M, Patel MV, Bodwell J, Kashuba ADM, Wira CR. Hormonal contraceptives differentially suppress TFV and TAF inhibition of HIV infection and TFV-DP in blood and genital tract CD4+ T-cells. Sci Rep. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shieh E, Marzinke MA, Fuchs EJ, et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J Int AIDS Soc. 2019;22(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiransuthikul A, Janamnuaysook R, Himmad K, et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J Int AIDS Soc. 2019;22(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 2015;10(4):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McPherson TD, Sobieszczyk ME, Markowitz M. Cabotegravir in the treatment and prevention of human immunodeficiency virus-1. Expert Opin Investig Drugs. 2018;27(4):413–420. [DOI] [PubMed] [Google Scholar]
- 10.Bowers GD, Culp A, Reese MJ, et al. Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica. 2016;46(2):147–162. [DOI] [PubMed] [Google Scholar]
- 11.Reese MJ, Bowers GD, Humphreys JE, et al. Drug interaction profile of the HIV integrase inhibitor cabotegravir: assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica. 2016;46(5):445–456. [DOI] [PubMed] [Google Scholar]
- 12.Landovitz R, Li S, Eron JJ, et al. Tail-phase safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected individuals [abstract OA15.06LB]. Presented at: HIV Research for Prevention; 2019; Madrid, Spain. [Google Scholar]
- 13.Food and Drug Administration. Clinical drug interaction studies - study design, data analysis, and clinical implications: guidance for industry. U.S. Department of Health and Human Services; 2017. Available at: https://www.fda.gov/media/82734/download.. [Google Scholar]
- 14.Acosta EP, Gerber JG. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res Hum Retroviruses. 2002;18(12):825–834. [DOI] [PubMed] [Google Scholar]
- 15.Scarsi KK, Darin KM, Chappell CA, Nitz SM, Lamorde M. Drug–drug Interactions, effectiveness, and safety of hormonal contraceptives in women living with HIV. Drug Saf. 2016;39(11):1053–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trezza C, Ford SL, Gould E, et al. Lack of effect of oral cabotegravir on the pharmacokinetics of a levonorgestrel/ethinyl oestradiol-containing oral contraceptive in healthy adult women. Br J Clin Pharmacol. 2017;83(7):1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radix A, Sevelius J, Deutsch MB. Transgender women, hormonal therapy and HIV treatment: a comprehensive review of the literature and recommendations for best practices. J Int AIDS Soc. 2016;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


