Abstract
Background
A minority of breast cancer (BC) patients suffer from severe reaction to adjuvant radiotherapy (RT). Although deficient DNA double‐strand break repair is considered the main basis for the reactions, pretreatment identification of high‐risk patients has been challenging.
Methods
To retrospectively determine the etiology of severe local reaction to RT in a 39‐year‐old woman with BC, we performed next‐generation sequencing followed by further clinical and functional studies.
Results
We found a −4 intronic variant (c.2251‐4A>G) in trans with a synonymous (c.3576G>A) variant affecting the ATM DNA‐repair gene (NG_009830.1, NM_000051.3) which is linked to autosomal recessive ataxia–telangiectasia (A–T). We verified abnormal transcripts resulting from both variants, next to a minor wild‐type transcript leading to a residual ATM kinase activity and genomic instability. Follow‐up examination of the patient revealed no classic sign of A–T but previously unnoticed head dystonia and mild dysarthria, a family history of BC and late‐onset ataxia segregating with the variants. Additionally, her serum level of alpha‐fetoprotein (AFP) was elevated similar to A–T patients.
Conclusion
Considering the variable presentations of A–T and devastating impact of severe reactions to RT, we suggest a routine measurement of AFP in RT‐candidate BC patients followed by next‐generation sequencing with special attention to non‐canonical splice site and synonymous variants in ATM.
Keywords: ataxia–telangiectasia, ATM, breast cancer, hypomorphic variants, normal tissue overreaction, radiotherapy, splice site variants
Severe local reaction to standard radiotherapy has devastating impact on breast cancer patients and reliable prediction has been difficult. We provide further evidence for hypomorphic biallelic variants in the DNA‐repair gene ATM as an important underlying cause for both, breast cancer and hypersensitivity to radiotherapy. We therefore discuss routine screening of serum alpha‐fetoprotein as a biomarker for ATM deficiency in radiotherapy‐candidate breast cancer patients followed by next‐generation sequencing with special attention to non‐canonical splice‐site and synonymous variants in the ATM gene.
1. INTRODUCTION
Breast cancer is the most frequent malignancy in women with an age‐adjusted annual incidence of 94.2/100'000 in Europe (Senkus et al., 2015). A significant milestone in the treatment of early stage breast cancer has been a shift from mastectomy toward breast‐conserving surgery over the last decades (Association of Breast Surgery at BASO, 2009, 2009). To reduce local relapse, postoperative radiotherapy (RT) is currently standard (Bartelink et al., 2015; Darby et al., 2011).
The success of RT in eradicating cancer cells depends significantly on sufficient radiation dosage which is limited by overreactions in the tumor‐surrounding normal tissues (OR). However, also patients treated with the same standard RT dosage may manifest with different degrees of mild to moderate OR, and a minority suffer from severe or even life threatening OR (Barnett et al., 2015; Granzotto et al., 2016).
Despite the efforts to anticipate the risk of OR by patient characteristics (such as age, hemoglobin level, comorbid conditions, etc.), cellular radiosensitivity or apoptosis testing, or evaluating common genetic polymorphisms, to date the risk cannot be unequivocally predicted (Andreassen, 2005; Barnett et al., 2009, 2015; Bergom et al., 2019; Dong, Cui, Tang, Cong, & Han, 2015; Story & Durante, 2018; Terrazzino et al., 2019). Nevertheless, deficient DNA double‐strand break recognition or repair responsible for genome stability and cell survival, have been proposed as an important underlying etiology of OR (Granzotto et al., 2016; West, 2003). Accordingly, rare biallelic germline variants in double‐strand break repair genes have been linked to radiosensitivity in recessive genetic disorders such as Fanconi anemia, Nijmegen breakage syndrome, and ataxia–telangiectasia (A–T) (Bentzen, 2006). Among them, A–T is remarkable for its broad clinical spectrum from "classical" with severe childhood‐onset cerebellar ataxia, multisystem involvement, predisposition primarily to lymphoreticular cancer, and radiosensitivity, to " mild" or “variant” with relatively milder neurological impairments and fewer systemic symptoms (van Os, Hensiek, et al., 2019; Sutton et al., 2004; Verhagen et al., 2012). Especially patients with atypical or milder adult‐onset presentations may remain undiagnosed for a long time (Claes et al., 2013; Dawson, Marles, Tomiuk, Riordan, & Gatti, 2015; van Os, Hensiek, et al., 2019). Defective DNA damage repair, impaired proteasome‐mediated protein degradation (Wood et al., 2011), or nuclear accumulation of histone deacetylase 4 (HDAC4) (Li et al., 2012) in neurons have been proposed as possible mechanisms of neurodegeneration following ATM deficiency. It is likely that some residual ATM activity and possibly other protective mechanisms delay neurodegeneration in some A–T patients. After all, impaired DNA damage repair and genomic instability are the most likely explanations for cancer susceptibility and radiosensitivity in ATM deficiency (Byrd et al., 2012; Choi, Kipps, & Kurzrock, 2016; Fang et al., 2010). In general, patients with classical A–T harbor biallelic truncating ATM (OMIM *607585) variants resulting in the absence of ATM kinase activity, while patients with mild A–T harbor pathogenic biallelic variants of which at least one is missense or affecting the splice site preserving some protein expression and residual kinase activity (Byrd et al., 2012; Verhagen et al., 2009, 2012). While heterozygous carriers of deleterious ATM variants are at increased risk of breast cancer, there is no clear clinical evidence for their higher risk of OR or manifesting A–T features (Bergom et al., 2019; Goldgar et al., 2011; Jerzak, Mancuso, & Eisen, 2018; Meyer et al., 2004; Modlin et al., 2019; Pinto et al., 2016).
Here, we detected two hypomorphic pathogenic variants in trans affecting ATM in a 39‐year‐old woman with unilateral breast cancer and severe acute and late OR to adjuvant RT (Figure 1A–C). This finding led to the diagnosis of mild A–T in the patient and her siblings (Figure 1D) after follow‐up examination and functional studies. Our findings further broaden the spectrum of A–T patients who remained undiagnosed and manifested with severe OR after RT creating an immense physical, psychological and financial burden. We therefore suggest considering the possible diagnosis of mild or variant A–T prior to the application of RT in breast cancer patients especially in those with younger age, neurological features, and/or a positive family history.
Figure 1.
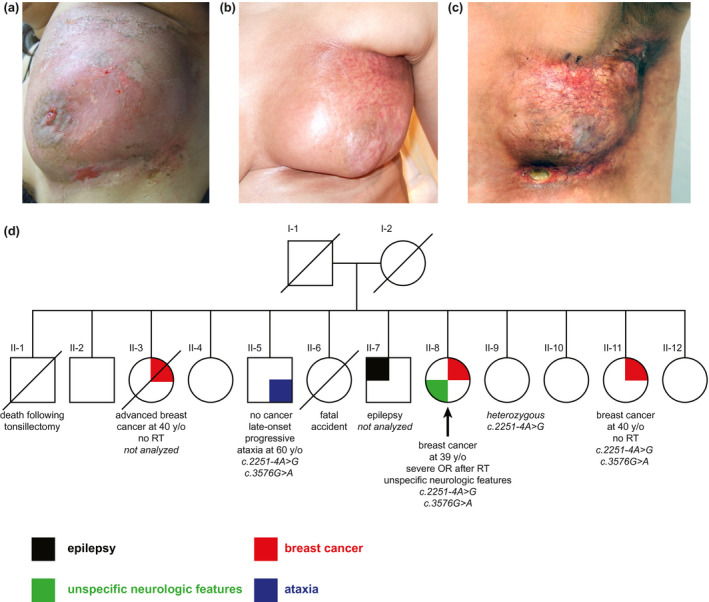
Local post‐radiotherapy reactions in the index patient (a–c), and her family pedigree (d). (a) Moist desquamation and skin abrasions (RTOG acute reaction grade 3) developing within few days after the last session of radiotherapy (RT, 66 Gy), (b) severe fibrosis and shrinkage together with telangiectasia after 1 year, and (c) marked telangiectasia, severe fibrosis, and necrosis (RTOG late reaction grade 3–4), requiring a surgical intervention 3 years after RT. (d) Pedigree of the family showing the segregation of ATM variants and clinical features. Index patient is indicated by the black arrow
2. MATERIALS AND METHODS
2.1. Genetic and transcriptional studies
Genetic testing was performed on a diagnostic basis with subsequent informed consents for publication.
Genomic DNA was extracted from EDTA blood of the index patient (II‐8) and other available siblings (II‐5, II‐9, II‐11) (Figure 1D). Next‐generation sequencing (NGS) of 4813 known disease genes was performed for the index patient using the TruSight™ One Sequencing Panel (Illumina, CA, USA) with paired‐end sequencing (MiSeq Reagent Kit v3, 200 Fwd‐200 Rev) on a MiSeq System (Illumina, CA, USA). The data were analyzed using NextGENe Software (SoftGenetics, PA, USA) for exonic and splice site variants considering both dominant and recessive modes of inheritance. The average depth of coverage was 198x, and ~95.7% of the targeted bases were assessed by ≥20 independent sequence reads.
Confirmation and segregation analysis of candidate ATM (NG_009830.1, NM_000051.3) variants were done after PCR amplification by Sanger sequencing using an ABI Genetic Analyzer 3730 (Applied Biosystems, Foster City, California).
For transcriptional analysis, total RNA was extracted from blood and fibroblast samples of the index patient (II‐8) and healthy controls using the PAXgene™ Blood RNA Kit 50 or RNeasy Plus Mini Kit 50 (Qiagen, Hilden, Germany), respectively. Reverse transcription was performed using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche, Mannheim, Germany). To evaluate the splicing effect of the variants, targeted Sanger sequencing was performed after PCR amplification of the cDNA using primers spanning exons 14–16, and 23–25 of ATM (NG_009830.1, NM_000051.3). Additionally, we performed allele‐specific RT‐PCR targeting either the wild‐type or mutated sequence of exon 14/15 and wild‐type sequences in exon 25. ATM mRNA expression levels were assessed in fibroblast cDNA of the patient, two healthy controls, and a primary melanoma culture (Cellosaurus M010817, as positive control) using customized SYBR green qPCR for exons 3 and 4 of the gene on a Roche LightCycler480. ATM mRNA expression levels were normalized to Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) levels. For statistical analyses, the means of three biological replicates were compared using one‐way ANOVA and Tukey's test for multiple comparisons.
Chromosomal analysis was performed using patient's peripheral lymphocytes stimulated with phytohemagglutinin for 72 hours followed by GTG banding at a 450–500 banding resolution.
2.2. Immunoblotting for ATM protein level and kinase activity
For protein evaluations, primary fibroblasts of the index patient (II‐8), two healthy controls and a primary melanoma culture (M010817, as positive control) were lysed in TNN buffer (50 mM Tris pH 7.5, 250 mM NaCl, 5 mM EDTA, 0.5% NP‐40, 50 mM NaF, 0.5 mM EGTA, 1x cOmplete™ and 1x PhosStop™ (both Roche). The protein concentration was determined using a bicinchoninic acid assay (Pierce BCA kit, Thermo SCIENTIFIC, Rockford, IL, USA). Samples were then boiled in 1x Laemmli Sample Buffer and run for SDS–PAGE using 4%–20% Mini‐PROTEAN TGX Stain‐Free Gels (Bio‐Rad). Gels were blotted using PVDF Trans‐Blot Turbo Transfer Packs, according to the manufacturer's protocols using a trans‐blot turbo blotting device (Bio‐Rad). The following antibodies were used: total ATM (D2E2) rabbit monoclonal antibody, recognizing the C‐terminal portion of human ATM (Cell Signaling Technology, cat. no. 2873), P‐ATM S1981 (EP1890Y) rabbit monoclonal antibody (Abcam, cat. no. ab81292), p44/42 MAPK (ERK1/2) (L34F12) mouse monoclonal antibody (Cell Signaling Technology, cat. no. 4696), and β‐actin (AC‐74) mouse monoclonal antibody (Sigma, cat. no. A5316). Signals were detected using Clarity™ ECL Western Blotting Substrate (Bio‐Rad) on a Fusion FX imager (Vilber Lourmat, Marne‐la‐Vallée, France).
To evaluate the ATM function, we performed a 24‐hr treatment course of oxaliplatin (SelleckChem, cat. no. S1224) which was obtained lyophilized, dissolved in DMSO to a stock solution of 10 mM, and then, used 1:1,000 in cell culture experiments. DMSO (1:1,000) was used as the vehicle control. Oxaliplatin is a platinum compound which provokes DSBs (Faivre, Chan, Salinas, Woynarowska, & Woynarowski, 2003), and therefore, activates ATM by increased autophosphorylation of ATM at S1981 (So, Davis, & Chen, 2009).
2.3. Cell cultures
Standard skin‐punch biopsies from the index patient (II‐8) with pathogenic ATM variants and healthy controls were obtained and cultured according to standard protocols.
Primary melanoma culture M010817 was obtained from R. Dummer and M. Levesque (Shakhova et al., 2012) and cultured in RPMI‐1640+GlutaMax, supplemented with 10% of FCS (both Gibco, Zug, Switzerland).
2.4. Radiosensitivity testing
Subcultures of patient fibroblasts were seeded at a density of 4000/cm2 in DMEM with stable glutamine and 15% of FCS (both Sigma, St. Louis, MO, USA) and DNA damage was elicited by exposure of cells to 6 MV X‐ray photons provided by a linear accelerator (Primus series, Siemens Healthcare, Erlangen, Germany) at a dose rate of 3 Gy/min at assay time 0. Matched cultures from treated and untreated fibroblasts were harvested 48 hr postirradiation using Accutase (PAN Biotech, Aidenbach, Germany). For cell cycle analysis, detached cells were pelleted and resuspended at a density of 106/ml in staining buffer containing 4 µg/ml of 4′,6‐diamidino‐2‐phenylindole (DAPI), 100 mM of Tris pH 7.4, 154 mM of NaCl, 1 mM of CaCl2, 0.5 mM of MgCl2, 0.2% of BSA, 0.1% of NP40 in dH2O (Sigma) for a minimum of 30 minutes at 4°C in the dark (Darzynkiewicz, Huang, & Zhao, 2017). Bivariate flow histograms were recorded on an analytical, triple laser‐equipped flow cytometer (LSRII, Becton Dickinson, Franklin Lakes, NJ, USA) using UV excitation/emission at 355/460 nm and FACSDiva software for data acquisition. The resulting cell cycle distributions reflecting cellular DNA content and cell cycle progression were quantified by means of the MPLUS AV software package to assess G2 phase accumulation (Phoenix Flow Systems, San Diego, CA, USA) (Schindler & Hoehn, 1999).
3. RESULTS
3.1. Patient's clinical course and family history
The index patient (II‐8, Figure 1D) was a 39‐year old female of Turkish origin diagnosed with multifocal invasive ductal carcinoma of the left breast (stage pT2 pN1(1/17) M0, G3, ER, and PR positive, HER2 negative). Her treatment consisted of a lumpectomy followed by four cycles of adjuvant chemotherapy with Epirubicin (130 mg) and Endoxan (900 mg) starting 1 month after the surgery, and adjuvant RT of the left breast starting 2 months after the last chemotherapy cycle. The RT protocol comprised percutaneous irradiation of the left breast with 25 fractions of 2 Gy (cumulative dose 50 Gy) and an additional boost dose of 16 Gy (8 × 2 Gy) to reach a final dose of 66 Gy during 45 days.
During RT, she developed acute skin reactions of grade 1–2 (according to the Radiation Therapy Oncology Group (RTOG) toxicity grading) including mild edema and erythema of the left breast, slight erythema in the left axilla, skin irritation, and hyperpigmentation under the left mammilla and axilla, as well as light pruritus and pain which were managed conservatively. These reactions were adequate for the dose, however, within few days after the last session (66 Gy), she began to develop moist desquamation and skin abrasions as well as strong stabbing pain and tenderness of the left breast (RTOG acute reaction grade 3, Figure 1A) which were managed with wound cleaning and topical analgesics. While the abrasions became smaller over time with no evidence of infection, skin fibrosis and granulation occurred which eventually led to severe fibrosis and shrinkage together with telangiectasia after a year (Figure 1B). Ultimately, she developed marked telangiectasia over the left breast, severe fibrosis, and necrosis (RTOG late reaction grade 3–4, Figure 1C) requiring a surgical intervention 3 years after RT.
Neurological examination of the index patient at this stage (age 43 years) revealed psychomotor restlessness, some parakinesia, and a dystonic posture of the head with intermittent irregular tremor and mild dysarthria, considered suggestive of a choreatiform movement disorder, but no ataxia or oculomotor abnormalities. She had neither oculocutaneous telangiectasia nor a history of pulmonary problems or recurrent infections. Her brain MRI as well as serum immunoglobulin levels (IgG 14 g/L (8.0–15.0 g/L), IgM 1.3 g/L (0.6–2.8 g/L), and IgA 3.0 g/L (0.8–3.4 g/L) were reported normal. Serum α‐fetoprotein (AFP) determined once at the age of 43 years was elevated (55 U/mL, reference value <10 U/mL).
She had two sisters (II‐3 and II‐11, Figure 1D) who were also diagnosed with breast cancer at the age of ~40 years. One of them (II‐3) deceased due to an advanced breast cancer without having received RT, and the other (II‐11) had bilateral breast cancer but did not undergo RT because of the severe OR in her sister. A brother of them (II‐5) was later reported by the patient to suffer from late‐onset progressive ataxia having started in his 30s, but no cancer at the age of 60 years, and another brother (II‐7) had a history of epilepsy since 20 years of age.
3.2. Genetic and transcriptional findings
Targeted exome sequencing in the index patient revealed two heterozygous variants in the ATM gene, an uncharacterized intronic variant located at −4 of exon 15 (c.2251‐4A>G) and a previously characterized synonymous variant of the last nucleotide of exon 24 (c.3576G>A). Our mRNA analysis revealed the inclusion of three nucleotides (TAG) in the ATM transcript by the −4 splice site variant causing an insertion of a premature stop codon (r.2250_2251instag, p.(Ser751*)) (Figure 2A and 2C), and an in‐frame skipping of exon 24 by the exonic synonymous variant resulting in a predicted deletion of 58 amino acids (r.3403_3576del, p.(Ser1135_Lys1192del)) (Figure 2A and 2C). By allele‐specific RT‐PCR, we observed a faint band indicating a wild‐type transcript resulting from one or two leaky alleles in the patient (Figure 2B and 2C). Notably, we did not observe a statistically significant reduction of the ATM mRNA levels in cultured skin fibroblasts of the index patient compared to two healthy controls (Figure 3A) possibly due to a transcriptional dosage compensation process. Compared to the fibroblasts, ATM mRNA levels were significantly higher in the primary melanoma cell cultures which were used as positive control (p < 0.01) (Figure 3A).
Figure 2.
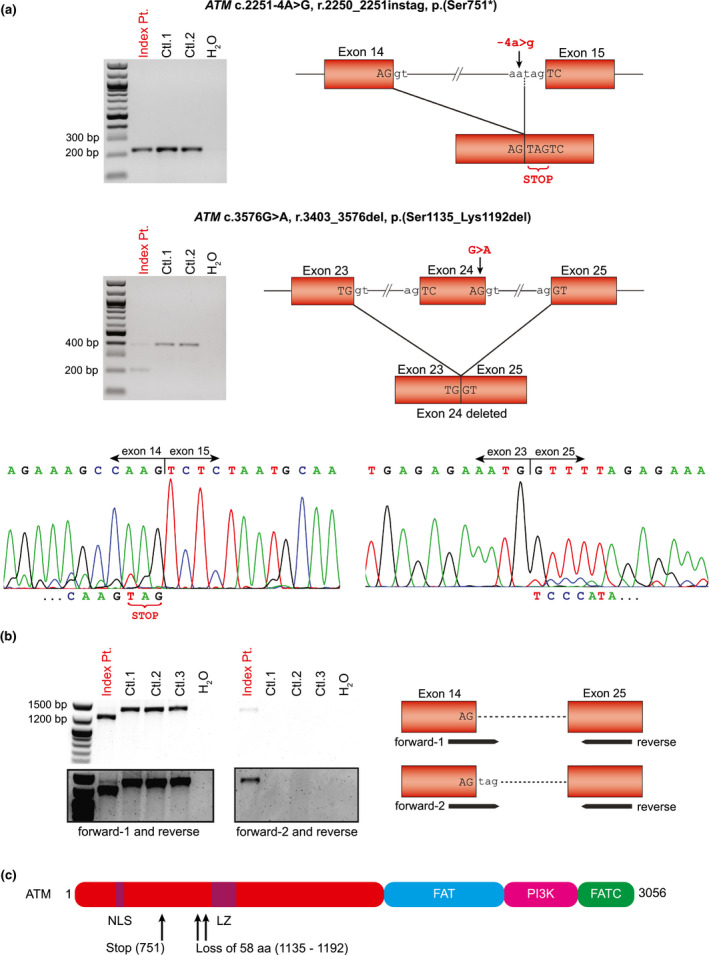
Splice site effects of the ATM (NG_009830.1, NM_000051.3, NP_000042.3) variants in fibroblast cells of the index patient: c.2251‐4A>G resulting in the insertion of a premature stop codon (r.2250_2251instag, p.(Ser751*)) and likely nonsense‐mediated mRNA decay, and c.3576G>A leading to an in‐frame deletion of exon 24 (r.3403_3576del, p.(Ser1135_Lys1192del)). (a) Upper agarose gel showing similar RT‐PCR bands sizing about 220 bp from primers spanning exons 14–16 of ATM encompassing the c.2251‐4A>G variant (‐4 of exon 15) in the index patient (Pt.) and controls (Ctl.1 and 2). Left electropherograms from Sanger sequencing of the patient's RT‐PCR product showing both the wild‐type sequence and a low level mutated sequence with the inclusion of a “TAG” codon (r.2250_2251instag, p.(Ser751*)) indicating the remnants of the aberrant allele undergoing nonsense‐mediated mRNA decay. Lower agarose gel showing RT‐PCR bands from primers spanning exons 23–25 of ATM encompassing the variant c.3576G>A (in exon 24) in the index patient and controls. Next to a faint band with the wild‐type fragment size (377 bp), a smaller aberrant band (203 bp) is visible in the patient lane. Right electropherograms from Sanger sequencing of the patient's RT‐PCR products showing the aberrant fragment to lack exon 24 corresponding to the loss of 174 bps (r.3403_3576del, p.(Ser1135_Lys1192del)). Low level wild‐type sequence (exon 24) consistent with the faint band of the expected size may indicate remnants of the decayed or leaky wild‐type alleles. (b) Agarose gel showing allele‐specific RT‐PCR bands from primers spanning exons 14–25 of ATM in the index patient and controls. Forward‐1 targets the wild‐type, forward‐2 contains the "TAG" inclusion at the 3′ end, and reverse targets the wild‐type. RT‐PCR with forward‐1 and reverse (left) shows a shorter product (1,262 bp) corresponding to the mutated allele with lack of exon 24, and a very faint band of the expected size (1,436 bp, better visible in the long exposure) likely indicating the expression of leaky wild‐type transcript in the patient. RT‐PCR with forward‐2 and reverse (right) shows a faint product of the expected size (1,436 bp) corresponding to the mutated allele with the inclusion of "TAG" seen in the patient, only. (c) Schematic structure of ATM protein with the affected sites highlighted. NLS: nuclear localization signal. LZ: leucine zipper. FAT: FRAP/ATM/TRRAP domain. PI3 K: PI3K‐related kinase domain. FATC: FAT C‐terminal
Figure 3.
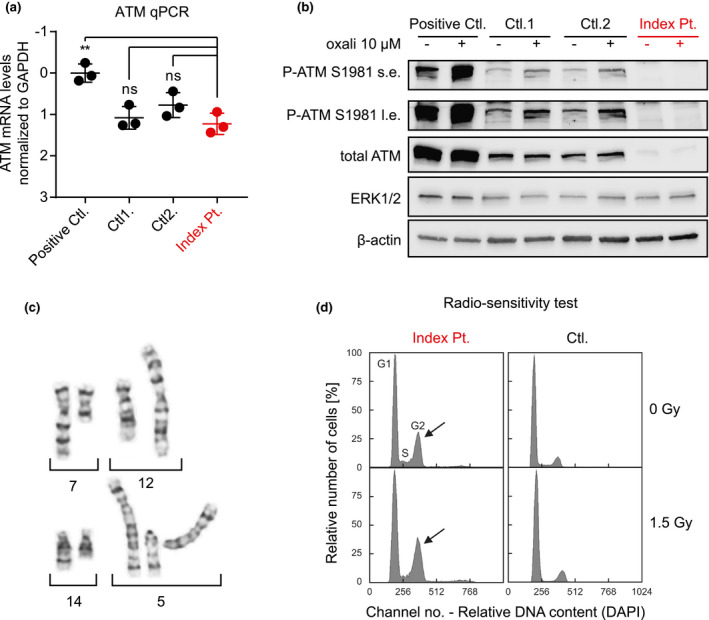
ATM mRNA and protein expression and kinase activity (a and b), chromosomal rearrangements (c), and radiosensitivity test in fibroblast cells of the index patient (d) indicating residual ATM activity and the consequent genomic instability, respectively. (a) ATM mRNA expression levels in the fibroblast cells of the index patient are compared to two healthy controls, and a primary melanoma culture (positive control). Values are expressed as ΔCt normalized to GAPDH with positive control set arbitrarily as 0 (a higher ΔCt value indicates lower mRNA abundance). While ATM mRNA levels were significantly higher in the positive control (p < 0.01), no statistically significant difference between the index patient and healthy controls was seen (three biological replicates). (b) Immunoblots of whole cell protein extracts from the same cultures as in (a) show significantly lower level of total ATM protein, and a very faint induction of phosphorylated ATM (at serine 1981) following oxaliplatin (oxali) treatment in the patient's fibroblasts compared to the controls. Cells were treated with 10 µM oxali (+) or vehicle control (−) for 24 hr. ERK1/2 and β‐actin were used as loading controls. The experiment was repeated two times and one representative blot is shown (s.e.: short exposure, l.e.: long exposure). (c) Examples of chromosomal breakage in three different metaphases including breakage on chromosome 7 (7q22‐qter terminal on chromosome 12p) in the upper panel, and breakage on chromosomes 14q and 5q in the lower panel are shown, which were detected in the patient's peripheral lymphocytes. (d) Radiosensitivity testing of patient's fibroblast cells toward ionizing radiation showing mildly increased G2 compartment in the absence of irradiation (upper left panel, G0/G1 56.4%, S 20.8%, G2 22.8% (arrow); normal G2 <16%) compared to an age‐matched control (upper right panel, G0/G1 77.4%, S 12.6%, G2 10.0%), and moderately elevated G2 phase accumulation after irradiation at a dosage of 1.5 Gy (lower left panel, G0/G1 53.4%, S 19.0%, G2 27.6% (arrow), normal <24%) compared to an age‐matched control (lower right panel, G0/G1 82.9%, S 4.8%, G2 12.3%)
Moreover, chromosome analyses of the patient's peripheral lymphocytes revealed rearrangements of chromosomes 5, 7, 12, and 14 in 6.5% of the 77 evaluated metaphases (Figure 3C) suggestive of chromosomal instability.
Among siblings of the index patient whose DNAs were available, we observed the presence of both sequence variants in the sister (II‐11) with a history of bilateral breast cancer, and in the brother (II‐5) with late‐onset progressive ataxia. One unaffected sister (II‐9) at the age of 45 was heterozygous for the −4 splice site variant. DNA samples from the deceased sister (II‐3) with a history of advanced breast cancer and the brother (II‐7) with epilepsy were not available for testing (Figure 1D). Accordingly the ATM variants were compound heterozygous in the index patient (II‐8), a sister (II‐11) and a brother (II‐5): NC_000011.9:g.[108128204A>G];[108151895G>A], NG_009830.1(NM_000051.3): c.[2251‐4A>G];[3576G>A], r.[2250_2251instag];[3403_3576del], NP_000042.3:p.[(Ser751*)];[(Ser1135_Lys1192del)].
3.3. ATM protein level and kinase activity
Total ATM protein levels, though still detectable, were significantly lower in the index patient's fibroblasts compared to the control fibroblast samples (Figure 3B, total ATM) indicating reduced amounts of detectable ATM protein despite the unchanged mRNA expression. This is possibly due to a misfolding or instability of the aberrant protein.
In addition, while the levels of phosphorylated ATM at serine 1981 were increased upon oxaliplatin treatment in control fibroblasts and the primary melanoma cell culture, we detected only a very faint induction in the patient's fibroblasts suggestive of a significantly reduced ATM kinase activity in her fibroblasts (Figure 3B, P‐ATM S1981).
3.4. Cellular radiosensitivity
Patient's fibroblasts demonstrated definitive elevation of the G2 phase compartment (Figure 3D) as manifestation of delay and arrest in cell cycle progression which is consistent with unresolved replication stress likely due to the genomic instability. Further disproportional increase of G2 phase accumulation after ionizing irradiation of fibroblasts (Figure 3D) confirmed the underlying genetic instability and cellular radiosensitivity (Branzei & Foiani, 2010; Seyschab, Sun, Friedl, Schindler, & Hoehn, 1993).
4. DISCUSSION
Our findings illustrate the importance of considering breast cancer before the age of 45–50 years as the first apparent sign of mild or variant, late‐onset A–T. Milder or unspecific neurological phenotypes such as extrapyramidal symptoms, significantly lesser presentations of telangiectasia or other A–T features, and less and later‐onset malignancies with a shift from lymphoreticular to solid cancers have been previously linked to the residual ATM kinase activity in patients with hypomorphic combinations of ATM variants (Gilad et al., 1998; Verhagen et al., 2009, 2012). Van Os, Hensiek, et al. (2019) defined six groups of neurological trajectories for mild forms of A–T with the mildest group showing a mean age at diagnosis of 35.8 years with extrapyramidal signs manifesting not later than the age of ~30 years, and ataxia and polyneuropathy not later than the age of ~40 years. Now our and the few published reports indicate that neurological signs may become apparent even later resulting in severe OR in unrecognized A–T patients presenting with breast cancer (Table 1) (Byrd et al., 2012; Fang et al., 2010; Stankovic et al., 1998). Retrospectively, family history of our patient with ataxia in an older brother and breast cancer in an older sister might have been suggestive of mild A–T, however, initially the brother's disease was not reported. Nevertheless, nowadays most pedigrees are rather small, and thus, family history becomes less informative which may delay or thwart the clinical suspicion of mild A–T. Serum AFP levels have repeatedly been reported to be elevated in patients with both classical and mild A–T (Gilad et al., 1998; van Os, Hensiek, et al., 2019; Verhagen et al., 2009, 2012) with biallelic pathogenic variants in ATM as held true for the index patient presented here. Therefore, serum AFP screening in women with breast cancer before the age of 45–50 years might be considered before starting adjuvant RT. However, since serum AFP levels above 10 U/ml have been also found in liver cancer, cirrhosis and gastric cancer, and to a lower extent in breast cancer (He, Lu, & Zhang, 2019), the definite diagnosis should be established by germline genetic testing. There are also a few examples of normal AFP levels in A–T patients and the possibility of false negative AFP or laboratory errors has been considered (Verhagen et al., 2012). Therefore, any neurological feature and/or positive family history should prompt genetic testing in patients with normal levels of AFP. Moreover, in patients with familial breast cancer, genetic testing should not be restricted to BRCA1 and 2 (OMIM *113705 and *600185) testing, but include at least ATM, too. This is especially important for genetic counseling of the extended family who may harbor heterozygous or biallelic ATM variants with different risk scores for developing breast cancer and OR after adjuvant RT. In addition, ATM testing might be of importance for considering the emerging targeted therapies in ATM‐deficient cancers (Choi et al., 2016).
Table 1.
Summary of patients diagnosed with mild ataxia–telangiectasia after OR a to adjuvant radiotherapy for breast cancer
| Study | Stankovic et al. (1998) | Fang et al. (2010) | Mandigers et al. (2011) | Byrd et al. (2012) | This study |
|---|---|---|---|---|---|
| Gender | Female | Female | Female | Female | Female |
| Age at A–T diagnosis (years) | 44 | 50 | 42 | 44 | 43 |
| Type of breast cancer | Right side invasive ductal carcinoma at 44 years, contra‐lateral intraductal carcinoma in situ at 48 years | Not reported | Ductal carcinoma (T2N2M0, ER+, Her2+) at 42 years | Right side carcinoma (T2N2, G2, ER+) at 44 years | Left side invasive ductal carcinoma (T2N1M0, G3, ER+, PR+, HER2‐) at 39 years |
| Type of OR a | Severe acute and late | Severe late | Severe acute | Severe acute and late | Severe acute and late |
|
Variants ATM (NG_009830.1, NM_000051.3, NP_000042.3) |
Homozygous: c.[7271T>G];[7271T>G], p.[(Val2424Gly)];[(Val2424Gly)] |
Bi‐allelic: c.[1918A>T];[1066‐6T>G], p.[(Lys640*)];[(?)] skipping of exon 11 and premature stop in exon 12 |
“single variant”: c.[8147T>C];[?] p.[(Val2716Ala)];[?] |
Bi‐allelic: c.[8672G>A];[1A>G], p.[(Gly2891Asp)];[(?)] |
Bi‐allelic: c.[2251‐4A>G];[3576G>A] r.[2250_2251instag];[3403_3576del], p.[(Ser751*)]; [(Ser1135_Lys1192del)] |
| Protein level | Normal | Reduced | Not reported | Reduced | Reduced |
| Kinase activity | Not reported | Residual | Not reported | Residual | Residual |
| Neurological features | Truncal ataxia and progressive dysarthria since early 20s, peripheral ataxia and oculomotor apraxia at 48 years | Not reported |
Unexplained, childhood‐onset choreoathetosis |
Only a mild axonal polyneuropathy | Psychomotor restlessness with some parakinesia, dystonic posture of the head with intermittent irregular tremor, mild dysarthria |
| Other features | Minimal telangiectasia, ulcerative proctitis | not reported |
Discrete oculocutaneous telangiectasias, chronic myeloid leukemia 3 years later (treated), she died a year after without evidence of cancer recurrence |
None | None |
| Serum level of alpha‐fetoprotein | Normal b | Not reported | Elevated (146.4 U/ml) | Not reported | Elevated (55 U/ml) |
| Family history | Mild childhood‐onset A–T in a brother, similar neurological course and invasive ductal carcinoma of the right breast at the age of 50 years in a sister, all harboring the same homozygous variant | Had relatives diagnosed with breast cancer | Not reported | Not reported | Severe late onset ataxia in a brother, breast cancer at the age of ~40 years in two sisters, the brother and a sister were tested and harbored the same bi‐allelic variants |
OR: overreactions in the surrounding normal tissues after radiotherapy.
The value and the age of sampling was not reported.
The final diagnosis of heterozygous carriers or affected individuals with (mild or variant) A–T should be attempted to be achieved by germline testing of ATM. However, ATM is a large gene and pathogenic variants may remain undetected by standard diagnostic approaches. The two germline ATM variants detected in trans in our patient were a previously reported synonymous splice site and a −4 intronic variant of unknown significance. The synonymous variant c.3576G>A was shown before (Gilad et al., 1998) and confirmed by us to cause exon 24 skipping and predicted in‐frame deletion of 58 amino acids. This variant has been previously reported in homozygous or compound heterozygous states in patients presenting with milder classic A–T presentations (Gilad et al., 1998; Jacquemin et al., 2012; van Os, Chessa, et al., 2019). The c.2251‐4A>G variant was shown by us to cause a premature stop codon with the transcript likely undergoing nonsense‐mediated decay with some leakiness. This variant has not been reported causative before, but was mentioned as a variant of uncertain significance in a large study of individuals who were referred for genetic testing with a hereditary cancer panel and the majority had family history of breast and/or ovarian cancer (Tsaousis et al., 2019). Co‐segregation with the phenotype in our family, aberrant splice site effects at mRNA level, profound reduction of ATM protein and its kinase activity, and considerable radiosensitivity detected in the fibroblasts of our index patient support the causality of these apparently hypomorphic variants.
The majority of earlier attempts of systematic searches for ATM germline variants in breast cancer patients with OR either did not reveal any ATM mutation likely because of using less‐sensitive methods compared to NGS, or detected variants with questionable pathogenicity considering current knowledge (Andreassen et al., 2006; Appleby et al., 1997; Bremer et al., 2003; Iannuzzi, Atencio, Green, Stock, & Rosenstein, 2002; Ramsay, Birrell, & Lavin, 1998). Nevertheless, Fang et al. (Fang et al., 2010) detected ATM protein levels of 8–45% in lymphoblastoid cell lines of 4/12 (33%) breast cancer patients with severe acute and/or late OR to adjuvant RT which could be explained by pathogenic biallelic ATM variants in only one of the four patients with the lowest ATM protein level (8%). This patient presented with breast cancer at the age of 50 years and severe late OR but no further details have been provided (Table 1).
Accordingly, the lack of NGS‐based studies with comprehensive assessment of all ATM variants may have led to an underestimation of biallelic pathogenic variants in ATM, which currently represents the strongest monogenic etiology of severe OR in breast cancer patients. In line with the finding in our index patient, ER and PR positive invasive ductal carcinomas have been more frequently observed in ATM‐associated breast tumors (Decker et al., 2017; Renault et al., 2018).
Taken together, our findings highlight the difficulty of recognizing mild or variant forms of A–T which may remain undiagnosed till presenting with breast cancer and severe OR to RT, thus, creating a massive burden for the patient and health care system. Therefore, we suggest a primary screening for elevated levels of serum AFP in RT‐candidate breast cancer patients followed by NGS‐based testing of cancer genes including ATM (Figure 4). To distinguish between heterozygous carriers and patients with biallelic variants, it is essential to consider all the ATM variants including the non‐canonical intronic and synonymous exonic variants for their mRNA splicing effects.
Figure 4.
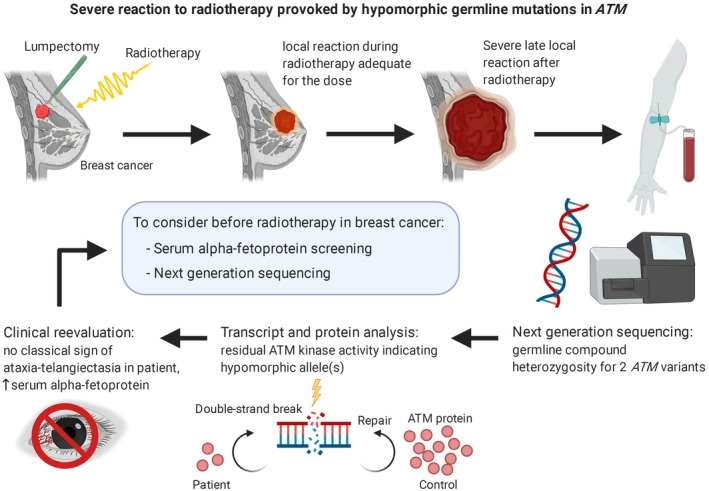
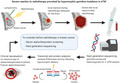
Overview of our investigation to find the etiology of a severe reaction to radiotherapy in a patient with breast cancer. We identified germline biallelic and hypomorphic pathogenic variants in the ATM DNA‐repair gene, as well as an increased serum level of alpha‐fetoprotein. We suggest a routine measurement of alpha‐fetoprotein in radiotherapy‐candidate breast cancer patients followed by next‐generation sequencing with special attention to ATM hypomorphic variants
CONFLICT OF INTEREST
Nothing to declare.
AUTHOR CONTRIBUTION
RA and CB designed and performed experiments, analyzed and interpreted experimental and clinical data and wrote the manuscript. PJ and BO supervised experiments and analyzed and interpreted data. DS supervised experiments, interpreted data and revised the manuscript. UM contributed clinical data. AR designed and supervised the investigations, contributed clinical data, interpreted the data, and wrote the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We sincerely thank the affected individuals for permission to publish their data. We would like to thank Dr Reinhard Dummer and Dr Mitchell Levesque from the Department of Dermatology, University of Zurich for providing the primary melanoma culture M010817. AR was supported by a grant from the von Sick Foundation.
Asadollahi R, Britschgi C, Joset P, et al. Severe reaction to radiotherapy provoked by hypomorphic germline mutations in ATM (ataxia–telangiectasia mutated gene. Mol Genet Genomic Med. 2020;8:e1409 10.1002/mgg3.1409
Reza Asadollahi and Christian Britschgi have contributed equally to this work.
DATA AVAILABILITY STATEMENT
Any additional data required are available on request.
REFERENCES
- Andreassen, C. N. (2005). Can risk of radiotherapy‐induced normal tissue complications be predicted from genetic profiles? Acta Oncologica, 44(8), 801–815. 10.1080/02841860500374513 [DOI] [PubMed] [Google Scholar]
- Andreassen, C. N. , Overgaard, J. , Alsner, J. , Overgaard, M. , Herskind, C. , Cesaretti, J. A. , … Rosenstein, B. S. (2006). ATM sequence variants and risk of radiation‐induced subcutaneous fibrosis after postmastectomy radiotherapy. International Journal of Radiation Oncology Biology Physics, 64(3), 776–783. 10.1016/j.ijrobp.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Appleby, J. M. , Barber, J. , Levine, E. , Varley, J. M. , Taylor, A. , Stankovic, T. , … Scott, D. (1997). Absence of mutations in the ATM gene in breast cancer patients with severe responses to radiotherapy. British Journal of Cancer, 76(12), 1546–1549. 10.1038/bjc.1997.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Breast Surgery at BASO . (2009). Surgical guidelines for the management of breast cancer. European Journal of Surgical Oncology, 35(Suppl 1), 1–22. 10.1016/j.ejso.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Barnett, G. C. , Kerns, S. L. , Noble, D. J. , Dunning, A. M. , West, C. M. , & Burnet, N. G. (2015). Incorporating genetic biomarkers into predictive models of normal tissue toxicity. Clinical Oncology, 27(10), 579–587. 10.1016/j.clon.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Barnett, G. C. , West, C. M. , Dunning, A. M. , Elliott, R. M. , Coles, C. E. , Pharoah, P. D. , & Burnet, N. G. (2009). Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nature Reviews Cancer, 9(2), 134–142. 10.1038/nrc2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelink, H. , Maingon, P. , Poortmans, P. , Weltens, C. , Fourquet, A. , Jager, J. , … Collette, L. (2015). Whole‐breast irradiation with or without a boost for patients treated with breast‐conserving surgery for early breast cancer: 20‐year follow‐up of a randomised phase 3 trial. The Lancet Oncology, 16(1), 47–56. 10.1016/s1470-2045(14)71156-8 [DOI] [PubMed] [Google Scholar]
- Bentzen, S. M. (2006). Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nature Reviews Cancer, 6(9), 702–713. 10.1038/nrc1950 [DOI] [PubMed] [Google Scholar]
- Bergom, C. , West, C. M. , Higginson, D. S. , Abazeed, M. E. , Arun, B. , Bentzen, S. M. , … Woodward, W. A. (2019). The implications of genetic testing on radiation therapy decisions: A guide for radiation oncologists. International Journal of Radiation Oncology Biology Physics, 105(4), 698–712. 10.1016/j.ijrobp.2019.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei, D. , & Foiani, M. (2010). Maintaining genome stability at the replication fork. Nature Reviews Molecular Cell Biology, 11(3), 208–219. 10.1038/nrm2852 [DOI] [PubMed] [Google Scholar]
- Bremer, M. , Klopper, K. , Yamini, P. , Bendix‐Waltes, R. , Dork, T. , & Karstens, J. H. (2003). Clinical radiosensitivity in breast cancer patients carrying pathogenic ATM gene mutations: No observation of increased radiation‐induced acute or late effects. Radiotherapy and Oncology, 69(2), 155–160. 10.1016/j.radonc.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Byrd, P. J. , Srinivasan, V. , Last, J. I. , Smith, A. , Biggs, P. , Carney, E. F. , … Taylor, A. M. (2012). Severe reaction to radiotherapy for breast cancer as the presenting feature of ataxia telangiectasia. British Journal of Cancer, 106(2), 262–268. 10.1038/bjc.2011.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, M. , Kipps, T. , & Kurzrock, R. (2016). ATM mutations in cancer: Therapeutic implications. Molecular Cancer Therapeutics, 15(8), 1781–1791. 10.1158/1535-7163.MCT-15-0945 [DOI] [PubMed] [Google Scholar]
- Claes, K. , Depuydt, J. , Taylor, A. M. R. , Last, J. I. , Baert, A. , Schietecatte, P. , … Vral, A. (2013). Variant ataxia telangiectasia: Clinical and molecular findings and evaluation of radiosensitive phenotypes in a patient and relatives. NeuroMolecular Medicine, 15(3), 447–457. 10.1007/s12017-013-8231-4 [DOI] [PubMed] [Google Scholar]
- Darby, S. , McGale, P. , Correa, C. , Taylor, C. , Arriagada, R. , Clarke, M. , … Peto, R. (2011). Effect of radiotherapy after breast‐conserving surgery on 10‐year recurrence and 15‐year breast cancer death: Meta‐analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet, 378(9804), 1707–1716. 10.1016/s0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz, Z. , Huang, X. , & Zhao, H. (2017). Analysis of cellular DNA content by flow cytometry. Current Protocols in Cytometry, 82, 7.5.1–7.5.20. 10.1002/cpcy.28 [DOI] [PubMed] [Google Scholar]
- Dawson, A. J. , Marles, S. , Tomiuk, M. , Riordan, D. , & Gatti, R. A. (2015). ataxia–telangiectasia with female fertility. American Journal of Medical Genetics. Part A, 167A(8), 1937–1939. 10.1002/ajmg.a.37084 [DOI] [PubMed] [Google Scholar]
- Decker, B. , Allen, J. , Luccarini, C. , Pooley, K. A. , Shah, M. , Bolla, M. K. , … Easton, D. F. (2017). Rare, protein‐truncating variants in ATM, CHEK2 and PALB2, but not XRCC2, are associated with increased breast cancer risks. Journal of Medical Genetics, 54(11), 732–741. 10.1136/jmedgenet-2017-104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, L. , Cui, J. , Tang, F. , Cong, X. , & Han, F. (2015). Ataxia telangiectasia‐mutated gene polymorphisms and acute normal tissue injuries in cancer patients after radiation therapy: A systematic review and meta‐analysis. International Journal of Radiation Oncology Biology Physics, 91(5), 1090–1098. 10.1016/j.ijrobp.2014.12.041 [DOI] [PubMed] [Google Scholar]
- Faivre, S. , Chan, D. , Salinas, R. , Woynarowska, B. , & Woynarowski, J. M. (2003). DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochemical Pharmacology, 66(2), 225–237. 10.1016/s0006-2952(03)00260-0 [DOI] [PubMed] [Google Scholar]
- Fang, Z. , Kozlov, S. , McKay, M. J. , Woods, R. , Birrell, G. , Sprung, C. N. , … Clarke, R. A. (2010). Low levels of ATM in breast cancer patients with clinical radiosensitivity. Genome Integr, 1(1), 9 10.1186/2041-9414-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad, S. , Chessa, L. , Khosravi, R. , Russell, P. , Galanty, Y. , Piane, M. , … Bar‐Shira, A. (1998). Genotype‐phenotype relationships in ataxia–telangiectasia and variants. American Journal of Human Genetics, 62(3), 551–561. 10.1086/301755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgar, D. E. , Healey, S. , Dowty, J. G. , Da Silva, L. , Chen, X. , Spurdle, A. B. , … Chenevix‐Trench, G. (2011). Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Research, 13(4), R73 10.1186/bcr2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzotto, A. , Benadjaoud, M. A. , Vogin, G. , Devic, C. , Ferlazzo, M. L. , Bodgi, L. , … Foray, N. (2016). Influence of nucleoshuttling of the ATM protein in the healthy tissues response to radiation therapy: Toward a molecular classification of human radiosensitivity. International Journal of Radiation Oncology Biology Physics, 94(3), 450–460. 10.1016/j.ijrobp.2015.11.013 [DOI] [PubMed] [Google Scholar]
- He, Y. , Lu, H. , & Zhang, L. (2019). Serum AFP levels in patients suffering from 47 different types of cancers and noncancer diseases. Progress in Molecular Biology and Translational Science, 162, 199–212. 10.1016/bs.pmbts.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Iannuzzi, C. M. , Atencio, D. P. , Green, S. , Stock, R. G. , & Rosenstein, B. S. (2002). ATM mutations in female breast cancer patients predict for an increase in radiation‐induced late effects. International Journal of Radiation Oncology Biology Physics, 52(3), 606–613. 10.1016/s0360-3016(01)02684-0 [DOI] [PubMed] [Google Scholar]
- Jacquemin, V. , Rieunier, G. , Jacob, S. , Bellanger, D. , d'Enghien, C. D. , Laugé, A. , … Stern, M.‐H. (2012). Underexpression and abnormal localization of ATM products in ataxia telangiectasia patients bearing ATM missense mutations. European Journal of Human Genetics, 20(3), 305–312. 10.1038/ejhg.2011.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzak, K. J. , Mancuso, T. , & Eisen, A. (2018). ataxia–telangiectasia gene (ATM) mutation heterozygosity in breast cancer: A narrative review. Current Oncology (Toronto, Ont.), 25(2), e176–e180. 10.3747/co.25.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Chen, J. , Ricupero, C. L. , Hart, R. P. , Schwartz, M. S. , Kusnecov, A. , & Herrup, K. (2012). Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nature Medicine, 18(5), 783–790. 10.1038/nm.2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandigers, C. M. , van de Warrenburg, B. P. , Strobbe, L. J. , Kluijt, I. , Molenaar, A. H. , & Schinagl, D. A. (2011). Ataxia telangiectasia: the consequences of a delayed diagnosis. Radiother Oncol, 99(1), 97–98. 10.1016/j.radonc.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Meyer, A. , John, E. , Dork, T. , Sohn, C. , Karstens, J. H. , & Bremer, M. (2004). Breast cancer in female carriers of ATM gene alterations: Outcome of adjuvant radiotherapy. Radiotherapy and Oncology, 72(3), 319–323. 10.1016/j.radonc.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Modlin, L. A. , Flynn, J. , Zhang, Z. , Mueller, B. , Khan, A. J. , Gillespie, E. F. , … Braunstein, L. Z. (2019). Breast radiotherapy among ATM‐mutation carriers. Journal of Clinical Oncology, 37(15_suppl), 1504 10.1200/JCO.2019.37.15_suppl.1504 [DOI] [Google Scholar]
- Pinto, P. , Paulo, P. , Santos, C. , Rocha, P. , Pinto, C. , Veiga, I. , … Teixeira, M. R. (2016). Implementation of next‐generation sequencing for molecular diagnosis of hereditary breast and ovarian cancer highlights its genetic heterogeneity. Breast Cancer Research and Treatment, 159(2), 245–256. 10.1007/s10549-016-3948-z [DOI] [PubMed] [Google Scholar]
- Ramsay, J. , Birrell, G. , & Lavin, M. (1998). Testing for mutations of the ataxia telangiectasia gene in radiosensitive breast cancer patients. Radiotherapy and Oncology, 47(2), 125–128. 10.1016/s0167-8140(98)00014-0 [DOI] [PubMed] [Google Scholar]
- Renault, A.‐L. , Mebirouk, N. , Fuhrmann, L. , Bataillon, G. , Cavaciuti, E. , Le Gal, D. , … Lesueur, F. (2018). Morphology and genomic hallmarks of breast tumours developed by ATM deleterious variant carriers. Breast Cancer Research, 20(1), 28 10.1186/s13058-018-0951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, D. , & Hoehn, H. (1999). Flow cytometric testing for syndromes with chromosomal instability, aplastic anemia and related hematological disorders In Wegner R.‐D. (Ed.), Diagnostic cytogenetics (pp. 269–281). Berlin, Heidelberg: Springer, Berlin Heidelberg. [Google Scholar]
- Senkus, E. , Kyriakides, S. , Ohno, S. , Penault‐Llorca, F. , Poortmans, P. , Rutgers, E. , … Cardoso, F. (2015). Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 26(Suppl 5), v8–v30. 10.1093/annonc/mdv298 [DOI] [PubMed] [Google Scholar]
- Seyschab, H. , Sun, Y. , Friedl, R. , Schindler, D. , & Hoehn, H. (1993). G2 phase cell cycle disturbance as a manifestation of genetic cell damage. Human Genetics, 92(1), 61–68. 10.1007/BF00216146 [DOI] [PubMed] [Google Scholar]
- Shakhova, O. , Zingg, D. , Schaefer, S. M. , Hari, L. , Civenni, G. , Blunschi, J. , … Sommer, L. (2012). Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nature Cell Biology, 14(8), 882–890. 10.1038/ncb2535 [DOI] [PubMed] [Google Scholar]
- So, S. , Davis, A. J. , & Chen, D. J. (2009). Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. Journal of Cell Biology, 187(7), 977–990. 10.1083/jcb.200906064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic, T. , Kidd, A. , Sutcliffe, A. , McGuire, G. M. , Robinson, P. , Weber, P. , … Taylor, A. (1998). ATM mutations and phenotypes in ataxia–telangiectasia families in the British Isles: Expression of mutant ATM and the risk of leukemia, lymphoma, and breast cancer. American Journal of Human Genetics, 62(2), 334–345. 10.1086/301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story, M. D. , & Durante, M. (2018). Radiogenomics. Medical Physics, 45(11), e1111–e1122. 10.1002/mp.13064 [DOI] [PubMed] [Google Scholar]
- Sutton, I. J. , Last, J. I. , Ritchie, S. J. , Harrington, H. J. , Byrd, P. J. , & Taylor, A. M. (2004). Adult‐onset ataxia telangiectasia due to ATM 5762ins137 mutation homozygosity. Annals of Neurology, 55(6), 891–895. 10.1002/ana.20139 [DOI] [PubMed] [Google Scholar]
- Terrazzino, S. , Cargnin, S. , Deantonio, L. , Pisani, C. , Masini, L. , Canonico, P. L. , … Krengli, M. (2019). Impact of ATM rs1801516 on late skin reactions of radiotherapy for breast cancer: Evidences from a cohort study and a trial sequential meta‐analysis. PLoS One, 14(11), e0225685 10.1371/journal.pone.0225685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaousis, G. N. , Papadopoulou, E. , Apessos, A. , Agiannitopoulos, K. , Pepe, G. , Kampouri, S. , … Nasioulas, G. (2019). Analysis of hereditary cancer syndromes by using a panel of genes: Novel and multiple pathogenic mutations. BMC Cancer, 19(1), 535 10.1186/s12885-019-5756-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os, N. J. H. , Chessa, L. , Weemaes, C. M. R. , van Deuren, M. , Fievet, A. , van Gaalen, J. , … Willemsen, M. (2019). Genotype‐phenotype correlations in ataxia telangiectasia patients with ATM c.3576G>A and c.8147T>C mutations. Journal of Medical Genetics, 56(5), 308–316. 10.1136/jmedgenet-2018-105635 [DOI] [PubMed] [Google Scholar]
- van Os, N. J. H. , Hensiek, A. , van Gaalen, J. , Taylor, A. M. R. , van Deuren, M. , Weemaes, C. M. R. , … van de Warrenburg, B. P. C. (2019). Trajectories of motor abnormalities in milder phenotypes of ataxia telangiectasia. Neurology, 92(1), e19–e29. 10.1212/WNL.0000000000006700 [DOI] [PubMed] [Google Scholar]
- Verhagen, M. , Abdo, W. F. , Willemsen, M. A. , Hogervorst, F. B. , Smeets, D. F. , Hiel, J. A. , … van Deuren, M. (2009). Clinical spectrum of ataxia–telangiectasia in adulthood. Neurology, 73(6), 430–437. 10.1212/WNL.0b013e3181af33bd [DOI] [PubMed] [Google Scholar]
- Verhagen, M. M. M. , Last, J. I. , Hogervorst, F. B. L. , Smeets, D. F. C. M. , Roeleveld, N. , Verheijen, F. , … Willemsen, M. A. A. P. (2012). Presence of ATM protein and residual kinase activity correlates with the phenotype in ataxia–telangiectasia: A genotype‐phenotype study. Human Mutation, 33(3), 561–571. 10.1002/humu.22016 [DOI] [PubMed] [Google Scholar]
- West, S. C. (2003). Molecular views of recombination proteins and their control. Nature Reviews Molecular Cell Biology, 4(6), 435–445. 10.1038/nrm1127 [DOI] [PubMed] [Google Scholar]
- Wood, L. M. , Sankar, S. , Reed, R. E. , Haas, A. L. , Liu, L. F. , McKinnon, P. , & Desai, S. D. (2011). A novel role for ATM in regulating proteasome‐mediated protein degradation through suppression of the ISG15 conjugation pathway. PLoS One, 6(1), e16422 10.1371/journal.pone.0016422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any additional data required are available on request.


