Abstract
Introduction
Severe hypoxia exists in placentas during early pregnancy, with reoxygenation during mid-gestation. Hypoxia-inducible factor-1α (Hif1α), an oxygen sensor, initiates placental vascular development. We have shown that the placental vasculature in Hmox1-deficient (Hmox1+/−, Het) pregnancies is impaired, with morphological defects similar to Hif1α-deficient placentas.
Materials and methods
Whole wild-type (WT) and Het mouse placentas were collected at E8.5 (1%–3% O2) and E9.5–15.5 (8%–10% O2). mRNA levels were determined using real-time RT-PCR or PCR arrays and protein levels using Western blot. Bone marrow-derived macrophages (BMDMs) from WT, Het, and Hmox1 knockout (KO) mice, representing different Hmox1 cellular levels, were generated to study the role of Hmox1 on Hif1α ′s response to hypoxia-reoxygenation and gestational age-specific placental lysates.
Results
Hif1α was expressed in WT and Het placentas throughout gestation, with protein levels peaking at E8.5 and mRNA levels significantly upregulated from E9.5–E13.5, but significantly lower in Het placentas. Genes associated with angiogenesis (Vegfa, Vegfr1, Mmp2, Cxcl12, Angpt1, Nos3), antioxidants (Sod1, Gpx1), and transcription factors (Ap2, Bach1, Nrf2) were significantly different in Het placentas. In response to in vitro hypoxia-reoxygenation and to WT or Het placental lysates, Hif1α transcription was lower in Het and Hmox1 KO BMDMs compared with WT BMDMs.
Discussion
These findings suggest that deficiencies in Hmox1 underlie the insufficient placental Hif1α response to hypoxia-reoxygenation during gestation and subsequently impair downstream placental vascular formation. Therefore, a dysregulation of Hif1α expression caused by any genetic defect or environmental influence in early pregnancy could be the root cause of pregnancy disorders.
Keywords: Angiogenesis, Spiral artery remodeling, Adverse pregnancy outcomes, Hypoxia, Antioxidant
Highlights
-
•
Expression of Hif1α in wild-type (WT) placentas is gestational age-dependent.
-
•
Hif1α expression is reduced in Hmox1-deficient placentas.
-
•
Expression of angiogenic genes is altered in Hmox1-deficient placentas.
-
•
Hypoxia-reoxygenation induces a differential expression of Hif1α in cells.
-
•
Adding placental lysates dysregulates expression of Hif1α in Hmox1-deficient cells.
Abbreviations
- ARNT
aryl hydrocarbon receptor nuclear translocator
- BM
bone marrow
- BMDMs
bone marrow-derived macrophages
- CO
carbon monoxide
- DFO
deferoxamine mesylate salt
- Hmox
heme oxygenase
- Het
Hmox1+/-
- Hif
hypoxia-inducible factor
- HRES
hypoxic response elements
- KO
knockout
- O2
oxygen
- ROS
reactive oxygen species
- SA
spiral artery
- uNK
uterine natural killer
- WT
wild-type
1. Introduction
In early pregnancy, a very critical window for placental development exists in both the human and mouse. During the 1st trimester, a severe hypoxic (1–3% oxygen [O2] or ~18 mmHg) microenvironment is present in the placenta due to the formation of a trophoblast plug within spiral arteries (SAs), which prevents the flow of maternal blood into the fetoplacental interface. During the 2nd trimester, the plug dissolves and maternal blood can now enter the intervillous spaces, thereby increasing O2 levels to 8% (60 mmHg) [[1], [2], [3]]. This normal physiological phenomenon, while resembling an ischemia-reperfusion- (or hypoxia-reoxygenation)-like injury, is crucial for normal placental vascular development. A hypoxic environment is thought to be required for mediating the attachment of the blastocyst to the uterine wall in order to initiate placental proliferation of invasive trophoblast cells and maintain the stem cell state of trophoblasts by preventing their differentiation [[4], [5], [6]]. When O2 levels increase (8%), the trophoblast stem cells begin to differentiate into their invasive phenotype, which can migrate and invade into SAs, and stimulate maternal and fetal blood vessel growth [[7], [8], [9], [10]].
The primary cellular molecule that senses and responds to O2 tensions is hypoxia-inducible factor (Hif) [[11], [12], [13]]. Hif is a global regulator, capable of transcriptionally controlling the expression of more than 1000 genes by binding to DNA sequences that contain hypoxic response elements (HRES) [14]. Therefore, HIF can affect many cellular processes in response to hypoxia, such as angiogenesis, migration/invasion, erythropoiesis, and cell metabolism [15,16]. Hif is a heterodimer protein composed of two subunits: an alpha subunit with two isoforms (Hif1α and Hif2α) and a beta subunit [Hif1β or aryl hydrocarbon receptor nuclear translocator (Arnt)]. While Hif1β is insensitive to O2, Hif1α is degraded rapidly in the presence of O2.
Hif1α is found at high levels at 7–9 weeks of gestation when the O2 level in the placental microenvironment is 2%–3% and at low levels at 10–12 weeks of gestation when the O2 level is 8% to 10% [2,3]. Studies using Hif1 knockout (KO) mice showed that the disruption of either the Hif1α or Hif1β subunit in fetal trophoblasts results in improper placental development, which is characterized by an insufficient chorion/allantois fusion and a diminished spongiotrophoblast and labyrinth layer [17,18]. Moreover, maternal Hif1α is also required for placentation by recruiting uterine natural killer (uNK) cells and trophoblasts into the maternal decidua and by affecting trophoblast function [17]. Therefore, insufficient Hif1α expression in either the maternal or fetal side of the placenta may be associated with impaired vascular development similar to that seen in preeclampsia [6].
Heme oxygenase (Hmox) is the enzyme that degrades heme to produce equimolar amounts of carbon monoxide (CO), iron, and biliverdin/bilirubin [19]. It is also a stress-response protein, since HO and its metabolites exhibit significant antioxidative [20], cytoprotective [21], pro-angiogenic [22], and anti-inflammatory [23] properties. Low expression of Hmox1, the inducible isoform, has been associated with a number of human pregnancy disorders [[24], [25], [26]]. In addition, our previous studies have shown that pregnant Hmox1-deficient (Hmox1+/−, Het) mice have preeclampsia-like features, such as hypertension and high sFlt 1 levels [27,28]. Het placentas also have insufficient SA remodeling and altered uNK cell differentiation and maturation [27,29,30]. Histological examination of Het placentas further revealed a markedly thinner spongiotrophoblast/giant cells layer compared with WT placentas [28,31]. Interestingly, many of these placental abnormalities resemble the phenotypes found in the pregnant Hif1α-deficient mouse.
Therefore, we hypothesized that a deficiency in Hmox1 induces the dysregulation of Hif1α, which subsequently causes placental malformation and dysfunction. To this end, we compared Hif1α protein and mRNA levels throughout gestation between wild-type (WT) and Het placentas and investigated the response of Hif1α to either hypoxia-reoxygenation or placental lysates in bone marrow-derived macrophages (BMDMs) generated from WT, Het, and Hmox1 KO mice.
2. Materials and methods
All studies were approved by Stanford University's Institutional Animal Care and Use Committee (Protocol #14525) and conducted in adherence to the National Institutes of Health Guidelines on the Use of Laboratory Animals.
2.1. Animals
Adult WT FVBn mice were purchased from Charles River Laboratories (Wilmington, MA). Adult female and male Hmox1 Het pairs were used from our mouse colony. The original Hmox1 KO (Hmox1−/−) mouse strain has a targeted deletion of a large portion of Hmox1 gene, and was created on a C57BL/6 background. To establish our Het colony, C57BL/6 Hmox1 mice were backcrossed with FVBn WT mice for >6 generations [28]. WT or Het mice were mated at 8–12 weeks of age. All animals were maintained and bred according to institutional guidelines of Stanford University. Gestational ages were calculated by the presence of a vaginal plug and recorded as E0.5. Het or Hmox1 KO mice were genotyped using PCR as described previously [28].
2.2. Gene analyses
To create a customized PCR array, primers were chosen from the PrimerBank-MGH-PGA (Harvard University, Cambridge, MA) and validated by PCR screening. Specific genes were selected that encode for proteins: Hmox1, Hmox2, Hif1α, Hif2α, angiogenic factors (placental growth factor [Pgf]; stromal cell-derived factor 1 [Cxcl12]; vascular endothelial growth factor A [Vegfa]; matrix metalloproteinase-9 [Mmp9]; and matrix metalloproteinase-2 [Mmp2], angiopoietin-1 [Angpt1], nitric oxide synthase [Nos2 and Nos3]), receptors (Vegr1, Vegf 2, C-X-C chemokine receptor 4 [Cxcr4]); antioxidant proteins (superoxide dismutase-1 [Sod1], −2 [Sod2]; glutathione peroxidase [Gpx1]), cyclin-dependent kinase inhibitor 1 (Cdkn1A, P21), transcription factors (Sp1, Ap2, Nrf2, and Bach1), and housekeeping genes (Actb and Gapdh) [32]. Primer sequences are shown in Table 1.
Table 1.
Primer sequences.
| Primers | |
|---|---|
| Hmox1 | (F) 5′-AAGCCGAGAATGCTGAGTTCA-3′ |
| (R) 5′-GCCGTGTAGATATGGTACAAGGA-3′ | |
| Hmox2 | (F) 5′-GGAGGGGGTAGATGAGTCAGA-3′ |
| (R) 5′-TCGGTCATGTGCTTCCTTGGT-3′ | |
| Hif1α | (F) 5′-TCTCGGCGAAGCAAAGAGTC-3′ |
| (R) 5′-AGCCATCTAGGGCTTTCAGATAA-3′ | |
| Hif2α | (F) 5′-GTGACATGATCTTTCTGTCGGAA-3′ |
| (R) 5′-CGCAAGGATGAGTGAAGTCAAA-3′ | |
| Pgf | (F) 5′-TGCTGGGAACAACTCAACAGAA-3′ |
| (R) 5′-TCTCCATGGGCCGACAGTAG-3′ | |
| Mmp2 | (F) 5′-ACCTGAACACTTTCTATGGCTG-3′ |
| (R) 5′-CTTCCGCATGGTCTCGATG-3′ | |
| Mmp9 | (F) 5′-GCAGAGGCATACTTGTACCG-3′ |
| (R) 5′-TGATGTTATGATGGTCCCACTTG-3′ | |
| Vegfa | (F) 5′-GCACATAGAGAGAATGAGCTTCC-3′ |
| (R) 5′-CTCCGCTCTGAACAAGGCT-3′ | |
| Vegfr1 | (F) 5′-AGGGATAACAGGCAATTCTGC-3′ |
| (R) 5′-GTGCATCTCTATGAAAGGACTCC-3′ | |
| Vegfr2 | (F) 5′-GTGATCCCAGATGACAGCCA-3′ |
| (R) 5′-GGTGAGCGCAGTGTGGTCC-3′ | |
| Cxcl12 | (F) 5′-TGCATCAGTGACGGTAAACCA-3′ |
| (R) 5′-CACAGTTTGGAGTGTTGAGGAT-3′ | |
| Cxcr4 | (F) 5′-GAAGTGGGGTCTGGAGACTAT-3′ |
| (R) 5′-TTGCCGACTATGCCAGTCAAG-3′ | |
| P21 | (F) 5′-CGAGAACGGTGGAACTTTGAC-3′ |
| (R) 5′-CCAGGGCTCAGGTAGACCTT-3′ | |
| Angpt1 | (F) 5′-ATCCCGACTTGAAATACAACTGC-3′ |
| (R) 5′-CTGGATGATGAATGTCTGACGAG-3′ | |
| Nos2 | (F) 5′-GGAGCCTTTAGACCTCAACAGA-3′ |
| (R) 5′-TGAACGAGGAGGGTGGTG-3′ | |
| Nos3 | (F) 5′-CCTTCCGCTACCAGCCAGA-3′ |
| (R) 5′-CAGAGATCTTCACTGCATTGGCTA-3′ | |
| Sod1 | (F) 5′-AACCAGTTGTGTTGTCAGGA-C3′ |
| (R) 5′-CCACCATGTTTCTTAGAGTGAGG-3′ | |
| Sod2 | (F) 5′-CCAAGGGAGATGTTACAACTCAG-3′ |
| (R) 5′-GGGCTCAGGTTTGTCCAGAA-3′ | |
| Gpx1 | (F) 5′-AGTCCACCGTGTATGCCTTCT-3′ |
| (R) 5′-GAGACGCGACATTCTCAATGA-3′ | |
| Ap2 | (F) 5′-TTTTTCAGCTATGGACCGTCAC-3′ |
| (R) 5′-GAAGTCGGCATTAGGGGTGTG-3′ | |
| Nrf2 | (F) 5′-TCTTGGAGTAAGTCGAGAAGTGT-3′ |
| (R) 5′-GTTGAAACTGAGCGAAAAAGGC-3′ | |
| Sp1 | (F) 5′-TGCAAACCAACAGATCATCCC-3′ |
| (R) 5′-TGACAGGTAGCAAGGTGATGT-3′ | |
| Bach1 | (F) 5′-GCCTGAAGAGGTAACGGTTAAA-3′ |
| (R) 5′-GCACACTTCGTCAACATTGTC-3′ | |
| Actb | (F) 5′-AAGGAGATTACTGCTCTGGCTCCTA-3′ |
| (R) 5′-ACTCATCGTACTCCTGCTTGCTGAT-3′ | |
| Gapdh | (F) 5′-TGACCTCAACTACATGGYCTACA-3′ |
| (R) 5′-CTTCCCATTCTCGGCCTTG-3′ |
Total RNA was extracted from whole WT, Het, and Hmox1 KO placentas (including deciduas, junction zones, and labyrinths) at different gestational ages using a Trizol Reagent (Thermo Scientific, Waltham, MA). cDNA was synthesized using a RT2 First Strand Kit (Qiagen, Redwood City, CA). Real-time RT-PCR was then performed using the RT2 Real-Time SYBR Green/ROX PCR Master Mix (Qiagen) on a Stratagene Mx3005P QPCR system (Agilent Technologies, Palo Alto, CA). Data were then analyzed using the ΔΔCt method, normalized to Actb and Gapdh. Fold change in gene expression over control levels were then calculated.
2.3. Western blots
Whole placental samples were sonicated and prepared as previously described [33]. 2 mM deferoxamine mesylate salt (DFO, Sigma-Aldrich, St. Louis, MO) was then added to prevent Hif1α degradation [34]. 100 μg of sonicate was boiled for 10 min in protein loading buffer and separated using 12% SDS-PAGE gel electrophoresis (BioRad, Hercules, CA). Proteins were transferred to a PDVF membrane (Bio-Rad) using a semidry transblotter (Bio-Rad). The membrane was probed with polyclonal antibodies raised against Hif1α (Invitrogen, Carlsbad, CA) and Actb (Santa Cruz Biotechnology, Santa Cruz, CA). Signals were detected using a SuperSignal™ West Pico PLUS chemiluminescent substrate (Thermofisher, Waltham, MA) and band intensities were quantitated using Image Studio Lite software (LI-COR Biosciences, Lincoln, NE). Coomassie blue staining was performed to confirm equal loading of samples.
2.4. In vitro establishment and stimulation of BMDMs
BMDMs were generated from bone marrow (BM) cells isolated from WT, Het, and Hmox1 KO mice as described [32]. In brief, 1 × 107 BM cells in 10 ml media (106 cells/ml) were seeded onto 100-mm Petri-dish plates (Fisher Scientific, Pittsburgh, PA) and maintained in 20% L929 conditional RPMI medium with β-mercaptoethanol. At day 3, half of the media was replaced with fresh media. At day 7, attached cells were treated with IL-4 (20 ng/ml, Miltenyi Biotec, Sunnyvale, CA) to differentiate M1 cells into M2a cells.
2.5. Study design
2.5.1. Hypoxia studies
M2a cells were exposed for 24 h at an O2 concentration of 21%, 5%, 1%, or 1%–21% using a hypoxia incubator (Thermo Scientific).
2.5.2. Placental lysate studies
Placentas from pregnant WT and Het mice at E8.5 or E10.5 were collected in RPMI medium. Tissues were homogenized, centrifuged, and filtered through 0.22-μm columns to yield crude lysates for treatment of BMDMs.
2.6. Statistical analyses
For comparisons of experimental groups, one-way ANOVAs were first performed for each set of experiments to determine significant differences when P < 0.05. To determine differences between individual experimental and control groups, Dunnett's tests were used which adjusts for multiple comparisons using the same control group.
3. Results
3.1. Expression of Hif1α in WT placentas is gestational age-dependent
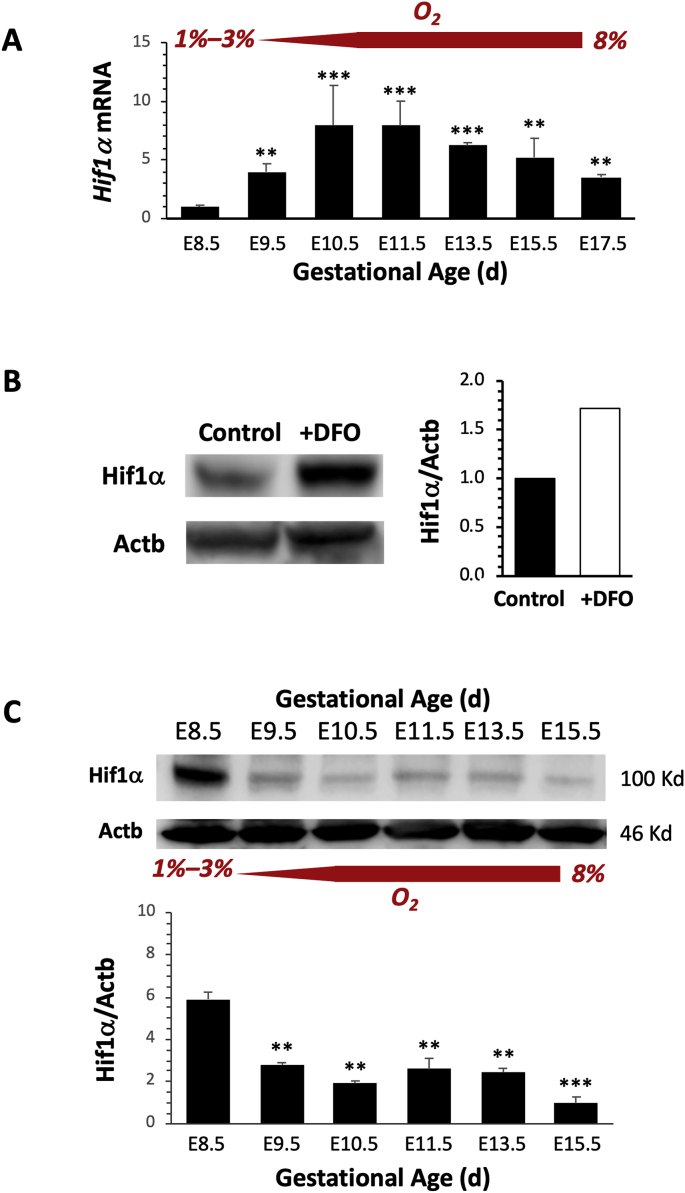
To investigate whether Hif1α expression in WT placentas change throughout gestation, we collected placentas at E8.5 to E17.5 and measured Hif1α mRNA levels using real-time RT-PCR. Significant upregulation of Hif1α mRNA was observed beginning at E9.5, when O2 levels are at 8% compared with O2 levels of 1%–3% at E8.5. Levels peaked between E10.5 to E11.5, and gradually decreased thereafter (Fig. 1A).
Fig. 1.
Expression of Hif1α is gestational age-dependent. (A) Hif1α mRNA levels at various gestational ages (n = 3 at each age) were measured using real-time RT-PCR and normalized to Gapdh and Actb levels. Significant upregulation was observed when the placental oxygen (O2) microenvironment increased from 3% to 8% at E9.5 to 10.5. (B) Addition of DFO to placental samples (white bar) helped to preserve Hif1α protein from degradation under ambient oxygen conditions as compared with control (no DFO) samples (black bar) as shown by Western blots. (C) Representative images of Western blots of Hif1α and Actb protein levels (top panel). Band intensities were quantified and normalized to Actb (n = 3). *P < 0.05; **P < 0.01; ***P < 0.005.
To quantitate Hif1α protein levels, WT placentas were collected at E8.5 to E15.5 and processed for Western blots. To prevent Hif1α degradation during ambient O2 exposure, DFO, an iron chelator, was added to the samples during homogenization [35]. For samples without DFO (controls), lysates were prepared as fast as possible to limit exposure to air (normally within 1 min or less) before the addition of loading buffer. We found that addition of DFO yielded up to 70%–80% more Hif1α protein compared with control samples (Fig. 1B). Therefore, for all placental samples processed for Western blot analysis, DFO was added. We found that Hif1α protein levels in WT placentas peaked at E8.5 when the placenta was under severe hypoxia, and decreased from E9.5 to E15.5 when O2 levels increased (Fig. 1C).
3.2. Hif1α expression is reduced in Hmox1-deficient placentas
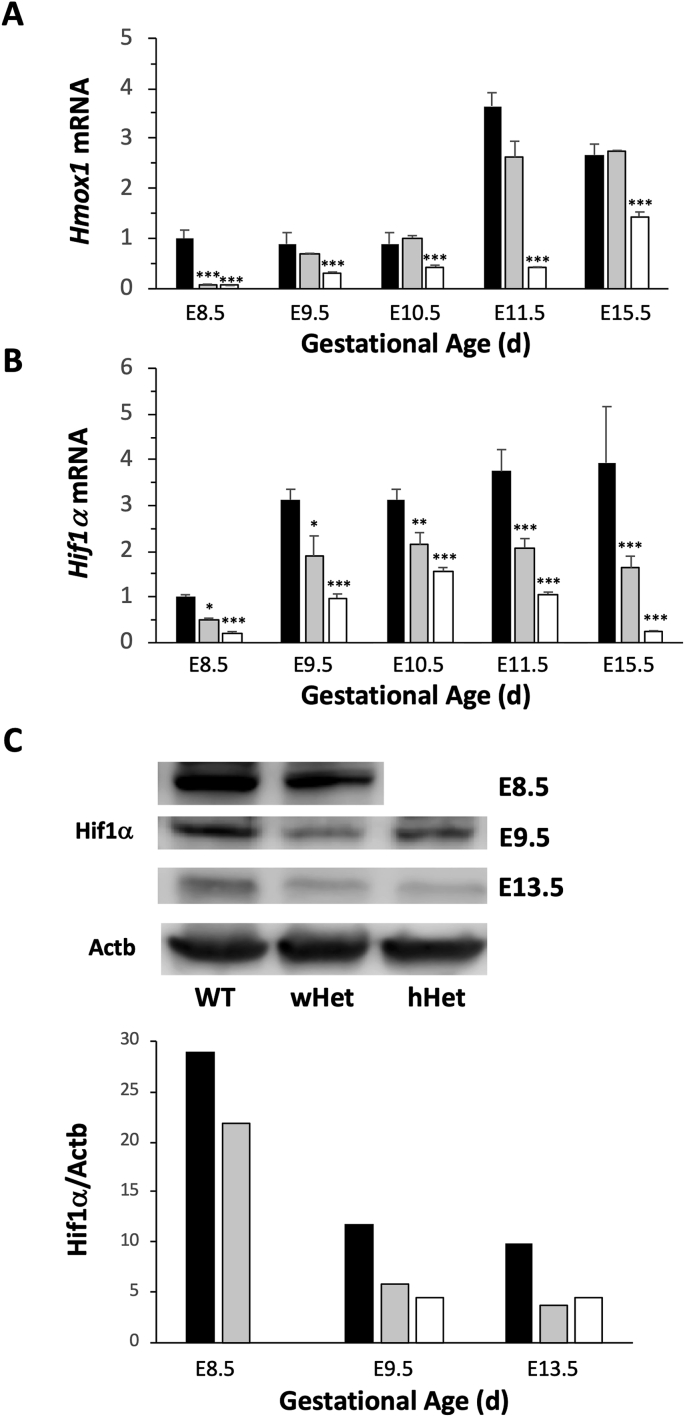
In Het pregnancies where Het females are mated with Het males, either WT or Het, but no Hmox1 KO, fetuses are present as observed previously [27,28]. After genotyping fetuses from Het pregnancies, placentas were then designated as follows: wHet for placentas from WT fetuses; hHet for placentas from Het fetuses; and WT for placentas from WT to WT breedings. Hmox1 deficiency was confirmed using RT-PCR, which showed significant reduction of Hmox1 in both wHet and hHet placentas at E8.5 compared with age-matched WT placentas (Fig. 2A). At E85, placentas are primarily composed of maternal uteri tissue or decidua. However, Hmox1 was significantly reduced only in hHet, but not in wHet, placentas at E10.5 to E15.5, when placentas are primarily composed of labyrinth tissue, the majority of which originate from the fetus, in wHet placentas.
Fig. 2.
Het placentas have significantly reduced Hif1α expression. (A) Hmox1 deficiency in placentas from Het pregnancies with WT fetuses (wHet, gray bars, n = 3 at each age) or Het fetuses (hHet, white bars, n = 3 at each age) was confirmed by measuring mRNA levels using real-time RT-PCR, normalized to Gapdh and Actb levels, and compared with those fetuses from WT pregnancies (WT, black bars, n = 3 at each age). (B) Hif1α mRNA expression was measured in placentas at various gestational ages harvested from WT pregnancies (WT, black bars, n = 3 at each age), Het pregnancies with WT fetuses (wHet, gray bars, n = 3 at each age) or Het fetuses (hHet, white bars, n = 3 at each age). Hif1α expression was normalized to Gapdh and Actb levels. (C) Hif1α protein levels in placentas at various gestational ages harvested from Het pregnancies with WT fetuses (wHet, gray bars) or Het fetuses (hHet, white bars) compared with placentas from WT pregnancies (black bars) are shown by Western blots. Hif1α expression was normalized to Actb levels. Due to the extremely small size of fetuses at E8.5, standard genotyping could not be performed. Therefore, Hmox1 mRNA levels were used to differentiate wHet and hHet placentas for mRNA studies, but was not feasible for Western blots. *P < 0.05; **P < 0.01; ***P < 0.005.
When we compared Hif1α mRNA expression in WT and Het placentas during pregnancy, we found a similar gestational age-dependent profile with an upregulation of transcription beginning at E9.5 and peaking around E10.5 to E11.5 (Fig. 2B). However, Hif1α mRNA levels in wHet placentas were significantly lower than those in WT placentas. Furthermore, Hif1α mRNA levels in hHet placentas were even lower than those in wHet placentas, which suggests that a reduction Hif1α mRNA is associated with Hmox1 deficiency. These findings were confirmed by quantification of Hif1α protein levels using Western blots, where we found decreased Hif1α protein levels in wHet and hHet placentas compared with those in WT placentas at E8.5, E9.5, and E13.5 (Fig. 2C).
3.3. Expression of angiogenic genes is altered in Hmox1-deficient placentas
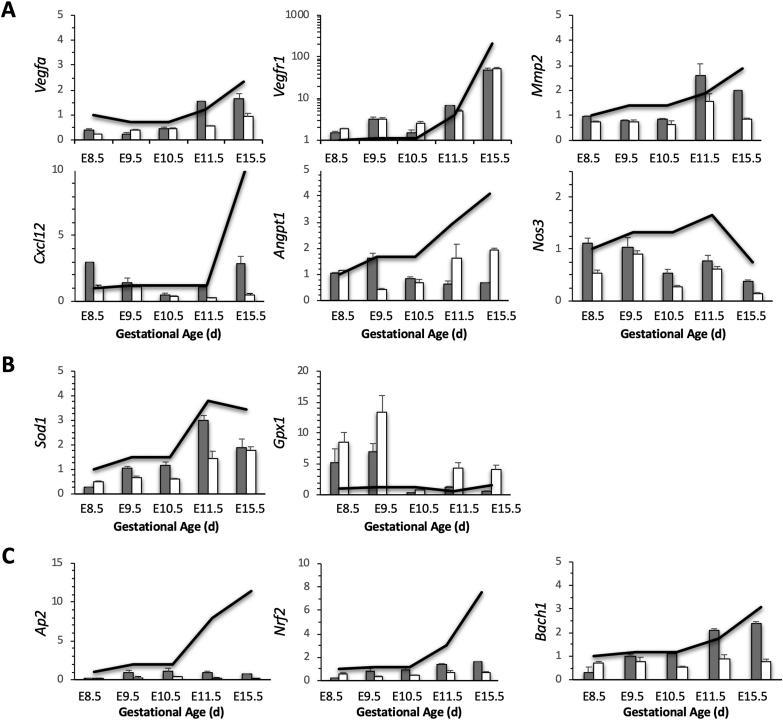
Using a custom-made PCR-array [32], we compared genes that are related to hypoxia-reoxygenation between WT and Het placentas. Expression of several genes associated with angiogenesis in wHet or hHet placentas were either suppressed (such as Vegfa, Mmp2, Cxcl12, Angpt1, and Nos3) or induced (e.g., Vegfr1) compared with WT placentas. The alterations in Vegfa, Vegfr1, and Mmp2 occurred early in pregnancy (E8.5 to E10.5), while changes in Cxcl12, Angpt1, and Nos3 were primarily observed at mid-to-late gestation (E11.5 to E15.5) (Fig. 3A). In addition, two antioxidant genes, Sod1 and Gpx1, were decreased or increased, respectively, especially in hHet placentas (Fig. 3B). Expression of transcription factors, Ap2 and Nrf2, were reduced in both wHet and hHet placentas, while Bach1 was down-regulated only in hHet, but not in wHet placentas (Fig. 3C). Hmox1 deficiency had no significant effects on the expression of Hif2α or Pgf (data not shown).
Fig. 3.
Differential gene expression profiles. mRNA levels of (A) angiogenic-associated genes; (B) antioxidant genes; and (C) transcription factors were measured using PCR arrays and compared between WT (black line, n = 3), wHet (gray bar, n = 3), and hHet (white bar, n = 3) placentas. All levels were normalized to Gapdh and Actb levels.
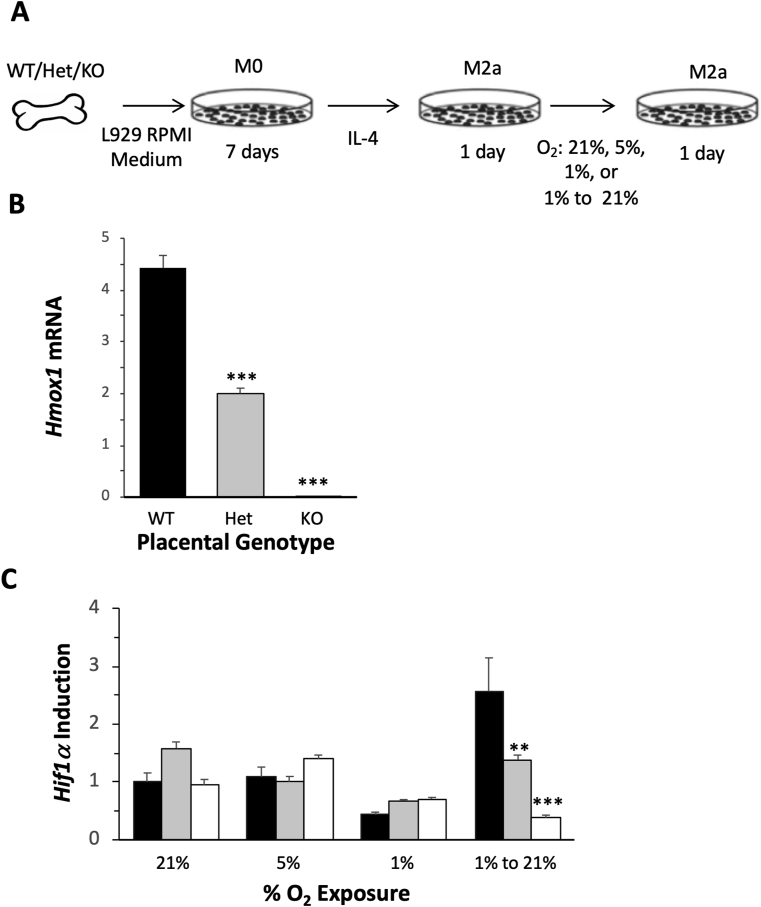
3.4. Hypoxia-reoxygenation induces the differential expression of Hif1α in BMDMs
To further investigate whether Hmox1 deficiency underlies the differential expression of Hif1α in response to hypoxia-reoxygenation, we first generated BMDMs from non-pregnant WT, Het, and Hmox1 KO mice and then treated these cells with IL-4 to differentiate these M0 cells into M2a cells. These cells were further exposed to different oxygen tensions: 21%, 5%, 1%, and 21% after 1% pretreatment. Our experimental design is outlined in Fig. 4A. After 24 h, cells were harvested and mRNA was isolated. Hmox1 deficiency in Het and Hmox1 KO cells were confirmed by RT-PCR (Fig. 4B). When cells were exposed to 21%, 5%, or 1% O2, no significant differences in Hif1α mRNA levels were observed between WT, Het, or Hmox1 KO cells. However, when cells that were initially exposed to O2 at 1% and then re-exposed to 21% to mimic the hypoxia-reoxygenation environment observed in early pregnancy, induction of Hif1α transcription was found to vary significantly between genotypes (Fig. 4C). Compared with mRNA levels at 1% O2, Hif1α was significantly upregulated in WT cells (~6-fold), but less increased in Het cells (~2-fold). However, Hif1α was not induced in Hmox1 KO cells (~0.5 fold), suggesting that the response of Hif1α to hypoxia-reoxygenation is regulated by the level of Hmox1 expression.
Fig. 4.
Differential expression of Hif1α in WT, Het, or Hmox1 KO cells in response to hypoxia-reoxygenation exposures. (A) Graphic of our study design. (B) Hmox1 expression in WT (black bar, n = 3), Het (gray bar, n = 3), and Hmox1 KO (white bar, n = 3) M2a cells. (C) Hif1α mRNA levels were measured after WT (black bars, n = 3 at each O2 level), wHet (gray bars, n = 3 at each O2 level), and hHet (white bars, n = 3 at each O2 level) M2a cells were exposed to 21%, 5%, 1%, or 1 to > 20% O2 levels. Hmox1 and Hif1α expression levels were normalized to Gapdh and Actb levels. *P < 0.05; **P < 0.01; ***P < 0.005.
3.5. Addition of placental lysates dysregulates expression of Hif1α in BMDMs
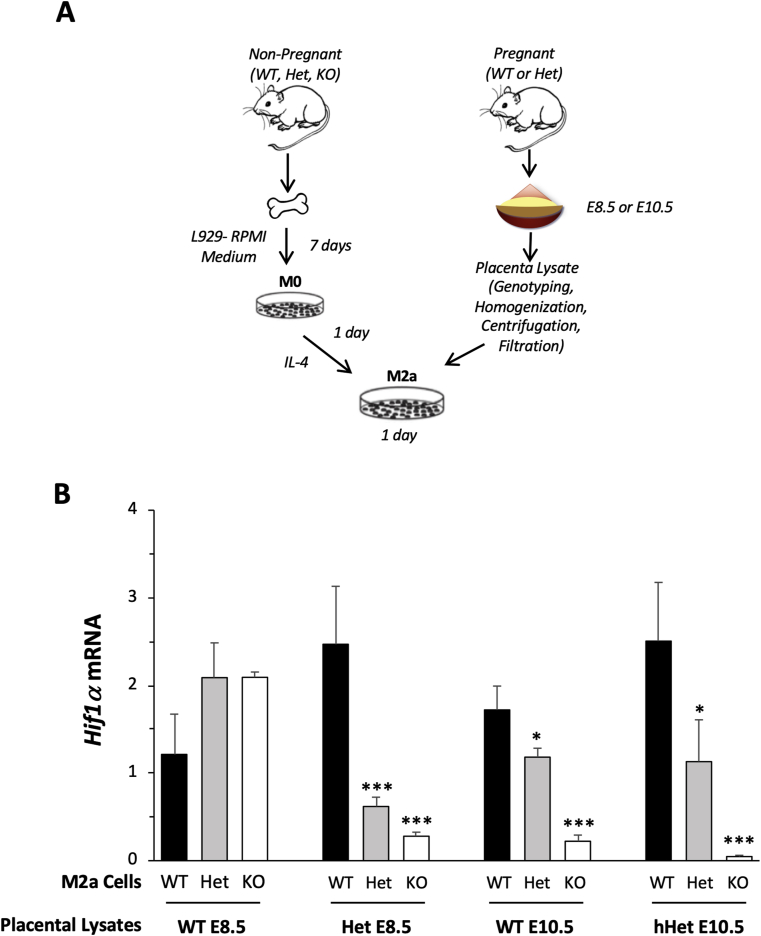
To investigate whether the dysregulation of Hif1α expression in Het placentas was due to insufficient Hmox1 expression in the cells or due to the Hmox1 deficiency-induced alterations in the placental microenvironment, we generated BMDMs from WT, Het, or Hmox1 KO mice and exposed these cells with lysates prepared from WT or Het placentas harvested at two gestational ages, E8.5 and E10.5, when O2 levels are low and high, respectively (Fig. 5A).
Fig. 5.
Differential Hif1α expression in response to the addition of placental lysates. (A) Graphic of our study design. (B) M2a cells with different Hmox1 genetic backgrounds (WT, black bars, n = 3; wHet, gray bars, n = 3; and hHet, white bars, n = 3) were treated with WT or Het lysates prepared from placentas harvested at E8.5 and E10.5. Hif1α mRNA levels were measured using real-time RT-PCR after 24 h of treatment and normalized to Gapdh and Actb levels. *P < 0.05; **P < 0.01; ***P < 0.005.
Hif1α induction was expressed as the relative fold change in Hif1α levels from the placental-lysate-treated cells over basal levels from non-treated control M2a cells. Differential expression of Hif1α in WT, Het, or Hmox1 KO M2a cells was not found after treatment with E8.5 WT placental lysates, but observed after treatment with WT placental lysates at E10.5 or Het placental lysates from E8.5 or E10.5, in a Hmox1 dose-dependent fashion (Fig. 5B). These data suggested that both cellular Hmox1 levels and extracellular factors could contribute to Hif1α expression in the placenta.
4. Discussion
We found that the severe hypoxic environment in the placenta during early mouse pregnancies can trigger Hif1α signaling, similar to that reported for human pregnancies [36]. Hif1α is regulated both at the transcriptional as well as post-translational levels. The half-life of Hif1α protein is short, being only about 5 min in ambient air [37], and likely contributes to the lack of correlation between Hif1α mRNA and Hif1α protein levels [36], as we have found in our studies (Fig. 1). We found that the severe hypoxic placental microenvironment at E8.5 sustained a high Hif1α protein level due to its low degradation at this O2 level. In contrast, higher O2 levels during E9.5 to 10.5 decreased protein levels, but increased Hif1α transcription (Fig. 1). This Hif1α transcriptional profile in the mouse placenta is consistent with the findings reported in human placental studies by Ietta et al., where Hif1α protein levels are highest around 7 to 10 gestational weeks when severe hypoxia is present, while mRNA levels peak between 14 to 18 gestational weeks when reoxygenation occurs [36].
The upregulation of Hif1α mRNA at E9.5 to E11.5 apparently was likely not due to hypoxia, but possibly due to the presence of other factors, such as reactive oxygen species (ROS), growth factors, and inflammatory cytokines. Due to their ability to induce Hif1α expression, these factors are also called “pseudo-hypoxia factors” [15]. We speculate that at the end of the 1st trimester, placental O2 levels rapidly increase, which can then lead to a transient period of oxidative stress as well as increased inflammatory cytokine levels. This is supported by our observations that certain antioxidant genes, such as Sod1 and Hmox1, significantly increase from E9.5 to E11.5 (Fig. 3B). In addition, when we treated M2a cells with different fractions of placental lysates, which were separated based on molecular weight, the highest induction of Hif1α was found in the < 3-kd fraction (unpublished data), which contains the highest levels of ROS.
Even though HIF1α has been shown to regulate Hmox1 expression [38], the effect of Hmox1 levels on Hif1α expression has not as yet been elucidated. Here, we show evidence that Hmox1 deficiency could induce the dysregulation of Hif1α in the placenta. Compared with WT placentas, significantly lower Hif1α mRNA and Hmox1 protein levels were found in Het placentas throughout all gestational ages (Fig. 2). This finding may possibly explain why Het placentas, especially hHet placentas, share similar morphological defects with those placentas from Hif1α-deficient pregnancies [39]. Since Hif1α also triggers the differentiation of trophoblast stem cells to an invasive phenotype [4], it is not surprising that Hmox1-deficient placentas have insufficient SA remodeling and impaired vascular development in the labyrinth due to lower Hif1α levels [27].
Hif1α is a global regulator that controls the expression of many genes, including Vegfa and Vegfr1 [32], which are critical for placental vasculogenesis and branching angiogenesis in early pregnancy. In our study, we found that placental Vegfa and Vegfr1 were significantly altered as early as E8.5 in Hmox1-deficient placentas (both wHet and hHet) compared with WT placentas (Fig. 3A), suggesting they may be controlled directly by Hif1α. Interestingly, no significantly changes were found in Hif2α as well as Pgf, another very important placental angiogenic factor that binds to Vegfr. These results support the finding that Pgf in trophoblasts is mostly regulated by Hif2α [40]. In addition, we also observed that the levels of other angiogenic-associated genes, such as Mmp2, Cxcl12, Angpt1, and Nos3, were decreased, but at a relatively later stage (E10 or so) (Fig. 3A), suggesting an indirect control by Hif1α, via a secondary event due to either a deficiency in Vegf expression or an impairment of SA remodeling.
To further elucidate whether the decrease in Hif1α expression in Hmox1 deficiency placenta is primarily due to the manipulation of the cellular molecular signaling or the changes of placental microenvironment, we used cultured WT, Het, and Hmox1 KO BMDMs treated with different O2 tensions or lysates from WT or Het placentas. BM and its derived cells (BMDMs) are relatively easier to collect, differentiate, and maintain compared with other primary cells, such as trophoblasts or endothelial cells. In these studies, we found that a deficiency of Hmox1 (either Het or KO) alone is not associated with the differential Hif1α expression at either normoxia or hypoxia; but only at the transition state from hypoxia to reoxygenation (Fig. 4C). It also explains the results of our placental lysate studies, which showed that lysates at E10.5, when the placentas are re-exposed to increased O2 levels caused a dysregulation of Hif1α in Hmox1-deficient M2a cells (Fig. 5B). In addition, Het placental lysates, but not WT, at E8.5 also induced the dysregulation in Het or Hmox1 KO BMDM cells, suggesting abnormal tissue stress in the Het placenta in early pregnancy. Taken together, these data suggested that failure to respond to the oxidative stress in Hmox1-deficient cells is associated with Hif1α suppression. Even though the molecular mechanism that cross-links Hmox1 and Hif1α is not yet understood, we speculate that it might be associated with oxidative stress and mitochondria function [[41], [42], [43], [44], [45], [46]].
We are also aware of the limitations of this study. Only whole placental tissues were used for measurements of Hif1α expression, and therefore the identification of the specific cell type(s) affected by Hmox1 deficiency was not possible. Previous work has shown that Hmox1 is expressed primarily by trophoblast cells, such as invasive trophoblasts and spongiotrophoblast cells [47]. Thus, we speculate that a down-regulation of Hif1α is caused by a deficiency in Hmox1, which could also occur in these trophoblast cells, and are well known for their role in SA remodeling, the failure of which is likely a contributing cause of preeclampsia.
The establishment of the fetomaternal interface is intricately regulated by O2 tensions in early pregnancy, followed by an increased in vascular formation via angiogenesis in later pregnancy. Therefore, the dysregulation of Hif1α, resulting in either a down- or upregulation, would have detrimental effects on SA remodeling and subsequent abnormal fetoplacental vascular formation [48]. Our findings reveal that a deficiency in Hmox1 could alter Hif1α expression in response to O2 tensions in the placental microenvironment and cause an alteration of the downstream genes associated with angiogenesis. Our model has provided an example of how pregnancy disorders can be affected by a dysregulation of Hif1α induced by other genetic mutations. Further research is needed to identify other gene or combinations of genes that can also affect Hif1α expression in response to hypoxia-reoxygenation, improving our understanding of placental pathology and possibly guiding therapeutic approaches to various pregnancy disorders involving placental vascular maldevelopment.
Statement of financial support
This work was supported by the Prematurity Research Fund; the March of Dimes Prematurity Research Center at Stanford University; the Charles B. and Ann L. Johnson Research Fund; the Christopher Hess Research Fund; the Providence Foundation Research Fund; the Roberts Foundation Research Fund; the Stanford Maternal and Child Health Research Institute; and the Bill and Melinda Gates Foundation.
Declaration of competing interest
All the authors confirm that they have the guidance on competing interests and none of the authors have any competing interests in the manuscript.
Acknowledgments
We like to thank Dr. Virginia D. Winn for providing technical/equipment support for these studies.
References
- 1.Hustin J., Schaaps J.P. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am. J. Obstet. Gynecol. 1987;157:162–168. doi: 10.1016/s0002-9378(87)80371-x. [DOI] [PubMed] [Google Scholar]
- 2.Jauniaux E., Watson A.L., Hempstock J., Bao Y.P., Skepper J.N., Burton G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss G., Sundl M., Glasner A., Huppertz B., Moser G. The trophoblast plug during early pregnancy: a deeper insight. Histochem. Cell Biol. 2016;146:749–756. doi: 10.1007/s00418-016-1474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gultice A.D., Kulkarni-Datar K., Brown T.L. Hypoxia-inducible factor 1alpha (HIF1A) mediates distinct steps of rat trophoblast differentiation in gradient oxygen. Biol. Reprod. 2009;80:184–193. doi: 10.1095/biolreprod.107.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myatt L., Cui X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 6.Fryer B.H., Simon M.C. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- 7.Caniggia I., Winter J., Lye S.J., Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 8.Caniggia I., Mostachfi H., Winter J., Gassmann M., Lye S.J., Kuliszewski M., Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J. Clin. Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genbacev O., Joslin R., Damsky C.H., Polliotti B.M., Fisher S.J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genbacev O., Zhou Y., Ludlow J.W., Fisher S.J. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 11.Semenza G.L., Nejfelt M.K., Chi S.M., Antonarakis S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G.L., Semenza G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E., Wykoff C.C., Pugh C.W., Maher E.R., Ratcliffe P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 14.Semenza G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi Y., Yokota A., Harada H., Huang G. Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1alpha in cancer. Canc. Sci. 2019;110:1510–1517. doi: 10.1111/cas.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb J.D., Coleman M.L., Pugh C.W. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell. Mol. Life Sci. 2009;66:3539–3554. doi: 10.1007/s00018-009-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenchegowda D., Natale B., Lemus M.A., Natale D.R., Fisher S.A. Inactivation of maternal Hif-1alpha at mid-pregnancy causes placental defects and deficits in oxygen delivery to the fetal organs under hypoxic stress. Dev. Biol. 2017;422:171–185. doi: 10.1016/j.ydbio.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adelman D.M., Gertsenstein M., Nagy A., Simon M.C., Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenhunen R., Marver H.S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 20.Turkseven S., Kruger A., Mingone C.J., Kaminski P., Inaba M., Rodella L.F., Ikehara S., Wolin M.S., Abraham N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- 21.Bach F.H. Heme oxygenase-1: a therapeutic amplification funnel. Faseb. J. 2005;19:1216–1219. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- 22.Cudmore M., Ahmad S., Al-Ani B., Fujisawa T., Coxall H., Chudasama K., Devey L.R., Wigmore S.J., Abbas A., Hewett P.W., Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 23.Cho R.L., Lin W.N., Wang C.Y., Yang C.C., Hsiao L.D., Lin C.C., Yang C.M. Heme oxygenase-1 induction by rosiglitazone via PKCalpha/AMPKalpha/p38 MAPKalpha/SIRT1/PPARgamma pathway suppresses lipopolysaccharide-mediated pulmonary inflammation. Biochem. Pharmacol. 2018;148:222–237. doi: 10.1016/j.bcp.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Denschlag D., Marculescu R., Unfried G., Hefler L.A., Exner M., Hashemi A., Riener E.K., Keck C., Tempfer C.B., Wagner O. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol. Hum. Reprod. 2004;10:211–214. doi: 10.1093/molehr/gah024. [DOI] [PubMed] [Google Scholar]
- 25.Kaartokallio T., Klemetti M.M., Timonen A., Uotila J., Heinonen S., Kajantie E., Kere J., Kivinen K., Pouta A., Lakkisto P., Laivuori H. Microsatellite polymorphism in the heme oxygenase-1 promoter is associated with nonsevere and late-onset preeclampsia. Hypertension. 2014;64:172–177. doi: 10.1161/HYPERTENSIONAHA.114.03337. [DOI] [PubMed] [Google Scholar]
- 26.Kaartokallio T., Utge S., Klemetti M.M., Paananen J., Pulkki K., Romppanen J., Tikkanen I., Heinonen S., Kajantie E., Kere J., Kivinen K., Pouta A., Lakkisto P., Laivuori H. Fetal microsatellite in the heme oxygenase 1 promoter is associated with severe and early-onset preeclampsia. Hypertension. 2018;71:95–102. doi: 10.1161/HYPERTENSIONAHA.117.10425. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H., Azuma J., Kalish F., Wong R.J., Stevenson D.K. Maternal heme oxygenase 1 regulates placental vasculature development via angiogenic factors in mice. Biol. Reprod. 2011;85:1005–1012. doi: 10.1095/biolreprod.111.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H., Wong R.J., Kalish F.S., Nayak N.R., Stevenson D.K. Effect of heme oxygenase-1 deficiency on placental development. Placenta. 2009;30:861–868. doi: 10.1016/j.placenta.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zenclussen M.L., Casalis P.A., El-Mousleh T., Rebelo S., Langwisch S., Linzke N., Volk H.D., Fest S., Soares M.P., Zenclussen A.C. Haem oxygenase-1 dictates intrauterine fetal survival in mice via carbon monoxide. J. Pathol. 2011;225:293–304. doi: 10.1002/path.2946. [DOI] [PubMed] [Google Scholar]
- 30.Zenclussen M.L., Linzke N., Schumacher A., Fest S., Meyer N., Casalis P.A., Zenclussen A.C. Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy. Front. Pharmacol. 2014;5:291. doi: 10.3389/fphar.2014.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong R.J., Zhao H., Stevenson D.K. A deficiency in haem oxygenase-1 induces foetal growth restriction by placental vasculature defects. Acta Paediatr. 2012;101:827–834. doi: 10.1111/j.1651-2227.2012.02729.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H., Kalish F.S., Wong R.J., Stevenson D.K. Hypoxia regulates placental angiogenesis via alternatively activated macrophages. Am. J. Reprod. Immunol. 2018;80 doi: 10.1111/aji.12989. [DOI] [PubMed] [Google Scholar]
- 33.Zhao H., Wong R.J., Nguyen X., Kalish F., Mizobuchi M., Vreman H.J., Stevenson D.K., Contag C.H. Expression and regulation of heme oxygenase isozymes in the developing mouse cortex. Pediatr. Res. 2006;60:518–523. doi: 10.1203/01.PDR.0000242374.21415.f5. [DOI] [PubMed] [Google Scholar]
- 34.Xiao H., Gu Z., Wang G., Zhao T. The possible mechanisms underlying the impairment of HIF-1alpha pathway signaling in hyperglycemia and the beneficial effects of certain therapies. Int. J. Med. Sci. 2013;10:1412–1421. doi: 10.7150/ijms.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melillo G. Hypoxia-inducible factor 1 inhibitors. Methods Enzymol. 2007;435:385–402. doi: 10.1016/S0076-6879(07)35020-9. [DOI] [PubMed] [Google Scholar]
- 36.Ietta F., Wu Y., Winter J., Xu J., Wang J., Post M., Caniggia I. Dynamic HIF1A regulation during human placental development. Biol. Reprod. 2006;75:112–121. doi: 10.1095/biolreprod.106.051557. [DOI] [PubMed] [Google Scholar]
- 37.Masoud G.N., Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenza G.L. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 1985;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. 2000. [DOI] [PubMed] [Google Scholar]
- 39.Soares M.J., Iqbal K., Kozai K. Hypoxia and placental development. Birth Defects Res. 2017;109:1309–1329. doi: 10.1002/bdr2.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii T., Nagamatsu T., Morita K., Schust D.J., Iriyama T., Komatsu A., Osuga Y., Fujii T. Enhanced HIF2alpha expression during human trophoblast differentiation into syncytiotrophoblast suppresses transcription of placental growth factor. Sci. Rep. 2017;7:12455. doi: 10.1038/s41598-017-12685-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto A., Sumi C., Tanaka H., Kusunoki M., Iwai T., Nishi K., Matsuo Y., Harada H., Takenaga K., Bono H., Hirota K. HIF-1-mediated suppression of mitochondria electron transport chain function confers resistance to lidocaine-induced cell death. Sci. Rep. 2017;7:3816. doi: 10.1038/s41598-017-03980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas L.W., Ashcroft M. Exploring the molecular interface between hypoxia-inducible factor signalling and mitochondria. Cell. Mol. Life Sci. 2019;76:1759–1777. doi: 10.1007/s00018-019-03039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hull T.D., Boddu R., Guo L., Tisher C.C., Traylor A.M., Patel B., Joseph R., Prabhu S.D., Suliman H.B., Piantadosi C.A., Agarwal A., George J.F. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullebner A., Dorighello G.G., Kozlov A.V., Duvigneau J.C. Interaction between mitochondrial reactive oxygen species, heme oxygenase, and nitric oxide synthase stimulates phagocytosis in macrophages. Front. Med. 2017;4:252. doi: 10.3389/fmed.2017.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandel N.S. Mitochondrial regulation of oxygen sensing. Adv. Exp. Med. Biol. 2010;661:339–354. doi: 10.1007/978-1-60761-500-2_22. [DOI] [PubMed] [Google Scholar]
- 46.Sferruzzi-Perri A.N., Higgins J.S., Vaughan O.R., Murray A.J., Fowden A.L. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc. Natl. Acad. Sci. U.S.A. 2019;116:1621–1626. doi: 10.1073/pnas.1816056116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyall F., Barber A., Myatt L., Bulmer J.N., Robson S.C. Hemeoxygenase expression in human placenta and placental bed implies a role in regulation of trophoblast invasion and placental function. Faseb. J. 2000;14:208–219. doi: 10.1096/fasebj.14.1.208. [DOI] [PubMed] [Google Scholar]
- 48.Albers R.E., Kaufman M.R., Natale B.V., Keoni C., Kulkarni-Datar K., Min S., Williams C.R., Natale D.R.C., Brown T.L. Trophoblast-specific expression of Hif-1alpha results in preeclampsia-like symptoms and fetal growth restriction. Sci. Rep. 2019;9:2742. doi: 10.1038/s41598-019-39426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]