ABSTRACT
The study of cancer has allowed researchers to describe some biological characteristics that tumor cells acquire during their development, known as the “hallmarks of cancer” but more research is needed to expand our knowledge about cancer biology and to generate new strategies of treatment. The role that RabGTPases might play in some hallmarks of cancer represents interesting areas of study since these proteins are frequently altered in cancer. However, their participation is not well known. Recently, Rab35was recognized as an oncogenic RabGTPase and and because of its association with different cellular functions, distinctly important in immune cells, a possible role of Rab35 in leukemia can be suggested. Nevertheless, the involvement of Rab35 in cancer remains poorly understood and its possible specific role in leukemia remains unknown. In this review, we analyze general aspects of the participation of RabGTPases in cancer, and especially, the plausible role of Rab35 in leukemia.
KEYWORDS: Arf6, RabGTPases, Rac1, Cdc42, Rab35, Epi64C, actin dynamics, cancer invasion, exosomes, immune evasion, leukemia, metastasis, vesicular trafficking
Introduction
Cancer and small GTPases
The first reference of cancer dates back to the Egyptians (1500–1600 BCE), who identified pathological conditions, tentatively, as cancer. Later, Hippocrates (c.460–360 BC) described a disease characterized by masses (onkos) with benign or malignant properties, for which, he coined the terms karkinos and karkinomas, respectively. Initial studies by Fibiger (1867–1928), Yamagiwa, and Ichikawa (1915) contributed to the identification of microorganisms and chemicals as causes of cancer. Harvey's study of Ras in 1964 constituted a major breakthrough in cancer research [1,2]. Ras was initially identified in rodents and human cancer cell lines as a viral gene with highly oncogenic properties. Subsequent studies observed frequent mutations in Ras in a wide spectrum of human cancers [1,3]. This prompted intensive research on the Ras structure, biochemistry, and biology. Ras was characterized as a small (molecular weight) GTP-binding protein and as a component of different signaling networks, such as Akt, epidermal growth factor receptor (EGFR), and phosphatidylinositol 3-kinase (PI3K). The focus on Ras allowed the discovery of the Ras superfamily, a group of related proteins comprising different subfamilies: RhoGTPases, ArfGTPases, RanGTPases, RasGTPases, and RabGTPases [1,3].
RabGTPases constitute the largest subfamily of the Ras superfamily of proteins. They are considered master regulators of vesicular trafficking, whose alteration is frequently associated with several complex aspects of cancer [4,5], Despite cancer's complexity, several common biological characteristics associated with tumor development have been defined as the hallmarks of cancer. These include: limitless replicative potential, apoptosis and immune destruction evasion, sustained angiogenesis, self-sufficiency in growth signals, insensitivity to antigrowth signals, reprogramming of energy metabolism tissue invasion and metastasis. (For more details, see refs [6,7]). These hallmarks of cancer address the complexity of cancer and highlight the role of many important players, as well as providing new targets of study, as is the case with the RabGTPases.
Cancer and RabGTPases
RabGTPases are frequently altered in cancer [4,5] and many times such alterations are associated with the hallmarks of cancer, invasion and metastasis, and are the main causes of death related to cancer [8] (see Table 1). Invasion and metastasis allow tumor cells to develop a motile and invasive phenotype required to escape from the primary tumor. In this process, migrating tumor cells use similar mechanisms to those in normal physiological functions, such as embryonic morphogenesis, wound healing, and immune-cell trafficking [9,10], which require actin cytoskeletal dynamics, vesicular transport, and recycling of adhesion molecules to modify the cellular shape and stiffness in the interaction with the surrounding tissue structures [6,7,11]. Also, endocytic pathway abnormalities are common in tumors [11] and accumulating evidence has suggested a link among endosome dynamics, cell migration, and invasion [12,13]. The RabGTPase Rab35 is of interest in these processes because of its implication in vesicular trafficking, its close link to actin dynamics, and its recent description as an oncogenic protein [14–17].
Table 1.
RabGTPase alterations in cancer are frequently associated with the hallmarks of cancer invasion and metastasis.
| Brain | Lung | Bladder | |
|---|---|---|---|
| Organs/RabGTPasesRab3 [101], Rab27 [102], Rab35 [32], | Rab6 [103], Rab11 [104], Rab35 [78], Rab37 [105], | (Rab11, Rab20, Rab23, Rab27) [106], Rab25 [107], | |
| Breast | |||
| Rab2 [108], Rab4 [109], Rab5 [110–112], Rab6 [113,114], Rab11 [115], | |||
| Rab25 [116–118], Rab27 [119–121], Rab31 [122,123], Rab35 [32,39,74], Rab40 [124], |
|
|
|
| Esophagus |
Colon/rectus |
Stomach |
|
| Rab25 [125], |
Rab1 [126], Rab3 [127], Rab5 [128], Rab25 [129], |
Rab23 [130], Rab40 [131], |
|
| Liver |
|
Pancreas |
|
| (Rab1, Rab4, Rab10, Rab22, Rab24, Rab25) [132], Rab5 [133], Rab23 [134], Rab27 [135], |
|
Rab20 [136], |
|
| Cervix |
Ovary |
Prostate |
Kidney |
| Rab5 [137], Rab35 [76], |
Rab25 [116,138], Rab35 [72] |
Rab7 [139], Rab25 [140] |
Rab25 [141], |
| Skin |
|
Blood |
|
| Rab7 [142], Rab11 [132], Rab35 [32], | Rab2 [143,144], | ||
| |
|
Rab4 [145], |
|
| Tongue |
Head, neck and oral squamous cell carcinoma |
Mesothelioma |
|
| Rab1 [146] | (Rab5, Rab7, Rab11) [147], Rab25 [148,149], | Rab7 [138] | |
Superscripts refer to reference. The table shows reports of RabGTPase alterations in different organs and tissues, many of which were reported to be related with metastasis.
The RabGTPase Rab35
Rab35 was originally discovered in a search for new RabGTPases in skeletal muscle and was described in 1994 [18]. Since then, Rab35 has been determined to be located mainly on the plasma membrane and on endosomes of different cell types. Multiple regulators of its activation and the description of many of its functions have also been found.
Regulation of Rab35´s activation
Rab35, similar to other small GTPases, cycles between a state of activation and inactivation regulated by different proteins (see Table 2). It is activated (bound to GTP) by the binding of GEF proteins (guanine-nucleotide exchange factor). This causes a conformational change in the GTPase structure increasing its affinity for GTP and reducing its affinity for GDP and GEF, followed by the release of both. Rab35's new conformational state, together with higher levels of GTP over GDP, favors the union of GTP. Once activated, Rab35 can interact temporarily with both effector proteins that associate with other elements. It can activate specific functional pathways and GAP proteins (GTPase-activating protein) that bind and increase the Rab35 GTPase activity and hence, the hydrolysis of GTP to GDP. Thus, Rab35 switches to the inactive state (bound to GDP). In turn, according to the cellular state, GEF proteins can either re-induce the activation of Rab35 or be maintained in the inactive state [14,19].
Table 2.
Regulators of Rab35 activation, inactivation and function: GEFs, GAPs and Effectors.
| GEFs | GAPs | Effectors |
|---|---|---|
| connecdenn1/DENND1A | TBC1D10A (Epi64A) | OCRL |
| TBC1D10B (Epi64B) | Fascin | |
| connecdenn1/DENND1B | TBC1D10C (Epi64C) | RUSC/NESCA |
| TBC1D13 | MICAL1 | |
| connecdenn1/DENND1C | TBC1D24 (Skywalker) | MICAL-L1 |
| ACAP2 | ||
| Podocalyxin |
Rab35´s functions
Figure 1 schematically summarizes many functions and models of study so far related to Rab35, among which are cytokinesis, and apico-basal polarity (Fig. 1a) Rab35 participates in different processes required for cytokinesis in HeLa cells. This GTPase regulates the PIP2 (Phosphatidylinositol 4,5-bisphosphate) and SEPT2 localization for bridge stability [20], as well as endocytic recycling for proper completion of cytokinesis [21]. A mechanism has even been proposed for daughter cell separation by involving the Rab35´s participation over the localization of OCRL and MICAL1 (effector proteins for Rab35) in the intercellular bridge, which promoted the hydrolysis of PIP2 [22] and actin depolymerization by oxidoreduction [23], respectively. Also, this GTPase is capable of linking cytokinesis to the initiation of apico-basal polarity (in MDCK cells), by controlling the localization of intracellular vesicles containing apical determinants (aPKC, Cdc42, Crumbs3, and podocalyxin) [24]. Recycling of plasma membrane components (Fig. 1b). Rab35 participates in recycling of many membrane components, among them, RME-2 yolk receptors [27], KCa2.3 (Ca2+ activated K+ Channel) [25], TfR, transferrin receptor (in different cell types, such as HeLa [20], HTB-9 [28], and Jurkat cells [26]), GLUT4 [29], M-cadherins [30], β1-integrin, EGF receptors, and N-cadherin [32]. Also, this GTPase is related to the recycling of plasma membrane components of the immune response, such as Major Histocompatibility Complex Class I (MHC I) [31], peptide-Major Histocompatibility Complex Class II (pMHC II) [33], and the zeta (ζ) chain [26], part of the TCR (T cell receptor) and Endocytosis (Fig. 1c). Rab35 participates in different types of endocytosis, in clathrin-mediated endocytosis (CME) and clathrin-independent endocytosis (CIE) [34], and in Phagocytosis of several particles: for example, E. coli by Drosophila [35], erythrocytes, by Entamoeba histolytica [36] and by Raw 264.7 cells [38], Raw cells also internalize zymosan [37]. Specifically, it is reported that Rab35 participates in different aspects of phagocytosis, such as phagosome formation (in E. histolytica [36] and in Raw 264.7 cells [37,38]), filopodia and lamellipodia generation [35], ROS production [39], as well as phagolysosome fusion (HeLa, Raw 264.7 cells [40] and in E. histolytica [36]). In addition, Rab35 participates in Autophagy [41], Exocytosis of Willebrand factor and P-selectin by Weibel-Palade bodies (WPB) [42], and Exosome release [43]. Rab35 is functionally altered by different microorganisms (Fig. 1d) such as Anaplasmaphagocytophilum [44], Uropathogenic E. coli [28], Enterohaemorrhagic E. coli [45], and Legionella pneumophila [46,47]. In Neurite outgrowth [48–53] (Fig. 1e) Rab35 is capable of promoting the formation of a complex between ACAP2 and MICAL-L1 (effector proteins for Rab35) to promote the recruitment of EHD1, which facilitates vesicle formation, to favor neurite outgrowth, bristle formation (in Drosophila S2 cells) [54], cell morphology (in BHK cells) [48], oligodendrocyte differentiation (in FBD-102b cells) [55], axon elongation (in rat primary neurons) [56], and trafficking of synaptic vesicles (in Drosophila) [57].
Figure 1.
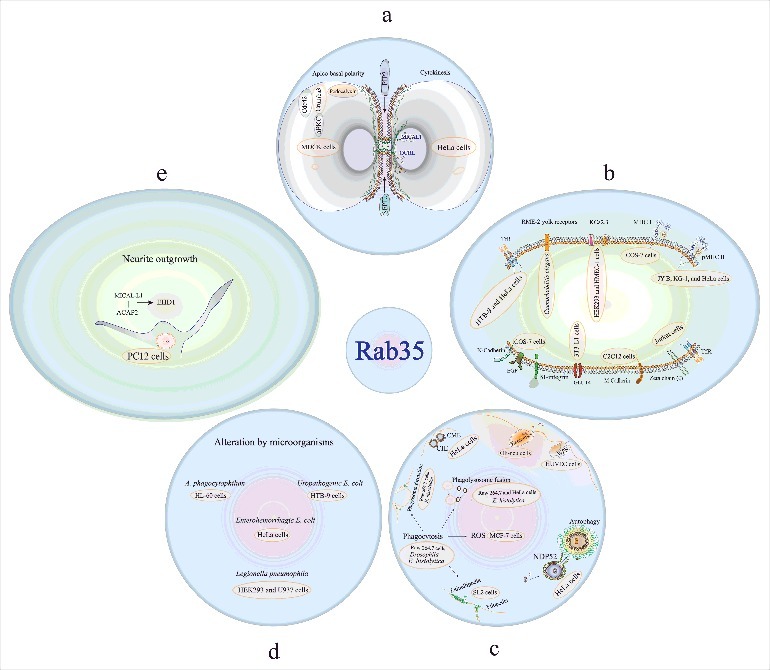
Summary of Cellular functions associated to Rab35. Most Rab35 functions are related to the endo/exocytic pathway (for details see text). Cytokinesis and apico-basal polarity (a), Recycling of plasma membrane components (b), Endocytosis and exocytosis (c), Rab35 is functionally altered by different microorganisms (d), In neurite outgrowth (e).
Because many of these functions of Rab35play prominent roles in actin dynamics and vesicular trafficking, and we know that Rab35 is altered in some cancers and now is recognized as an oncogenic protein, similar behavior to Ras leads us to infer that alteration of Rab35 might be involved in cancer development in an important way. In this paper we discuss the possible implication of Rab35 in some hallmarks of cancer, such as invasion and metastasis, with particular emphasis on immune evasion and leukemia.
Rab35 and cancer: Invasion and metastasis
Several Rab35 associated functions cited in this review have been related to different aspects of cancer development but direct implications have not been shown, such as the alterations for clathrin-mediated/independent endocytosis [59], apical-basal polarity [60], and cytokinesis [61]. Major histocompatibility complex class I (MHC I) [62] and II (MHC II) [63] molecules are downregulated in cancer [62,63]. Zeta chain expression (in the TCR, T cell receptor) is modified in T lymphocytes taken from cancer patients [64]. Different aspects of phagocytosis, such as filopodia, lamellipodia, and ROS generation (reactive oxygen species) [66–68] have been related to increased cell motility and aggressiveness. Because phagocytosis is altered in cancer [68] and is key in the elimination of tumor cells [69] and in the intake of exosomes [70], this can contribute to changes in tumor microenvironment and metastasis [71]. These studies, in addition to the prominent roles that Rab35 play in actin dynamics and vesicular trafficking, support the hypothesis that functional abnormalities in this GTPase could contribute to different aspects of cancer development, perhaps in part, through membrane trafficking dysregulation. For more details see ref [16].
On the other hand, few reports directly associate Rab35 alteration in cancer. In 2009, it was shown that the expression of Rab35 (mRNA) was upregulated in ovarian cancer OVCAR3, OSEC2 cells, and in epithelial ovarian cancer tissue under androgen treatment, which then correlates Rab35 with androgen receptor (AR) expression [72]. Interestingly, androgens are related to changes in actin remodeling [73]. In 2013, a functional interplay between Rab35 and Arf6 was suggested in cell migration and cell–cell adhesion by a mechanism whereby Rab35 negatively regulated Arf6 activity and β1-integrin recycling by promoting cadherin recycling and cell adherence. Also Rab35 was reported to be downregulated in other human tumors (gliomas and squamous cancers) where Arf6 showed hyperactivity [32]. Also in 2013, Rab35 was associated to migration of tumor cells (breast cancer) by a different mechanism, one that included the Dvl2/Rab35/Rac1 signaling pathway promoted by Wnt5a [74] (the protein involved in cancer progression) [75]. In 2015, Rab35 was identified in ovarian cancer as a direct target of miR-720 (HeLa cells), suggesting that miR-720 promoted cell migration by downregulating Rab35 [76], and we know that this microRNA, miR-720, is implicated in cancer aggressiveness [77]. Although these reports associated Rab35´s alteration to different types of cancer cells, it was in 2015 when Rab35 was described as an oncogenic protein, with oncogenic potential comparable to Ras in human cancer. Some mutations found on Rab35 were similar to those in Ras (S22N, A151T and F161L). Likewise, some of those mutations (A151T and F161L) were capable of activating the PI3K/AKT pathway and were shown to be oncogenic in the NIH-3T3 cell focus-formation assay, which is used to evaluate Ras transformation capability. In addition, it was shown that the Q67L mutation on Rab35, that turns the GTPase into a constitutively activated state, was oncogenic too [17].
Other studies continued reporting the association of Rab35 with cancer. In 2016, it was shown that the silencing of this GTPase correlated to decreased cell migration in non-small cell lung cancer, NSCLC [78]. Also, Rab35 was capable of enhancing breast cancer cell invasion, through the activation of MICAL1 and ROS generation [39].
Despite several lines of evidence that relate Rab35 to cancer, many questions remain unanswered about its implication in cancer development, particularly in invasion and metastasis. It is possible that the Rab35´s close link to actin dynamics, vesicular trafficking [15,16,35,48,54,79,80], and its close functional relationship with Rac1, Cdc42 as well asArf6 [21,32,34,35,37,38,48,50,52,53,55] could be playing important roles in those hallmarks of cancer. Alteration of Rab35 could impact tumor establishment and the tumor microenvironment in different ways. To begin with, actin dynamics dysfunction could cause cellular morphological changes, which could affect mechano-transduction signaling and how cells sense and interact with the microenvironment and perhaps influence invasion by means of actin protrusions [35,48,80]. In turn, those protrusions could be favoring the release of exosomes and metastasis [71,81]. Additionally, altered vesicular trafficking could support the release of tumor cells from the primary tumor and aid their survival during migration, perhaps by modulating the recycling of particular membrane proteins (β1-integrin, EGF receptors and cadherins), according to cellular needs. Specifically, the altered vesicular trafficking of β1-integrin, EGF receptors and cadherins has also been suggested recently. For more details see Ref [16]. The crosstalk between Rab35 and other small GTPases, such as Rac1, Cdc42 and Arf6 could also be contributing to developing and potentially altering actin dynamics and vesicular trafficking. Altered vesicular trafficking associated to Rab35 or Rac1, Cdc42 and Arf6 could cause mutual functional abnormalities, thus potentiating important biological changes, among them; proteome membrane composition and dysregulated cellular signaling events. Dysregulation of CME and CIE could affect the normal trafficking of proteins [34] giving rise to increased receptor signaling through internalized vesicles and improper recycling of proteins. Additionally, those defects could be affecting local physical membrane properties such as membrane tension, which is also associated to invasion [82].
Alterations in the phagocytic capabilities of tumor and/or tumor related cells could be associated with modifications in exosome intake [68,70], constituting another way to promote transformation and malignity [83,84], in addition to immune evasion [85]. Thus, Rab35 alteration could contribute to cancer development and aggressiveness (Fig. 2a). On the other hand, the presence of Rab35 in released exosomes [86,87], its participation in the regulation of exosome release [43], its participation in TCR recycling [26], as well as in TCR modulation [58] suggests that Rab35 could be playing an important role in leukemia as well as in immune evasion.
Figure 2.
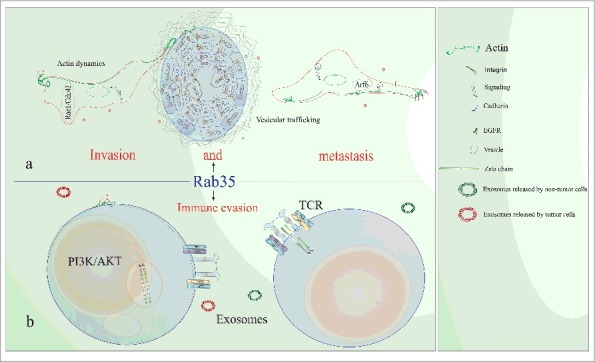
The role of the oncogenic Rab35 in cancer invasion, metastasis, immune evasion and Leukemia. Given the prominent role that Rab35 plays in actin dynamics, vesicular trafficking, as well as in functions of lymphocytes such as TCR (T cell receptor) modulation, we hypothesize that Rab35 could be participating in some hallmarks of cancer. These include cancer invasion, metastasis, and immune evasion. In (a) we highlight; actin dynamics, vesicular trafficking, and the close functional relationship between Rab35 and other GTPases (Arf6, Rac1, and Cdc42), as important elements in the potential role of Rab35in cancer invasion and metastasis. On the other hand, in (b) we suggest that Rab35 could play an important role in leukemia development and immune evasion. Given that Rab35 mediates the PI3K/AKT pathway activation and that such a pathway is important in the biology of leukemic cells, we infer that Rab35 could be mediating the activation of such a pathway in lymphocytes. In addition, it is feasible that alteration of Rab35 (by mutations or dysregulated expression) could promote leukemia development, perhaps by contributing to cell survival. Because the TCR (T cell receptor) is frequently altered in diverse types of cancer (including in leukemic T cells) and considering that alterations in the TCR could be a way whereby tumor cells evade immune response (anti-cancer immune response) and considering that Rab35 has modulating roles at the TCR level, we hypothesize that Rab35 alteration could be contributing to leukemia development by affecting lymphocyte development, activation, and proliferation. The role of Rab35 in exosome release as well as its identification in secreted exosomes could be another way to limit immune response and to modify the microenvironment.
Rab35: An outlook in leukemia and immune evasion
In our work, we point out different lines of evidence that support the hypothesis that Rab35 could be implicated in leukemia.
First: we found that Rab35 seems to be overexpressed in leukemia according to our analysis in Oncomine [88]. The oncogenic mutation (A151T) on Rab35, capable of activating the PI3K/AKT pathway, has been identified in an acute lymphoblastic leukemia (ALL) sample and reported in the Cosmic database [89]. The PI3K/AKT pathway is critical for the biology of leukemia, as reported by different studies. One study described constitutive hyperactivation of that pathway in T-Acute lymphoblastic leukemia (T-ALL) specimens, even though those cells expressed higher levels of the main negative regulator PTEN (Phosphatase and tensin homolog) compared to normal T cell precursors. This effect was associated, in part, to the role of Casein Kinase 2 (CK2) in activating the PI3K/AKT pathway [90]. In another study, the expression and activity levels of CK2 were higher than their counterparts (in healthy thymocytes) in primary T-ALL (γδ T-ALL and αβ T-ALL) cells. Furthermore, it was shown that CK2 activity (modulated by stimulating the TCR, T cell receptor) was capable of promoting the AKT signaling in thymocytes (γδ), all of which suggested a link between the PI3K/AKT pathway and TCR in leukemia [91]. The importance of upstream regulators of the PI3K/AKT pathway in leukemia can be concluded from this evidence and that of CK2, in accordance with the last two articles cited. At the same time, it is feasible to think that Rab35, which is an upstream activator of the PI3K/AKT pathway, in some cells [17], with TCR modulating roles [26,58], could be contributing to leukemia development in this way.
Second: Rab35 participates in characteristic cellular functions associated to the immune response of lymphocytes, in zeta (ζ) chain recycling for immunological synapse formation [26], TCR modulation [58], and exosome release [43]. Specifically at the TCR level, in 1992 researchers reported alterations in TCR of lymphocytes from tumor-bearing mice. The alteration was related to the expression of an unusual TCR that lacked zeta chain. Notably, that phenotype was also documented in peripheral blood T cells in human cancer patients. It is possible to think that those abnormalities could have been associated to defective zeta chain recycling [64]. Later, several works described similar alterations in the TCR for different types of cancer, such as in infiltrating T-cells isolated from patients with colorectal carcinomas, which expressed significantly less zeta chain than T-cells in the peripheral blood of the same patients, and even less than in the healthy controls [92]. Also, tumor-infiltrating lymphocytes from patients with renal cell carcinoma (in 10 of 11 cases) showed marked decrease in the expression of the same chain [93]. Later, a similar phenotype (decreased levels of zeta chain) in tumor-associated lymphocytes was reported in ascitic fluids of women with ovarian carcinoma (OvCA) [94]. All these reports have provided insights on the pathophysiology of leukemia, specifically at the TCR level, and have even allowed the suggestion that tumor growth may induce a functional state in T cells characterized by low zeta chain content in the TCR [95]. TCR alterations have also been described in cancer where lymphocytes per se are transformed. The zeta chain expression on T lymphocytes from patients with B cell chronic lymphocytic leukemia (CLL) was significantly reduced compared to normal controls [96], as were the peripheral blood T lymphocytes from patients with untreated Hodgkin‘s disease [97]. The phenotype associated to TCR dysregulation in cancer has led to the suggestion that the downregulation of zeta chain in T cells in tumor-bearing patients might be a widespread phenomenon as an escape from the immune response against cancer [97]. In part, this suggestion came from the observation that this chain was expressed at very low levels in peripheral blood T cells of the patient at the time of diagnosis, but the expression of this molecule rose to almost normal levels after successful treatment [95,97]. Remarkably, the phenotype described, the TCR defects in cancer, have also has been reported in leukemic T lymphocytes (T-ALL samples), which showed marked reduction in zeta chain expression [98]. It seems that alteration in the TCR could be a mechanism that the transformed tumor lymphocyte cells use to evade immune destruction.
Third: it has been reported that Rab35 participates in TCR modulation. This alteration was related to enhancement of TCR signaling in TH2 cell [58]. The participation of Rab35 in TH2 cells supposes that this GTPase could play different roles in different lymphocyte subpopulations.
Fourth: we found that in Jurkat cells, Rab35 regulates zeta chain recycling and the immunological synapse formation. These processes were altered by the S22N mutation on Rab35, which takes the GTPase to the inactive state. Those lymphocytes also showed defects at the vesicular level related to the development of big vacuoles, which supposed a role of such negative regulators for Rab35 activity as Epi64C [26] (a hematopoietic restricted Rab35 GAP) [99] that inactivates Rab35. We showed that Epi64C caused similar defects in the same cells. In lymphocytes, we also found that the oncogenic mutation on Rab35 (Rab35 Q67L) was associated with the generation of membrane protrusions in those cells. This highlights the capacity of Rab35 to influence actin dynamics [26], thus resembling morphological changes induced by the overexpression of Rac1 and Cdc42 [35]. These membrane protrusion could be a way to favor the release of exosomes and malignity [83,84], as well as another path for immune evasion [85].
Different reports relate Rab35 to exosomes. Rab35 was identified in secreted exosomes from colorectal adenocarcinoma (Human CRC cells, HT29) [86] and transformed B cells (human B-cell line RN; HLA-DR15) [87]. Rab35 is also reported as participating in the regulation of exosome release (Oli-neu cells), along with its GAP Epi64C [43], which was later related to the same process in adenocarcinoma cells [100]. These suggest that Rab35´s alteration could impact exosomes at different levels, such as cargo composition and rate of release. The alteration of Rab35 associated to abnormal rate of exosome release [43] could affect intercellular communication and perhaps favor the generation of bone marrow niches. Since Rab35 is part of the content of exosome release by different cell types [86,87], it is possible that alterations of Rab35 could affect the levels of Rab35 in exosomes. This is turn would alter Rab35 expression on recipient cells, and could unbalance many functions in which Rab35 participates. Given the intracellular localization of Rab35 (plasma membrane mainly), such abnormalities could be changing many physical plasma membrane properties, such as membrane tension and related functions [82].
Taken together, these studies suggest a possible link between leukemia and some Rab35 related functions, such as the PI3K/AKT pathway, TCR modulation, and exosomes, which could be contributing to leukemia development and/or immune evasion. Accordingly, some important and fundamental questions need to be answered in order to support or reject such a hypothesis about Rab35 and leukemia. Some of those questions are: Is Rab35 activating the PI3K/AKT pathway in lymphocytes? Is there and association between Rab35 and TCR alteration in leukemic samples? Is Rab35 regulating exosome release in lymphocytes?
We hypothesize that Rab35 could be contributing to leukemia development through various mechanisms. For example, since Rab35 is capable of activating the PI3K/AKT pathway in different cell types [17], it is possible that the Rab35 participation in that pathway could be operating in lymphocytes too, which could be favoring leukemic cell survival. The TCR alteration observed in different types of cancer, including leukemia, and the roles of Rab35 on TCR modulation suppose Rab35 as a protein target of study in leukemia development by means of TCR alteration. Perhaps, the Rab35 alteration in its expression (with or without oncogenic mutations) might be altering proper TCR-dependent processes, such as lymphocyte selection (during lymphocyte development), TCR signaling associated with lymphocyte activation, and clonal expansion (proliferation). Also, TCR defects could be related to immune evasion by an as yet unrecognized mechanism, as suggested above. In addition, improper Rab35 function could be altering the rate of exosome release on lymphocytes as well as their cargo content. Perhaps, more proteins that limit the anti-leukemic immune response could be present in those exosomes, which could contribute to the generation of bone marrow niches and immune evasion (Fig. 2b).
Concluding remarks
The frequent alterations of RabGTPases in cancer imply that these proteins can contribute importantly to cancer development. Specifically, the oncogenic potential of the RabGTPase Rab35 and its prominent roles in actin dynamics and vesicular trafficking in the endo/exocytic pathway, suggest that this protein might be playing important roles in some hallmarks of cancer, including invasion and metastasis, which are the main causes of death in cancer patients. The possible Rab35 participation in the PI3K/AKT pathway in lymphocytes, its role on TCR modulation, as well as its possible implication in exosome release, in the same cells, suppose that Rab35 might be acting in leukemia development and in immune evasion. As we proposed, Rab35 could be participating in the hallmarks of cancer invasion, metastasis, and immune evasion by different mechanisms, some of which could be related to its participation on vesicular trafficking, actin dynamics, as well as close functional relationship with Rac1, Cdc42 and Arf6.
In spite of the stated hypothesis about Rab35 and some hallmarks of cancer, it is necessary to investigate important basic questions about them, such as whether it is known if Rab35 and its immune GAP regulator Epi64C regulate exosome release as well as regulating the PI3K/AKT pathway in lymphocytes. In summary, Rab35 research in the context of cancer is a novel and exciting field where Rab35 might become a possible therapeutic target.
Acknowledgments
We wish to thank Dr. Juan Daniel Diaz-Valencia and Dr. Leopoldo Santos-Argumedo for insightful discussions and critical reading of this manuscript.
Funding Statement
FRV is funded by CONACYT through the PhD Fellowship #257893. GPL work is funded through Federal Funds from the Hospital Infantil de México, grants HIM-2014/003 SSA 1129 and HIM-2015/005 SSA 1182. HIM-2016/012 SSA 1233.
Disclosure potential conflicts of interest
No potential conflicts of interest.
References
- [1].Malumbres M, Barbacid M.. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–65. [DOI] [PubMed] [Google Scholar]
- [2].Tsuchida N, Murugan AK, Grieco M. Kirsten Ras* oncogene: significance of its discovery in human cancer research. Oncotarget. 2016;7:46717–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stephen AG, Esposito D, Bagni RK, et al. Dragging ras back in the ring. Cancer Cell. 2014;25:272–81. [DOI] [PubMed] [Google Scholar]
- [4].Cheng KW, Lahad JP, Gray JW, et al. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–9. [DOI] [PubMed] [Google Scholar]
- [5].Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795:110–6. [DOI] [PubMed] [Google Scholar]
- [6].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- [7].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [8].Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bozzuto G, Ruggieri P, Molinari A. Molecular aspects of tumor cell migration and invasion. Ann Ist Super Sanita. 2010;46:66–80. [DOI] [PubMed] [Google Scholar]
- [10].Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. [DOI] [PubMed] [Google Scholar]
- [11].Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–50. [DOI] [PubMed] [Google Scholar]
- [12].Porther N, Barbieri MA. The role of endocytic Rab GTPases in regulation of growth factor signaling and the migration and invasion of tumor cells. Small GTPases. 2015;6:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tzeng HT, Wang YC. Rab-mediated vesicle trafficking in cancer. J Biomed Sci. 2016;23:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaineau M, Ioannou MS, McPherson PS. Rab35: GEFs, GAPs and effectors. Traffic. 2013;14:1109–17. [DOI] [PubMed] [Google Scholar]
- [15].Klinkert K, Echard A. Rab35 GTPase: A central regulator of Phosphoinositides and F-actin in endocytic recycling and beyond. Traffic. 2016;17:1063–77. [DOI] [PubMed] [Google Scholar]
- [16].Shaughnessy R, Echard A. Rab35 GTPase and cancer: Linking membrane trafficking to tumorigenesis. Traffic. 2018;19(4):247–252. [DOI] [PubMed] [Google Scholar]
- [17].Wheeler DB, Zoncu R, Root DE, et al. Identification of an oncogenic RAB protein. Science. 2015;350:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu AX, Zhao Y, Flier JS. Molecular cloning of two small GTP-binding proteins from human skeletal muscle. Biochem Biophys Res Commun. 1994;205:1875–82. [DOI] [PubMed] [Google Scholar]
- [19].Gavriljuk K, Itzen A, Goody RS, et al. Membrane extraction of Rab proteins by GDP dissociation inhibitor characterized using attenuated total reflection infrared spectroscopy. Proc Natl Acad Sci U S A. 2013;110:13380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kouranti I, Sachse M, Arouche N, et al. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–25. [DOI] [PubMed] [Google Scholar]
- [21].Chesneau L, Dambournet D, Machicoane M, et al. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr Biol. 2012;22:147–53. [DOI] [PubMed] [Google Scholar]
- [22].Dambournet D, Machicoane M, Chesneau L, et al. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol. 2011;13:981–8. [DOI] [PubMed] [Google Scholar]
- [23].Fremont S, Hammich H, Bai J, et al. Oxidation of F-actin controls the terminal steps of cytokinesis. Nat Commun. 2017;8:14528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Klinkert K, Rocancourt M, Houdusse A, et al. Rab35 GTPase couples cell division with initiation of epithelial apico-basal polarity and lumen opening. Nat Commun. 2016;7:11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gao Y, Balut CM, Bailey MA, et al. Recycling of the Ca2+-activated K+ channel, KCa2.3, is dependent upon RME-1, Rab35/EPI64C, and an N-terminal domain. J Biol Chem. 2010;285:17938–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Patino-Lopez G, Dong X, Ben-Aissa K, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sato M, Sato K, Liou W, et al. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. EMBO J. 2008;27:1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dikshit N, Bist P, Fenlon SN, et al. Intracellular Uropathogenic E. coli exploits host Rab35 for iron acquisition and survival within urinary bladder cells. PLoS Pathog. 2015;11:e1005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Davey JR, Humphrey SJ, Junutula JR, et al. TBC1D13 is a RAB35 specific GAP that plays an important role in GLUT4 trafficking in adipocytes. Traffic. 2012;13:1429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Charrasse S, Comunale F, De Rossi S, Echard A, et al. Rab35 regulates cadherin-mediated adherens junction formation and myoblast fusion. Mol Biol Cell. 2013;24:234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Allaire PD, Marat AL, Dall'Armi C, et al. The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol Cell. 2010;37:370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Allaire PD, Seyed Sadr M, Chaineau M, et al. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci. 2013;126:722–31. [DOI] [PubMed] [Google Scholar]
- [33].Walseng E, Bakke O, Roche PA. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dutta D, Donaldson JG. Sorting of Clathrin-independent cargo proteins depends on Rab35 delivered by Clathrin-Mediated Endocytosis. Traffic. 2015;16:994–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shim J, Lee SM, Lee MS, et al. Rab35 mediates transport of Cdc42 and Rac1 to the plasma membrane during phagocytosis. Mol Cell Biol. 2010;30:1421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Verma K, Datta S. The Monomeric GTPase Rab35 regulates phagocytic cup formation and phagosomal maturation in Entamoeba histolytica. J Biol Chem. 2017;292:4960–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Egami Y, Fujii M, Kawai K, et al. Activation-inactivation cycling of Rab35 and ARF6 is required for Phagocytosis of Zymosan in RAW264 Macrophages. J Immunol Res. 2015;2015:429439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Egami Y, Fukuda M, Araki N. Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during FcgammaR-mediated phagocytosis. J Cell Sci. 2011;124:3557–67. [DOI] [PubMed] [Google Scholar]
- [39].Deng W, Wang Y, Gu L, et al. MICAL1 controls cell invasive phenotype via regulating oxidative stress in breast cancer cells. BMC Cancer. 2016;16:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith AC, Heo WD, Braun V, et al. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J Cell Biol. 2007;176:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Minowa-Nozawa A, Nozawa T, Okamoto-Furuta K, et al. Rab35 GTPase recruits NPD52 to autophagy targets. EMBO J. 2017;36:2790–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Biesemann A, Gorontzi A, Barr F, et al. Rab35 protein regulates evoked exocytosis of endothelial Weibel-Palade bodies. J Biol Chem. 2017;292:11631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hsu C, Morohashi Y, Yoshimura S, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang B, Hubber A, McDonough JA, et al. The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cell Microbiol. 2010;12:1292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Furniss RC, Slater S, Frankel G, et al. Enterohaemorrhagic E. coli modulates an ARF6:Rab35 signaling axis to prevent recycling endosome maturation during infection. J Mol Biol. 2016;428:3399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Allgood SC, Romero Duenas BP, Noll RR, et al. Legionella effector AnkX disrupts host cell endocytic recycling in a Phosphocholination-dependent manner. Front Cell Infect Microbiol. 2017;7:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mukherjee S, Liu X, Arasaki K, et al. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 2011;477:103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chevallier J, Koop C, Srivastava A, et al. Rab35 regulates neurite outgrowth and cell shape. FEBS Lett. 2009;583:1096–101. [DOI] [PubMed] [Google Scholar]
- [49].Kobayashi H, Fukuda M. Rab35 establishes the EHD1-association site by coordinating two distinct effectors during neurite outgrowth. J Cell Sci. 2013;126:2424–35. [DOI] [PubMed] [Google Scholar]
- [50].Kobayashi H, Etoh K, Fukuda M. Rab35 is translocated from Arf6-positive perinuclear recycling endosomes to neurite tips during neurite outgrowth. Small GTPases. 2014;5:e29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kobayashi H, Etoh K, Ohbayashi N, et al. Rab35 promotes the recruitment of Rab8, Rab13 and Rab36 to recycling endosomes through MICAL-L1 during neurite outgrowth. Biol Open. 2014;3:803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kobayashi H, Fukuda M. Rab35 regulates Arf6 activity through centaurin-beta2 (ACAP2) during neurite outgrowth. J Cell Sci. 2012;125:2235–43. [DOI] [PubMed] [Google Scholar]
- [53].Rahajeng J, Giridharan SS, Cai B, et al. MICAL-L1 is a tubular endosomal membrane hub that connects Rab35 and Arf6 with Rab8a. Traffic. 2012;13:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang J, Fonovic M, Suyama K, et al. Rab35 controls actin bundling by recruiting fascin as an effector protein. Science. 2009;325:1250–4. [DOI] [PubMed] [Google Scholar]
- [55].Miyamoto Y, Yamamori N, Torii T, et al. Rab35, acting through ACAP2 switching off Arf6, negatively regulates oligodendrocyte differentiation and myelination. Mol Biol Cell. 2014;25:1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Villarroel-Campos D, Henriquez DR, Bodaleo FJ, et al. Rab35 Functions in Axon Elongation Are Regulated by P53-Related Protein Kinase in a Mechanism That Involves Rab35 Protein Degradation and the Microtubule-Associated Protein 1B. J Neurosci. 2016;36:7298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Uytterhoeven V, Kuenen S, Kasprowicz J, et al. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell. 2011;145:117–32. [DOI] [PubMed] [Google Scholar]
- [58].Yang CW, Hojer CD, Zhou M, et al. Regulation of T cell receptor signaling by DENND1B in TH2 cells and allergic disease. Cell. 2016;164:141–55. [DOI] [PubMed] [Google Scholar]
- [59].Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Khursheed M, Bashyam MD. Apico-basal polarity complex and cancer. J Biosci. 2014;39:145–55. [DOI] [PubMed] [Google Scholar]
- [61].Fujiwara T, Bandi M, Nitta M, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–7. [DOI] [PubMed] [Google Scholar]
- [62].Leone P, Shin EC, Perosa F, et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Institute. 2013;105:1172–87. [DOI] [PubMed] [Google Scholar]
- [63].Shi B, Vinyals A, Alia P, et al. Differential expression of MHC class II molecules in highly metastatic breast cancer cells is mediated by the regulation of the CIITA transcription Implication of CIITA in tumor and metastasis development. Int J Biochem Cell Biol. 2006;38:544–62. [DOI] [PubMed] [Google Scholar]
- [64].Mizoguchi H, O'Shea JJ, Longo DL, et al. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–8. [DOI] [PubMed] [Google Scholar]
- [65].Jacquemet G, Hamidi H, Ivaska J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr Opinion Cell Biol. 2015;36:23–31. [DOI] [PubMed] [Google Scholar]
- [66].Kato T, Kawai K, Egami Y, et al. Rac1-dependent lamellipodial motility in prostate cancer PC-3 cells revealed by optogenetic control of Rac1 activity. PLoS One. 2014;9:e97749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Luanpitpong S, Talbott SJ, Rojanasakul Y, et al. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem. 2010;285:38832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cron J, Jansa P. Role of phagocytic cells in cancer. Folia Haematologica. 1981;108:481–527. [PubMed] [Google Scholar]
- [69].Gul N, van Egmond M. Antibody-dependent phagocytosis of tumor cells by macrophages: A potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res. 2015;75:5008–13. [DOI] [PubMed] [Google Scholar]
- [70].Feng D, Zhao WL, Ye YY, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–87. [DOI] [PubMed] [Google Scholar]
- [71].Hoshino A, Costa- B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sheach LA, Adeney EM, Kucukmetin A, et al. Androgen-related expression of G-proteins in ovarian cancer. Br J Cancer. 2009;101:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Montt-Guevara MM, Shortrede JE, Giretti MS, et al. Androgens regulate T47D cells motility and invasion through actin cytoskeleton remodeling. Front Endocrinol (Lausanne). 2016;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhu Y, Shen T, Liu J, et al. Rab35 is required for Wnt5a/Dvl2-induced Rac1 activation and cell migration in MCF-7 breast cancer cells. Cell Signal. 2013;25:1075–85. [DOI] [PubMed] [Google Scholar]
- [75].Asem MS, Buechler S, Wates RB, et al. Wnt5a signaling in cancer. Cancers (Basel). 2016;8:79. doi: 10.3390/cancers8090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tang Y, Lin Y, Li C, et al. MicroRNA-720 promotes in vitro cell migration by targeting Rab35 expression in cervical cancer cells. Cell Biosci. 2015;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Das SG, Romagnoli M, Mineva ND, et al. miR-720 is a downstream target of an ADAM8-induced ERK signaling cascade that promotes the migratory and invasive phenotype of triple-negative breast cancer cells. Breast Cancer Res. 2016;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Duan B, Cui J, Sun S, et al. EGF-stimulated activation of Rab35 regulates RUSC2-GIT2 complex formation to stabilize GIT2 during directional lung cancer cell migration. Cancer Lett. 2016;379:70–83. [DOI] [PubMed] [Google Scholar]
- [79].Chua CE, Lim YS, Tang BL. Rab35–a vesicular traffic-regulating small GTPase with actin modulating roles. FEBS Lett. 2010;584:1–6. [DOI] [PubMed] [Google Scholar]
- [80].Marat AL, Ioannou MS, McPherson PS. Connecdenn 3/DENND1C binds actin linking Rab35 activation to the actin cytoskeleton. Mol Biol Cell. 2012;23:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hoshino D, Kirkbride KC, Costello K, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Holst MR, Vidal-Quadras M, Larsson E, et al. Clathrin-independent endocytosis suppresses cancer cell blebbing and invasion. Cell Rep. 2017;20:1893–905. [DOI] [PubMed] [Google Scholar]
- [83].Keerthikumar S, Gangoda L, Liem M, et al. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget. 2015;6:15375–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci. 2015;72:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Choi DS, Lee JM, Park GW, et al. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res. 2007;6:4646–55. [DOI] [PubMed] [Google Scholar]
- [87].Buschow SI, van Balkom BW, Aalberts M, et al. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88:851–6. [DOI] [PubMed] [Google Scholar]
- [88].OncomineTM Research Edition : Rab35 and leukemia(11/27/2017). https://www.oncomine.org/resource/main.html#a%3A1484%3Bd%3A673%3Bdso%3AgeneOverex%3Bdt%3ApredefinedClass%3Bec%3A%5B2%5D%3Bepv%3A150001.151078%2C3508%3Bet%3Aover%3Bf%3A26922%3Bg%3A11021%3Bp%3A200001080%3Bpg%3A1%3Bpvf%3A3123%2C32821%3Bscr%3Adatasets%3Bss%3Aanalysis%3Bv%3A18
- [89].Cosmic Catalogue Of Somatic Mutations In Cancer: Rab35 (11/27/2017). http://cancer.sanger.ac.uk/cosmic/gene/analysis?all_data=&coords=AA%3AAA&dr=&end =202&gd=&id=1130&ln=RAB35&seqlen=202&sn=haematopoietic_and_lymphoid_tissue&start=1#ts
- [90].Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ribeiro ST, Tesio M, Ribot JC, et al. Casein kinase 2 controls the survival of normal thymic and leukemic gammadelta T cells via promotion of AKT signaling. Leukemia. 2017;31:1603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nakagomi H, Petersson M, Magnusson I, et al. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–2. [PubMed] [Google Scholar]
- [93].Finke JH, Zea AH, Stanley J, et al. Loss of T-cell receptor zeta chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–6. doi: [PubMed] [Google Scholar]
- [94].Rabinowich H, Reichert TE, Kashii Y, et al. Lymphocyte apoptosis induced by Fas ligand- expressing ovarian carcinoma cells. Implications for altered expression of T cell receptor in tumor-associated lymphocytes. J Clin Invest. 1998;101:2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Massaia M, Attisano C, Beggiato E, et al. Correlation between disease activity and T-cell CD3 zeta chain expression in a B-cell lymphoma. Br J Haematol. 1994;88:886–8. [DOI] [PubMed] [Google Scholar]
- [96].Rossi E, Matutes E, Morilla R, et al. Zeta chain and CD28 are poorly expressed on T lymphocytes from chronic lymphocytic leukemia. Leukemia. 1996;10:494–7. [PubMed] [Google Scholar]
- [97].Renner C, Ohnesorge S, Held G, et al. T cells from patients with Hodgkin's disease have a defective T-cell receptor zeta chain expression that is reversible by T-cell stimulation with CD3 and CD28. Blood. 1996;88:236–41. [PubMed] [Google Scholar]
- [98].Torelli GF, Paolini R, Tatarelli C, et al. Defective expression of the T-cell receptor-CD3 zeta chain in T-cell acute lymphoblastic leukaemia. Br J Haematol. 2003;120:201–8. [DOI] [PubMed] [Google Scholar]
- [99].Pan F, Sun L, Kardian DB, et al. Feedback inhibition of calcineurin and Ras by a dual inhibitory protein Carabin. Nature. 2007;445:433–6. [DOI] [PubMed] [Google Scholar]
- [100].Li W, Hu Y, Jiang T, et al. Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: involvement of EPI64. APMIS. 2014;122:1080–7. [DOI] [PubMed] [Google Scholar]
- [101].Kim JK, Lee SY, Park CW, et al. Rab3a promotes brain tumor initiation and progression. Mol Biol Rep. 2014;41:5903–11. [DOI] [PubMed] [Google Scholar]
- [102].Wu X, Hu A, Zhang M, et al. Effects of Rab27a on proliferation, invasion, and anti-apoptosis in human glioma cell. Tumour Biol. 2013;34:2195–203. [DOI] [PubMed] [Google Scholar]
- [103].Huang H, Jiang Y, Wang Y, et al. miR-5100 promotes tumor growth in lung cancer by targeting Rab6. Cancer Lett. 2015;362:15–24. [DOI] [PubMed] [Google Scholar]
- [104].Dong Q, Fu L, Zhao Y, et al. Rab11a promotes proliferation and invasion through regulation of YAP in non-small cell lung cancer. Oncotarget. 2017;8:27800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wu CY, Tseng RC, Hsu HS, et al. Frequent down-regulation of hRAB37 in metastatic tumor by genetic and epigenetic mechanisms in lung cancer. Lung Cancer. 2009;63:360–7. [DOI] [PubMed] [Google Scholar]
- [106].Ho JR, Chapeaublanc E, Kirkwood L, et al. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS One. 2012;7:e39469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang J, Wei J, Lu J, et al. Overexpression of Rab25 contributes to metastasis of bladder cancer through induction of epithelial-mesenchymal transition and activation of Akt/GSK-3beta/Snail signaling. Carcinogenesis. 2013;34:2401–8. [DOI] [PubMed] [Google Scholar]
- [108].Luo ML, Gong C, Chen CH, et al. The Rab2A GTPase promotes breast cancer stem cells and tumorigenesis via Erk signaling activation. Cell Rep. 2015;11:111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Frittoli E, Palamidessi A, Marighetti P, et al. A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J Cell Biol. 2014;206:307–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mendoza P, Ortiz R, Diaz J, et al. Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells. J Cell Sci. 2013;126:3835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Porther N, Barbieri M. Growth factor influences Rab5 function in breast cancer. FASEB J. 2015;29:1_supplement. (Abstract 893.1). [Google Scholar]
- [112].Yang PS, Yin PH, Tseng LM, et al. Rab5A is associated with axillary lymph node metastasis in breast cancer patients. Cancer Sci. 2011;102:2172–8. [DOI] [PubMed] [Google Scholar]
- [113].Shan J, Yuan L, Budman DR, et al. WTH3, a new member of the Rab6 gene family, and multidrug resistance. Biochim Biophys Acta. 2002;1589:112–23. doi: [DOI] [PubMed] [Google Scholar]
- [114].Tian K, Jurukovski V, Yuan L, et al. WTH3, which encodes a small G protein, is differentially regulated in multidrug-resistant and sensitive MCF7 cells. Cancer Res. 2005;65:7421–8. [DOI] [PubMed] [Google Scholar]
- [115].Palmieri D, Bouadis A, Ronchetti R, et al. Rab11a differentially modulates epidermal growth factor-induced proliferation and motility in immortal breast cells. Breast Cancer Res Treat. 2006;100:127–37. [DOI] [PubMed] [Google Scholar]
- [116].Cheng KW, Lahad JP, Kuo WL, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–6. [DOI] [PubMed] [Google Scholar]
- [117].Cheng JM, Ding M, Aribi A, et al. Loss of RAB25 expression in breast cancer. Int J Cancer. 2006;118:2957–64. [DOI] [PubMed] [Google Scholar]
- [118].Cheng JM, Volk L, Janaki DK, et al. Tumor suppressor function of Rab25 in triple-negative breast cancer. Int J Cancer. 2010;126:2799–812. [DOI] [PubMed] [Google Scholar]
- [119].Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30. [DOI] [PubMed] [Google Scholar]
- [120].Wang JS, Wang FB, Zhang QG, et al. Enhanced expression of Rab27A gene by breast cancer cells promoting invasiveness and the metastasis potential by secretion of insulin-like growth factor-II. Mol Cancer Res. 2008;6:372–82. [DOI] [PubMed] [Google Scholar]
- [121].Zhang JX, Huang XX, Cai MB, et al. Overexpression of the secretory small GTPase Rab27B in human breast cancer correlates closely with lymph node metastasis and predicts poor prognosis. J Transl Med. 2012;10:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Grismayer B, Solch S, Seubert B, et al. Rab31 expression levels modulate tumor-relevant characteristics of breast cancer cells. Mol Cancer. 2012;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jin C, Rajabi H, Pitroda S, et al. Cooperative interaction between the MUC1-C oncoprotein and the Rab31 GTPase in estrogen receptor-positive breast cancer cells. PLoS One. 2012;7:e39432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Jacob A, Jing J, Lee J, et al. Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells. J Cell Sci. 2013;126:4647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tong M, Chan KW, Bao JY, et al. Rab25 is a tumor suppressor gene with antiangiogenic and anti-invasive activities in esophageal squamous cell carcinoma. Cancer Res. 2012;72:6024–35. [DOI] [PubMed] [Google Scholar]
- [126].Thomas JD, Zhang YJ, Wei YH, et al. Rab1A Is an mTORC1 Activator and a Colorectal Oncogene. Cancer Cell. 2016;30:181–2. [DOI] [PubMed] [Google Scholar]
- [127].Chang YC, Su CY, Chen MH, et al. Secretory RAB GTPase 3C modulates IL6-STAT3 pathway to promote colon cancer metastasis and is associated with poor prognosis. Mol Cancer. 2017;16:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Diaz J, Mendoza P, Ortiz R, et al. Rab5 is required in metastatic cancer cells for Caveolin-1-enhanced Rac1 activation, migration and invasion. J Cell Sci. 2014;127:2401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Nam KT, Lee HJ, Smith JJ, et al. Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest. 2010;120:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hou Q, Wu YH, Grabsch H, et al. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 2008;68:4623–30. [DOI] [PubMed] [Google Scholar]
- [131].Yang Q, Jie Z, Cao H, et al. Low-level expression of let-7a in gastric cancer and its involvement in tumorigenesis by targeting RAB40C. Carcinogenesis. 2011;32:713–22. [DOI] [PubMed] [Google Scholar]
- [132].He H, Dai F, Yu L, et al. Identification and characterization of nine novel human small GTPases showing variable expressions in liver cancer tissues. Gene Expr. 2002;10:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Fukui K, Tamura S, Wada A, et al. Expression of Rab5a in hepatocellular carcinoma: Possible involvement in epidermal growth factor signaling. Hepatol Res. 2007;37:957–65. [DOI] [PubMed] [Google Scholar]
- [134].Liu YJ, Wang Q, Li W, et al. Rab23 is a potential biological target for treating hepatocellular carcinoma. World J Gastroenterol. 2007;13:1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Dong WW, Mou Q, Chen J, et al. Differential ex-pression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol. 2012;18:1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Amillet JM, Ferbus D, Real FX, et al. Characterization of human Rab20 overexpressed in exocrine pancreatic carcinoma. Hum Pathol. 2006;37:256–63. [DOI] [PubMed] [Google Scholar]
- [137].Liu SS, Chen XM, Zheng HX, et al. Knockdown of Rab5a expression decreases cancer cell motility and invasion through integrin-mediated signaling pathway. J Biomed Sci. 2011;18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Davidson B, Zhang Z, Kleinberg L, et al. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2006;12:5944–50. [DOI] [PubMed] [Google Scholar]
- [139].Steffan JJ, Dykes SS, Coleman DT, et al. Supporting a role for the GTPase Rab7 in prostate cancer progression. PLoS One. 2014;9:e87882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Calvo A, Xiao N, Kang J, et al. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325–35. [PubMed] [Google Scholar]
- [141].Liu L, Ding G. Rab25 expression predicts poor prognosis in clear cell renal cell carcinoma. Exp Therapeutic Med. 2014;8:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Alonso-Curbelo D, Riveiro-Falkenbach E, Perez-Guijarro E, et al. RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell. 2014;26:61–76. [DOI] [PubMed] [Google Scholar]
- [143].Culine S, Honore N, Tavitian A, et al. Overexpression of the ras-related rab2 gene product in peripheral blood mononuclear cells from patients with hematological and solid neoplasms. Cancer Res. 1992;52:3083–8. [PubMed] [Google Scholar]
- [144].Culine S, Honore N, Closson V, et al. A small GTP-binding protein is frequently overexpressed in peripheral blood mononuclear cells from patients with solid tumours. Eur J Cancer. 1994;30A:670–4. [DOI] [PubMed] [Google Scholar]
- [145].Ferrandiz-Huertas C, Fernandez-Carvajal A, Ferrer-Montiel A. Rab4 interacts with the human P-glycoprotein and modulates its surface expression in multidrug resistant K562 cells. Int J Cancer. 2011;128:192–205. [DOI] [PubMed] [Google Scholar]
- [146].Shimada K, Uzawa K, Kato M, et al. Aberrant expression of RAB1A in human tongue cancer. Br J Cancer. 2005;92:1915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].da Silva SD, Marchi FA, Xu B, et al. Predominant Rab-GTPase amplicons contributing to oral squamous cell carcinoma progression to metastasis. Oncotarget. 2015;6:21950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Amornphimoltham P, Rechache K, Thompson J, et al. Rab25 regulates invasion and metastasis in head and neck cancer. Clin Cancer Res. 2013;19:1375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Seven D, Dogan S, Kilic E, et al. Downregulation of Rab25 activates Akt1 in head and neck squamous cell carcinoma. Oncol Lett. 2015;10:1927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


