ABSTRACT
The small GTPase RhoA is a master regulator of signalling in cell-extracellular matrix interactions. RhoA signalling is critical to many cellular processes including migration, mechanotransduction, and is often disrupted in carcinogenesis. Investigating RhoA activity in a native tissue environment is challenging using conventional biochemical methods; we therefore developed a RhoA-FRET biosensor mouse, employing the adaptable nature of intravital imaging to a variety of settings. Mechanotransduction was explored in the context of osteocyte processes embedded in the calvaria responding in a directional manner to compression stress. Further, the migration of neutrophils was examined during in vivo “chemotaxis” in wound response. RhoA activity was tightly regulated during tissue remodelling in mammary gestation, as well as during mammary and pancreatic carcinogenesis. Finally, pharmacological inhibition of RhoA was temporally resolved by the use of optical imaging windows in fully developed pancreatic and mammary tumours in vivo. The RhoA-FRET mouse therefore constitutes a powerful tool to facilitate development of new inhibitors targeting the RhoA signalling axis.
KEYWORDS: biosensors, breast cancer, extracellular matrix, FLIM, FRET, Intravital imaging, pancreatic cancer, RhoA, Rho-GTPases
The small GTPase RhoA has been linked to a wide range of cellular processes such as adhesion, migration, cell cycle progression, apoptosis and mechanotransduction [1]. RhoA is activated by the hydrolysis of GTP and in turn modulates a number of downstream effectors [2]. The rapid, and often fleeting, nature of this process makes it challenging to assess using conventional biochemical methods (Fig. 1A). Förster Resonance Energy Transfer (FRET) biosensors have become a vital tool to study in vivo protein interactions with high spatial and temporal resolution [3–5] and several biosensors of RhoA regulation have been demonstrated to date [6–10]. These FRET-biosensors have played a critical role in the investigation of RhoA signalling dynamics in vitro. Here, using fluorescence lifetime microscopy (FLIM) we monitored the changes in FRET when RhoA was activated, however, it should be noted that intensity based approaches to monitor FRET in vivo have also been employed recently. This type of analysis can further be performed using this biosensor mouse as previously achieved with other FRET biosensor mice [11–15]. To provide a spatio-temporal read-out of RhoA activity in native tissues we created a new RhoA-FRET mouse [15] using a modified version of the Raichu-RhoA biosensor [10]. Here, the original CFP/YFP fluorophore pair was replaced by EGFP and mRFP respectively [15,16] in order to avoid potential problems with recombination from tandem repeats of related fluorescent protein sequences during mouse generation [17] (Fig 1). In the RhoA-FRET ‘OFF’ mouse, the biosensor construct is flanked by a lox-stop-lox site (LSL), which allows for conditional expression of the reporter using tissue specific Cre recombinases. We then created a RhoA-FRET ‘ON’ mouse that expresses the biosensor constitutively by removing the LSL site with a deleter CMV-Cre.
Figure 1.
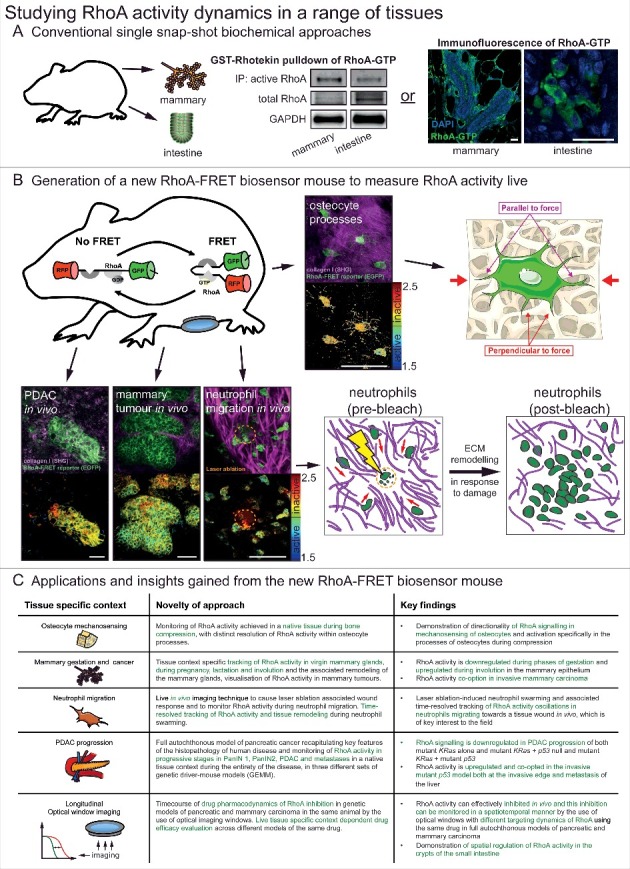
Studying RhoA activity dynamics in a range of tissues. (A) Conventional single snap-shot based biochemical approaches to analysing RhoA activity in two examples tissues of the mammary gland and intestine. These included bead-based pulldown of RhoA-GTP in tissue lysate and a recently developed immunofluorescence of fixed tissue samples using a RhoA-GTP specific antibody [15,19]. scale bars, 25 μm (B) With the generation of the new RhoA-FRET biosensor mouse RhoA activity could be monitored live in osteocytes of the calvaria, in vivo in pancreatic ductal adenocarcinomas, mammary tumours and during neutrophil migration (RhoA-FRET biosensor, green; collagen-derived second harmonic generation (SHG) signal, magenta) with corresponding fluorescence lifetime imaging microscopy (FLIM) images of RhoA activity (high RhoA activity: blue to green; low RhoA activity: yellow to red). scale bars, 50 μm (C) A summary of the new insights gained by the use of the new RhoA-FRET biosensor mouse in a variety of tissues and applications. Adapted from Nobis et al. 2017, Cell Reports and adapted from Servier Medial Art, licensed under the Creative Commons Attribution 3.0 Unported license (https://creativecommons.org/licenses/by/3.0/).
RhoA has been shown to transduce mechanical stimuli from the surrounding extra-cellular matrix (ECM) via attachment sites, such as integrins, to downstream intracellular signalling pathways e.g.: ROCK [18,19]. In particular, RhoA has been implicated in the cellular response to mechanical loading in the bone [20]. To explore its involvement in mechanotransduction we examined RhoA activity in osteocyte processes in their native environment embedded in canaliculi of the mouse calvaria (Fig. 1B). The application of ∼1% lateral compression by an in-house compression apparatus to freshly excised calvaria of Col1a1.3.6-Cre;RhoA-FRET mice revealed activation of RhoA in processes oriented perpendicular to the direction of applied force but not in processes oriented parallel to the force or in uncompressed samples (for further details of the apparatus and compression procedure see Nobis et al. 2017 [15]). This illustrates an active role for RhoA in directional signal transduction of mechanostimuli, potentially mediated by the differential shear forces experienced by the dendritic processes in the fluid-filled lacunae upon application of pressure [21,22].
RhoA has been shown to be actively involved in the migration of cells at both the leading and retracting edges of cells [23–25]. RhoA and another small GTPase Rac1 are thought to be reciprocally active at the edge of moving cells [26]. RhoA and Rac1 are mutually inhibitory and modelling has predicted that the RhoA-Rac1 signalling network can exhibit bistability [27,28] with stable states associated with different modes of migration such as mesenchymal-like migration, generally driven by Rac1 and amoeboid-like migration, typically driven by RhoA depending on their surrounding microenvironment [27,29–32]. Having previously explored the oscillatory activation of Rac1 during migration in isolated neutrophils of the Rac-FRET mouse [14], we characterized the activity of RhoA here during chemotaxis in vivo in LysM-Cre;RhoA-FRET mice by intravital imaging. Neutrophil egression to and regression from a site of microbial infection have been described recently [33], highlighting the role of neutrophils as primary infection responders. Following enrichment of the local neutrophil population by bacterial particle inoculation of the ear, a resident dendritic cell was laser-ablated creating a damage site to which neutrophils swarm (Fig. 1B). Oscillations of RhoA activity were observed [15] and noted to be similar to those measured for Rac1 in an in vitro chemotaxis assay [14], demonstrating the active role both small GTPases play during coordinated neutrophil migration. Furthermore, active rearrangement of the collagen network by the neutrophils during this acute damage phase was observed, suggesting a role for immune-based ECM remodelling in addition to the known role fibroblasts play in this process [34,35]. For further and concise overview of the other applications of the biosensor in distinct tissue settings achieved previously [15] see Fig. 1C.
RhoA activity also plays a key role during a variety of tissue remodelling processes and during disease progression such as cancer. In mammary tissue the downstream effector of RhoA PKN1 has been shown to play a role during gestation and lactation [36]. Using the conditional RhoA-FRET biosensor mouse crossed to a mammary specific Cre-driver line (MMTV-Cre), we tracked RhoA activity through the gestation cycle. This revealed an initially high RhoA activity during virgin branching morphogenesis, which progressively decreased during alveoli formation in pregnancy and in the mature milk-producing alveoli during lactation. During involution following weaning, RhoA activity was upregulated again as the alveoli break down and the gland returns to the pre-pregnancy state [15]. RhoA activity was further examined during cancerous transformation of the mammary gland in a genetic polyoma-middle-T antigen (PyMT) driven breast cancer model. In this model, RhoA activity was upregulated compared to the wildtype virgin mammary gland during tumourigenesis, pointing to a potential co-option of RhoA in invasive and metastatic breast cancer [15]. RhoA activity can further be tracked in PyMT driven mammary cancer during progressive stages from early adenoma, late adenoma, carcinoma and in metastases of the lung. This revealed a progressive down-regulation of RhoA activity during tumour progression (Fig. 2A). An increase of RhoA activity, however, was revealed at the invasive edges of primary PyMT tumours, again pointing to its potential co-option in the metastatic cascade of this tumour type (Fig. 2B). This is in line with similar discoveries in invasive pancreatic cancer, illustrating the role of RhoA in invasion [15,16]. Finally, by intravenous injection of the contrast dye (Qtracker655), as previously achieved [37] the local tumour vasculature was visualized and RhoA activity in cells mapped within distinct tissue regions in relation to the local vessels (Fig. 2C).
Figure 2.
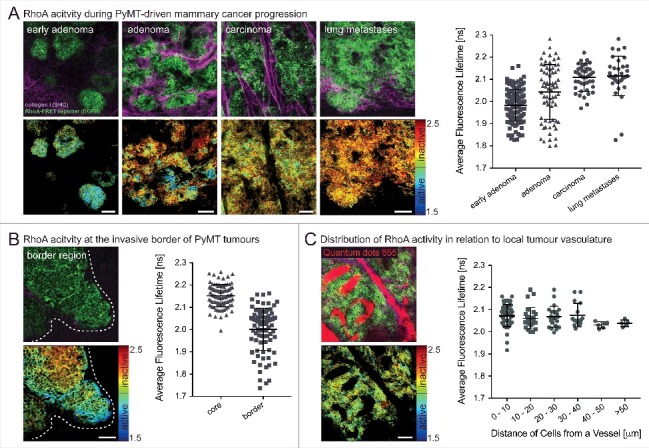
Spatially defined RhoA acitivty during the progression of PyMT-driven mammary carcinomas. (A) RhoA-FRET mice crossed to MMTV-polyoma-middle-T antigen (PyMT) mice allow for the tracking of RhoA activity during the progression of invasive mammary carcinoma (n = 1 mouse, 280 cells). (B) RhoA activity is increased at the invasive borders of primary PyMT tumours (white dashed line) compared to tumour core regions (n = 1 mouse, 180 cells). (C) Intravenous injeciton of a contrast dye (Qtracker655) allows for monitoring of RhoA activity in cancer cells in relation to their proximity to local vasculature (n = 2 mice, 130 cells). Dots, single cells; line, mean; error bars, SD; scale bars, 50 µm.
RhoA has also been shown to be activated at both the rear and leading edge of cells in a mutant p53R172H driven invasive pancreatic cancer model in vivo [16]. Activation of RhoA via its upstream regulators, such as RhoA GEF-H1, has been implicated in the progression and metastasis of pancreatic cancer, amplifying MAPK signalling [38]. Recently a mutant isoform Δ133 of p53 has been linked to pancreatic tumour cell invasion and metastasis via interleukin-6 activation of the JAK-STAT and RhoA-ROCK signalling pathways [39]. This led to the utilization of the conditional RhoA-FRET biosensor mouse in tracking RhoA activity in a genetic model of pancreatic cancer with the initiating mutation of KRasG12D and mutant p53R172H driven by Pdx1-Cre (KPC), resulting in invasive pancreatic ductal adenocarcinoma (PDAC) [40,41]. This model closely recapitulates the human histopathology, where the initiating KRAS mutation is found in up to 90% of patients, while loss of P53 or mutant P53 occurs in 50–75% of tumours [42,43]. Intravital imaging of pancreatic tissue as the disease advanced revealed a progressive inactivation of RhoA from pancreatic intraepithelial neoplasms (PanINs) to fully developed PDAC [15]. In the invasive mutant p53 driven KPC model RhoA activation was observed both at the invasive edges of the primary tumour as well as at distant metastatic sites of the liver, revealing a spatially coordinated switching of activity that may facilitate cancer cell movement [15]. Comparing the invasive fronts of both non-invasive KPflC (p53 null) and invasive KPC tumours revealed that an increase in RhoA activity was confined to mice carrying the mutant gain of function (GOF) p53 allele (p53R172H/+) and, this was absent in mice with non-invasive PDAC driven by loss of p53 (Fig. 3A+B) [41,44,45].
Figure 3.
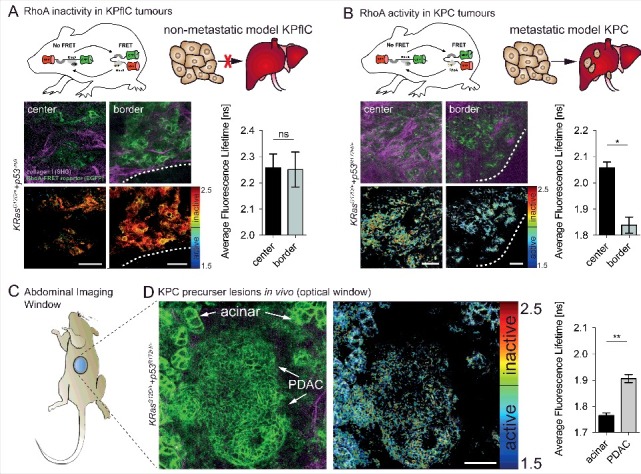
In vivo imaging of RhoA activity in the pancreas and KPC tumours reveals spatial activation at the invasive border of mutant p53 driven KPC tumours. (A) RhoA is inactive in non-invasive p53-null PDACs both at the tumour center and borders (white dashed line) (n = 2 mice, 163 cells). (B) RhoA acitvity is increased at the invasive border of p53 mutant (p53R172H/+) tumours compared to tumour center regions (n = 2 mice, 77 cells). (C) Schematic of an abdominal imaging window (AIW) to examine RhoA activity in the pancreas and in primary pancreatic tumours. (D) RhoA activity during tumour progression of primary mutant p53 driven PDACs imaged intravitally through optical windows (n = 3 mice, 293 cells). Columns, mean; error bars, SEM; *p < 0.05; **p < 0.01; scale bars, 50 µm.
RhoA is a promising potential therapeutic target as it plays an active role in the progression and invasive potential in both mammary and pancreatic cancer models. Therapeutic intervention targeting RhoA indirectly has been shown previously, particularly targeting downstream effectors of RhoA such as ROCK1 and ROCK2, which are upregulated and associated with poor prognosis in pancreatic cancer [46–50]. In our biosensor mouse study, we tracked RhoA activity using optical windows [51,52] where we could see a sub-organ resolution distinct difference in RhoA activation states in acinar versus PDAC cells (Fig. 3C+D). This allows for possible future studies on the role of RhoA in acinar to ductal metaplasia (ADM), which is thought to play a partial role in early phases of this disease [53]. We administered small molecule drugs which affect the ECM-tumour cell feedback loop, such as Src activity, integrin engagement and EGFR signalling and monitored the effect on RhoA activity. For example, RhoA activity in response to Src inhibition via the administration of the Src/Abl kinase inhibitor dasatinib was monitored over a 24 h period [15]. After spontaneous pancreatic tumour development at approximately 125 days in the genetically engineered KPC pancreatic cancer model, mice were engrafted with an abdominal imaging window positioned above the primary tumour. Repeated intravital imaging of the RhoA-FRET biosensor in the pancreatic KPC model upon dasatinib treatment revealed effective inhibition of RhoA activity 7 h hours after the final dasatinib administration. In the PyMT-driven mammary cancer model, mammary imaging windows were implanted in the skin above the developing primary tumours after an average of 85 days. In this model the dynamics of RhoA modulation were very different from the pancreatic cancer model with RhoA inhibition observed as little as 2 h post-administration [15]. These differences underline the importance of pre-clinical optimisation of drug targeting in the native tissue microenvironment [4]. The delayed RhoA inhibition in the pancreatic model may be due to delayed drug penetrance of the dense desmoplasia often found in pancreatic cancer [35,54]. Recent studies in pancreatic cancer have aimed to reduce this fibrosis by targeting FAK, YAP/TAZ, Cdk4, PAK1, JAK/STAT and hyaluronic acid [55–60] as well as characterized the cross-talk of tumour-stroma interactions of patient derived xenografts revealing potential new targets [61]. The RhoA-FRET biosensor mouse could prove an indispensable tool to optimise targeting of these pathways by providing a live readout of the effect of drug targeting on ECM-cancer cell reciprocity via RhoA activity. More recently, a study investigating Crohn's disease demonstrated that fibrosis in the intestinal tract caused by RhoA-ROCK pathway upregulation in myofibroblasts in Crohn's disease can be effectively reversed by ROCK inhibition [62]. Fine-tuning of this targeting could potentially be achieved in the future with the RhoA-FRET mouse, which we previously used to observe spatial regulation of RhoA in intestinal crypts by in vivo imaging [15].
With the use of the RhoA-FRET biosensor mouse, new insights into several key aspects of this prototypical small GTPase may be obtained in in vivo settings ranging from mechanotransduction, migration, ECM remodelling, cancer progression to the spatiotemporal response to drug targeting. This new biosensor mouse therefore lends itself to a wide range of applications exploring the activity of RhoA in native tissue contexts in the future and may reveal new insights into the switch-like rapid behaviour of this small GTPase in vivo. Other elegant approaches to imaging RhoA activation have been reported previously, such as using optogenetic activation of RhoA using the CRY2/CIBN light-gated dimerizer system. This allowed for light induced control of traction and tension within cells and their surrounding tissue [63]. Future applications in an intravital setting of other RhoA-biosensors that are available, such as cytoplasmic DORA sensors with RhoA binding to a PKN1 domain [9] or the RhoA-2G biosensor that can report on GDI activity [6,8], have the potential to reveal intricate changes of this vital signalling node in normal and disease settings.
Funding Statement
MN, DH, SCW, TRC and PT were funded by an NHMRC project grant and fellowship, an ARC Future and Len Ainsworth Pancreatic Cancer Fellowship, Cancer Institute New South Wales Early Carrer Fellowship, Cancer Council NSW, Sydney Catalyst, Tour de Cure and a National Breast Cancer Foundation (NBCF) grant. KIA and DS were funded by a CRUK core grant. This project was made possible by an Avner Pancreatic Cancer Foundation Grant.
Disclosure of potential conflicts of interest
The authors report no financial interest or potential conflicts of interest.
References
- [1].Karlsson R, Pedersen ED, Wang Z, et al. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–8. doi: 10.1016/j.bbcan.2009.03.003. PMID:19327386. [DOI] [PubMed] [Google Scholar]
- [2].Hodge RG, Ridley AJ.. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. PMID:27301673. [DOI] [PubMed] [Google Scholar]
- [3].Conway JRW, Warren SC, Timpson P. Context-dependent intravital imaging of therapeutic response using intramolecular FRET biosensors. Methods. 2017;128:78–94. doi: 10.1016/j.ymeth.2017.04.014. PMID:28435000. [DOI] [PubMed] [Google Scholar]
- [4].Conway JRW, Carragher NO, Timpson P. Developments in preclinical cancer imaging: innovating the discovery of therapeutics. Nat Rev Cancer. 2014;14:314–28. doi: 10.1038/nrc3724. PMID:24739578. [DOI] [PubMed] [Google Scholar]
- [5].Nobis M, Warren SC, Lucas MC, et al. Moving molecular mobility and activity to an intravital imaging setting: implications for cancer modelling and targeting. J Cell Sci. 2018. doi: 10.1242/jcs.206995. [DOI] [PubMed] [Google Scholar]
- [6].Fritz RD, Letzelter M, Reimann A, et al. A versatile toolkit to produce sensitive FRET biosensors to visualize signaling in time and space. Sci Signal. 2013;6:rs12. doi: 10.1126/scisignal.2004135. PMID:23882122. [DOI] [PubMed] [Google Scholar]
- [7].Kardash E, Reichman-Fried M, Maître J-L, et al. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12:47-53-11. doi: 10.1038/ncb2003. [DOI] [PubMed] [Google Scholar]
- [8].Pertz O, Hodgson L, Klemke RL, et al. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–72. doi: 10.1038/nature04665. PMID:16547516. [DOI] [PubMed] [Google Scholar]
- [9].van Unen J, Reinhard NR, Yin T, Wu YI, et al. Plasma membrane restricted RhoGEF activity is sufficient for RhoA-mediated actin polymerization. Sci Rep. 2015;5:14693. doi: 10.1038/srep14693. PMID:26435194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoshizaki H, Ohba Y, Kurokawa K, et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–32. doi: 10.1083/jcb.200212049. PMID:12860967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kumagai Y, Naoki H, Nakasyo E, et al. Heterogeneity in ERK activity as visualized by in vivo FRET imaging of mammary tumor cells developed in MMTV-Neu mice. Oncogene. 2015;34:1051–7. doi: 10.1038/onc.2014.28. PMID:24632612. [DOI] [PubMed] [Google Scholar]
- [12].Yamauchi F, Kamioka Y, Yano T, et al. In vivo FRET imaging of tumor endothelial cells highlights a role of low PKA activity in vascular hyperpermeability. Cancer Res. 2016;76:5266–76. doi: 10.1158/0008-5472.CAN-15-3534. PMID:27488524. [DOI] [PubMed] [Google Scholar]
- [13].Hiratsuka T, Fujita Y, Naoki H, et al. Intercellular propagation of extracellular signal-regulated kinase activation revealed by in vivo imaging of mouse skin. Elife. 2015;4:e05178. doi: 10.7554/eLife.05178. PMID:25668746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johnsson A-KE, Dai Y, Nobis M, et al. The Rac-FRET mouse reveals tight spatiotemporal control of Rac activity in primary cells and tissues. Cell Rep. 2014;6:1153–64. doi: 10.1016/j.celrep.2014.02.024. PMID:24630994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nobis M, Herrmann D, Warren SC, et al. A RhoA-FRET Biosensor Mouse for Intravital Imaging in Normal Tissue Homeostasis and Disease Contexts. Cell Rep. 2017;21:274–88. doi: 10.1016/j.celrep.2017.09.022. PMID:28978480. [DOI] [PubMed] [Google Scholar]
- [16].Timpson P, McGhee EJ, Morton JP, et al. Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Res. 2011;71:747–57. doi: 10.1158/0008-5472.CAN-10-2267. PMID:21266354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Komatsubara AT, Matsuda M, Aoki K. Quantitative analysis of recombination between YFP and CFP genes of FRET biosensors introduced by lentiviral or retroviral gene transfer. Sci Rep. 2015;5:13283. doi: 10.1038/srep13283. PMID:26290434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lawson CD, Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases. 2014;5:e27958. doi: 10.4161/sgtp.27958. PMID:24607953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boyle ST, Kular J, Nobis M, et al. Acute compressive stress activates RHO/ROCK-mediated cellular processes. Small GTPases. 2018. doi: 10.1080/21541248.2017.1413496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Noble BS. The osteocyte lineage. Arch Biochem Biophys. 2008;473:106–11. doi: 10.1016/j.abb.2008.04.009. PMID:18424256. [DOI] [PubMed] [Google Scholar]
- [21].Burra S, Nicolella DP, Francis WL, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107:13648–53. doi: 10.1073/pnas.1009382107. PMID:20643964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thi MM, Suadicani SO, Schaffler MB, et al. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αVβ3 integrin. Proc Natl Acad Sci U S A. 2013;110:21012–7. doi: 10.1073/pnas.1321210110. PMID:24324138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Timpson P, Jones GE, Frame MC, et al. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001;11:1836–46. doi: 10.1016/S0960-9822(01)00583-8. PMID:11728306. [DOI] [PubMed] [Google Scholar]
- [24].Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–12. doi: 10.1016/j.ceb.2015.08.005. PMID:26363959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].O'Connor K, Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 2013;4:141–7. doi: 10.4161/sgtp.25131. PMID:24025634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Machacek M, Hodgson L, Welch C, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. PMID:19693013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Byrne KM, Monsefi N, Dawson JC, et al. Bistability in the Rac1, PAK, and RhoA Signaling Network Drives Actin Cytoskeleton Dynamics and Cell Motility Switches. Cell Syst. 2016;2:38–48. doi: 10.1016/j.cels.2016.01.003. PMID:27136688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hetmanski JHR, Zindy E, Schwartz J-M, et al. A MAPK-Driven Feedback Loop Suppresses Rac Activity to Promote RhoA-Driven Cancer Cell Invasion. PLoS Comput Biol. 2016;12:e1004909. doi: 10.1371/journal.pcbi.1004909. PMID:27138333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McGhee EJ, Morton JP, Von Kriegsheim A, Schwarz JP, et al. FLIM-FRET imaging in vivo reveals 3D-environment spatially regulates RhoGTPase activity during cancer cell invasion. Small GTPases. 2011;2:239–44. doi: 10.4161/sgtp.2.4.17275 doi: 10.4161/sgtp.2.4.17275. PMID:22145098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hirata E, Yukinaga H, Kamioka Y, et al. In vivo fluorescence resonance energy transfer imaging reveals differential activation of Rho-family GTPases in glioblastoma cell invasion. J Cell Sci. 2012;125:858–68. doi: 10.1242/jcs.089995. PMID:22399802. [DOI] [PubMed] [Google Scholar]
- [31].Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. PMID:12635176. [DOI] [PubMed] [Google Scholar]
- [32].Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. PMID:22118458. [DOI] [PubMed] [Google Scholar]
- [33].Hampton HR, Bailey J, Tomura M, et al. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat Commun. 2015;6:7139. doi: 10.1038/ncomms8139. PMID:25972253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. PMID:25415508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vennin C, Murphy KJ, Morton JP, et al. Reshaping the tumor stroma: emerging therapies in pancreatic cancer. Gastroenterology. 2017. pii: S0016-5085(17)36738-0. doi: 10.1053/j.gastro.2017.11.280. PMID:29287624. [DOI] [PubMed] [Google Scholar]
- [36].Fischer A, Stuckas H, Gluth M, et al. Impaired tight junction sealing and precocious involution in mammary glands of PKN1 transgenic mice. J Cell Sci. 2007;120:2272–83. doi: 10.1242/jcs.03467. PMID:17591691. [DOI] [PubMed] [Google Scholar]
- [37].Nobis M, McGhee EJ, Morton JP, et al. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of Src in pancreatic cancer. Cancer Res. 2013;73:4674–86. doi: 10.1158/0008-5472.CAN-12-4545. PMID:23749641. [DOI] [PubMed] [Google Scholar]
- [38].Cullis J, Meiri D, Sandi MJ, et al. The RhoGEF GEF-H1 is required for oncogenic RAS signaling via KSR-1. Cancer Cell. 2014;25:181–95. doi: 10.1016/j.ccr.2014.01.025. PMID:24525234. [DOI] [PubMed] [Google Scholar]
- [39].Campbell H, Fleming N, Roth I, et al. Δ133p53 isoform promotes tumour invasion and metastasis via interleukin-6 activation of JAK-STAT and RhoA-ROCK signalling. Nat Commun. 2017; accepted. doi: 10.1038/s41467-017-02408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. PMID:15894267. [DOI] [PubMed] [Google Scholar]
- [41].Morton JP, Timpson P, Karim SA, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:246–51. doi: 10.1073/pnas.0908428107. PMID:20018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. PMID:12459728. [DOI] [PubMed] [Google Scholar]
- [43].Biankin A V., Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. PMID:23103869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Muller PAJ, Caswell PT, Doyle B, et al. Mutant p53 Drives Invasion by Promoting Integrin Recycling. Cell. 2009;139:1327–41. doi: 10.1016/j.cell.2009.11.026. PMID:20064378. [DOI] [PubMed] [Google Scholar]
- [45].Trinidad AG, Muller PAJ, Cuellar J, et al. Interaction of p53 with the CCT complex promotes protein folding and wild-type p53 activity. Mol Cell. 2013;50:805–17. doi: 10.1016/j.molcel.2013.05.002. PMID:23747015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vennin C, Chin VT, Warren SC, et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med. 2017;9:eaai8504. doi: 10.1126/scitranslmed.aai8504. PMID:28381539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rath N, Morton JP, Julian L, et al. ROCK signaling promotes collagen remodeling to facilitate invasive pancreatic ductal adenocarcinoma tumor cell growth. EMBO Mol Med. 2017;9:198–218. doi: 10.15252/emmm.201606743. PMID:28031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–8. doi: 10.1038/embor.2012.127. PMID:22964758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Whatcott CJ, Ng S, Barrett MT, et al. Inhibition of ROCK1 kinase modulates both tumor cells and stromal fibroblasts in pancreatic cancer. PLoS One. 2017;12:e0183871. doi: 10.1371/journal.pone.0183871. PMID:28841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu S, Goldstein RH, Scepansky EM, et al. Inhibition of Rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742–51. doi: 10.1158/0008-5472.CAN-09-1541. PMID:19887617. [DOI] [PubMed] [Google Scholar]
- [51].Ritsma L, Steller EJA, Ellenbroek SIJ, et al. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat Protoc. 2013;8:583–94. doi: 10.1038/nprot.2013.026. PMID:23429719. [DOI] [PubMed] [Google Scholar]
- [52].Ritsma L, Steller EJA, Beerling E, et al. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Sci Transl Med. 2012;4:158ra145. doi: 10.1126/scitranslmed.3004394. PMID:23115354. [DOI] [PubMed] [Google Scholar]
- [53].Wauters E, Sanchez-Arévalo Lobo VJ, Pinho A V., et al. Sirtuin-1 regulates acinar-to-ductal metaplasia and supports cancer cell viability in pancreatic cancer. Cancer Res. 2013;73:2357–67. doi: 10.1158/0008-5472.CAN-12-3359. PMID:23370328. [DOI] [PubMed] [Google Scholar]
- [54].Neesse A, Krug S, Gress TM, et al. Emerging concepts in pancreatic cancer medicine: targeting the tumor stroma. Onco Targets Ther. 2013;7:33–43. doi: 10.2147/OTT.S38111. PMID:24379681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–60. doi: 10.1038/nm.4123. PMID:27376576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chakraborty S, Njah K, Pobbati A V., et al. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017;18:2464–79. doi: 10.1016/j.celrep.2017.02.041. PMID:28273460. [DOI] [PubMed] [Google Scholar]
- [57].Chou A, Froio D, Nagrial AM, et al. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut. 2017; pii: gutjnl–2017––315144.. doi: 10.1136/gutjnl-2017-315144. PMID:29080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yeo D, Phillips P, Baldwin GS, et al. Inhibition of group 1 p21-activated kinases suppresses pancreatic stellate cell activation and increases survival of mice with pancreatic cancer. Int J cancer. 2017;140:2101–11. doi: 10.1002/ijc.30615. PMID:28109008. [DOI] [PubMed] [Google Scholar]
- [59].Wörmann SM, Song L, Ai J, et al. Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology. 2016;151:180–193.e12. doi: 10.1053/j.gastro.2016.03.010. PMID:27003603. [DOI] [PubMed] [Google Scholar]
- [60].Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359–66. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- [61].Nicolle R, Blum Y, Marisa L, et al. Pancreatic Adenocarcinoma Therapeutic Targets Revealed by Tumor-Stroma Cross-Talk Analyses in Patient-Derived Xenografts. Cell Rep. 2017;21:2458–70. doi: 10.1016/j.celrep.2017.11.003. PMID:29186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Holvoet T, Devriese S, Castermans K, et al. Treatment of Intestinal Fibrosis in Experimental Inflam-matory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology. 2017;153:1054–67. doi: 10.1053/j.gastro.2017.06.013. PMID:28642198. [DOI] [PubMed] [Google Scholar]
- [63].Valon L, Marín-Llauradó A, Wyatt T, et al. Optogenetic control of cellular forces and mechanotransduction. Nat Commun. 2017;8:14396. doi: 10.1038/ncomms14396. PMID:28186127. [DOI] [PMC free article] [PubMed] [Google Scholar]


