ABSTRACT
Actin remodeling plays an essential role in diverse cellular processes such as cell motility, vesicle trafficking or cytokinesis. The scaffold protein and actin nucleation promoting factor Cortactin is present in virtually all actin-based structures, participating in the formation of branched actin networks. It has been involved in the control of endocytosis, and vesicle trafficking, axon guidance and organization, as well as adhesion, migration and invasion. To migrate and invade through three-dimensional environments, cells have developed specialized actin-based structures called invadosomes, a generic term to designate invadopodia and podosomes. Cortactin has emerged as a critical regulator of invadosome formation, function and disassembly. Underscoring this role, Cortactin is frequently overexpressed in several types of invasive cancers. Herein we will review the roles played by Cortactin in these specific invasive structures.
KEYWORDS: ARP2/3, actin cytoskeleton, Cortactin, invadopodia, invasion, migration, podosome, Rac1, Rho GTPases
Introduction
The actin cytoskeleton is involved in multiple cellular processes such as cell division, migration, or exocytosis. Monomeric globular actin (G-actin) is a 42kDa protein that polymerizes into filaments to form actin stress fibers or branched actin networks, which are required for the formation of various cellular structures including lamellipodia and invadopodia. G-actin polymerization in filaments is initiated by the association of three actin monomers forming a nucleus. This process, called actin nucleation, is highly unfavorable and requires actin nucleators such as formins or the ARP2/3 complex to allow the formation of unbranched or branched actin filaments, respectively.1 Conversely, actin nucleation is inhibited by proteins like Profilin or Thymosin-β4. The intrinsic nucleation activity of ARP2/3 is very low and the complex requires binding to other proteins, the Nucleation Promoting Factors (NPF), for activation.2,3 NPFs have been subdivided in two classes1: class I NPFs include WASP (Wiskott-Aldrich Syndrome Protein) and SCAR/WAVE, they bind to both monomeric actin and to the Arp2/3 complex. On the other hand, class II NPFs such as Cortactin bind to actin filaments and are thought to recruit ARP2/3 to these filaments allowing branched network assembly.1,4 Cortactin only weakly promotes the nucleation activity of ARP2/3 but stabilizes newly generated actin branching points, preventing disassembly of the network.1,4
Cortactin (p80/p85) was identified in 1991 as a new Src substrate colocalizing with F-actin in cellular protrusions and podosomes.5 p80 and p85 are encoded by a single mRNA5 and the presence of two bands is caused by post-translational modifications, likely multiple phosphorylations, with phosphorylated S418 only found in p80 and S405 phosphorylation only found in p856,7. The conversion of p80 to p85 is associated with the relocalization of Cortactin from the cytoplasm to the cell cortex and sites of cell/matrix contacts.6,7 The associated gene (originally called EMS1, now CTTN) was cloned in 1992 and is located on chromosome 11q13. This region is frequently amplified in human breast cancer and in head and neck squamous cell carcinomas (HNSCC) and is associated with unfavorable clinical outcome.8 The interaction between Cortactin and F-actin was confirmed in 1993, with the identification of F-actin-binding repeats in the amino-terminal half of the protein and of an SH3 domain in the carboxyl-terminal part of Cortactin.9 Given its enrichment in cortical structures such as membrane ruffles and lamellipodia, and its binding to F-actin, the name of Cortactin was coined. The same year came the realization that EMS1, amplified in several cancers, and Cortactin are in fact the same protein that may promote the invasive potential of tumors, owing to Cortactin localization in cellular structures dedicated to invasion and migration.10 Since this work, Cortactin function in cell motility and invasion, as well as in other cellular processes, has been intensely investigated and Cortactin is widely used as a marker of invadosomes. Given the domain structure of Cortactin (Fig. 1) and the many interactors and regulators identified, Cortactin is considered as a scaffold protein.
Figure 1.
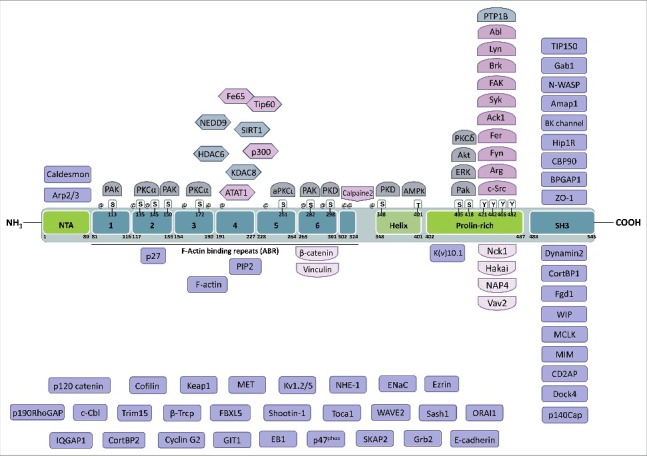
Domain structure of Cortactin and interacting partners. Cortactin is composed of an N-terminal acidic domain that allows the interaction with Arp2/3, followed by six and a half F-actin binding repeats of 32 amino acids that mediate binding to F-actin and can be acetylated on lysines or phosphorylated on serines. On its C-terminal part, Cortactin has a helical domain, a proline-rich domain that is extensively regulated by phosphorylation and whose tyrosines are targeted, among others, by Src-family kinases. Finally, Cortactin has an SH3 domain at its C-terminal end, which binds many different proteins mostly involved in the regulation of actin cytoskeleton dynamics, including N-WASP. The known binding partners of Cortactin are indicated close to the domain to which they bind to or target, when known, or in the lower part of the figure when unknown. Lysines targeted for acetylation are indicated by @ (amino acids 87, 124, 144, 161, 181, 198, 235, 272, 295, 304, 309, 346). The amino acid numbering refers to the mouse protein (NP_031829.2).
Regulation of Cortactin expression and stability
Human Cortactin is encoded by the CTTN gene, located on the long arm of chromosome 11 and is expressed ubiquitously, except in hematopoietic cells, which instead express the Cortactin paralog HS1.11 Only few studies have investigated the regulation of Cortactin expression. As far as we know, Cortactin mRNA level is increased after activation of CD44 hyaluronan receptor activation via NFκB signaling12 and after binding of phospho-STAT3 to the Cortactin promoter.13 Conversely, Cortactin expression is decreased by miR-542-3p14 and miR-32615. Thus, Cortactin might be mostly regulated by post-translational modifications and interactions with others proteins.
Cortactin has three isoforms generated by alternative splicing.16,17 The SV-1 and SV-2 splice variants are deleted for exon 11 or exon 10 and 11, respectively, corresponding to the 6th or 5th and 6th actin binding repeats (Fig. 1). The SV-1 variant is co-expressed with full-length Cortactin in all tissues whereas the SV-2 variant is absent from several tissues and has a decreased ability to bind F-actin and to induce actin filament polymerization.16,17 Alternative splicing of Cortactin mRNA is regulated by the RNA binding protein Rbfox2, which induces exon 11 exclusion after induction of epithelial-mesenchymal transition (EMT) by TGF-β.18 PTBP1, another RNA-binding protein, is also involved in Cortactin mRNA splicing. Indeed, PTBP1 induces the inclusion of exon 11, favoring the expression of full-length Cortactin and in this way tumor cell migration and invasion.19 However, the specific contribution of each Cortactin isoform to the different functions of Cortactin remains to be elucidated.
Cortactin degradation was reported to be induced by its phosphorylation by ERK on Ser405 and Ser418, which induces the interaction between Cortactin and β-TrCP, an E3 ubiquitin-ligase, and its degradation by the proteasome.20 The E3 ubiquitin-ligase Hakai binds tyrosine-phosphorylated Cortactin via a novel phosphotyrosine-binding domain.21 It remains unclear however whether Hakai targets Cortactin for destruction by the proteasome. The sites phosphorylated by ERK and Src have been shown to promote Cortactin activity towards actin polymerization and cell migration/invasion22,23 and targeting Cortactin phosphorylated on these sites for degradation could constitute a means to downregulate its activity. Another means to repress Cortactin activity is via cleavage by Calpain.24
Functions and post-translational modifications of Cortactin
As far as we know, Cortactin is mainly regulated by post-translational modifications. Cortactin is a substrate of Src family tyrosine kinases, which phosphorylate Cortactin on tyrosines located in its proline-rich region (Fig. 1),25-27 notably after homophilic interactions between E-cadherin or N-cadherin.28,29 Tyrosines in the proline-rich domain are also targeted by Arg and Abl, two Abl-family tyrosine kinases, after PDGF30,31 or EGFR22 stimulation or β1 integrin activation.32 Cortactin can also be phosphorylated on serines and threonines mainly located in the F-actin binding repeats and proline-rich region, regulating Cortactin function, including actin polymerization.33 Acetyltransferases and deacetylases control acetylation on several lysines within the F-actin binding repeats, also regulating Cortactin localization and activity.34-41
The main function of Cortactin is to promote the formation of branched actin networks. Several studies have shown that the Arp2/3 complex binds Cortactin on its N-terminal acidic (NTA) domain and this interaction promotes actin nucleation, supporting a NPF function for Cortactin.4,42 The interaction between Cortactin, Arp2/3 and F-actin takes place at the cell periphery and induces branched actin generation, thereby promoting migration and invasion.42,43 On the other hand, the protein MIM (Missing-in-Metastasis, MTSS1), which binds to the SH3 domain of Cortactin, inhibits actin polymerization and cell migration, possibly by opposing N-WASP activity.44 This antagonistic relation was found to play a critical role during ciliogenesis, where MIM inhibited Src-mediated Cortactin phosphorylation, thereby promoting cilia formation.45
Cortactin binds to N-WASP and WIP (WASP-interacting protein) via its SH3 domain and since Cortactin is an Arp2/3 complex activator, it was first believed that Cortactin activated Arp2/3 via its interaction with N-WASP,46,47 but it appears that the mechanism is more complex than initially thought.1,4,48,49 Indeed, Cortactin can promote the interaction of Arp2/3 with F-actin,4,42 which activates Arp2/3. In addition, Cortactin directly stabilizes branched actin networks by remaining at newly formed branch points with Arp2/3, unlike N-WASP that is released, preventing the dissociation of branched actin.4 Cortactin also binds WAVE2, another WASP-family protein member, and this interaction induces actin polymerization.50 The Cortactin/WAVE2 interaction is inhibited by SKAP2 (Src Kinase-Associated Phosphoprotein 2), a Src substrate, and promoted by Cortactin phosphorylation on Ser405 and Ser418 by PKCδ.51,52 Phosphorylation of these serines by ERK also promotes N-WASP activation and actin polymerization.53 Due to its role in branched actin polymerization, Cortactin has a major function in invadopodia regulation which is described below.
Numerous studies have shown that Cortactin promotes cell migration in different cell types and by different mechanisms,16,42,54,55 often following phosphorylation by various kinases. Cortactin phosphorylation on Tyr421 and Tyr466 by Src, Fyn and Fer kinases,25-27,56 as well as on Thr401/Ser405 and Ser417/Ser418 by Akt or ERK53,57 is pro-migratory. Although Cortactin is used as a lamellipodia marker and regulates actin nucleation in lamellipodia, it is not required for lamellipodia formation.54,55,58,59 As a matter of fact, Cortactin, whose localization in lamellipodia is regulated by several proteins (p120 catenin,60 BPGAP1,61 NEDD9,36 Rac1,62 Arp2/343 and GIT-163), is required for lamellipodia persistence54,58,60 and for the regulation of actin dynamics in lamellipodia downstream of Rho GTPases and Dynamin.54,59,64-66 This regulation could be mediated by the interaction of Cortactin with different Guanine-nucleotide Exchange Factors (GEFs) and GTPase-Activating Proteins (GAPs) such as Fgd1 and BPGAP1.61,67
Recent work supports an important role for lysine acetylation of Cortactin in the regulation of cell migration. The histone deacetylases HDAC6 (class II) and Sirtuin1 (class III) modulate the acetylation levels of Cortactin within its actin-binding repeats and consequently promote the interaction between Cortactin and F-actin and cell motility.34,35 Furthermore, the scaffold protein NEDD9 recruits HDAC6 on Cortactin, promoting its deacetylation and localization at lamellipodia.36 This effect could be explained by a recent study providing evidence that deacetylated Cortactin binds KEAP1 (Kelch-Like ECH-Associated Protein 1) to be exported from the nucleus, allowing its transport to lamellipodia.37 In endothelial cells, HDAC6-mediated Cortactin deacetylation increases cell migration and is required for angiogenesis.38 Cortactin can also be deacetylated by KDAC8 (HDAC8) in vascular smooth muscle cells but the specific effects of this deacetylation remain to be investigated.39 Cortactin acetylation is mediated by several acetyltransferases, including p300,35 CBP when Cortactin is nuclear,37 ATAT1,40 as well as Tip60, which is recruited on Cortactin by Fe65.41 Most studies support an inhibition of cell migration by Cortactin acetylation.
The many functions of Cortactin
Aside from its role in migration and invasion, Cortactin is involved in the regulation of a wide variety of cellular processes that will be briefly described here. Another function of Cortactin is its involvement in vesicle trafficking. First, Cortactin regulates clathrin-dependent endocytosis and Golgi transport in association with Dynamin2.68,69 Cortactin/Dynamin2 interaction is promoted by Cortactin phosphorylation on Ser261 by aPKC⍳ and allows MT1-MMP trafficking.70 Cortactin function in clathrin-dependent endocytosis involves branched actin polymerization, which was negatively regulated by Hip1R (Huntingtin Interacting Protein 1 Related)71 and PIP272 and positively by Ack1 (Tyrosine Kinase Non Receptor 2, TNK2) and CD2AP during EGFR endocytosis.73,74 Cortactin also regulates clathrin-independent endocytosis and has been involved in IL-2 receptor endocytosis via a Rac1/PAK1-2/P-Ser405-Ser418-Cortactin pathway, which increased Cortactin/N-WASP binding and actin polymerization required for endocytosis.75,76 A recent study has shown that Cortactin promotes exosome secretion by controlling both trafficking and plasma membrane docking of multivesicular late endosomes with the Arp2/3 complex.77 This role of Cortactin in promoting vesicle trafficking is important in the context of invadosome function, which, when mature, secrete proteases in the intercellular space to digest matrix. By controlling vesicle trafficking, Cortactin also regulates autophagy under control of HDAC6 and INPP5E by inducing actin remodeling and stabilization, allowing autophagosome-lysosome fusion and substrate degradation.78,79
Cortactin is also involved in cell-cell contact formation. Cortactin is recruited and phosphorylated by Src-family kinases after homophilic interaction between E-cadherins. Cortactin directly binds to the cytoplasmic tail of E-cadherin, allowing the recruitment of Arp2/3 and WAVE2 and actin reorganization at junctions.29,50,80 Similarly, Cortactin is recruited by Rac1 to cell junctions involving N-cadherins, where it is phosphorylated by Fer kinase, strengthening intercellular adhesion.28 Cortactin is also involved in endothelial barrier remodeling via recruitment to the cell periphery by IQGAP1.81 Cortactin also plays a role in the formation of new focal adhesions.54 Cortactin binds to and is phosphorylated by FAK, and this interaction promotes focal adhesion turnover and cell motility.82
Cortactin is involved in the response against different stresses that induce cytoskeletal remodeling to protect cells. For example, shear stress in vascular endothelial cells activates AMPK, which phosphorylates Cortactin on Thr401, priming Cortactin for deacetylation by Sirtuin1 and allowing actin remodeling.83 During hyperosmotic stress inducing cell shrinkage, Cortactin is phosphorylated by Fyn and Fer kinases to mediate cytoskeletal rearrangements needed for osmoprotection.84,85 The phosphatase PTP1B also controls Cortactin tyrosine phosphorylation to prevent apoptosis induced by hyperosmotic stress.86
Finally, Cortactin also regulates membrane excitability by linking the actin cytoskeleton and several ion channels, such as calcium-activated (BK) channels,87 potassium channels Kv1.5,88 Kv10.189 and sodium channels ENaC90 or by regulating channel endocytosis, as reported for potassium channel Kv1.2.91
During neurogenesis Cortactin appears to plays several roles: it regulates the balance between stable neurite shaft and the formation of new growth cone,24 the collateral branching of axons via the control of filopodia formation,92 dendritic spine formation93,94 and traction force generation in axonal growth cones via an interaction with Shootin1.95
Cortactin in cancer
Overexpression of Cortactin is frequently observed in many types of cancers, including head and neck squamous cell carcinoma (HNSCC),96 hepatocellular carcinoma,97 breast cancer,98 bladder cancer,99 renal cell carcinoma,100 esophageal squamous cell carcinoma,101 colorectal adenocarcinoma,102 melanoma,103 osteosarcoma,104 prostate cancer,105 non-small cell lung cancer,106 glioma,107 epithelial ovarian cancer,108 thyroid cancer109 and B-cell chronic lymphocytic leukemia.110 This overexpression is partially caused by the amplification of chromosome 11q13 where the CTTN gene is located. For instance, CTTN amplification is found in 60–68% of esophageal cancer,101,111 20–37% of HNSCC112,113 and almost 60% in oral squamous cell carcinoma, a HNSCC subtype,114 18% of hepatocellular carcinoma,115 15–26% of breast cancer116,117 and 11% of bladder cancer.99 Nevertheless, several studies showed that CTTN or 11q13 amplification does not always explain Cortactin overexpression, suggesting that it may be caused by other mechanisms. For example, in esophageal cancer, Cortactin expression is induced by VEGF-C, which decreases Dicer-mediated maturation of miR-326, thereby relieving the suppressive effect of miR-326 on Cortactin expression.15 Furthermore, in a wide variety of human tumors, the frequent constitutive activation of STAT3, which targets the CTTN promoter,13 may underlie Cortactin overexpression.118
Cortactin overexpression is consistently associated with poor prognosis and decreased patient survival in most cancers.98,100,102-105,108,110 Some studies suggest that phospho-Y421-Cortactin levels are also elevated in cancer and associated with poor prognosis.119,120 Recently, two studies focused on deciphering the involvement of each Cortactin isoform in carcinogenesis and this work suggest that full length Cortactin is overexpressed compared to the SV-1 isoform and may be responsible for the oncogenic role of Cortactin.19,110 This could be explained by the overexpression of PTBP1, which regulates alternative splicing of Cortactin.19 However, the specific contribution of each Cortactin isoform to oncogenesis is still unclear and needs to be investigated.
A major conclusion from clinical studies is that Cortactin overexpression is associated with local invasion, lymph node metastasis and/or distal metastasis in almost every cancer in which it is overexpressed.19,96,97,99,101,103,106,109,113 Moreover, several mouse models have provided evidence that Cortactin promotes the metastatic process.97,101,121 Altogether, these findings indicate that Cortactin plays an important role in promoting tumor invasion and metastasis, consistent with the major role of Cortactin in regulating lamellipodia and invadopodia formation.
Cortactin in invadosomes
During embryogenesis or wound healing, cells have to move. They also move during tumor invasion. Cell movement requires that they invade into their surroundings formed by a dense network of extracellular matrix (ECM) proteins. For this, the cell first attaches to the ECM, degrades it, and moves into the newly liberated space. Invadosomes, which designates invadopodia made by cancer cells and podosomes made by normal cells, are specialized cellular structures that enable all these steps.122 Invadosomes are very dynamic, with a half-life of a few minutes. After a stimulus, they first assemble at sites of ECM interaction with the cell, they then release proteins that degrade the matrix, such as matrix metalloproteases (MMPs), and finally disassemble again, allowing cell movement (Fig. 2A). Invadopodia often become activated in cancer cells, which allows tumor cells to invade either locally or throughout the body to form metastases. Many proteins regulating invadopodia formation have been identified, including receptors to growth factors or ECM, scaffold proteins, kinases or GTPases. Amongst them, Cortactin plays a major role in all steps of the invadosome lifecycle.
Figure 2.
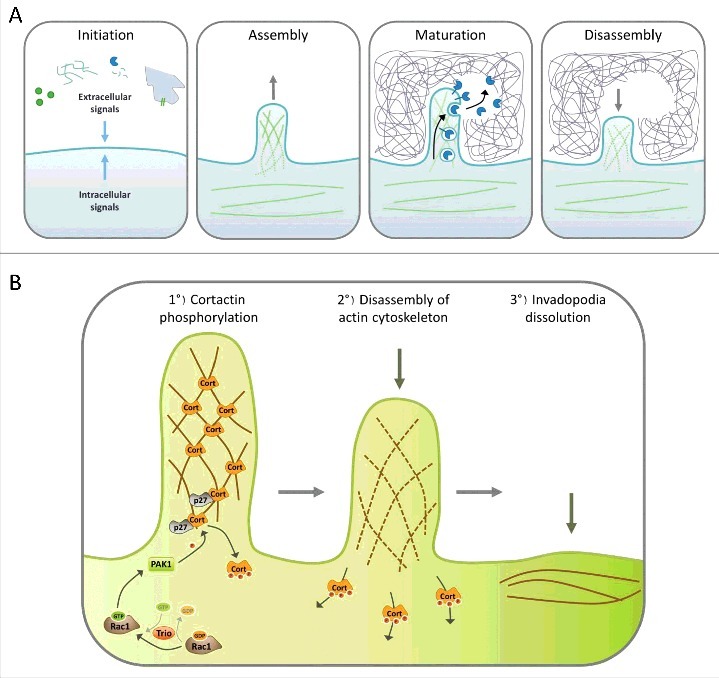
Steps of invadopodia formation. A. The invadopodia lifecycle can be divided in four steps: Initiation, assembly, maturation and disassembly. Cells begin to form invadopodia in response to activating signals transmitted by growth factor or matrix receptors, MMP activity, heterotypic cell interaction, EMT or oncogenic transformation. These signals activate different signaling pathways that induce branched actin polymerization and formation of a cellular protrusion. Once invadopodia are mature, different proteases are secreted, allowing degradation of the surrounding matrix. The final step consists in the dissolution of the invadopodia, which includes branched actin disassembly. B. Role of Cortactin in invadopodia disassembly. Invadopodia dissolution is triggered by the activation of Rac1 by its GEF Trio; in turn Rac1 activates PAK1, which is recruited by p27 on Cortactin. Then, PAK1 phosphorylates Cortactin on S113, S150 and/or S282, which probably induces the release of Cortactin from branched F-actin, destabilizing the branched actin network which disassembles, allowing the return to a basal situation.
Initiation
Invadosome formation is induced by different cellular signals which can be divided into 5 types: (i) growth factors such as EGF, PDGF or TGF-β123 activating their receptors; (ii) oncogenic transformation induced by oncogenes like v-src or Ras124,125; (iii) EMT induction126; (iv) cellular environment, including matrix composition,122 heterotypic cell interaction with macrophages127 or hypoxia128; and (v) metalloproteases activity.123 After one of these stimuli, several signaling pathways are activated. Among them, Src seems to play a central function by activating Cdc42 GEFs required for invadosome formation (Vav-1, β-PIX and Fgd1,123 but also Tuba, which regulates linear invadosomes129) and by phosphorylating scaffold proteins like Tks5 and Cortactin. Cortactin plays a central role by scaffolding several proteins required for invadopodia assembly at the initiation site.
Several signaling pathways like Src, PAK or Erk130 converge to Cortactin to regulate its function in invadopodia via phosphorylations. Nevertheless, Cortactin phosphorylation by PAK1 may have antagonistic effects depending on cell context. Indeed, Cortactin phosphorylation on Ser113 by PAK1 increases ECM degradation130 but also induces invadopodia disassembly.131,132 Stimulation of c-Met by HGF induces invadopodia formation and cell invasion mediated by an interaction of Cortactin with Grb2 and Gab1.133,134 Furthermore, c-Met directly binds Cortactin and induces its phosphorylation, but whether c-Met directly phosphorylates Cortactin is still unclear.133,134
Assembly
Invadopodia assembly is driven by branched F-actin generation and the formation of a cellular protrusion. The first step is the assembly of the invadopodial core, with the recruitment of Cortactin, which scaffolds Arp2/3, Cofilin and N-WASP, to the membrane. Even if Tyr421 and Tyr466 of Cortactin can be phosphorylated by Src, at the invadopodia, it seems that they are mainly targeted by the tyrosine kinase Arg.22 Arg is activated both by β1-integrin and the EGFR/Src pathway.32,135 However, Arg activation seems to have opposite roles according to the cellular context. Indeed, in mammary tumor cells, Arg promotes invadopodia formation, whereas it has an inhibitory role in squamous cell carcinoma cells.32,135 Cortactin phosphorylation on Y421 and Y466 allows its association with Nck1, which recruits N-WASP to Cortactin, as well as Vav2,136 promoting the generation of free actin barbed ends and branched actin polymerization.23
Inside the invadopodial core, Cofilin plays a major role. Cofilin, an actin filament-severing protein, generates new free actin barbed ends, promoting branched actin formation by the Arp2/3 complex.137 Cortactin inhibits Cofilin's severing activity inside the invadopodia.138 This inhibition is relieved when Cortactin is phosphorylated on tyrosines (Y421, Y466 and Y482), allowing the recruitment of NHE1, which induces a local increase of pH.138,139 This increase promotes the dissociation of Cortactin and Cofilin, allowing Cofilin to sever actin filaments and to promote Arp2/3 activity. Cortactin scaffolds ARP2/3, F-actin barbed ends and N-WASP to promotes actin nucleation.4 After invadopodia formation, Cortactin is dephosphorylated, inhibiting again Cofilin to allow invadopodia growth and stabilization.138,139 Cofilin activity is also spatially regulated by RhoC, whose active form surrounds invadopodia and activate the ROCK/LIMK pathway, maintaining Cofilin in its Ser3-phosphorylated (a target of LIMK), inhibited form, thus concentrating Cofilin activity within the invadopodial core.140 The regulation of RhoC activation state is mediated by p190RhoGEF and p190RhoGAP140 as well as Podoplanin.141 Cofilin function in invadopodia is also regulated after transient biomechanical forces via β3-integrin signaling.142
Invadopodia maturation is promoted by the Mena isoform MenaINV, an actin barbed-end capping protein antagonist. MenaINV is recruited just after invadopodia formation initiates and promotes Tyr421 phosphorylation of Cortactin, possibly by displacing PTP1B from the invadopodial core and preventing Cortactin dephosphorylation on Tyr421.143 By displacing actin barbed end capping proteins, Mena also promotes branched actin polymerization in invadopodia. The phosphatase SHIP2 is also involved in invadopodia assembly by controlling Mena recruitment as well as PI(3,4)P2 accumulation, which promotes Tks5 recruitment via its phox homology (PX) domain.144,145
Even if branched actin generation by Arp2/3, N-WASP, Cortactin and Cofilin is required for invadopodia formation, several studies found that unbranched actin is also present in invadopodia. Indeed, different F-actin bundling proteins such as α-Actinin, Fimbrin or Fascin are present in invadopodia and Fascin knockdown decreases invadopodia formation and ECM degradation.146,147 Moreover, formins from the DRF family are also involved in these two processes, confirming that unbranched F-actin is important for invadopodia formation and activity.148
Finally, Cortactin function in invadosome is also regulated by Caldesmon, which inhibits invadopodia formation by an unknown mechanism,149-151 but could involve a competition with Arp2/3 complex binding to Cortactin, since Caldesmon also binds to the NTA domain of Cortactin,149 or a direct inhibition of Arp2/3 in invadopodia.152
Maturation
During the maturation stage of the invadopodia lifecycle, the structure is transiently stabilized and the surrounding ECM is degraded by proteases. Several proteases degrade ECM at invadopodia, including MMPs, ADAMs, Cathepsins and serine proteinases.153 MT1-MMP (also called MMP-14) has a preponderant function at invadopodia to degrade ECM.154 Proteases recruitment involves kinesin activity along the microtubule network to bring them via vesicles from the Golgi apparatus, which often localizes close to invadopodia, and then vesicles merge with the plasma membrane.153,155 ECM stiffness and rigidity also promote degradation activity at invadopodia.156-158 Several studies investigating MT1-MMP delivery to plasma membrane have shown that it is regulated by the exocyst complex, an 8-protein complex involved in vesicle trafficking regulated by IQGAP1 (under control of Cdc42 and RhoA) and the WASH complex.159,160 MT1-MMP delivery is also regulated by the v-SNARE VAMP-7 and negatively regulated by CIP4 and SNX9, two Src-substrates.161-163 Tks5 promotes Rab40b-mediated transport of MMP-2 and MMP-9 to invadopodia.164 Cortactin has a major function in matrix degradation and MMP-2, MMP-9 and MT1-MMP secretion.154,165,166 Indeed, the cytoplasmic tail of MT1-MMP is phosphorylated by LIMK, allowing its interaction with Cortactin which is required for its trafficking to invadopodia.167 A recent study shows that Cortactin phosphorylation by PKCι allows its association with Dynamin-2, promoting trafficking of MT1-MMP containing endosomes.70 Cortactin acetylation levels also appear to regulate its role in MT1-MMP transport and ECM degradation.40 Recent evidence has shown a key role for Cortactin in late endosomal vesicle trafficking and exosome secretion.77 The function of Cortactin in trafficking of others proteases, such as MMP-2 or MMP-9 that also have an important function in invadosomes, still needs to be investigated. Together, evidence suggests that Cortactin acts as a hub for both invadopodia formation and function.
Disassembly
Invadopodia disassembly is clearly the least understood step of the invadopodia lifecycle. Nevertheless, recent studies seem to involve two different pathways in the regulation of this critical step. The first pathway begins with the activation of Rac1 by one of its GEFs, Trio, whose upstream activators are not described yet. In turn, active Rac1 activates its effector PAK1, which is recruited by p27 on Cortactin and phosphorylates Cortactin on Ser113, S150 and/or S282131,132 (Fig. 2B). Inhibiting this pathway increases invadopodia lifetime, thereby increasing invadopodia number and matrix degradation. Surprisingly, cell invasion is negatively affected by inhibition of this pathway, showing that invadopodia turnover is required for an efficient invasion.131,132 The mechanism involved in invadopodia dissolution after PAK-mediated Cortactin phosphorylation is still unclear but may be due to a decreased affinity of Cortactin phosphorylated within its actin-binding repeats for F-actin, which may destabilize invadopodia.168
A second pathway inducing invadopodia disassembly has been described recently. RhoG, a Rho GTPase of the Rac subfamily, promotes the phosphorylation of Paxillin.169 Then, phosphorylated Paxillin induces invadosome dissolution by an ERK/Calpain pathway.170 Calpain is a cysteine protease that promotes podosome disassembly by cleaving Talin, Pyk2 and WASP.171 However, since Talin and Pyk2 functions have mainly been described in podosomes, it is unclear if these proteins are also Calpain substrates in invadopodia. As Cortactin is a substrate of Calpain,172 it would be interesting to investigate if Calpain function in invadopodia disassembly could be mediated by Cortactin cleavage.
The pathway involved in invadopodia disassembly seems dependent on the upstream pathway activated or on the cell type investigated. Indeed, in cells where RhoG induces invadopodia disassembly, Rac1 is involved in invadopodia initiation.169 Reciprocally, RhoG knockdown in cells where Rac1/PAK1/Cortactin promotes invadopodia disassembly does not affect invadopodia turnover.131 Further investigations are needed to fully understand the pathways that lead to invadopodia disassembly.
Conclusion and perspectives
Since its identification, numerous studies have described roles for Cortactin in a wide variety of cellular processes including cell migration, vesicle trafficking or neurite outgrowth. Most of these functions appear to relate to its ability to regulate actin cytoskeleton dynamics, either directly or via the scaffolding of proteins involved in signaling pathways that impinge on the actin cytoskeleton. An outstanding question to be addressed is the contribution of each splice variants of Cortactin in its different functions. The best characterized function of Cortactin is in the regulation of cell migration and invasion, which probably underlies its frequent overexpression in several metastatic cancers, as Cortactin is involved in every step of the invadopodia lifecycle, allowing matrix degradation and tumor invasion.
Similarly, Cortactin is present in podosomes in different types of cells, such as v-Src transformed fibroblasts,173 vascular smooth muscle cells,174 osteoclasts175 and dendritic cells.176 Cortactin is recruited to podosomes via Tks5177 and acts as a scaffold to recruit several proteins required for podosome formation, including N-WASP,173 Fgd1,178 AFAP1L1179 or ZO-1.180 Unlike what is described in invadopodia, Cortactin also regulates microtubule dynamics181 and actomyosin contractility in podosomes.174 It is interesting to notice that HS1, the Cortactin homologue expressed in hematopoietic cells, has a function in the regulation of podosome organization in dendritic cells.182 Overall, Cortactin is involved in the formation,183 maturation175 and matrix degradation176 at podosomes but a potential role of Cortactin in podosome disassembly remains to be investigated.
Despite the numerous articles characterizing invadosome function in vitro, there are much less reports studying them in vivo, undoubtedly due to the technical difficulty to observe such ephemeral structures within tissues or organisms. A first line of evidence of invadosomes' existence in vivo is provided by the fact that in different cancers, many proteins playing a major role in invadopodia formation or maturation are associated with poor prognosis or/and metastasis, including Cortactin, Fascin, Fgd1, MT1-MMP or Tks5.184 In mouse models, the use of an shRNA against N-WASP dramatically reduces the ability of cells to form cellular protrusions, intravasate or metastasize.185 Similarly, mammary tumor cells in which Tks5 is knocked-down lose their ability to invade locally or to form distant metastases.126 Moreover, primary cells derived from patients with different types of cancer form invadopodia enriched in Cortactin in culture, which are structurally identical to those observed in cell lines.185
Different studies have directly observed invadosomes in in vivo models. When they intravasate, tumor cells form protrusions which resemble invadopodia186 and this phenomenon is also observed in zebrafish.187 In the nematode C. elegans, anchor cells generate invasive protrusions to break down the basement membrane in response to inducing signals.188 In mice, Cortactin- and Tks5-enriched cellular protrusions have been observed in xenografted tumor cells by immunohistofluorescence, suggesting invadopodia presence in vivo.126,185 Finally, in recent work using the chorioallantoic membrane model in chicken embryos, human tumor cells injected in capillaries were observed extravasating by intravital microscopy.189 This approach has shown that, during extravasation, cells form membrane protrusions enriched in Cortactin, Tks4 and Tks5 allowing them to breakdown blood vessel walls.189 Knockdown of Cortactin with shRNA inhibited tumor cells extravasation. This study validates in vivo the presence of invadopodia in tumor cells and their role in extravasation.189 Nevertheless, further investigations are still required to fully understand the function of invadopodia in tumor cell invasion and the specific roles of Cortactin in this process.
Acknowledgments
The Besson group is supported by funds from the Fondation ARC pour la Recherche sur le Cancer and by an “FRM Equipes” grant (DEQ20170336707) from the Fondation pour la Recherche Médicale.
Funding
Fondation pour la Recherche Medicale, DEQ20170336707.
References
- 1.Goley ED, Welch MD.. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–26. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 2.Nürnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat Rev Cancer. 2011;11:177–87. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- 3.Pollard TD. Actin and Actin-Binding Proteins. Cold Spring Harb Perspect Biol. 2016;8(8):a018226. doi: 10.1101/cshperspect.a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver AM, Karginov AV, Kinley AW, et al. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol CB. 2001;11:370–4. doi: 10.1016/S0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Reynolds AB, Kanner SB, et al. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol 1991;11:5113–24. doi: 10.1128/MCB.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Damme H, Brok H, Schuuring-Scholtes E, et al. The redistribution of cortactin into cell-matrix contact sites in human carcinoma cells with 11q13 amplification is associated with both overexpression and post-translational modification. J Biol Chem. 1997;272:7374–80. doi: 10.1074/jbc.272.11.7374. [DOI] [PubMed] [Google Scholar]
- 7.Tegtmeyer N, Wittelsberger R, Hartig R, et al. Serine phosphorylation of cortactin controls focal adhesion kinase activity and cell scattering induced by Helicobacter pylori. Cell Host Microbe. 2011;9:520–31. doi: 10.1016/j.chom.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Schuuring E, Verhoeven E, Mooi WJ, et al. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–61. [PubMed] [Google Scholar]
- 9.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–26. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuuring E, Verhoeven E, Litvinov S, et al. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol Cell Biol. 1993;13:2891–8. doi: 10.1128/MCB.13.5.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rossum AGSH, Schuuring-Scholtes E, van Buuren-van Seggelen V, et al. Comparative genome analysis of cortactin and HS1: the significance of the F-actin binding repeat domain. BMC Genomics. 2005;6:15. doi: 10.1186/1471-2164-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill A, McFarlane S, Mulligan K, et al. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–91. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- 13.Du X-L, Yang H, Liu S-G, et al. Calreticulin promotes cell motility and enhances resistance to anoikis through STAT3-CTTN-Akt pathway in esophageal squamous cell carcinoma. Oncogene. 2009;28:3714–22. doi: 10.1038/onc.2009.237. [DOI] [PubMed] [Google Scholar]
- 14.Long H-C, Gao X, Lei C-J, et al. miR-542-3p inhibits the growth and invasion of colorectal cancer cells through targeted regulation of cortactin. Int J Mol Med. 2016;37:1112–8. doi: 10.3892/ijmm.2016.2505. [DOI] [PubMed] [Google Scholar]
- 15.Hong C-C, Chen P-S, Chiou J, et al. miR326 maturation is crucial for VEGF-C-driven cortactin expression and esophageal cancer progression. Cancer Res. 2014;74:6280–90. doi: 10.1158/0008-5472.CAN-14-0524. [DOI] [PubMed] [Google Scholar]
- 16.van Rossum AGSH, de Graaf JH, Schuuring-Scholtes E, et al. Alternative splicing of the actin binding domain of human cortactin affects cell migration. J Biol Chem. 2003;278:45672–9. doi: 10.1074/jbc.M306688200. [DOI] [PubMed] [Google Scholar]
- 17.Ohoka Y, Takai Y. Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes Cells Devoted Mol Cell Mech. 1998;3:603–12. [DOI] [PubMed] [Google Scholar]
- 18.Braeutigam C, Rago L, Rolke A, et al. The RNA-binding protein Rbfox2: an essential regulator of EMT-driven alternative splicing and a mediator of cellular invasion. Oncogene. 2014;33:1082–92. doi: 10.1038/onc.2013.50. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z-N, Liu D, Yin B, et al. High expression of PTBP1 promote invasion of colorectal cancer by alternative splicing of cortactin. Oncotarget. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Wei J, Mialki R, Zou C, Mallampalli RK, Zhao Y. Extracellular signal-regulated kinase (ERK) regulates cortactin ubiquitination and degradation in lung epithelial cells. J Biol Chem. 2012;287:19105–14. doi: 10.1074/jbc.M112.339507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee M, Chow SY, Yusoff P, et al. Structure of a novel phosphotyrosine-binding domain in Hakai that targets E-cadherin. EMBO J. 2012;31:1308–19. doi: 10.1038/emboj.2011.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mader CC, Oser M, Magalhaes MA, et al. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–41. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oser M, Mader CC, Gil-Henn H, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–73. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mingorance-Le Meur A, O'Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2009;28:248–60. doi: 10.1038/emboj.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Liu J, Haudenschild CC, et al. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998;273:25770–6. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Asawa T, Takato T, et al. Cooperative roles of Fyn and cortactin in cell migration of metastatic murine melanoma. J Biol Chem. 2003;278:48367–76. doi: 10.1074/jbc.M308213200. [DOI] [PubMed] [Google Scholar]
- 27.Sangrar W, Gao Y, Scott M, et al. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol Cell Biol. 2007;27:6140–52. doi: 10.1128/MCB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Sayegh TY, Arora PD, Fan L, et al. Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol Biol Cell. 2005;16:5514–27. doi: 10.1091/mbc.E05-05-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren G, Helwani FM, Verma S, et al. Cortactin is a functional target of E-cadherin-activated Src family kinases in MCF7 epithelial monolayers. J Biol Chem. 2009;284:18913–22. doi: 10.1074/jbc.M109.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle SN, Michaud GA, Schweitzer B, et al. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol CB. 2007;17:445–51. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 31.Lapetina S, Mader CC, Machida K, et al. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009;185:503–19. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaty BT, Sharma VP, Bravo-Cordero JJ, et al. β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol Biol Cell. 2013;24:1661–75, S1-11. doi: 10.1091/mbc.E12-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macgrath SM, Koleske AJ. Cortactin in cell migration and cancer at a glance. J Cell Sci. 2012;125:1621–6. doi: 10.1242/jcs.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Yuan Z, Zhang Y, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhang M, Dong H, et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–60. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 36.Kozyreva VK, McLaughlin SL, Livengood RH, et al. NEDD9 regulates actin dynamics through cortactin deacetylation in an AURKA/HDAC6-dependent manner. Mol Cancer Res MCR. 2014;12:681–93. doi: 10.1158/1541-7786.MCR-13-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito A, Shimazu T, Maeda S, et al. The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1. Sci Signal. 2015;8:ra120. doi: 10.1126/scisignal.aad0667. [DOI] [PubMed] [Google Scholar]
- 38.Kaluza D, Kroll J, Gesierich S, et al. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J. 2011;30:4142–56. doi: 10.1038/emboj.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen A, Karolczak-Bayatti M, Sweeney M, et al. Lysine deacetylase inhibition promotes relaxation of arterial tone and C-terminal acetylation of HSPB6 (Hsp20) in vascular smooth muscle cells. Physiol Rep. 2013;1:e00127. doi: 10.1002/phy2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro-Castro A, Janke C, Montagnac G, et al. ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur J Cell Biol. 2012;91:950–60. doi: 10.1016/j.ejcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Sun J, Lungchukiet P, et al. Fe65 Suppresses Breast Cancer Cell Migration and Invasion through Tip60 Mediated Cortactin Acetylation. Sci Rep. 2015;5:11529. doi: 10.1038/srep11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uruno T, Liu J, Zhang P, et al. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–66. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 43.Weed SA, Karginov AV, Schafer DA, et al. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Liu J, Wang Y, et al. Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene. 2005;24:2059–66. doi: 10.1038/sj.onc.1208412. [DOI] [PubMed] [Google Scholar]
- 45.Bershteyn M, Atwood SX, Woo W-M, et al. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell. 2010;19:270–83. doi: 10.1016/j.devcel.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinley AW, Weed SA, Weaver AM, et al. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr Biol CB. 2003;13:384–93. doi: 10.1016/S0960-9822(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 47.Weaver AM, Heuser JE, Karginov AV, et al. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol CB. 2002;12:1270–8. doi: 10.1016/S0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 48.Siton O, Ideses Y, Albeck S, et al. Cortactin releases the brakes in actin- based motility by enhancing WASP-VCA detachment from Arp2/3 branches. Curr Biol CB. 2011;21:2092–7. doi: 10.1016/j.cub.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Uruno T, Liu J, Li Y, et al. Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with neural Wiscott-Aldrich syndrome protein (N-WASP) and cortactin during branched actin filament network formation. J Biol Chem. 2003;278:26086–93. doi: 10.1074/jbc.M301997200. [DOI] [PubMed] [Google Scholar]
- 50.Han SP, Gambin Y, Gomez GA, et al. Cortactin scaffolds Arp2/3 and WAVE2 at the epithelial zonula adherens. J Biol Chem. 2014;289:7764–75. doi: 10.1074/jbc.M113.544478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimamura S, Sasaki K, Tanaka M. The Src substrate SKAP2 regulates actin assembly by interacting with WAVE2 and cortactin proteins. J Biol Chem. 2013;288:1171–83. doi: 10.1074/jbc.M112.386722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janjanam J, Chandaka GK, Kotla S, et al. PLCβ3 mediates cortactin interaction with WAVE2 in MCP1-induced actin polymerization and cell migration. Mol Biol Cell. 2015;26:4589–606. doi: 10.1091/mbc.E15-08-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Quiles N, Ho H-YH, Kirschner MW, et al. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–80. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryce NS, Clark ES, Leysath JL, et al. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol CB. 2005;15:1276–85. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 55.van Rossum AGSH, Moolenaar WH, Schuuring E. Cortactin affects cell migration by regulating intercellular adhesion and cell spreading. Exp Cell Res. 2006;312:1658–70. [DOI] [PubMed] [Google Scholar]
- 56.Fujimura A, Michiue H, Cheng Y, et al. Cyclin G2 promotes hypoxia-driven local invasion of glioblastoma by orchestrating cytoskeletal dynamics. Neoplasia N Y N. 2013;15:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X, Renuse S, Sahasrabuddhe NA, et al. Activation of diverse signalling pathways by oncogenic PIK3CA mutations. Nat Commun. 2014;5:4961. doi: 10.1038/ncomms5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelley LC, Hayes KE, Ammer AG, et al. Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PloS One. 2010;5:e13847. doi: 10.1371/journal.pone.0013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai FPL, Szczodrak M, Oelkers JM, et al. Cortactin promotes migration and platelet-derived growth factor-induced actin reorganization by signaling to Rho-GTPases. Mol Biol Cell. 2009;20:3209–23. doi: 10.1091/mbc.E08-12-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boguslavsky S, Grosheva I, Landau E, et al. p120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc Natl Acad Sci U S A. 2007;104:10882–7. doi: 10.1073/pnas.0702731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lua BL, Low BC. BPGAP1 interacts with cortactin and facilitates its translocation to cell periphery for enhanced cell migration. Mol Biol Cell. 2004;15:2873–83. doi: 10.1091/mbc.E04-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weed SA, Du Y, Parsons JT. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J Cell Sci. 1998;111 (Pt 16):2433–43. [DOI] [PubMed] [Google Scholar]
- 63.Majumder S, Sowden MP, Gerber SA, et al. G-protein-coupled receptor-2-interacting protein-1 is required for endothelial cell directional migration and tumor angiogenesis via cortactin-dependent lamellipodia formation. Arterioscler Thromb Vasc Biol. 2014;34:419–26. doi: 10.1161/ATVBAHA.113.302689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Binamé F, Bidaud-Meynard A, Magnan L, et al. Cancer-associated mutations in the protrusion-targeting region of p190RhoGAP impact tumor cell migration. J Cell Biol. 2016;214:859–73. doi: 10.1083/jcb.201601063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krueger EW, Orth JD, Cao H, et al. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell. 2003;14:1085–96. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McNiven MA, Kim L, Krueger EW, et al. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–98. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim K, Hou P, Gorski JL, et al. Effect of Fgd1 on cortactin in Arp2/3 complex-mediated actin assembly. Biochemistry (Mosc). 2004;43:2422–7. doi: 10.1021/bi036173t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao H, Orth JD, Chen J, et al. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–70. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao H, Weller S, Orth JD, et al. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat Cell Biol. 2005;7:483–92. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- 70.Rossé C, Lodillinsky C, Fuhrmann L, et al. Control of MT1-MMP transport by atypical PKC during breast-cancer progression. Proc Natl Acad Sci U S A. 2014;111:E1872–1879. doi: 10.1073/pnas.1400749111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Clainche C, Pauly BS, Zhang CX, et al. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J. 2007;26:1199–210. doi: 10.1038/sj.emboj.7601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong NH, Qi A, Weaver AM. PI(3,5)P2 controls endosomal branched actin dynamics by regulating cortactin-actin interactions. J Cell Biol. 2015;210:753–69. doi: 10.1083/jcb.201412127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelley LC, Weed SA. Cortactin is a substrate of activated Cdc42-associated kinase 1 (ACK1) during ligand-induced epidermal growth factor receptor downregulation. PloS One. 2012;7:e44363. doi: 10.1371/journal.pone.0044363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lynch DK, Winata SC, Lyons RJ, et al. A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J Biol Chem. 2003;278:21805–13. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- 75.Grassart A, Dujeancourt A, Lazarow PB, et al. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 2008;9:356–62. doi: 10.1038/embor.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grassart A, Meas-Yedid V, Dufour A, et al. Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic Cph Den. 2010;11:1079–91. [DOI] [PubMed] [Google Scholar]
- 77.Sinha S, Hoshino D, Hong NH, et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214:197–213. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hasegawa J, Iwamoto R, Otomo T, et al. Autophagosome-lysosome fusion in neurons requires INPP5E, a protein associated with Joubert syndrome. EMBO J. 2016;35:1853–67. doi: 10.15252/embj.201593148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee J-Y, Koga H, Kawaguchi Y, et al. HDAC6 controls autophagosome maturation essential for ubiquitin‐selective quality‐control autophagy. EMBO J. 2010;29:969–80. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Helwani FM, Kovacs EM, Paterson AD, et al. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian Y, Tian X, Gawlak G, et al. IQGAP1 regulates endothelial barrier function via EB1-cortactin cross talk. Mol Cell Biol. 2014;34:3546–58. doi: 10.1128/MCB.00248-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomar A, Lawson C, Ghassemian M, et al. Cortactin as a target for FAK in the regulation of focal adhesion dynamics. PloS One. 2012;7:e44041. doi: 10.1371/journal.pone.0044041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shentu T-P, He M, Sun X, et al. AMP-Activated Protein Kinase and Sirtuin 1 Coregulation of Cortactin Contributes to Endothelial Function. Arterioscler Thromb Vasc Biol. 2016;36:2358–68. doi: 10.1161/ATVBAHA.116.307871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kapus A, Szászi K, Sun J, et al. Cell shrinkage regulates Src kinases and induces tyrosine phosphorylation of cortactin, independent of the osmotic regulation of Na+/H+ exchangers. J Biol Chem. 1999;274:8093–102. doi: 10.1074/jbc.274.12.8093. [DOI] [PubMed] [Google Scholar]
- 85.Kapus A, Di Ciano C, Sun J, et al. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites. The role of Fyn and FER kinases. J Biol Chem. 2000;275:32289–98. doi: 10.1074/jbc.M003172200. [DOI] [PubMed] [Google Scholar]
- 86.Stuible M, Dube N, Tremblay ML. PTP1B regulates cortactin tyrosine phosphorylation by targeting Tyr446. J Biol Chem. 2008;283:15740–6. doi: 10.1074/jbc.M710534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian L, Chen L, McClafferty H, et al. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J Off Publ Fed Am Soc Exp Biol. 2006;20:2588–90. [DOI] [PubMed] [Google Scholar]
- 88.Cheng L, Yung A, Covarrubias M, et al. Cortactin is required for N-cadherin regulation of Kv1.5 channel function. J Biol Chem. 2011;286:20478–89. doi: 10.1074/jbc.M111.218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herrmann S, Ninkovic M, Kohl T, et al. Cortactin controls surface expression of the voltage-gated potassium channel K(V)10.1. J Biol Chem. 2012;287:44151–63. doi: 10.1074/jbc.M112.372540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ilatovskaya DV, Pavlov TS, Levchenko V, et al. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:2688–99. [DOI] [PubMed] [Google Scholar]
- 91.Williams MR, Markey JC, Doczi MA, et al. An essential role for cortactin in the modulation of the potassium channel Kv1.2. Proc Natl Acad Sci U S A. 2007;104:17412–7. doi: 10.1073/pnas.0703865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu J, Bai X, Bowen JR, et al. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol CB. 2012;22:1109–15. doi: 10.1016/j.cub.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin Y-C, Yeckel MF, Koleske AJ. Abl2/Arg controls dendritic spine and dendrite arbor stability via distinct cytoskeletal control pathways. J Neurosci Off J Soc Neurosci. 2013;33:1846–57. doi: 10.1523/JNEUROSCI.4284-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ueda S, Negishi M, Katoh H. Rac GEF Dock4 interacts with cortactin to regulate dendritic spine formation. Mol Biol Cell. 2013;24:1602–13. doi: 10.1091/mbc.E12-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kubo Y, Baba K, Toriyama M, et al. Shootin1-cortactin interaction mediates signal-force transduction for axon outgrowth. J Cell Biol. 2015;210:663–76. doi: 10.1083/jcb.201505011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rothschild BL, Shim AH, Ammer AG, et al. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017–25. doi: 10.1158/0008-5472.CAN-05-4490. [DOI] [PubMed] [Google Scholar]
- 97.Chuma M, Sakamoto M, Yasuda J, et al. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–36. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 98.Hui R, Ball JR, Macmillan RD, et al. EMS1 gene expression in primary breast cancer: relationship to cyclin D1 and oestrogen receptor expression and patient survival. Oncogene. 1998;17:1053–9. doi: 10.1038/sj.onc.1202023. [DOI] [PubMed] [Google Scholar]
- 99.Bringuier PP, Tamimi Y, Schuuring E, et al. Expression of cyclin D1 and EMS1 in bladder tumours; relationship with chromosome 11q13 amplification. Oncogene. 1996;12:1747–53. [PubMed] [Google Scholar]
- 100.Wang G-C, Hsieh P-S, Hsu H-H, et al. Expression of cortactin and survivin in renal cell carcinoma associated with tumor aggressiveness . World J Urol. 2009;27:557–63. doi: 10.1007/s00345-009-0376-2. [DOI] [PubMed] [Google Scholar]
- 101.Luo M-L, Shen X-M, Zhang Y, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006;66:11690–9. doi: 10.1158/0008-5472.CAN-06-1484. [DOI] [PubMed] [Google Scholar]
- 102.Lee Y-Y, Yu C-P, Lin C-K, et al. Expression of survivin and cortactin in colorectal adenocarcinoma: association with clinicopathological parameters. Dis Markers. 2009;26:9–18. doi: 10.1155/2009/821543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu X-Z, Garcia MV, Li T, et al. Cytoskeleton alterations in melanoma: aberrant expression of cortactin, an actin-binding adapter protein, correlates with melanocytic tumor progression. Mod Pathol. 2009;23:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Folio C, Zalacain M, Zandueta C, et al. Cortactin (CTTN) overexpression in osteosarcoma correlates with advanced stage and reduced survival. Cancer Biomark Sect Dis Markers. 2011;10:35–41. [DOI] [PubMed] [Google Scholar]
- 105.Hou H, Chen W, Zhao L, et al. Cortactin is associated with tumour progression and poor prognosis in prostate cancer and SIRT2 other than HADC6 may work as facilitator in situ. J Clin Pathol. 2012;65:1088–96. doi: 10.1136/jclinpath-2012-200940. [DOI] [PubMed] [Google Scholar]
- 106.Noh SJ, Baek HA, Park HS, et al. Expression of SIRT1 and cortactin is associated with progression of non-small cell lung cancer. Pathol Res Pract. 2013;209:365–70. doi: 10.1016/j.prp.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Wang L, Zhao K, Ren B, et al. Expression of cortactin in human gliomas and its effect on migration and invasion of glioma cells. Oncol Rep. 2015;34:1815–24. doi: 10.3892/or.2015.4156. [DOI] [PubMed] [Google Scholar]
- 108.Li A, Zhang L, Zhang X, et al. Expression and clinical significance of cortactin protein in ovarian neoplasms. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2016;18:220–7. [DOI] [PubMed] [Google Scholar]
- 109.Watkins RJ, Imruetaicharoenchoke W, Read ML, et al. Pro-invasive Effect of Proto-oncogene PBF Is Modulated by an Interaction with Cortactin. J Clin Endocrinol Metab. 2016;101:4551–63. doi: 10.1210/jc.2016-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gattazzo C, Martini V, Frezzato F, et al. Cortactin, another player in the Lyn signaling pathway, is over-expressed and alternatively spliced in leukemic cells from patients with B-cell chronic lymphocytic leukemia. Haematologica. 2014;99:1069–77. doi: 10.3324/haematol.2013.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu X, Moon JW, Li S, et al. Amplification and overexpression of CTTN and CCND1 at chromosome 11q13 in Esophagus squamous cell carcinoma (ESCC) of North Eastern Chinese Population. Int J Med Sci. 2016;13:868–74. doi: 10.7150/ijms.16845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodrigo JP, García LA, Ramos S, et al. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin Cancer Res Off J Am Assoc Cancer Res. 2000;6:3177–82. [PubMed] [Google Scholar]
- 113.Rodrigo JP, García-Carracedo D, García LA, et al. Distinctive clinicopathological associations of amplification of the cortactin gene at 11q13 in head and neck squamous cell carcinomas. J Pathol. 2009;217:516–23. doi: 10.1002/path.2462. [DOI] [PubMed] [Google Scholar]
- 114.Xia J, Chen Q, Li B, et al. Amplifications of TAOS1 and EMS1 genes in oral carcinogenesis: association with clinicopathological features. Oral Oncol. 2007;43:508–14. doi: 10.1016/j.oraloncology.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Yuan B-Z, Zhou X, Zimonjic DB, et al. Amplification and overexpression of the EMS 1 oncogene, a possible prognostic marker, in human hepatocellular carcinoma. J Mol Diagn JMD. 2003;5:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Callagy G, Pharoah P, Chin S-F, et al. Identification and validation of prognostic markers in breast cancer with the complementary use of array-CGH and tissue microarrays. J Pathol. 2005;205:388–96. doi: 10.1002/path.1694. [DOI] [PubMed] [Google Scholar]
- 117.Hui R, Campbell DH, Lee CS, et al. EMS1 amplification can occur independently of CCND1 or INT-2 amplification at 11q13 and may identify different phenotypes in primary breast cancer. Oncogene. 1997;15:1617–23. doi: 10.1038/sj.onc.1201311. [DOI] [PubMed] [Google Scholar]
- 118.Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim Biophys Acta BBA - Rev Cancer. 2014;1845:136–54. [DOI] [PubMed] [Google Scholar]
- 119.Matsuo T, Miyata Y, Watanabe S, et al. Pathologic significance and prognostic value of phosphorylated cortactin expression in patients with sarcomatoid renal cell carcinoma. Urology. 2011;78:476.e9–15. doi: 10.1016/j.urology.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 120.Radhakrishnan VM, Kojs P, Young G, et al. pTyr421 cortactin is overexpressed in colon cancer and is dephosphorylated by curcumin: involvement of non-receptor type 1 protein tyrosine phosphatase (PTPN1). PloS One. 2014;9:e85796. doi: 10.1371/journal.pone.0085796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y, Tondravi M, Liu J, et al. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001;61:6906–11. [PubMed] [Google Scholar]
- 122.Di Martino J, Henriet E, Ezzoukhry Z, et al. The microenvironment controls invadosome plasticity. J Cell Sci. 2016;129:1759–68. doi: 10.1242/jcs.182329. [DOI] [PubMed] [Google Scholar]
- 123.Beaty BT, Condeelis J. Digging a little deeper: the stages of invadopodium formation and maturation. Eur J Cell Biol. 2014;93:438–44. doi: 10.1016/j.ejcb.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980;77:6687–91. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Neel NF, Rossman KL, Martin TD, et al. The RalB small GTPase mediates formation of invadopodia through a GTPase-activating protein-independent function of the RalBP1/RLIP76 effector. Mol Cell Biol. 2012;32:1374–86. doi: 10.1128/MCB.06291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eckert MA, Lwin TM, Chang AT, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–86. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33:4203–12. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Díaz B, Yuen A, Iizuka S, et al. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol. 2013;201:279–92. doi: 10.1083/jcb.201209151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Juin A, Martino JD, Leitinger B, et al. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol. 2014;207:517–33. doi: 10.1083/jcb.201404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ayala I, Baldassarre M, Giacchetti G, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–78. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 131.Moshfegh Y, Bravo-Cordero JJ, Miskolci V, et al. A Trio-Rac1-Pak1 signalling axis drives invadopodia disassembly. Nat Cell Biol. 2014;16:574–86. doi: 10.1038/ncb2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jeannot P, Nowosad A, Perchey RT, et al. p27Kip1 promotes invadopodia turnover and invasion through the regulation of the PAK1/Cortactin pathway. eLife. 2017;6:e22207. doi: 10.7554/eLife.22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Crostella L, Lidder S, Williams R, et al. Hepatocyte Growth Factor/scatter factor-induces phosphorylation of cortactin in A431 cells in a Src kinase-independent manner. Oncogene. 2001;20:3735–45. doi: 10.1038/sj.onc.1204474. [DOI] [PubMed] [Google Scholar]
- 134.Rajadurai CV, Havrylov S, Zaoui K, et al. Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia. J Cell Sci. 2012;125:2940–53. doi: 10.1242/jcs.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hayes KE, Walk EL, Ammer AG, et al. Ableson kinases negatively regulate invadopodia function and invasion in head and neck squamous cell carcinoma by inhibiting an HB-EGF autocrine loop. Oncogene. 2013;32:4766–77. doi: 10.1038/onc.2012.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosenberg BJ, Gil-Henn H, Mader CC, et al. Phosphorylated cortactin recruits Vav2 guanine nucleotide exchange factor to activate Rac3 and promote invadopodial function in invasive breast cancer cells. Mol Biol Cell. 2017;28:1347–60. doi: 10.1091/mbc.E16-12-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ichetovkin I, Grant W, Condeelis J. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr Biol CB. 2002;12:79–84. doi: 10.1016/S0960-9822(01)00629-7. [DOI] [PubMed] [Google Scholar]
- 138.Oser M, Yamaguchi H, Mader CC, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Magalhaes MAO, Larson DR, Mader CC, et al. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 2011;195:903–20. doi: 10.1083/jcb.201103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bravo-Cordero JJ, Oser M, Chen X, et al. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–44. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martín-Villar E, Borda-d'Agua B, Carrasco-Ramirez P, et al. Podoplanin mediates ECM degradation by squamous carcinoma cells through control of invadopodia stability. Oncogene. 2015;34:4531–44. doi: 10.1038/onc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gasparski AN, Ozarkar S, Beningo KA. Transient mechanical strain promotes the maturation of invadopodia and enhances cancer cell invasion in vitro. J Cell Sci. 2017;130:1965–78. doi: 10.1242/jcs.199760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weidmann MD, Surve CR, Eddy RJ, et al. Mena(INV) dysregulates cortactin phosphorylation to promote invadopodium maturation. Sci Rep. 2016;6:36142. doi: 10.1038/srep36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma VP, Eddy R, Entenberg D, et al. Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells. Curr Biol CB. 2013;23:2079–89. doi: 10.1016/j.cub.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rajadurai CV, Havrylov S, Coelho PP, et al. 5′-Inositol phosphatase SHIP2 recruits Mena to stabilize invadopodia for cancer cell invasion. J Cell Biol. 2016;214:719–34. doi: 10.1083/jcb.201501003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li A, Dawson JC, Forero-Vargas M, et al. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol CB. 2010;20:339–45. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schoumacher M, Goldman RD, Louvard D, et al. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lizárraga F, Poincloux R, Romao M, et al. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009;69:2792–800. doi: 10.1158/0008-5472.CAN-08-3709. [DOI] [PubMed] [Google Scholar]
- 149.Huang R, Cao G-J, Guo H, et al. Direct interaction between caldesmon and cortactin. Arch Biochem Biophys. 2006;456:175–82. doi: 10.1016/j.abb.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Quintavalle M, Elia L, Price JH, et al. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci Signal. 2011;4:ra49. doi: 10.1126/scisignal.2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshio T, Morita T, Kimura Y, et al. Caldesmon suppresses cancer cell invasion by regulating podosome/invadopodium formation. FEBS Lett. 2007;581:3777–82. doi: 10.1016/j.febslet.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 152.Yamakita Y, Oosawa F, Yamashiro S, et al. Caldesmon inhibits Arp2/3-mediated actin nucleation. J Biol Chem. 2003;278:17937–44. doi: 10.1074/jbc.M208739200. [DOI] [PubMed] [Google Scholar]
- 153.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 154.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, et al. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 155.Murphy DA, Courtneidge SA. The “ins” and “outs” of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parekh A, Ruppender NS, Branch KM, et al. Sensing and modulation of invadopodia across a wide range of rigidities. Biophys J. 2011;100:573–82. doi: 10.1016/j.bpj.2010.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alexander NR, Branch KM, Parekh A, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol CB. 2008;18:1295–9. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parekh A, Weaver AM. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adhes Migr. 2009;3:288–92. doi: 10.4161/cam.3.3.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sakurai-Yageta M, Recchi C, Le Dez G, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–98. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Monteiro P, Rossé C, Castro-Castro A, et al. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J Cell Biol. 2013;203:1063–79. doi: 10.1083/jcb.201306162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu J, Mukhopadhyay A, Truesdell P, et al. Cdc42-interacting protein 4 is a Src substrate that regulates invadopodia and invasiveness of breast tumors by promoting MT1-MMP endocytosis. J Cell Sci. 2011;124:1739–51. doi: 10.1242/jcs.078014. [DOI] [PubMed] [Google Scholar]
- 162.Steffen A, Le Dez G, Poincloux R, et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol CB. 2008;18:926–31. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 163.Bendris N, Stearns CJS, Reis CR, et al. Sorting nexin 9 negatively regulates invadopodia formation and function in cancer cells. J Cell Sci. 2016;129:2804–16. doi: 10.1242/jcs.188045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jacob A, Linklater E, Bayless BA, et al. The role and regulation of Rab40b-Tks5 complex during invadopodia formation and cancer cell invasion. J Cell Sci. 2016;129:4341–53. doi: 10.1242/jcs.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Clark ES, Whigham AS, Yarbrough WG, et al. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 166.Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. Eur J Cell Biol. 2008;87:581–90. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lagoutte E, Villeneuve C, Lafanechère L, et al. LIMK Regulates Tumor-Cell Invasion and Matrix Degradation Through Tyrosine Phosphorylation of MT1-MMP. Sci Rep. 2016;6:24925. doi: 10.1038/srep24925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Webb BA, Zhou S, Eves R, et al. Phosphorylation of cortactin by p21-activated kinase. Arch Biochem Biophys. 2006;456:183–93. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 169.Goicoechea SM, Zinn A, Awadia SS, et al. A RhoG-mediated signaling pathway that modulates invadopodia dynamics in breast cancer cells. J Cell Sci. 2017;130:1064–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Badowski C, Pawlak G, Grichine A, et al. Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol Biol Cell. 2008;19:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Calle Y, Carragher NO, Thrasher AJ, et al. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci. 2006;119:2375–85. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- 172.Perrin BJ, Amann KJ, Huttenlocher A. Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration. Mol Biol Cell. 2006;17:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mizutani K, Miki H, He H, et al. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–74. [PubMed] [Google Scholar]
- 174.Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci. 2004;117:223–31. doi: 10.1242/jcs.00839. [DOI] [PubMed] [Google Scholar]
- 175.Luxenburg C, Parsons JT, Addadi L, et al. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–88. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- 176.Bañón-Rodríguez I, Monypenny J, Ragazzini C, et al. The cortactin-binding domain of WIP is essential for podosome formation and extracellular matrix degradation by murine dendritic cells. Eur J Cell Biol. 2011;90:213–23. doi: 10.1016/j.ejcb.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 177.Crimaldi L, Courtneidge SA, Gimona M. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp Cell Res. 2009;315:2581–92. doi: 10.1016/j.yexcr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 178.Daubon T, Buccione R, Génot E. The Aarskog-Scott syndrome protein Fgd1 regulates podosome formation and extracellular matrix remodeling in transforming growth factor β-stimulated aortic endothelial cells. Mol Cell Biol. 2011;31:4430–41. doi: 10.1128/MCB.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Snyder BN, Cho Y, Qian Y, et al. AFAP1L1 is a novel adaptor protein of the AFAP family that interacts with cortactin and localizes to invadosomes. Eur J Cell Biol. 2011;90:376–89. doi: 10.1016/j.ejcb.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kremerskothen J, Stölting M, Wiesner C, et al. Zona occludens proteins modulate podosome formation and function. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25:505–14. [DOI] [PubMed] [Google Scholar]
- 181.Biosse Duplan M, Zalli D, Stephens S, et al. Microtubule dynamic instability controls podosome patterning in osteoclasts through EB1, cortactin, and Src. Mol Cell Biol. 2014;34:16–29. doi: 10.1128/MCB.00578-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dehring DAK, Clarke F, Ricart BG, et al. Hematopoietic lineage cell-specific protein 1 functions in concert with the Wiskott-Aldrich syndrome protein to promote podosome array organization and chemotaxis in dendritic cells. J Immunol Baltim Md 1950. 2011;186:4805–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tehrani S, Faccio R, Chandrasekar I, et al. Cortactin has an essential and specific role in osteoclast actin assembly. Mol Biol Cell. 2006;17:2882–95. doi: 10.1091/mbc.E06-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. Eur J Cell Biol. 2012;91:902–7. doi: 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 185.Gligorijevic B, Wyckoff J, Yamaguchi H, et al. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–34. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–64. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 187.Stoletov K, Kato H, Zardouzian E, et al. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123:2332–41. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ziel JW, Hagedorn EJ, Audhya A, et al. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol. 2009;11:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Leong HS, Robertson AE, Stoletov K, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8:1558–70. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]


