Abstract
In the recent years, using genetically modified T cells has been known as a rapid developing therapeutic approach due to the heartwarming results of clinical trials with patients suffering from relapsed or refractory (R/R) hematologic malignancies such as R/R Acute Lymphoblastic Leukemia (R/R ALL). One of these renowned approaches is Chimeric antigen receptors (CARs). CARs are synthetic receptors with the ability to be expressed on the surface of T lymphocytes and are specifically designed to target a tumor-associated antigen (TAA) of interest. CAR-expressing T cells have the capability of proliferating and maintaining their immunological functionality in the recipient body but like any other therapeutic approach, the safety, effectiveness, and specificity enhancement of CAR T cells still lingers in the ambiguity arena. Genetic manipulation methods, expansion protocols, infusion dosage, and conditioning regimens are all among crucial factors which can affect the efficacy of CAR T cell-based cancer therapy. In this article, we discuss the studies that have focused on various aspects that affect the efficacy and persistence of CAR T-cell therapy for ALL treatment and provide a widespread overview regarding the practical approaches capable of elevating the effectiveness and lessening the relative toxicities attributed to it.
Keywords: Chimeric antigen receptor, CD19, Acute lymphoblastic leukemia, Immunotherapy
Introduction
Acute lymphoblastic leukemia (ALL) has been known as the most common cancer in children and the most frequent cause of cancer-related death in patients with less than 20 years of age [1]. In the United States, approximately 6000 cases of ALL are diagnosed annually, half of which comprised children and teenager cases [2]. Thrombocytopenia-related bruising or bleeding, infections caused by neutropenia, and anemia-related pallor and fatigue are all among common symptoms of ALL [2]. Spleen, liver, lymph node, and mediastinum leukemic infiltration have also been known as common signs during diagnosis [2]. Currently, there are several treatment options available for various leukemia subtypes because of their genetic heterogeneity. However, the outcome of these therapeutic methods is not satisfactory as a result of resistance development by the cancer cells [3]. Recently, cancer treatments based on immunotherapy have gained considerable clinical success and they have achieved several FDA-approvals [4]. Allogeneic bone marrow transplantation (BMT) or hematopoietic stem cell transplantation (HSCT) is a type of immune-based therapy for leukemia which is capable of mediating prolonged survival rates in about 50% of the patients [5]. Nevertheless, there are some serious concerns that limit their broad application. Relapsing after the treatment and lack of suitable donors in addition to several clinical complications make HSCT not an optimum gold standard treatment option for these patients [6]. Therefore, there is a need to find more efficient and safer therapeutic strategies to improve the treatment outcome of leukemia patients. Recently, chimeric antigen receptor (CAR) T cell-based therapy has been known as an effective immunotherapeutic tool that could be used for the treatment of disorders that are refractory or resistant to the available treatment options [7]. For instance, CAR T cells that target the CD19 antigen molecule have been shown to mediate complete remission (CR) in relapsed or refractory acute lymphoblastic leukemia (R/R ALL) patients. These CAR T cells have shown prolonged persistence of even 6 months after infusion [8]. Scientists in Memorial Sloan Kettering Cancer Center (MSKCC) reported that patients with R/R ALL, who did not receive HSCT, had prolonged disease-free survival of more than 12 months after treatment by CAR T cells. These results hypothesize the possibility that HSCT therapy can be replaced with CAR T-cell therapy in patients with R/R ALL [9]. Recent improvements with the purpose of having more effective T-cell therapies have been achieved by the progression of CAR T-cell manufacturing process alongside using conditioning regimens before and after the administration of CAR T cells [10]. In this review, we discuss various aspects that affect the efficacy and persistence of CAR T-cell therapy and then we focus on different practical strategies for the aim of having more effective and less toxic CAR T cells.
Clinical trial history and development of CAR T-cell therapeutics
The Leukemia and Lymphoma Society reported about 54,270 new leukemia patients and 24,450 leukemia-related deaths in the United States in 2015 [11]. The different overall survival rates in various leukemia types were also reported by this organization, with a rate of 70% for ALL [12]. Almost a quarter-century ago, the remission duration in ALL patients who had received BMT and suffered from graft versus host disease (GVHD) demonstrated the significant role of grafted T cells in long-term remission induction after the treatment [13]. Based on these findings, researchers theorized that tumor cells could be targeted and eliminated by the administration of genetically manipulated autologous T cells capable of recognizing malignant cells without causing further development of GVHD [10]. Since then, CAR T cells have been considered as dynamic and intelligent medications that have the potential to proliferate and provide strong tumoricidal effects against a particulate target after their systemic administration into patients [14].
To this date, more than 57,889 oncology trials have been registered on Clinical Trials.gov. Some of these trials can be categorized as CAR T-cell therapy, most of which have been conducted in the United States and/or the European Union and China [15]. Studies in the field of adoptive T-cell therapy in cancer treatment are taking rapid steps around the world. More than 200 protocols were recorded only in December 2015 [16], around 40% of which were related to CAR T-cell therapy [17]. Surprisingly, about 65% of these CAR T-cell therapies were designed for the treatment of hematological malignancies by targeting CD19 as a common antigen involved in the pathogenesis of various B cell malignancies (more than 80%) [18]. It is interesting to know that although researchers introduced CAR T-cell therapy for targeting solid tumors in the beginning [19], its clinical response in the treatment of B cell malignancies has been more successful [20].
For the first time, the efficacy of adoptive cell therapy (ACT) was proved by the initiation of molecular remission, a complete remission with no PCR-detectable trace of the disease in the blood cells or bone marrow, after T-cell infusion in hematological malignancies [21]. The quick responses in patients with refractory malignancies induced through the administration of CAR T cells make them a suitable option for the treatment of such malignancies [22]. For instance, in the first published trial from the University of Pennsylvania (UPenn), 90% of R/R-ALL patients enrolled in the trial experienced complete remission (CR) shortly (1 month) after the administration of CAR T cells [8]. Nevertheless, the clinical outcomes attributed to CAR T-cell therapy exhibit variations in different clinical centers which can be influenced by various factors such as the manufacturing process of CAR T cells and their management procedure before and after infusion [2]. Furthermore, CAR T production methods and the type of conditioning regimen are also among important factors affecting the clinical outcomes of CAR T-cell therapy [23].
Currently, researchers have focused on strategies that could lead to the improvement of CAR T-cell therapy effectiveness. Strategies such as using retroviral and plasmid vectors to generate a broader population of CAR-expressing T cells since the number of T cells are important in determining the final clinical response [24]. Based on previous findings, patients who received CAR T cells with a higher potential of continuous expansion showed more promising clinical responses. Furthermore, these CAR T cells managed to keep their cytotoxic functionality for more than 4 years in the patient’s body who achieved CR without any relapse [25]. Another encouraging report on CD19-redirected T cells has been issued by the research groups at UPenn and MSKCC reporting 70 to 90% CR rates in almost 65 patients with R/R ALL between 3 different trials [26]. Also, another report from the Great Ormond Street Hospital Biomedical Research Centre/Institute of Child Health, University College London, United Kingdom, has shown successful treatment of R/R-ALL patients with CD19-specific allogeneic CAR T cells [27]. Kochenderfer et al. showed additional promising clinical outcomes after CD19-specific CAR T-cell infusion in patients with B cell lymphoma which included 57% CR [28]. In another clinical trial by Gauthier et al., twelve months of CR was achieved after autologous anti-CD19 CAR T-cell therapy in 75 R/R-ALL patients [29]. Table 1 summarizes some of the CAR T-cell therapy clinical trials using various targets for the treatment of ALL.
Table 1.
Several clinical trials using various targets for CAR T cell-based ALL treatment in the United States and China
| Targeted Antigen | CAR Construct Signaling Domain | Gene-transfer Method | Phase | Number of patient | ClinicalTrials.gov Identifier | Responsible party | Starting date | Status |
|---|---|---|---|---|---|---|---|---|
| CD19 | CD28-CD3ζ | Retroviral | 1 | 14 | NCT00586391 | Baylor College of Medicine | February 2009 | Completed |
| CD28-CD3ζ | Retroviral | 1 | 68 | NCT00840853 | Baylor College of Medicine | April 2009 | Completed | |
| 4-1BB-CD3ζ | Lentiviral | 1 | 26 | NCT01029366 | University of Pennsylvania | March 2010 | Completed | |
| 4-1BB-CD3ζ | Lentiviral | 1 & 2 | 73 | NCT01626495 | University of Pennsylvania | August 2011 | Completed | |
| CD28-CD3ζ / 4-1BB-CD3ζ | Lentiviral / Retroviral | 1 & 2 | 50 | NCT00466531 | Memorial Sloan Kettering Cancer Center | March 2007 | Completed | |
| CD28-CD3ζ | Retroviral | 1 | 53 | NCT01593696 | National Cancer Institute | June 2012 | Completed | |
| CD20 | 4-1BB-CD3ζ | Lentiviral | 1 & 2 | 50 | NCT01735604 | Chinese PLA General Hospital | January 2013 | Completed |
| CD22 | 4-1BB-CD3ζ | Lentiviral | 1 | 126 | NCT02315612 | National Cancer Institute | December 2014 | Ongoing |
| CD133 | 4-1BB-CD3ζ | Retroviral | 1 & 2 | 20 | NCT02541370 | Chinese PLA General Hospital | June 2015 | Completed |
| ROR1 | 4-1BB-CD3ζ | Lentiviral | 1 | 60 | NCT02706392 | Fred Hutchinson Cancer Research Center | March 2016 | Ongoing |
Various aspects affecting the CAR T-cell therapy efficacy and persistence
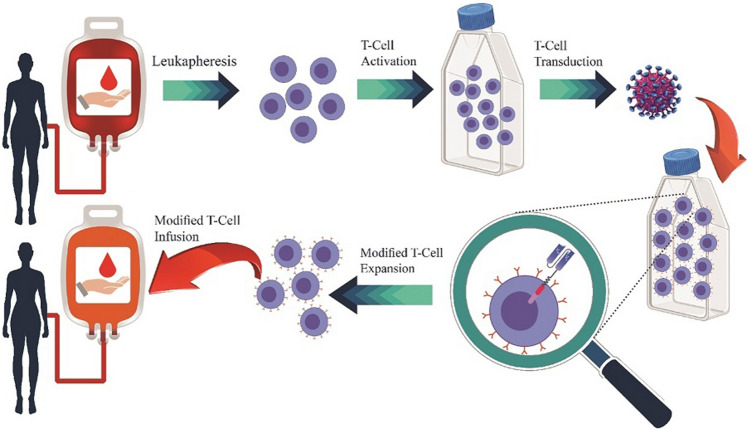
Adoptive T-cell therapy is based on creating an immune-mediated anti-tumor response by ex vivo T-cell manipulation via transferring a CAR gene into them [20]. The main principles for the generation of CAR T cells for adoptive immunotherapy [30] are presented in Fig. 1. The first clinical trial regarding the treatment of an ALL patient (1 patient) using CAR T cells was published by Brentjens et al. in 2011 which reported the achievement of CR [31]. In 2013, they repeated the experiment but this time with an increased number of ALL patients (5 patients) [32]. In clinical trials conducted at MSKCC [9], UPenn [33], NCI [34], and Fred Hutchinson Cancer Research Center (FHCRC), researchers achieved similar response rates. Also, Grupp et al. reported CR in two children with R/R ALL treated by second-generation CAR T cells with just one of them having undergone prior conditioning chemotherapy [35]. In another study conducted by Cruz et al. 25% of ALL patients (4 patients) that were treated by second generation of CAR T cells without preconditioning therapy showed CR for 3 months [36]. In 2014, Davila et al. reported CR of 75% of R/R-ALL patients (16 patients) who had undergone preconditioning chemotherapy before the administration of second-generation CD19-targeting CAR T cells [32]. Another study reported 70% CR of ALL patients in a clinical trial including 20 patients who were treated with second-generation CD19 CAR T cells [26]. Different clinical effectiveness and safety variations observed in these different institutions can result from differences in the experimental design such as the population of patients, conditioning regimens, tumor burden, administered dosage of CAR T cells, the generation of the CAR, and gene transfer methods [37, 38].
Fig. 1.
Main principles for the generation of CAR T cells for adoptive immunotherapy. Made in ©BioRender—biorender.com
Target discovery
Preferably, an antigen targeted by CAR-modified T cells should be tumor-specific and ubiquitously expressed only by tumor cells, but not normal cells. For instance, CD19 which is a B cell-specific surface marker expressed throughout B-cell development stages could be considered as a suitable CAR T therapy target in B-ALL [39]. Furthermore, the management of B-ALL with CD19-targeted CAR T cells is known as the most advanced engineered T-cell therapeutic approach that has been experienced so far [32].
Alongside CD19, various other antigens could be targeted for the treatment of ALL by CAR T cells. For instance, almost 5 to 15% of patients that suffer from B-ALL show a high expression profile of thymic stromal lymphopoietin receptor (TSLPR) [40]. This marker is known as a JAK and STAT signaling cascade inducer that supports the proliferation and growth of immature B cells. Abnormal activation of the TSLP-TSLPR axis could promote the metastasis of B lineage in ALL [41]. Researchers have shown the potential influence of TSLPR-targeting CAR T cells on the elimination of TSLPR-positive leukemic cells in human ALL [42]. Additionally, CD20 and CD22 are also cell surface markers that are specifically expressed on B cells and can be targeted for the elimination of leukemic cells in B-ALL [43].
As mentioned earlier, the specific expression of CD19 in various B cell differentiation stages from pro-B cells to memory B cells, but not on hematopoietic stem cells and other cell types, make the CD19 molecule as the most suitable antigen for targeting by CAR T cells in the treatment of R/R B-ALL [26]. Many clinical trials which have been conducted in USA research centers such as the NCI [26], MSKCC [9], UPenn [8], FHCRC [44], and the MD Anderson Cancer Center [45] have used CD19 as their CAR T cell-targeted antigen for the treatment of B-ALL. Despite the initial poor clinical outcomes of CAR T-cell therapy in clinical trials [26], the therapeutic effectiveness of this novel adoptive cell therapy platform has experienced an increase in complete remission rates from 70 to 90%, during the recent years [26]. A report from UPenn and Children’s Hospital of Philadelphia (CHOP) showed a 90% CR rate (out of 30 patients) in R/R-ALL patients in phase 1 trials [8]. Clinical trial results from MSKCC (16 patients) [32] and NCI (20 patients) showed 88% and 70% CR rates in R/R-ALL patients, respectively [26], and also in 2016, Grupp et al. reported the improvement of CR rates to 92% [46].
CAR design and structure
CAR design and structure, for example, the presence and type of the co-stimulatory domains, can have a substantial impact on the efficacy and tumoricidal functionality of CAR T cells [47, 48].
The importance of co-stimulatory domains was introduced by Savoldo et al. in 2011 as they observed a significant enhancement in CAR T-cell persistence after using a combination of first and second-generation CAR T cells for the treatment of lymphoma patients [49]. Several studies showed enhancement of T-cell expansion, activation, cytokine production, and antitumor responses in patients with hematologic malignancies through the introduction of co-stimulatory signaling domains such as CD28 or 4-1BB to form the second generation of CAR T cells [26]. Most of the clinical trials that used the second-generation CARs composed of either CD28 or 4-1BB co-stimulatory signaling domains have stated that CAR T cells containing CD28 exhibit faster expansion and shorter persistence, on the other hand, CARs with 4-1BB as their co-stimulatory domain display longer persistence [10]. It is worth mentioning that 4-1BB is known as a ‘late’ co-stimulatory signaling domain which could have substantial effects on CAR T-cell persistence [50]. Clinical studies that used the second generation of CAR T cells have reported high efficacy and potential of this CAR design [20]. Maude et al. [8] and Riches et al. [51], respectively, reported 90% (out of 30 patients) and 83% (out of 70 patients) CR in ALL patients receiving second-generation CD19-targeting CAR T cells. On the other hand, clinical trials that utilized the first-generation of CAR T cells with no co-stimulatory signaling domains suffered from insufficient cytokine production, T-cell proliferation, and also insufficient antitumor response [8].
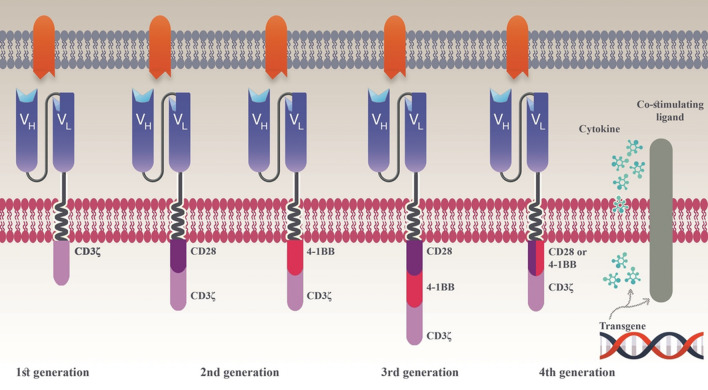
Third-generation CAR T cells contain two co-stimulatory signaling domains which can be the combination of CD28 and OX40, CD28 and 4-1BB, or other co-stimulatory signaling domains that can mediate continuous activation of T cells [52–54]. Alongside sustained expansion and tumoricidal activity, third-generation CAR T cells have shown reduced activation-triggered cell death [55, 56]. In 2015, Zhao et al. reported promising clinical outcomes while they used the combination of CD28 and 4-1BB signaling domains in their CAR construct [57]. However, to this date, different studies have evaluated the pros and cons of third-generation CAR T cells and have compared them to their second-generation counterparts. Toxicity-related safety concerns are pointed at the fact that the three signaling domains used in the construct of third-generation CAR T cells may synergistically contribute to lowering the threshold of CAR T-cell activation to a point that it can be triggered even when there is no target-antigen engagement. However, the use of two co-stimulatory domains can be utilized as a tool for engineering various properties of CAR T cells including T-cell memory development, metabolic pathways, and tonic signaling regardless of antigen engagement [55, 58, 59]. In this regard, CD28 and OX40 co-stimulatory domains can be used to inhibit the production and secretion of anti-inflammatory T-cell activity compromising cytokines such as interleukin-10 (IL-10) [60]. Moreover, increased in vivo persistence of CAR T cells can be achieved by using CD28 or 4-1BB coupled with the inducible T-cell co-stimulator (ICOS) co-stimulatory domain. Also, the utilization of MyD88/CD40 co-stimulatory domains has been known to enhance the in vivo proliferation of third-generation CAR T cells [61, 62]. Various CAR generations and the details of their signaling domains are shown in Fig. 2.
Fig. 2.
Various CAR generations classified by their intracellular signaling domains. The first-generation of CAR is composed of an scFv, a transmembrane domain, and the CD3ζ chain as a signaling domain. This molecule provides activation signal only to T cells which encounter T-cell anergy upon repeated antigen stimulation [98]. The second-generation of CAR has a co-stimulatory signaling domain which can be CD28 or 4-1BB [8, 99]. These co-stimulatory signaling domains are responsible for the activation signal which can be provided through the engagement of the targeting domain with the target antigen [99]. The third generation of CARs contains two co-stimulatory signaling domains which can be either the combination of CD28 and 4-1BB or CD28 and OX40 [99]. The fourth generation of CARs (also known as TRUCKs or armored CARs), use the combination of a second-generation CAR alongside factors such as co-stimulatory ligands, cytokines, etc., that enhance the anti-tumoral activity of CAR T cells [100]. Made in ©BioRender—biorender.com
Furthermore, enhancement of the effectiveness of CAR T-cell therapy can also be achieved through targeting more than one antigen. Multiple targeting strategies may reduce the possibility of tumor escape variants or tumor relapse [63].
T-cell isolation, expansion, and activation method
The methods applied for the isolation of defined T-cell subsets are also important factors that affect the success rate of CAR T-cell therapy. Many clinical trials have used peripheral blood as a source to isolate mononuclear cells (PBMC), as well as purifying and expanding the T cells in vitro. Utilization of CD3/CD28 Dynabeads and the Dyna-Magnet has been reported to be popular methods for the stimulation and activation of T cells in several clinical trials [64].
Lately, numerous studies have focused on improving T-cell isolation methods through providing good manufacturing (GMP) conditions [65]. For instance, using a combination of CAR T cells derived from naive and memory T cells in animal models could induce a more effective anti-tumor response [66]. Memory T cells that have a potential of higher proliferation rate, as compared to more differentiated T cells, are responsible for the long-term maintenance of CAR T-cell effectiveness which could eventually result in the improvement of CAR T-cell therapy [67].
Immune checkpoint blockade
The blockade of the negative immune regulators is also among other factors that can improve the clinical outcome of CAR T-cell therapy. Blocking the negative regulators such as CTLA-4 and PD-1/PD1-L to control their inhibitory effects can mediate the enhancement of CAR T-cell functionality [68]. Kobold et al. confirmed this hypothesis by targeting PD-1 and disrupting its immunosuppressive effects which resulted in the augmentation of CAR T-cell efficacy [69].
Conditioning regimen
Conditioning regimen before the infusion of CAR T cells is another critical factor that affects the clinical outcome of CAR T-cell therapy [70]. Lymphodepleting chemotherapy is a type of conditioning regimen that provides an appropriate environment for the infused T cells before their administration through the elimination of regulatory and suppressive components of the immune system [71]. The effects of conditioning regimens on the efficacy and persistence of CAR T cells have been demonstrated by the encouraging clinical outcome improvements in FHCRC [44]. Even before the clinical trial experiments, preclinical studies had shown the role of lymphodepletion in CAR T-cell efficacy [72]. Clinical trials conducted without conditioning chemotherapy at MD Anderson have shown considerably lower response rates in CAR T-cell therapy [45]. Using chemotherapeutic drugs such as fludarabine in B-ALL patients in clinical trials before or after the infusion of CAR T cells is a particulate example of a conditioning regimen that controls the result of the treatment [44]. Furthermore, Turtle et al. have shown the effect of fludarabine on the enhancement of T-cell expansion and persistence. They found a lower CAR T-cell population 28 days after the administration in patients who had not received fludarabine in comparison to those who had undergone lymphodepletion chemotherapy [44].
Aging parameter
Aging is also another parameter that could significantly affect the response rate to CAR T-cell therapy. Immunotherapy by CAR T cells has been more promising in pediatric R/R ALL as compared to adult R/R ALL [2]. Long-term follow-up reports from B-ALL patients after treatment with CD19 CAR T cells have stated longer median event-free survival in children and young adult patients (more than 11 months) in comparison with adult patients (more than 6.1 months) suggesting a considerable difference between the survival rates of pediatric and adult patients which can be resulted from age-related changes of the collected T cells. In detail, T cells (later genetically modified to express CAR) derived from aged donors exhibit enhanced cytotoxicity but less memory-like phenotypes and shorter persistence. It has been demonstrated that age-dependent CAR T-cell phenotype characterized by the unique secretory profile, gene expression pattern, and/or transcription factor balance can substantially cause differences in the clinical outcome of CAR T-cell therapy between younger patients and older ones [73]. Taken together, T-cell collection from younger donors for CAR T-cell therapy can result in longer persistence and memory-like phenotype of the T cells leading to the improvement of the clinical outcome.
GVHD management
GVHD is an immune response that can have adverse effects on the recipient’s vital organs and it may require the administration of immunosuppressive drugs which in their way increase the risks of infectious diseases and other immunosuppression-related complications. Since the early days of considering this therapy for the treatment of ALL, GVHD has not been a famous complication in the post-transplant patients [74]. One example for chronic GVHD development was in a patient with previous stage 1 acute skin GVHD, which had happened three months after the beginning of the therapy, for which subsequent treatment with corticosteroid was considered [74]. Furthermore, it has been demonstrated that donor-derived CAR T cells can substantially cause GVHD (grade 2–3) 3–4 weeks after cell infusions [75]. Nevertheless, researchers could manage GVHD by manufacturing T cells deficient in the expression of both their αβ T-cell receptors using multiplex genome-editing strategies [76].
CAR T-cell therapy toxicity management
Although treatment with CD19-targeting CAR T cells has reached almost 70–80% complete remission in R/R-ALL patients, it has several serious complications that require meticulous clinical management [7]. As mentioned earlier, the effectiveness of CAR T cells in clinical trials is associated with various factors. Possibilities of providing an uncomplicated manufacturing process as well as producing a safe cellular product are two important criteria that must be taken into consideration. Toxicity management of this therapeutic approach could be achieved by improving our knowledge about the probable immunological responses that happen after the infusion of CAR T cells [13]. The most potential toxicity symptoms that happen as the consequences of CAR T-cell therapy are cytokine release syndrome (CRS), neurological toxicities, on-target/off-tumor recognition, and anaphylaxis.
Cytokine release syndrome (CRS)
For having a more effective CAR T therapy, clinical outcome optimization must be taken into consideration through serious adverse event (SAE) management. Since CAR T cell-based treatment has been known as a promising method of therapy in pediatric R/R ALL, SAE management has become a subject of paramount importance [32]. CRS is an acute systemic inflammatory syndrome characterized by outrageous multi-cytokine release caused by an immune system hyperactivation resulting from the rapid stimulation and proliferation of the infused T cells. CRS is usually associated with fever [77], tachycardia [77], hypotension [77], acute respiratory distress syndrome [77], and various other life-threatening complications such as multi-organ failure. The altered cytokine profile pattern is characterized by the elevated levels of soluble interleukin-1 receptor (IL-1R), interleukin-2 (IL-2), interleukin-2 receptor (IL-2R) [8], interleukin-6 (IL-6) [74], interleukin-10 (IL-10) [74], interferon-γ (INF-γ) [74], granulocyte-macrophage colony-stimulating factor (GM-CSF) [74], tumor necrosis factor-α (TNF-α) [35], C-reactive protein [77], lactate dehydrogenase (LDH) [77], and hyperferritinemia [77]. Based on the results obtained from clinical trials, there is a direct relationship between the CRS severity and effectiveness of CAR T-cell therapy [8]. In reports, between 19 and 43% of R/R B-ALL patients have shown CRS after treatment with CD19-targeting CAR T cells [8]. Studies in MSKCC, UPenn, NCI, and FHCRC have reported 44%, 27%, 29%, and 23% of their patients with high-severity CRS resultant from the administration of CAR T cells, respectively [78].
Since elevated levels of cytokines are known as hallmarks of CRS, anti-cytokine therapy seems like a suitable first-line agent choice for its treatment [77]. Clinical administration of a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R) called tocilizumab has provided considerable satisfaction due to its rapid response and effectiveness without altering the antileukemic activity or expansion rate of the infused cells [35]. Moreover, there has been some slight positive effects in the resolution of CRS and CRS-related toxicities using a combination of corticosteroids and tocilizumab [32].
Neurological toxicity
The migration of the genetically manipulated T cells into the cerebrospinal fluid and subsequent secretion of high levels of cytokines such as IL-6, IFN-γ, and TNF-α make neurotoxicity as an associated adverse event of this type of therapy engaging the nervous system which is reportedly unpreventable by the previously mentioned anti-cytokine therapy [79]. Encephalopathy, cognitive disturbance, dysphasia, tremors, ataxia, myoclonus, seizures, and cerebral edema are all among mentionable neurological adverse events attributable to cancer therapy by CAR T cells [80]. Based on a report from CHOP/Penn, 20% of ALL patients treated with CAR T cells have shown signs of encephalopathy syndrome after the occurrence of CRS [32]. In another study from MSKCC, R/R B-ALL patients with lesser disease severity showed a lower risk of neurotoxicity in phase I clinical trial of CD19-targeting CAR T-cell therapy [7]. Furthermore, neurotoxicity resolution has been achieved in preclinical animal models manifesting lethal meningeal inflammation (which tocilizumab failed to resolve) using an IL-1R antagonist, called anakinra, which blocks the biologic activity of interleukin-1 (IL-1) by competitively inhibiting it from binding IL-1R thus offering a novel therapeutic strategy which enables us to abolish CAR T cell-mediated neurotoxicity [79].
On-target off-tumor recognition
Selecting the targeted antigen in CAR T-cell therapy is extremely crucial because the most suitable antigen should be the one specifically expressed by the tumor cells of our interest. Expression of the target antigen by other types of cells or normal cells could cause “on-target off-tumor” toxicity in the recipients. For example, in CD19-targeting CAR T-cell therapy, normal B cells can be affected alongside cancerous cells. This phenomenon leads to the depletion of normal B cells, known as B cell aplasia, and consequent reduction in the level of gamma-globulin (hypogammaglobulinemia) thus rendering patients susceptible to potentially opportunistic life-threatening infections [80]. Reconstituting the patient's immunoglobulin G (IgG) level via intravenous (IV) or subcutaneous injection of IgG obtained from the plasma of healthy donors is a clinical strategy to address this issue which offers the patients protection against a wide variety of pathogens [80].
Anaphylaxis
Another toxicity syndrome intertwined with the platform of CAR T-cell therapy is the clinical rejection of the infused cells if the antigen recognition domain of the CAR construct is based on animal-derived antibodies such as murine or camelid antibodies [81]. So, the hope to achieve prolonged persistence and consequently better anti-tumor responses in patients by repeating the rounds of infusion is terminated due to immune-mediated rejection [82]. Administration of autologous T cells equipped with a murine antibody-decorated CAR results in the occurrence of anaphylaxis within minutes following the infusion which is most likely through IgE antibodies against the targeting domain of the CAR. This phenomenon strongly accentuates the potential immunogenicity of using these animal-origin antibodies as targeting domains [83]. Construction of CAR constructs using a fully human targeting domain is a simple strategy to overcome the issues of immunogenicity. Also, humanization techniques such as grafting of the heavy and light chain complementary determining regions onto acceptor human germline frameworks for the development of humanized antibodies can be viewed as an immunogenicity-tackling guideline [82].
Practical strategies for having a more effective and less toxic CAR T-cell therapy
Dual targeting receptor strategies (Logic-gated CARs)
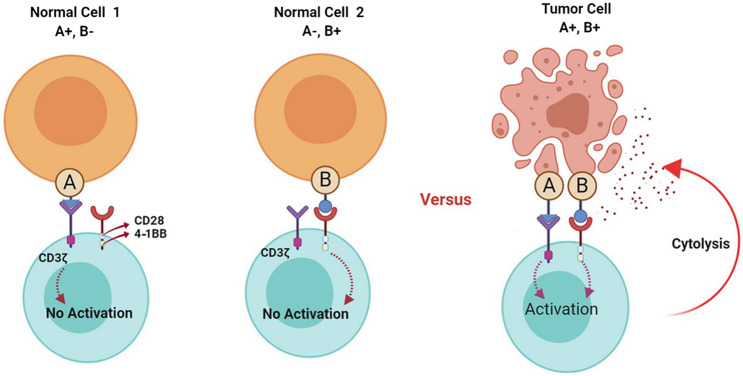
Dual targeting receptor strategies use two different CAR constructs with each targeting a particulate antigen. One CAR construct only harbors the CD3ζ signaling domain and the other CAR construct is designed to contain the co-stimulatory signaling domains necessary for the cytotoxic functionality of the CAR T cells. Conceptually, genetically modified T cells benefiting from this strategy will not enforce cytotoxic effects unless they encounter tumor cells that simultaneously express both of the antigens recognized by those two CARs. In conclusion, this strategy which simultaneously targets two dissimilar tumor-associated antigens has been developed for the improvement of CAR T-cell therapy specificity and safety index (Fig. 3).
Fig. 3.
Dual targeting CAR T cells and their mechanism of action. As shown in the figure, CAR T cells cannot achieve the signals necessary for their activation and cytotoxic functionality upon their encounter with normal cells that only express one of the antigens. On the other hand, upon their interaction with tumor cells simultaneously expressing both of the antigens, they receive the necessary activation signals resulting in their activation and tumoricidal effects. Made in ©BioRender—biorender.com
CARs affinity management
Commonly targeted tumor antigens are usually expressed by healthy tissues as well. The T cell-mediated killing of normal cells alongside malignant cells, as referred to as on-target off-tumor toxicity, has put obstacles in the way of wider clinical application of adoptive T-cell therapy. The mentioned phenomenon could lead to B cell aplasia in the case of CAR T-cell therapy for the treatment of CD19+ ALL which results in unwanted elimination of normal CD19-expressing B cells alongside leukemic B cells.
Adjusting the level of target-antigen affinity of the CAR T cells can be used as a means to discriminate target-antigen overexpressing tumor cells from normal tissues expressing it at physiologic levels. Affinity-tuned cells show sufficient antitumor efficacy, similar to that of high-affinity cells, only towards tumor cells and manage to refrain killing normal cells expressing the target antigen at physiologic levels. This elaborate strategy can be viewed to further validate the use of T cells equipped with affinity-tuned CARs as a means by which it is possible to target tumors and minimize the unwanted damages to normal tissues in the meantime [84].
Using a combination of CD4+ and CD8+ CAR T cells
To increase the persistence of CAR T cells, in addition to their in vivo expansion, a combination of CD4+ and CD8+ CAR T cells have been used in several studies. This strategy decreases the requirement of a high number of cells and leads to a more effective and less toxic CAR T therapy in B-ALL patients [74].
Inhibitory CAR (iCAR) and switchable CARs
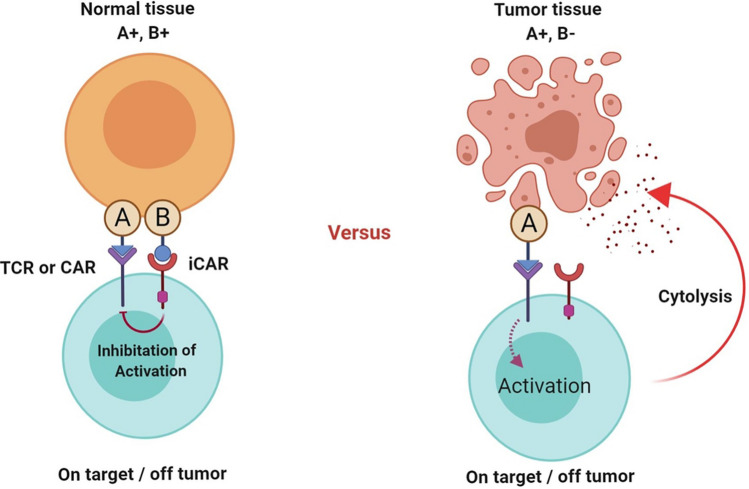
Inhibitory CAR (iCAR) and switchable CARs can also play important roles in the improvement of CAR T-cell therapy efficacy. CAR T cells benefiting from iCAR strategy express two separate CAR constructs. One CAR construct is a conventional CAR that is capable of recognizing a tumor antigen of interest and the other one is designed by attaching the signaling domains of exogenous T-cell inhibitory receptors such as PD-1 to an antigen recognition domain that recognizes an antigen expressed by healthy cells. Conceptually, cells that simultaneously express these two antigens will not be eliminated by CAR T cells equipped with an iCAR. This strategy helps CAR T cells discriminate between tumor cells that only express the tumor antigen and healthy cells that express both of them at the same time (Fig. 4) [85].
Fig. 4.
Inhibitory CARs (iCARs) and their mechanism of action. Upon the interaction of iCAR T cells with healthy cells expressing the antigen recognized by the targeting domain of the iCAR construct, the inhibitory signaling cascades suppress the activation of the CAR T cells leading to minimization of on-target/off-tumor toxicities. On the other hand, upon the encounter of iCAR T cells with tumor cells and subsequent engagement with the activatory antigen (in the absence of the inhibitory antigen), genetically modified T cells achieve the necessary activation signals and eliminate the tumor cells. Made in ©BioRender—biorender.com
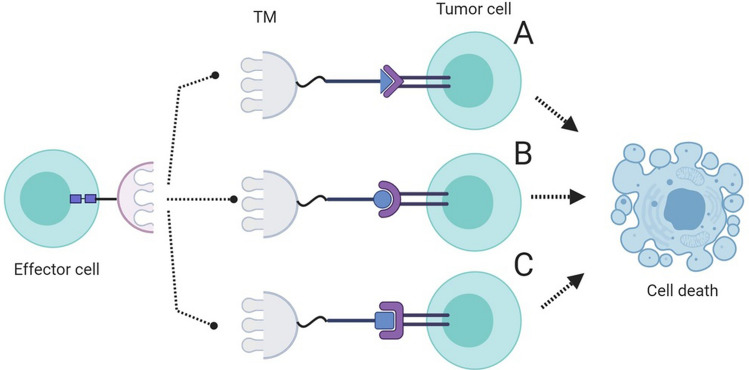
Furthermore, switchable CARs can also be utilized to help CAR T cells eliminate a broad spectrum of tumor cells through the administration of a small target module (TM). Switchable CAR T cells can be redirected towards different antigen-expressing tumor cells via a TM. The targeting domain of the CAR construct recognizes one part of the TM and attaches to it. On the other hand, the other part of the TM is specific for a tumor antigen of interest (Fig. 5). In conclusion, this strategy can be used for broadening the cytotoxic effects of CAR T cells towards different tumor cells without the need for developing separate CAR constructs for them [86].
Fig. 5.
Switchable CAR T cells and their mechanism of action. Different target modules (TM) specific for different antigen molecules can be used to redirect CAR T cells against different types of tumors. Made in ©BioRender—biorender.com
Suicide genes
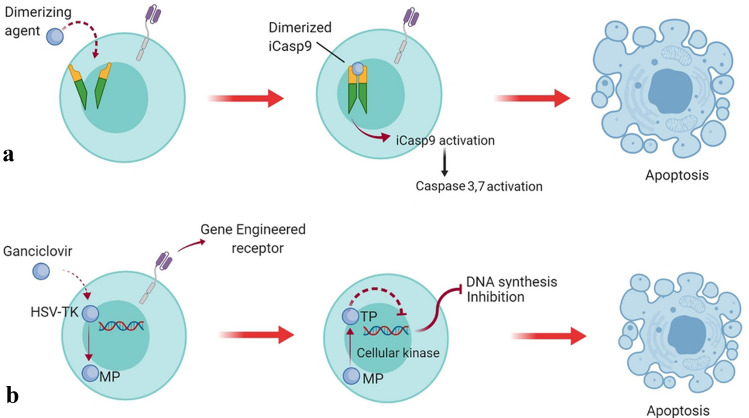
Using suicide genes is another strategy that can be used for reducing CAR T-cell therapy toxicities [87]. Inducible suicide genes are utilized to control the cytotoxic effects of the infused CAR T cells in vivo since they can be activated upon the introduction of a small non-therapeutic chemical inducer of dimerization (CID) such as inducible death molecules of Fas or Caspase9 (iCasp9) which trigger downstream cellular apoptotic pathways resulting in the selective elimination of the infused T cells in situations of emergency (Fig. 6a) [87]. Another example of a safety switch is the use of herpes simplex virus thymidine kinase (HSV-TK) which can be implemented for selective CAR T-cell depletion since it has successfully achieved encouraging results in clinics (Fig. 6b) [87].
Fig. 6.
Safety switch strategies for increasing the safety index of CAR T cells and their mechanism of action. a iCasp9 safety suicide and its mechanism of action. The activation of inducible caspase 9 is mediated upon the introduction of a small synthetic dimerizing agent leading to the activation of downstream apoptotic pathways and selective elimination of the CAR T cells equipped with this switch. b Herpes simplex virus thymidine kinase (HSV-TK) safety switch and its mechanism of action. Upon the introduction of ganciclovir, HSV-TK catalyzes it to ganciclovir-monophosphate (MP) which will eventually be modified to ganciclovir-trisphosphate (TP). Furthermore, TP will mediate the inhibition of DNA synthesis leading to the selective elimination of the T cells harboring this safety switch. Made in ©BioRender—biorender.com
Bispecific antibodies
Bispecific antibodies have been widely investigated because of their ability to recruit cytolytic T cells to eliminate cancer cells. A bispecific antibody named Blinatumomab which is an anti-CD19/CD3 bispecific T-cell engager (BiTE) has shown promising clinical results in B cell leukemia and lymphoma patients. Despite this, disadvantages such as possible immunogenicity, manufacturing-related challenges, and non-favorable pharmacokinetics are all among reasons limiting the clinical-level application of these bispecific antibodies. Taken together, the utilization of bispecific antibodies such as anti-CD20/CD3 is another strategy that can improve the clinical outcome of CAR T-cell therapy by simultaneous targeting of the tumor-associated antigen expressed by the cancer cells and CD3 presented on polyclonal T cells [88].
VHH-based CAR T cells
Heavy-chain antibodies (HCAbs) are structurally unique antibodies since they are composed of only heavy chains and they lack light chains and CH1 domain in comparison to a conventional IgG [89]. As naturally occurring antibodies in camelids, each heavy chain is composed of a single-variable domain that is called “VHH” or “nanobody®”, which is considered as the smallest intact functional antigen-binding fragment of HCAbs, and two constant domains called CH2 and CH3 [89]. Recently, VHHs have been successfully applied in various experimental and clinical settings such as their implementation in CAR T cells because of their favorable properties such as their ability to target uncommon or hidden epitopes which is due to their relatively longer complementarity-determining regions 3 (CDR3) loop compared with those in human and mouse antibodies, their small size which is about 15 kDa and half of the molecular weight of a conventional single-chain variable fragment (scFv), their solubility and high stability profile at high temperatures of between 80 and 92 °C and extreme of pH conditions, their low immunogenicity profile and the easy task of their humanization because they share more than 80% similarity with family III of the human variable heavy chain, and their binding kinetics which is comparable to those of conventional antibodies [37, 47, 90–92]. It is worth mentioning that Cablivi (caplacizumab) has been the first nanobody® approved for adults with acquired thrombotic thrombocytopenic purpura (aTTP), even though in combination with plasma exchange and immunosuppressive therapy, by the European Union and FDA [93]. In this section, we briefly discuss the use of VHHs as targeting domains of CAR constructs used in the CAR T-cell therapy of solid tumors and hematologic malignancies, review our own long-time laboratory experience in this field, and try to demonstrate why the use of VHHs in the field of CAR T-cell therapy is associated with several therapeutic benefits.
The utilization of VHH as the extracellular domain and the targeting domain of CARs is not broadly investigated in comparison to scFv. Our lab team is among the first groups that have used this novel strategy for the development of CAR T cells. About almost a decade ago, we generated CAR-expressing T cells equipped with anti-MUC1 VHH as the targeting domain [92]. Later, we incorporated the OX40 (CD134) co-stimulatory domain into the mentioned CAR construct and we showed that it effectively optimizes the T-cell activation signaling process alongside improving its IL-2 production level [91]. Our efforts for achieving a more optimized third-generation VHH-based CAR with a safety switch even went further. As a proof of concept, we used a regulated dimerization system with caspase 8 that acts as a suicide gene. In an in vitro experiment, we proved that this system shows considerable efficacy [91].
More recently, researches generated VHH-based CAR T cells against CD38 for the treatment of multiple myeloma (MM). They showed that these VHH-based CD38-targeting CAR T cells proliferate efficiently and produce more inflammatory cytokines, such as IL-2, IFN-γ, and TNF-α, upon activation [94]. The mentioned CAR T cells effectively lysed CD38+ MM cell lines and primary MM cells from multiple myeloma patients. These cells did not demonstrate any cytotoxicity against CD38− cells. Additionally, the in vivo results of this study showed that these CAR T cells inhibited tumor growth in NOD/SCID mice that were subcutaneously inoculated with a MM cell line [94]. These results strongly demonstrate that VHH-based CAR T cells can be promising for the treatment of hematologic malignancies.
In other studies, Munter et al. have generated bispecific VHH-based CAR T cells against CD20 and HER2. As a proof of concept, they showed that these CAR T cells can induce T-cell activation, cytokine production, and tumor lysis when incubated with transgenic Jurkat cells expressing either antigen or both antigens simultaneously. The results of this study also showed that using VHH technology for the production of CARs allows for the production of compact CARs with dual specificity and predefined affinity [95].
Furthermore, polyclonal and oligoclonal antibodies can recognize and bind to several different epitopes of a single antigen on a given tumor cell, therefore, it is believed that this property of the mentioned antibodies can minimize the possibility of the occurrence of escape variants [96]. This fact is mainly because it is unlikely that a tumor cell loses all of its target epitopes at once [96]. It is also believed that CAR T cells that target different antigenic epitopes can mediate a more effective cytotoxic response in comparison with CAR T cells that only target a single antigenic epitope [38]. In brief, polyclonal and oligoclonal CAR T cells might prevent the development of tumor cell escape variants alongside generating a more effective cytotoxic effect [38]. Different studies have investigated oligoclonal CAR T cells as proof-of-concept studies to demonstrate that epitope-distinct VHHs grant CAR T cells the ability to recognize different epitopes on a specific antigen which subsequently leads to lysing the antigen-expressing cells in a specific manner [38, 97]. Our previous works are among these studies where we generated HER2 epitope-distinct oligoclonal VHHs and then we incorporated them in CAR constructs and generated HER2-targeting oligoclonal VHH-based CAR T cells [38, 97]. Our results showed that these CAR T cells had higher proliferation rate, cytokine secretion, and cytotoxicity index in comparison with each individual VHH-based CAR T cells and we concluded that using oligoclonal VHHs with third-generation CAR constructs can substantially enhance the function of engineered T cells [38].
Recently, we established a novel second-generation anti-MUC1 VHH-based CAR composed of a camelid-derived anti-MUC1, IgG3 hinge, a CD28 transmembrane domain, and the intracellular signaling domains of CD28 and CD3ζ. Later on, we transduced human primary T cells with lentiviral vectors harboring this gene fragment and evaluated the CAR cell surface expression alongside with the cytokine secretion and tumoricidal activity of the anti-MUC1 VHH-based CAR T cells [90]. Our results indicated that these anti-MUC1 VHH-based CAR T cells had high levels of CAR expression. Furthermore, these CAR T cells significantly increased their cytotoxic activity and IL-2, TNF-α, and IFN-γ secretion upon the recognition of MUC1-expressing tumor cells [90]
Eventually, to use these promising proof-of-concept level results for the field of CAR T-cell therapy of hematologic malignancies especially ALL, lately, we have been working on CD19-targeting VHHs incorporated in CAR constructs for the generation of CD19-targeting VHH-based CAR T cells. The aforementioned CAR T cells demonstrated encouraging in vitro and in vivo results (in animal xenograft models) with considerable proliferation rate, cytokine secretion, and antitumor activity against CD19-positive targets which are comparable to those of CD19-targeting scFv-based CAR T cells (under publication). These promising results propose that CD19-targeting VHH-based CAR T cells can be considered as alternatives to conventional anti-CD19 CAR T cells for the treatment of R/R ALL as well as other hematologic malignancies associate with the CD19 tumor marker.
Conclusion
The expansion of our knowledge regarding the relationship between the immune system of the recipient of CAR T-cell therapy and the type of cancer and its targetable biomarkers has led to significant improvements in the field of cancer immunotherapy. The emergence of engineered T cells that could target the CD19 antigen expressed on the surface of cancerous B cells has been known as a revolution in the world of cancer immunotherapy. These engineered T cells which can express anti-CD19 CAR have shown promising results in the treatment of patients suffering from R/R ALL. So far, the data from the completed CAR T-cell therapy clinical trials have shown that a great percentage (more than 90%) of ALL patients have responded to the treatment by anti-CD19 CAR T cells, a number which is very higher than any other approach of cancer immunotherapy. Later, researchers achieved even more improvements in the results of clinical studies by combining CAR T-cell therapy and other therapeutic approaches such as immune checkpoint blockade. Optimizing the basic elements of CAR T-cell therapy such as CAR design, delivery methods of the engineered cells, CAR gene introduction methods, and the design of clinical trials as well as improving our understanding about the immunological responses following CAR T-cell infusion could lead to significant improvements in clinical responses and toxicity management of the future studies. Moreover, considering various practical strategies such as logic-gated CARs, iCARs, and switchable CARs alongside the use of bispecific antibodies and VHH-based CAR T cells enable us to achieve a more effective CAR T-cell therapy. Other innovative strategies such as the deployment of suicide switches can guarantee a shorter list of toxicities attributed to this therapy platform. It is worth mentioning that studying the results of the completed CAR T-cell therapy clinical trials will help us to achieve these new creativities.
Acknowledgements
This work was partly supported by the Iranian Council for Stem Cell Sciences and Technologies reference [Rep 393] and National Institute for Medical Research Development [Grant No. 984179].
Funding
Not applicable
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/jco.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373(16):1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 3.Knoechel B, Aster JC. Metabolic mechanisms of drug resistance in leukemia. Cell Metab. 2015;22(5):759–760. doi: 10.1016/j.cmet.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 6.Mashreghi M, Azarpara H, Bazaz MR, Jafari A, Masoudifar A, Mirzaei H, et al. Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J Cell Physiol. 2018;233(4):2949–2965. doi: 10.1002/jcp.26049. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Riviere I, Wang X, Senechal B, Wang Y, Mead E, et al. Durable long-term survival of adult patients with relapsed B-ALL after CD19 CAR (19–28z) T-cell therapy. J Clini Oncol. 2017;35(15):7008. doi: 10.1200/JCO.2017.35.15_suppl.7008. [DOI] [Google Scholar]
- 8.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Riviere I, Wang X, Bernal Y, Purdon T, Halton E, et al. Efficacy and safety of CD19-targeted 19–28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. J Clin Oncol. 2015;33(15):7010–7010. doi: 10.1200/jco.2015.33.15_suppl.7010. [DOI] [Google Scholar]
- 10.Van Der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discovery. 2015;14(7):499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics. A Cancer J Clinic. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 12.Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 13.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orlowski RJ, Porter DL, Frey NV. The promise of chimeric antigen receptor T cells (CART s) in leukaemia. Br J Haematol. 2017;177(1):13–26. doi: 10.1111/bjh.14475. [DOI] [PubMed] [Google Scholar]
- 15.Feinberg BA, Fillman J, Simoncini J, Nabhan C. CAR-T cells: the next era in immuno-oncology. Am J Manag Care. 2017;23(2 Spec No.):Sp48-sp52. [PubMed]
- 16.Aranda F, Buqué A, Bloy N, Castoldi F, Eggermont A, Cremer I, et al. Trial Watch: Adoptive cell transfer for oncological indications. Oncoimmunology. 2015;4(11):e1046673. doi: 10.1080/2162402X.2015.1046673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo C, Wei J, Han W. Spotlight on chimeric antigen receptor engineered T cell research and clinical trials in China. Science China Life Sciences. 2016;59(4):349–359. doi: 10.1007/s11427-016-5034-5. [DOI] [PubMed] [Google Scholar]
- 18.Berger C, Sommermeyer D, Hudecek M, Berger M, Balakrishnan A, Paszkiewicz PJ, et al. Safety of Targeting ROR1 in Primates with Chimeric Antigen Receptor-Modified T Cells. Cancer Immunol Res. 2015;3(2):206–216. doi: 10.1158/2326-6066.CIR-14-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 20.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123(17):2625–2635. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev. 2010;36(7):528–538. doi: 10.1016/j.ctrv.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almåsbak H, Aarvak T, Vemuri MC. CAR T Cell Therapy: A Game Changer in Cancer Treatment. J Immunol Res. 2016;2016:5474602. doi: 10.1155/2016/5474602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turtle CJ, Berger C, Sommermeyer D, Hanafi L-A, Pender B, Robinson EM, et al. Anti-CD19 Chimeric Antigen Receptor-Modified T Cell Therapy for B Cell Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Fludarabine and Cyclophosphamide Lymphodepletion Improves In Vivo Expansion and Persistence of CAR-T Cells and Clinical Outcomes. Blood. 2015;126(23):184–1844. doi: 10.1182/blood.V126.23.184.184. [DOI] [Google Scholar]
- 24.Yee C, Lizee G, Schueneman AJ. Endogenous T-cell therapy: clinical experience. The Cancer Journal. 2015;21(6):492–500. doi: 10.1097/PPO.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 25.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117–1127. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. The Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374):2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 28.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2014;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauthier J, Yakoub-Agha I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: clinical data to date, current limitations and perspectives. Current Research in Translational Medicine. 2017;65(3):93–102. doi: 10.1016/j.retram.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Makita S, Yoshimura K, Tobinai K. Clinical development of anti-CD 19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci. 2017;108(6):1109–1118. doi: 10.1111/cas.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Science Translational Medicine. 2014;6(224):224ra25–224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beatty GL, O'Hara MH, Nelson AM, McGarvey M, Torigian DA, Lacey SF, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. J Clin Oncol. 2015;33(15):3007–3007. doi: 10.1200/jco.2015.33.15_suppl.3007. [DOI] [Google Scholar]
- 34.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz CRY, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iri-Sofla FJ, Rahbarizadeh F, Ahmadvand D, Rasaee MJ. Nanobody-based chimeric receptor gene integration in Jurkat cells mediated by PhiC31 integrase. Exp Cell Res. 2011;317(18):2630–2641. doi: 10.1016/j.yexcr.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Jamnani FR, Rahbarizadeh F, Shokrgozar MA, Mahboudi F, Ahmadvand D, Sharifzadeh Z, et al. T cells expressing VHH-directed oligoclonal chimeric HER2 antigen receptors: towards tumor-directed oligoclonal T cell therapy. Biochim Biophys Acta. 2014;1840(1):378–386. doi: 10.1016/j.bbagen.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 39.Scheuermann R, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leukemia & lymphoma. 1995;18(5–6):385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 40.Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci. 2010;107(1):252–257. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuan EL, Ziegler SF. Thymic stromal lymphopoietin and cancer. J Immunol. 2014;193(9):4283–4288. doi: 10.4049/jimmunol.1400864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin H, Cho M, Haso W, Zhang L, Tasian SK, Oo HZ, et al. Eradication of B-ALL using chimeric antigen receptor–expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126(5):629–639. doi: 10.1182/blood-2014-11-612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fry TJ, Stetler-Stevenson M, Shah NN, Yuan CM, Yates B, Delbrook C, et al. Clinical Activity and Persistence of Anti-CD22 Chimeric Antigen Receptor in Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL) Blood. 2015;126(23):1324–1324. doi: 10.1182/blood.V126.23.1324.1324. [DOI] [Google Scholar]
- 44.Turtle CJ, Berger C, Sommermeyer D, Budiarto T, Hanafi L-A, Melville K, et al. Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition. J Clin Oncol. 2015;33(15):3006–3006. doi: 10.1200/jco.2015.33.15_suppl.3006. [DOI] [Google Scholar]
- 45.Kebriaei P, Huls H, Singh H, Olivares S, Figliola M, Maiti S, et al. Adoptive Therapy Using Sleeping Beauty Gene Transfer System and Artificial Antigen Presenting Cells to Manufacture T Cells Expressing CD19-Specific Chimeric Antigen Receptor. Blood. 2014;124(21):311–311. doi: 10.1182/blood.V124.21.311.311. [DOI] [Google Scholar]
- 46.Grupp SA, Maude SL, Shaw P, Aplenc R, Barrett DM, Callahan C, et al. T Cells Engineered with a Chimeric Antigen Receptor (CAR) Targeting CD19 (CTL019) Have Long Term Persistence and Induce Durable Remissions in Children with Relapsed. Refractory ALL. Blood. 2014;124(21):380–380. doi: 10.1182/blood.V124.21.380.380. [DOI] [Google Scholar]
- 47.Sharifzadeh Z, Rahbarizadeh F, Shokrgozar MA, Ahmadvand D, Mahboudi F, Jamnani FR, et al. Genetically engineered T cells bearing chimeric nanoconstructed receptors harboring TAG-72-specific camelid single domain antibodies as targeting agents. Cancer Lett. 2013;334(2):237–244. doi: 10.1016/j.canlet.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J Clin Investig. 2011;121(5):1822. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 51.Riches JC, Gribben JG. Understanding the Immunodeficiency in Chronic Lymphocytic Leukemia: Potential Clinical Implications. Hematology/Oncology Clinics. 2013;27(2):207–235. doi: 10.1016/j.hoc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson H, Svensson E, Gigg C, Jarvius M, Olsson-Strömberg U, Savoldo B, et al. Evaluation of intracellular signaling downstream chimeric antigen receptors. PLoS ONE. 2015;10(12):e0144787. doi: 10.1371/journal.pone.0144787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pircher M, Schirrmann T, Petrausch U. T Cell Engineering Prog Tumor Res. 2015;42:110–135. doi: 10.1159/000437180. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Z, Condomines M, van der Stegen SJ, Perna F, Kloss CC, Gunset G, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28(4):415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawalekar OU, Fraietta JA, Guo L, McGettigan SE, Posey AD, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T Cells. Immunity. 2016;44(3):712. doi: 10.1016/j.immuni.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 59.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1(4):458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collinson-Pautz MR, Chang WC, Lu A, Khalil M, Crisostomo JW, Lin PY, et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia. 2019;33(9):2195–2207. doi: 10.1038/s41375-019-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guedan S, Posey AD, Jr, Shaw C, Wing A, Da T, Patel PR, et al. Enhancing CAR T cell persistence through ICOS and 4–1BB costimulation. JCI Insight. 2018 doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS et al. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Molecular Therapy—Nucleic Acids. 2013;2(7): 105. [DOI] [PMC free article] [PubMed]
- 64.Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32(2):169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riddell SR, Sommermeyer D, Berger C, Liu LS, Balakrishnan A, Salter A, et al. Adoptive therapy with chimeric antigen receptor modified T cells of defined subset composition. Cancer journal (Sudbury, Mass) 2014;20(2):141. doi: 10.1097/PPO.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35(9):651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 69.Kobold S, Grassmann S, Chaloupka M, Lampert C, Wenk S, Kraus F, et al. Impact of a new fusion receptor on PD-1–mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst. 2015 doi: 10.1093/jnci/djv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Investig. 2016;126(6):2123. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4(11):e1027469. doi: 10.1080/2162402X.2015.1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poirot L, Philip B, Schiffer-Mannioui C, Le Clerre D, Chion-Sotinel I, Derniame S, et al. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Can Res. 2015;75(18):3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 77.Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2):e124. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davila ML, Sadelain M. Biology and clinical application of CAR T cells for B cell malignancies. Int J Hematol. 2016;104(1):6–17. doi: 10.1007/s12185-016-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 80.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. The journal of gene medicine. 2012;14(6):405–415. doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maude SL, Barrett DM, Ambrose DE, Rheingold SR, Aplenc R, Teachey DT, et al. Efficacy and Safety of Humanized Chimeric Antigen Receptor (CAR)-Modified T Cells Targeting CD19 in Children with Relapsed/Refractory ALL. Blood. 2015;126(23):683–683. doi: 10.1182/blood.V126.23.683.683. [DOI] [Google Scholar]
- 83.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1(1):26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Can Res. 2015;75(17):3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Can Res. 2016;76(6):1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci. 2016;113(4):E459–E468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol. 2014;5:254. doi: 10.3389/fphar.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee YG, Chu H, Low PS. Abstract LB-187: New methods for controlling CAR T cell-mediated cytokine storms. Can Res. 2017;77(13):LB-187. doi: 10.1158/1538-7445.Am2017-lb-187. [DOI] [Google Scholar]
- 89.Iezzi ME, Policastro L, Werbajh S, Podhajcer O, Canziani GA. Single-domain antibodies and the promise of modular targeting in cancer imaging and treatment. Frontiers in immunology. 2018;9:273. doi: 10.3389/fimmu.2018.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajabzadeh A, Rahbarizadeh F, Ahmadvand D, Kabir SM, Hamidieh AA. A VHH-Based Anti-MUC1 Chimeric Antigen Receptor for Specific Retargeting of Human Primary T Cells to MUC1-Positive Cancer Cells. Cell journal. 2021;22(4):502. doi: 10.22074/cellj.2021.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khaleghi S, Rahbarizadeh F, Ahmadvand D, Rasaee MJ, Pognonec P. A caspase 8-based suicide switch induces apoptosis in nanobody-directed chimeric receptor expressing T cells. Int J Hematol. 2012;95(4):434–444. doi: 10.1007/s12185-012-1037-6. [DOI] [PubMed] [Google Scholar]
- 92.Bakhtiari SHA, Rahbarizadeh F, Hasannia S, Ahmadvand D, Iri-Sofla FJ, Rasaee MJ. Anti-MUC1 nanobody can redirect T-body cytotoxic effector function. Hybridoma. 2009;28(2):85–92. doi: 10.1089/hyb.2008.0079. [DOI] [PubMed] [Google Scholar]
- 93.Duggan S. Caplacizumab: First Global Approval. Drugs. 2018;78(15):1639–1642. doi: 10.1007/s40265-018-0989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.An N, Hou YN, Zhang QX, Li T, Zhang QL, Fang C, et al. Anti-multiple myeloma activity of nanobody-based anti-CD38 chimeric antigen receptor T cells. Mol Pharm. 2018;15(10):4577–4588. doi: 10.1021/acs.molpharmaceut.8b00584. [DOI] [PubMed] [Google Scholar]
- 95.De Munter S, Ingels J, Goetgeluk G, Bonte S, Pille M, Weening K, et al. Nanobody Based Dual Specific CARs. Int J Mol Sci. 2018;19(2):403. doi: 10.3390/ijms19020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haurum JS. Recombinant polyclonal antibodies: the next generation of antibody therapeutics? Drug Discovery Today. 2006;11(13–14):655–660. doi: 10.1016/j.drudis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 97.Jamnani FR, Rahbarizadeh F, Shokrgozar MA, Ahmadvand D, Mahboudi F, Sharifzadeh Z. Targeting high affinity and epitope-distinct oligoclonal nanobodies to HER2 over-expressing tumor cells. Exp Cell Res. 2012;318(10):1112–1124. doi: 10.1016/j.yexcr.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 98.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci. 1989;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(18):5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 100.Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology. 2012;1(4):458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]