ABSTRACT
The majority of eukaryotic messenger RNA precursors (pre-mRNAs) undergo cleavage and polyadenylation at their 3′ end. This canonical 3′-end processing depends on sequence elements in the pre-mRNA as well as a mega-dalton protein machinery. The cleavage site in mammalian pre-mRNAs is located between an upstream poly(A) signal, most frequently an AAUAAA hexamer, and a GU-rich downstream sequence element. This review will summarize recent advances from the studies on this canonical 3′-end processing machinery. They have revealed the molecular mechanism for the recognition of the poly(A) signal and provided the first glimpse into the overall architecture of the machinery. The studies also show that the machinery is highly dynamic conformationally, and extensive re-arrangements are necessary for its activation. Inhibitors targeting the active site of the CPSF73 nuclease of this machinery have anti-cancer, anti-inflammatory and anti-protozoal effects, indicating that CPSF73 and pre-mRNA 3′-end processing in general are attractive targets for drug discovery.
Abbreviations
APA: alternative polyadenylation; β-CASP: metallo-β-lactamase-associated CPSF Artemis SNM1/PSO2; CTD: C-terminal domain; CF: cleavage factor; CPF: cleavage and polyadenylation factor; CPSF: cleavage and polyadenylation specificity factor; CstF: cleavage stimulation factor; DSE: downstream element; HAT: half a TPR; HCC: histone pre-mRNA cleavage complex; mCF: mammalian cleavage factor; mPSF: mammalian polyadenylation specificity factor; mRNA: messenger RNA; nt: nucleotide; NTD: N-terminal domain; PAP: polyadenylate polymerase; PAS: polyadenylation signal; PIM: mPSF interaction motif; Poly(A): polyadenylation, polyadenylate; Pol II: RNA polymerase II; pre-mRNA: messenger RNA precursor; RRM: RNA recognition module, RNA recognition motif; snRNP: small nuclear ribonucleoprotein; TPR: tetratricopeptide repeat; UTR: untranslated region; ZF: zinc finger
KEWORDS: Cleavage and polyadenylation, alternative polyadenylation, canonical 3′-end processing, endonuclease, CPSF3, JTE-607
Introduction
The majority of eukaryotic messenger RNA precursors (pre-mRNAs) are cleaved and polyadenylated at their 3′ end, which is important for the nuclear export, stability and translation of the mRNAs [1–7] as well as transcription termination of RNA polymerase II (Pol II) [8–10]. This 3′-end processing occurs co-transcriptionally, and is controlled by sequence elements in the pre-mRNA as well as a large, mega-dalton protein machinery, the canonical pre-mRNA 3′-end processing machinery (the canonical machinery in short here onward) (Figure 1). This canonical 3′-end cleavage and polyadenylation of the majority of pre-mRNAs is in contrast to the replication-dependent histone pre-mRNAs in metazoans, which are cleaved at their 3′ end but not polyadenylated. In addition, these histone pre-mRNAs contain different sequence elements and a distinct machinery (the histone machinery for short) is required for their 3′-end processing [11–13].
Figure 1.
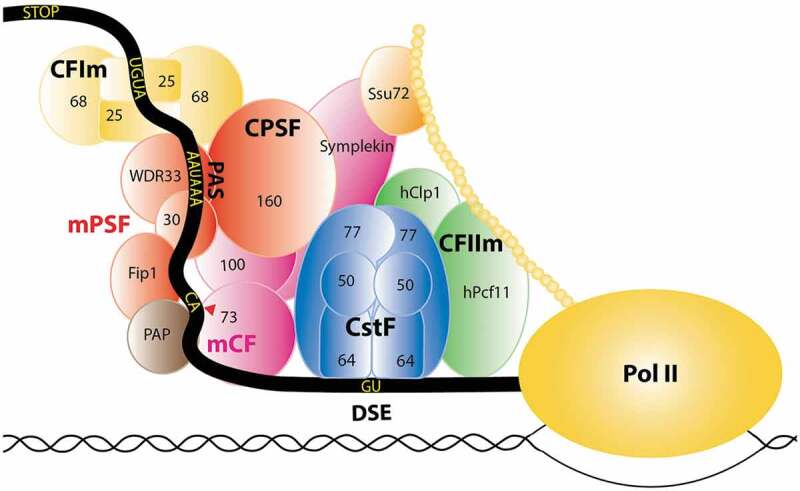
Schematic of the human canonical pre-mRNA 3′-end processing machinery.
mPSF is colored in red, mCF in magenta, CstF in blue, CFIm in yellow and CFIIm in green. The CTD of Pol II is indicated by the beaded chain in gold.
For canonical 3′-end processing in mammals, the cleavage site is located between an upstream polyadenylation signal (PAS), most frequently an AAUAAA hexamer, and a GU-rich downstream element (DSE) (Figure 1). Classical biochemical experiments have identified several complexes within the mammalian canonical machinery, for example CPSF (cleavage and polyadenylation specificity factor), CstF (cleavage stimulation factor), CFIm (cleavage factor Im) and CFIIm, while additional protein factors have been identified through proteomic approaches [14]. The yeast canonical machinery contains homologs of many of the mammalian protein factors, as well as unique components and sequence elements in the pre-mRNAs.
Recent studies showed that CPSF is composed of two modules: mammalian polyadenylation specificity factor (mPSF) that recognizes the PAS and recruits the poly(A) polymerase (PAP) to catalyze the polyadenylation, and mammalian cleavage factor (mCF) that catalyzes the cleavage reaction [15,16]. mPSF contains four subunits: CPSF160 (also known as CPSF1), CPSF30 (CPSF4), WDR33 and hFip1, while mCF contains three subunits: CPSF73 (CPSF3), CPSF100 (CPSF2) and symplekin (Figure 1). Therefore, CPSF functionally contains seven subunits, while five subunits (CPSF160, CPSF100, CPSF73, CPSF30, and hFip1) were identified originally. CPSF73 is the endonuclease for the cleavage reaction [17]. It is a member of the metallo-β-lactamase superfamily [18] and contains an additional domain, the β-CASP domain, that controls access to the active site [17]. mCF is equivalent to the histone pre-mRNA cleavage complex (HCC) in the histone machinery [19,20], and therefore the two machineries share equivalent cleavage modules.
The structures of various proteins, their domains and complexes in these machineries have been determined by X-ray crystallography over the years [2,21]. The recent revolution in cryo-electron microscopy has enabled larger complexes to be studied at the structural level [22–28], including the structure of a reconstituted, active human histone machinery [29], which have provided significant new information about these machineries. This review will focus on recent molecular insights into the canonical machinery.
The poly(A) signal
The AAUAAA hexamer was first recognized nearly 50 years ago as a motif located about 20 nucleotides upstream of the poly(A) tail [30,31]. Subsequent analyses based on cDNAs and ESTs (expressed sequence tags) showed that this hexamer is the most common PAS, present in ~60% of mammalian pre-mRNAs, while AUUAAA is present in about 15% [32,33]. Other PAS hexamers, primarily single-nucleotide variants of AAUAAA, are much less frequent, typically present in <2% of pre-mRNAs. These observations are also supported by an analysis [34] of the RefSeq database [35].
We carried out an analysis of the 3′-end non-poly(A) nucleotides (nts) of ~40,000 known human mRNAs in RefSeq (GRCh38 release). 60% of the mRNAs has only AAUAAA, 14% has only AUUAAA, and 7.6% has both motifs in the last 120 nts. These motifs are predominantly located 21 or 22 nts upstream of the cleavage site (Figure 2), measured from the first nt of the hexamer motif, with a range of 15 to 39 nts. 15% of the mRNAs contain single-nucleotide variants of the AAUAAA or AUUAAA hexamer, and these motifs are also primarily located 21 or 22 nts upstream of the cleavage site (Figure 2), suggesting that they likely also contribute to 3′-end processing. The most common such hexamers include AGUAAA, AUAAAA, AAUAUA, AAUACA, UAUAAA, and AAGAAA, essentially the same as those identified before [32,33]. In comparison, hexamers with two or more variations from the AAUAAA motif are evenly distributed in this 120 nt region (not shown). 3.5% of the mRNAs contain no AAUAAA, AUUAAA or their single-nucleotide variants. The PAS for these mRNAs may be located outside of this 120 nt region, as secondary-structure elements in the pre-mRNA between the PAS and the cleavage site can allow a larger separation between the two [34].
Figure 2.

Distribution of 3′-end processing sequence elements in human pre-mRNAs. The positions of AAUAAA (red), AUUAAA (yellow), single-nucleotide variants of AAUAAA or AUUAAA (green, labeled as AAUAAA–1), and UGUA (blue) motifs in the last 120 nts of known human mRNAs are shown, as a function of the distance to the cleavage site.
An upstream UGUA motif in pre-mRNAs can enhance 3′-end processing [36,37], which is recognized by the CFIm25 subunit (also known as Nudt21, CPSF5) [38]. 56% of the mRNAs analyzed contain at least one copy of this motif in this 120 nt region, although its distribution in the 3′-end region is much more diffuse, showing some abundance ~50 nts upstream of the cleavage site (Figure 2). Among the mRNAs with UGUA, 60% has only 1, 28% has 2, 9% has 3, and 2.6% has 4 copies of the motif. Therefore, 76% of the known mRNAs have no or only 1 UGUA motif in the last 120 nts.
The cleavage site is typically considered as being after a CA dinucleotide [39–41]. Among the ~32,000 mRNAs with at least one A at the 3′ end, the nucleotide preceding the A has a slight preference for pyrimidines, 36% C, 35% U, and 29% G. This lack of a CA motif at the cleavage site is consistent with a recent genomic study based on all mammalian conserved poly(A) sites [42]. Among the remaining 17% of mRNAs that do not have an A at the 3′ end, there is a slight preference for C (37%) over G (33%) and U (30%) as the last nt.
Recognition of the AAUAAA poly(A) signal by mPSF
CPSF160 contains three β-propellers (BPA, BPB and BPC) and a C-terminal domain (CTD) (Figure 3(a)). WDR33 contains a WD40 domain near the N-terminus, and the rest of the protein (including a collagen repeat region) is expected to be highly flexible. CPSF30 contains five zinc fingers (ZF1-5) and a zinc knuckle. hFip1 interacts with CPSF30 and recruits the poly(A) polymerase (PAP) for polyadenylation [43], but is not involved in PAS recognition. hFip1 also interacts with U-rich sequences [43], which are found upstream and downstream of the poly(A) site [37].
Figure 3.
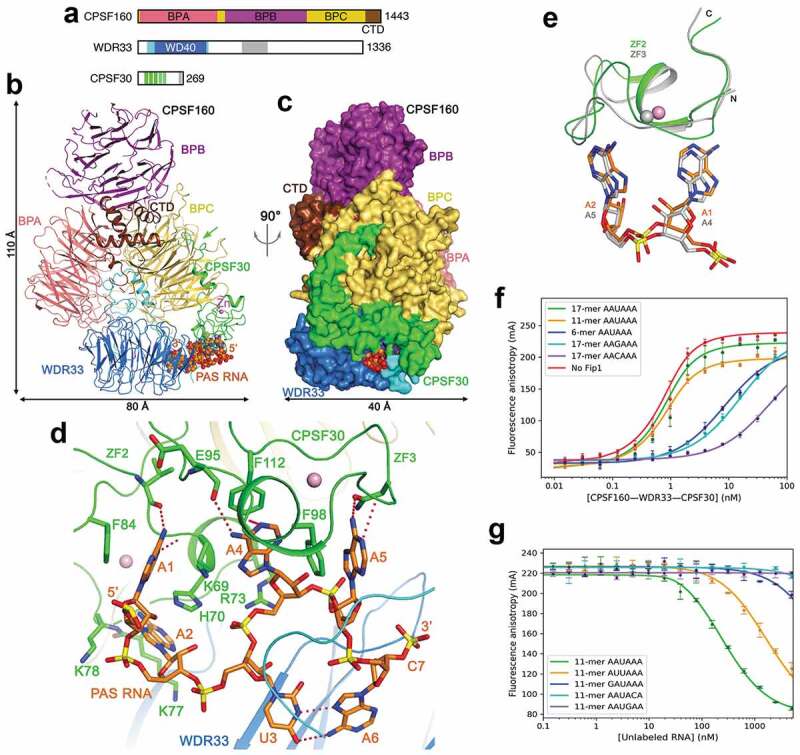
Recognition of the AAUAAA PAS by mPSF. (a). Domain organization of human CPSF160, WDR33 and CPSF30. The segments just before and after the WD40 domain of WDR33 are in cyan, and the collagen repeat region in gray. The ZFs of CPSF30 are in green, and the zinc knuckle in gray. (b). Schematic drawing of the structure of the human CPSF160-WDR33-CPSF30-PAS RNA quaternary complex [23]. The proteins are colored as in panel a, and the PAS RNA in orange. The green arrow points to the interaction between the N-terminal segment of CPSF30 and BPC of CPSF160. (c). The structure of the complex, viewed after a 90° rotation around the vertical axis. The proteins are shown as molecular surface. (d). Interactions between the AAUAAA hexamer and CPSF30 and WDR33. Hydrogen-bonding interactions are shown as dashed lines in red. (e). Overlay of the binding mode of A1-A2 to ZF2 (in color) with that of A4-A5 to ZF3 of CPSF30 (in gray). (f). Fluorescence anisotropy binding curves of various RNAs to CPSF160-WDR33-CPSF30-hFip1 [47]. (g). Fluorescence anisotropy competition binding curves of various PAS hexamers to CPSF160-WDR33-CPSF30-hFip1. Structure figures were produced with PyMOL (www.pymol.org) unless otherwise noted.
In the structure of CPSF160-WDR33-CPSF30-AAUAAA PAS RNA quaternary complex [23,24], the three β-propellers of CPSF160 are shaped like a trefoil, and WDR33 has tight interactions with a deep groove between BPA and BPC, mediated primarily by segments just before and after its WD40 domain (Figure 3(b,c)). The N-terminal segment and ZF1 of CPSF30 contacts BPC of CPSF160, with the first 7 residues of CPSF30 located in a prominent depression in the face of BPC opposite to the binding site for WDR33. A CPSF30 mutant missing the first 32 residues cannot interact with CPSF160-WDR33 [23], confirming the importance of this interface.
The PAS RNA is bound between CPSF30 and WDR33, while CPSF160 has no direct contact with the RNA (Figure 3(b)). Only the PAS hexamer and the phosphate group of the following nt are well ordered in the complex (Figure 3(d)), supporting the notion that mPSF primarily recognizes only the PAS hexamer. The backbone of the PAS assumes an S shape, allowing the six bases to be organized into three pairs, pointing toward three different directions. The A1-A2 pair interacts with ZF2 of CPSF30, while the A4-A5 pair interacts with ZF3 (Figure 3(d)). The adenine bases have hydrogen-bonding interactions through their N1 and/or N6 atoms as well as π-stacking and van der Waals interactions with the ZFs. In fact, the binding mode of A1-A2 to ZF2 is remarkably similar to that of A4-A5 to ZF3 (Figure 3(e)), indicating that these two ZFs use a conserved mechanism to recognize dinucleotides.
U3 and A6 of the PAS RNA form a Hoogsteen base pair (Figure 3(d)), π-stacked between Phe43 and Phe153 of WDR33. The segment near residue 43 partly covers the PAS RNA, and is disordered in the structure CPSF160-WDR33 alone [23,44]. Besides this segment, there are no large conformational changes in CPSF160-WDR33 upon binding CPSF30 and RNA.
The nonstructural protein 1 (NS1) of some influenza virus strains interferes with host canonical pre-mRNA 3′-end processing during infection [45]. The effector domain of NS1 forms a complex with ZF2 and ZF3 of CPSF30 [46], and the structure of ZF2 and ZF3 in this complex is essentially the same as that in the PAS complex. The NS1 effector domain directly competes with PAS for binding to CPSF30, thereby explaining its mechanism of suppressing host antiviral response.
ZF4, ZF5 and the rest of CPSF30 are disordered in the structure, consistent with the fact that they are not involved in PAS binding. In the structure of the yeast equivalent of mPSF, ZF3 of Yth1 (CPSF30 homolog) is also disordered as there is no RNA in the complex [22]. On the other hand, the overall structure of the yeast complex is similar to that of mPSF, indicating the conservation in pre-mRNA 3′-end processing between yeast and humans.
High affinity of mPSF for the AAUAAA poly(A) signal
mPSF has remarkably high affinity for RNAs containing the AAUAAA PAS, with Kd of ~0.5 nM [44,47]. The length of the RNA has a substantial impact on the affinity (Figure 3(f)), suggesting that nucleotides outside the AAUAAA hexamer contribute nonspecifically to the binding [47]. The AAUAAA hexamer itself has very low binding affinity while including a single nt at the 3′-end improves the Kd to 110 nM, consistent with the phosphate group of this nt being ordered in the structure (Figure 3(d)). Compared to AAUAAA, an RNA with the AUUAAA hexamer has ~6-fold lower affinity, while the affinities of the other PAS hexamers are at least 50-fold lower (Figure 3(g)). Therefore, the frequencies of the PAS hexamers are consistent with their affinity for mPSF. The hexamer AAGAAA would break the U3-A6 Hoogsteen base pair, but the corresponding RNA can bind mPSF albeit with lower affinity. Other components of the canonical machinery, for example CstF and CFIm [36,48], also contribute to the overall affinity for the pre-mRNA, which may be especially important in cases where the pre-mRNA carries single-nucleotide variations at the PAS.
Even though CPSF160 has no direct contact with the RNA, it is a crucial scaffold, recruiting both WDR33 and CPSF30 and pre-organizing them into the correct configuration for RNA binding. Both WDR33 and CPSF30 are required for high-affinity binding of the RNA [23,47]. Mutations of CPSF30 residues that contact the RNA can substantially reduce the affinity.
mCF is tethered to mPSF to form CPSF
CPSF73 contains an N-terminal catalytic module and a CTD (Figure 4(a)). The structure of this catalytic module alone is in a closed state [17], with the metallo-β-lactamase and β-CASP domains positioned close together near the active site such that there is no access by the RNA substrate. Nonetheless, a sulfate is coordinated to the two zinc ions in the active site, suggesting that it is a mimic of the scissile phosphate of the substrate. CPSF100 is a weak sequence homolog of CPSF73 (Figure 4(a)), with a similar structure for the yeast homolog Ydh1 [17], but its catalytic module has mutations in the zinc ligands and is unlikely to bind zinc. Symplekin contains an N-terminal domain (NTD) [49] that interacts with Ssu72 [50–52], a middle segment that interacts with CstF64 [53], and a CTD (Figure 4(a)). Ssu72 has Pol II CTD Ser5 [51,54–56] and weaker Ser7 [52,57,58] phosphatase activities.
Figure 4.
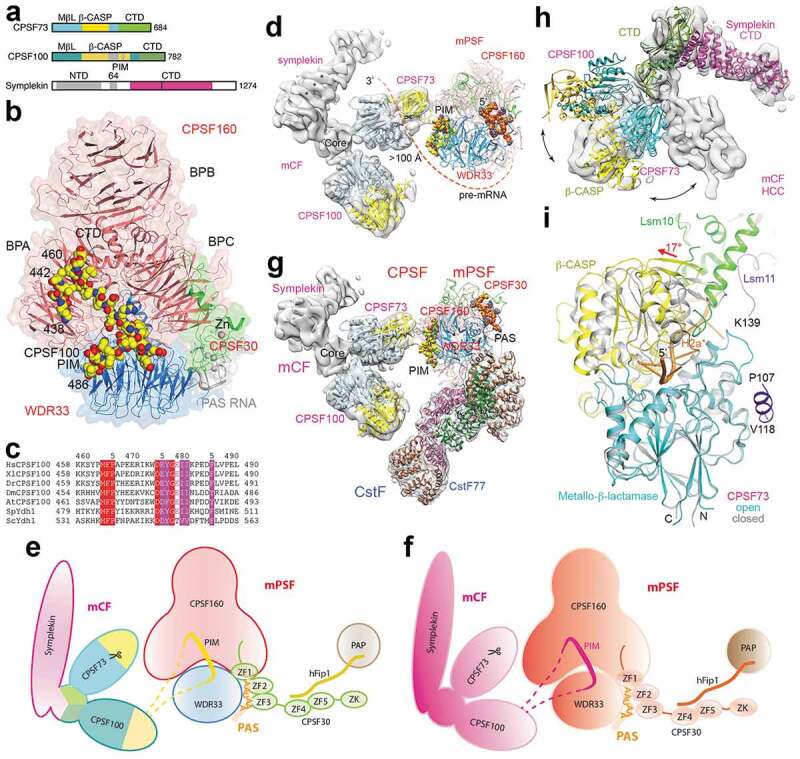
Overall architecture of the human canonical machinery. (a). Domain organization of human CPSF73, CPSF100 and symplekin. The highly hydrophilic segment in the β-CASP domain of CPSF100 is in gray, and the PIM is located in this segment. (b). Schematic drawing of the structure of the human mPSF in complex with the CPSF100 PIM [28]. (c). Conservation of the PIM sequences in CPSF100 homologs from yeast to humans. (d). A low-resolution structure of mCF in the complex with mPSF, showing a conformational state of CPSF [28]. The mPSF subunits are labeled in red, and mCF subunits in magenta. (e). A schematic drawing of the observed conformational state of CPSF. The proteins are colored individually. PAP is also shown. (f). A schematic drawing of CPSF. The proteins are colored according to Figure 1. (g). Composite structure of mPSF in complex with mCF and CstF, showing an overall architecture of the mammalian canonical machinery. (h). Conformational differences between the structure of mCF in an inactive state (gray surface) and the structure of active HCC [29]. (i). Conformational change in the β-CASP domain of CPSF73 for activation. The inactive, closed state is in gray. Panels d, gand hproduced with Chimera [59].
In contrast to mPSF, the structure of mCF alone is highly dynamic [28]. It has a trilobal shape, but the relative positions of the three lobes are highly variable. A mixture of recombinant mCF and mPSF readily leads to the formation of CPSF, but the structure of mCF in this complex is still highly dynamic. Nonetheless, the core of CPSF has a stable structure, and it contains mPSF and a 27-residue segment (residues 460–486) from CPSF100, which has been named the mPSF interaction motif (PIM) (Figure 4(b)) [28]. The PIM is located in a highly hydrophilic, disordered, and generally poorly conserved segment of ~100 residues in the β-CASP domain of CPSF100 (Figure 4(a)), consistent with the dynamic nature in the association between mCF and mPSF. This segment is ~200 residues long in the yeast homolog Ydh1, which was removed by a protease during crystallization [17,60].
On the other hand, residues in the PIM itself are highly conserved among CPSF100 homologs in yeasts, plants and humans (Figure 4(c)), and the PIM has extensive interactions with both CPSF160 (BPA and CTD) and WDR33 (Figure 4(b)). A loop in the CTD of CPSF160, disordered in the structure of mPSF alone, becomes ordered in the presence of mCF and contributes to PIM binding. Mutations in the PIM or its deletion abolish the formation of CPSF, demonstrating its important role in tethering mCF to mPSF to form CPSF and also revealing a function for CPSF100 in the canonical machinery.
Because of the dynamic behavior of mCF, only a low-resolution structure (7 Å) has been obtained for it in the context of CPSF (Figure 4(d)), representing the first glimpse into one conformational state of this factor (Figure 4(e,f)) [28]. The core of mCF is formed by the CTDs of the three proteins, while the three lobes belong to the catalytic module of CPSF73, the equivalent module of CPSF100 and the rest of the symplekin CTD. The NTD of symplekin is disordered in this structure. However, the distance from the PAS in mPSF to the active site of CPSF73 in mCF is >100 Å, too long for the ~20 nts between the PAS and the cleavage site (Figure 2), suggesting that this is an inactive state of CPSF.
CstF is recruited by mPSF
CstF is required for cleavage but dispensable for polyadenylation. It contains three subunits, CstF50 (also known as CstF1), CstF64 (CstF2) and CstF77 (CstF3) (Figure 1). CstF64 has an N-terminal RNA recognition module (RRM), which mediates the binding of the DSE. CstF77 has HAT-N (half a TPR [61]) and HAT-C domains, which form a bow-shaped dimer through the HAT-C domain [62,63], suggesting that CstF may function as a dimer in the canonical machinery (Figure 1). A segment in CstF77 (residues 581–594) just after the HAT domain is bound to the side of the C-terminal WD40 domain of CstF50 (Figure 5(a)) [48]. The N-terminal segment of CstF50 forms a dimer on its own (Figure 5(b)) [64], consistent with the dimerization of CstF. The C-terminal 30 residues of CstF77 can modulate RNA binding by CstF64 RRM, thereby enhancing cleavage and polyadenylation [65].
Figure 5.

Structures of CstF components. (a). Structure of WD40 domain of CstF50 in complex with a segment of CstF77 [48]. (b). Structure of the dimer of N-terminal segment of CstF50 [64]. (c). Structure of Kluyveromyces lactis Rna14-Rna15 complex [66]. The HAT domain of Rna14 is shown in cartoon, while the Rna14-Rna15 complex is in surface (Rna14 in red and Rna15 in light green).
The structure of CstF alone is also highly dynamic, with the positions of CstF50 and CstF64 relative to the HAT domain dimer of CstF77 being highly variable [28]. This dynamic behavior is also seen in the yeast complex of Rna14-Rna15 (homologs of CstF77 and CstF64) (Figure 5(c)) [66,67]. A stable mPSF-CstF complex is formed by mixing the recombinant components, but only the HAT dimer can be observed in the structure (Figure 4(g)) [28]. The HAT-C dimer has contacts with both CPSF160 (BPA) and WDR33, while the HAT-N domains are somewhat flexible. Intriguingly, this complex does not follow the 2-fold symmetry of the CstF77 HAT dimer, even though the dimer could associate with two copies of mPSF without any steric clashes. Further studies are needed to illuminate how the stoichiometry between mPSF and CstF is determined.
Activation of mCF for cleavage
Extensive conformational changes are required for the activation of mCF, as visualized by structural differences between mCF in an inactive state in the canonical machinery [28] and the equivalent HCC in the active state in the histone machinery [29]. These include re-arrangements in the positions of the three subunits relative to each other (Figure 4(h)) as well as conformational changes in the active site region of CPSF73 to assume an open state for RNA binding and cleavage (Figure 4(i)). While mCF in the inactive state is trilobal, with no contacts among the subunits outside of its core, HCC in the active state has extensive contacts between the catalytic module of CPSF73 and the equivalent module of CPSF100. In fact, these two modules form a pseudo-dimer, consistent with their sequence conservation. In the active site region of CPSF73, a 17° rotation of the β-CASP domain relative to the metallo-β-lactamase domain creates a deep canyon between the two domains. The pre-mRNA is captured in this canyon, poised for the cleavage reaction. The binding mode of the pre-mRNA provides exquisite molecular insight into the catalytic mechanism of the reaction, and confirms that the scissile phosphate is located near the sulfate observed earlier in the closed state of CPSF73 [17]. The adenine base at the cleavage site has hydrogen-bonding interactions with CPSF73, while the base preceding it is poorly disordered and is not recognized by CPSF73, consistent with the sequence analysis at the cleavage site of pre-mRNAs (see above).
In the histone machinery, the activation of HCC is triggered through the recognition of a structure motif in the RNA by the symplekin NTD, which is required for the cleavage reaction and Ssu72 can inhibit this cleavage [29]. The CPSF73-CPSF100 pseudo-dimer is also involved in the recognition and may be important for the activation as well, suggesting another role for CPSF100 in the two machineries. The β-CASP domain of CPSF73 is positioned against the Lsm10 subunit of the U7 snRNP (Figure 4(i)), a component unique to the histone machinery, and this contact is important for the activation of CPSF73. The active state of mCF in the canonical machinery is likely similar to that of HCC observed in the histone machinery, although a different mechanism of activation is required as Lsm10 is not in the canonical machinery.
The conformational dynamics of the human canonical machinery is also observed for the yeast canonical machinery, and an overall structure of that machinery could not be obtained [25]. Since the PIM is conserved in yeast (Figure 4(c)), it may have a similar role in tethering components in that machinery. A co-expressed 8-protein core of the yeast cleavage and polyadenylation factor [1] (CPFcore) has very weak but specific endonuclease activity, and good cleavage and polyadenylation activity is obtained when CPFcore is supplemented with CF IA and CF IB [25]. CF IA contains Rna14 (homolog of human CstF77), Rna15 (CstF64), Clp1 (hClp1), and Pcf11 (hPcf11), and therefore is equivalent to CstF and CFIIm in the mammalian canonical machinery, except that CstF50 does not have a homolog in CF IA. CF IB contains one protein, Hrp1, which does not have a clear homolog in the human canonical machinery. hPcf11 contains a Pol II CTD interaction domain (CID) at the N terminus, which is dispensable for cleavage activity, while the C-terminal segment, including the FEGP repeats, is required for activity [68].
CPFcore contains Yhh1/Cft1 (homolog of CPSF160), Pfs2 (WDR33), Yth1 (CPSF30), Fip1 (hFip1), Pap1 (PAP), Ysh1 (CPSF73), Ydh1/Cft2 (CPSF100), and Mpe1 (RBBP6), and therefore is roughly equivalent to CPSF. An important difference is that Pta1 (symplekin) is not required for activity in this reconstituted yeast canonical machinery, while symplekin is essential for stable association of CPSF73 and CPSF100 as well as for activity of the histone machinery [28,29]. The N-terminal ubiquitin-like domain of Mpe1 is bound to the metallo-β-lactamase domain of Ysh1, although the Ysh1 catalytic module is also in a closed state in this complex [25]. The mammalian homolog of Mpe1, RBBP6, is in the canonical machinery [14,69]. Interestingly, the binding site in Ysh1 for Mpe1 is utilized in CPSF73 for binding a helix in the N-terminal extension of Lsm11 in the histone machinery (Figure 4(i)) [29].
CPSF160-WDR33 is the core of the canonical machinery
By combining the structures of the mPSF-mCF and mPSF-CstF complexes, a composite model for an mPSF-mCF-CstF complex can be produced, providing insights into the overall architecture of the human canonical machinery (Figure 4(g)) [28]. There are no steric clashes among the components in this model, and a mixture of the three components can form a stable ternary complex.
The structural observations indicate that CPSF160-WDR33 is the core of the canonical machinery. It recruits CPSF30 for recognition of the PAS, CstF for recognition of the DSE, and mCF for cleaving the pre-mRNA. Both CPSF160 and WDR33 are required for these interactions, and the overall structure of CPSF160-WDR33 is essentially the same in the various complexes. CPSF30 in turn recruits hFip1 and thereby PAP for polyadenylating the mRNA after cleavage.
The overall structure of CPSF160-WDR33 is similar to that of the DDB1-DDB2 complex that is important for sensing and repairing DNA damage [70–72]. DDB1 can also interact with other proteins using its three β-propeller domains [73–77]. The BPA and BPC domains and the CTD of CPSF160 are involved in many interactions in the structures determined so far. A binding partner for the BPB domain has not been observed yet, but it could mediate interactions with other factors in the machinery.
Future perspectives
The highly dynamic behaviors of the pre-mRNA 3′-end processing machineries may be a natural property for them, enabling them to process a wide range of nascent mRNAs. This conformational flexibility makes it very difficult to study these machineries at near atomic resolution. It may be possible that the machineries are only transiently organized in the correct architecture for the cleavage reaction, as was trapped for the histone machinery [29]. CPSF73 is only activated upon the recognition of the genuine pre-mRNA substrate, an important regulatory mechanism that prevents unwanted activity toward other RNAs.
While an active reconstituted human histone machinery [29] and yeast canonical machinery [25] have been obtained, so far there are no reports of an active reconstituted mammalian canonical machinery, illustrating a significant difference between the human and yeast canonical machineries. A mixture of recombinant factors with fractionated nuclear extract is required for activity [68]. Additional protein factors and/or post-translational modifications may be important for producing an active reconstituted human canonical machinery. Having such a machinery is likely a critical first step in determining its structure and elucidating the molecular mechanism for the activation of CPSF73 in the canonical machinery. It will also allow detailed studies of alternative polyadenylation (APA) [5,41,78–80], especially how the various protein factors contribute to the selection of different poly(A) sites. APA is widespread among human pre-mRNAs, and may contribute to cancer development through global 3′ UTR shortening [81]. CFIm is an important regulator of this APA, by promoting processing at the distal PAS [82–85] due to an enrichment of UGUA motifs there [42,83].
The overall organization of CstF in the canonical machinery remains to be determined. CstF64 has a hinge region immediately after the RRM in the primary sequence, and CstF77 and symplekin may compete for binding to this region [53,86]. CstF64 is a component of HCC through its association with symplekin, but it is not required for the cleavage activity in vitro [29]. The functional importance (if any) of CstF64 in the histone machinery remains to be characterized. On the other hand, CstF64 is not a component of mCF, possibly because CstF77 is required for the canonical machinery. CstF64τ is a conserved paralog of CstF64 in mammals [87,88]. It has overlapping functions with CstF64 but can only form a complex with CstF77, lacking the ability to interact with symplekin [89]. Further studies on these two proteins in the canonical machinery are required to dissect their functional roles. CstF64τ may also be involved in alternative processing of small nuclear RNAs [90].
Defects and dysregulation of pre-mRNA 3′-end processing, both polyadenylation and APA, are linked to various diseases in humans [91–93]. Some of these diseases are caused by mutations in the PAS, indicating its functional importance. Recently CPSF73 was discovered as the target of the compound JTE-607 (Figure 6(a)) [94,95], which was originally identified for its anti-inflammatory activity [96]. JTE-607 is an ester pro-drug, and is activated in cells by carboxylesterase 1 (CES1) to its acid form. It is bound in the active site of CPSF73 in the closed form (Kd of 370 nM) but does not directly contact the zinc ions (Figure 6(b)). It inhibits the activity of the reconstituted yeast canonical machinery as well as pre-mRNA 3′-end processing in cells where the pro-drug can be activated. A subset of acute myeloid leukemia (AML) and Ewing’s sarcoma cell lines are susceptible to the treatment by this compound, indicating that CPSF73 and mRNA processing in general are potential targets for anti-cancer agents [97]. The anti-inflammatory effect of JTE-607 suggests that CPSF73 may also be involved in that disease area. In addition, oxaborole compounds (Figure 6(c)) are potent inhibitors of Plasmodium falciparum growth by targeting its CPSF73 [98], and they are also active toward other parasites such as Toxoplasma gondii [99], Trypanosoma brucei [100,101], and the intestinal pathogen Cryptosporidium [102]. The compound is bound in the active site of CPSF73, with the oxaborole moiety coordinated to the zinc ions (Figure 6(d)) [102], in contrast to JTE-607. Overall, these recent developments demonstrate that CPSF73 is an attractive target for drug discovery against cancer, inflammation, protozoan infections, and possibly other diseases.
Figure 6.
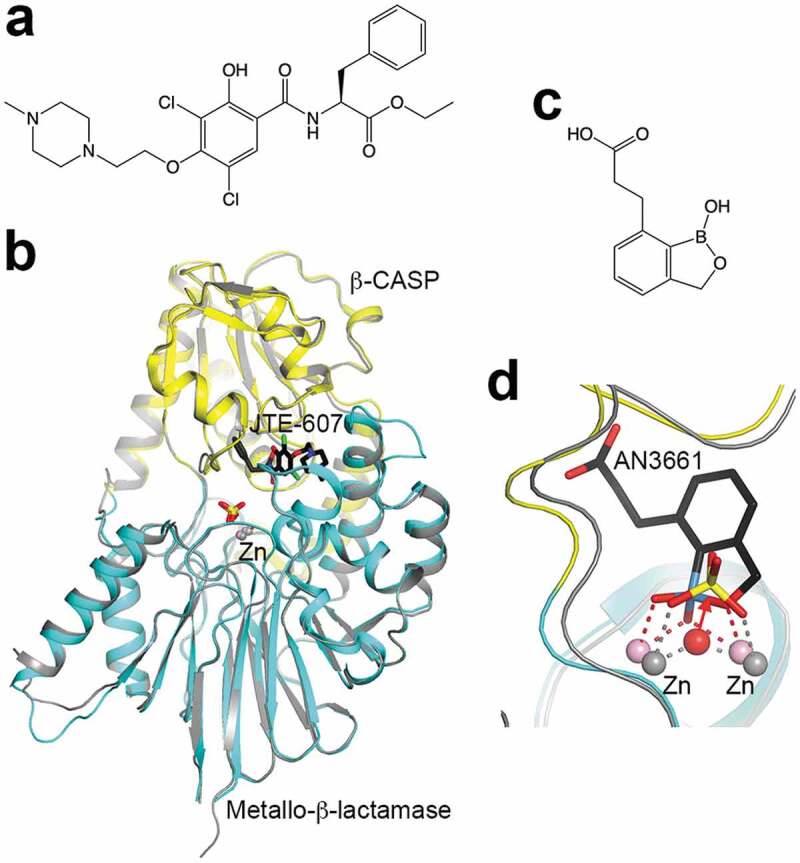
CPSF73 is a drug discovery target.
(a). Chemical structure of JTE-607. (b). Structure of human CPSF73 catalytic module (in color) in complex with the acid form of JTE-607 (in black) [95], overlaid with that in complex with sulfate in a closed form [17]. (c). Chemical structure of the anti-protozoal benzoxaborole AN3661. (d). Binding mode of AN3661 in the active site of C. huminis CPSF73 [102]. The oxaborole moiety competes with the scissile phosphate of the substrate and replaces the bridging ligand between the two zinc ions, which is the nucleophile for the cleavage reaction. The boron atom is in light blue.
Acknowledgments
We thank former members of the Tong laboratory Wei Shen Aik, Yun Bai, Ashley Jurado, Corey Mandel, Dazhi Tan, Kehui Xiang and Hailong Zhang for their contributions to this project.
Funding Statement
This research is supported by NIH grant [R35GM118093] (to LT).
Disclosure statement
The authors declare no competing interests.
References
- [1].Zhao J, Hyman L, Moore CL.. Formation of mRNA 3′ ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNA synthesis. Microbiol Mol Biol Rev. 1999;63:405–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mandel CR, Bai Y, Tong L. Protein factors in pre-mRNA 3′-end processing. Cell Mol Life Sci. 2008;65:1099–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xiang K, Tong L, Manley JL. Delineating the structural blueprint of the pre-mRNA 3′ end processing machinery. Mol Cell Biol. 2014;34:1894–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi Y, Manley JL. The end of the message: multiple protein-RNA interactions define the mRNA polyadenylation site. Genes Dev. 2015;29:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Neve J, Patel R, Wang Z, et al. Cleavage and polyadenylation: ending the message expands gene regulation. RNA Biol. 2017;14:865–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tudek A, Lloret-Llinares M, Jensen TH. The multitasking polyA tail: nuclear RNA maturation, degradation and export. Philos Trans R Soc Lond B Biol Sci. 2018;373:20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stewart M. Polyadenylation and nuclear export of mRNAs. J Biol Chem. 2019;294:2977–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Proudfoot NJ. Transcriptional termination in mammals: stopping the RNA polymerase II juggernaut. Science. 2016;352:aad9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Porrua O, Boudvillain M, Libri D. Transcription termination: variations on Common Themes. Trends Genet. 2016;32:508–522. [DOI] [PubMed] [Google Scholar]
- [10].Eaton JD, Francis L, Davidson L, et al. A unified allosteric/torpedo mechanism for transcriptional termination on human protein-coding genes. Genes Dev. 2020;34:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dominski Z, Marzluff WF. Formation of the 3′ end of histone mRNA: getting closer to the end. Gene. 2007;396:373–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Romeo V, Schumperli D. Cycling in the nucleus: regulation of RNA 3′ processing and nuclear organization of replication-dependent histone genes. Curr Opin Cell Biol. 2016;40:23–31. [DOI] [PubMed] [Google Scholar]
- [13].Marzluff WF, Koreski KP. Birth and death of histone mRNAs. Trends Genet. 2017;33:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shi Y, Di Giammartino DC, Taylor D, et al. Molecular architecture of the human pre-mRNA 3′ processing complex. Mol Cell. 2009;33:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chan SL, Huppertz I, Yao C, et al. CPSF30 and Wdr33 directly bind to AAUAAA in mammalian mRNA 3′ processing. Genes Dev. 2014;28:2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schonemann L, Kuhn U, Martin G, et al. Reconstitution of CPSF active in polyadenylation: recognition of the polyadenylation signal by WDR33. Genes Dev. 2014;28:2381–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mandel CR, Kaneko S, Zhang H, et al. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature. 2006;444:953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Callebaut I, Moshous D, Mornon J-P, et al. Metallo-b-lactamase fold within nucleic acids processing enzymes: the b-CASP family. Nucleic Acid Res. 2002;30:3592–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang X-C, Burch BD, Yan Y, et al. FLASH, a proapoptotic protein involved in activation of caspase-8, is essential for 3′ end processing of histone pre-mRNAs. Mol Cell. 2009;36:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang X-C, Xu B, Sabath I, et al. FLASH is required for the endonucleolytic cleavage of histone pre-mRNAs but is dispensable for the 5′ exonucleolytic degradation of the downstream cleavage product. Mol Cell Biol. 2011;31:1492–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang Q, Doublie S. Structural biology of poly(A) site definition. Wiley Interdiscip Rev RNA. 2011;2:732–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Casanal A, Kumar A, Hill CH, et al. Architecture of eukaryotic mRNA 3′-end processing machinery. Science. 2017;358:1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun Y, Zhang Y, Hamilton K, et al. Molecular basis for the recognition of the human AAUAAA polyadenylation signal. Proc Natl Acad Sci USA. 2018;115:E1419–E1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Clerici M, Faini M, Muckenfuss LM, et al. Structural basis of AAUAAA polyadenylation signal recognition by the human CPSF complex. Nat Struct Mol Biol. 2018;25:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hill CH, Boreikaite V, Kumar A, et al. Activation of the endonuclease that defines mRNA 3′ ends requires incorporation into an 8-subunit core cleavage and polyadenylation factor complex. Mol Cell. 2019;73:1217–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thore S, Fribourg S. Structural insights into the 3′-end mRNA maturation machinery: snapshot on polyadenylation signal recognition. Biochimie. 2019;164:105–110. [DOI] [PubMed] [Google Scholar]
- [27].Kumar A, Clerici M, Muckenfuss LM, et al. Mechanistic insights into mRNA 3′-end processing. Curr Opin Struct Biol. 2019;59:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Y, Sun Y, Shi Y, et al. Structural insights into the human pre-mRNA 3′-end processing machinery. Mol Cell. 2020;77:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun Y, Zhang Y, Aik WS, et al. Structure of an active human histone pre-mRNA 3′-end processing machinery. Science. 2020;367:700–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Proudfoot NJ, Brownlee GG. Sequence at the 3′ end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974;252:359–362. [DOI] [PubMed] [Google Scholar]
- [31].Proudfoot NJ, Brownlee GG. 3′ non-coding region sequences in eukaryotic messenger RNA. Nature. 1976;263:211–214. [DOI] [PubMed] [Google Scholar]
- [32].Beaudoing E, Freier S, Wyatt JR, et al. Patterns of variant polyadenylation signal usage in human genes. Genome Res. 2000;10:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tian B, Hu J, Zhang H, et al. A large-scale analysis of mRNA polyadenylation of human and mouse genes. NucleicAcid Res. 2005;33:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu X, Bartel DP. Widespread influence of 3′-end structures on mammalian mRNA processing and stability. Cell. 2017;169:905–917.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].O’Leary NA, Wright MW, Brister JR, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Venkataraman K, Brown KM, Gilmartin GM. Analysis of a noncanonical poly(A) site reveals a tripartite mechanism for vertebrate poly(A) site recognition. Genes Dev. 2005;19:1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hu J, Lutz CS, Wilusz J, et al. Bioinformatic identification of candidate cis-regulatory elements involved in human mRNA polyadenylation. RNA. 2005;11:1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang Q, Gilmartin GM, Doublie S. Structural basis of UGUA recognition by the Nudix protein CFI(m)25 and implications for a regulatory role in mRNA 3′ processing. Proc Natl Acad Sci USA. 2010;107:10062–10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sheets MD, Ogg SC, Wickens MP. Point mutations in AAUAAA and the poly(A) addition site: effects on the accuracy and efficiency of cleavage and polyadenylation in vitro. Nucleic Acid Res. 1990;18:5799–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen F, MacDonald CC, Wilusz J. Cleavage site determinants in the mammalian polyadenylation signal. Nucleic Acid Res. 1995;23:2614–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bogard N, Linder J, Rosenberg AB, et al. A deep neural network for predicting and engineering alternative polyadenylation. Cell. 2019;178:91–106.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang R, Zheng D, Yehia G, et al. A compendium of conserved cleavage and polyadenylation events in mammalian genes. Genome Res. 2018;28:1427–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kaufmann I, Martin G, Friedlein A, et al. Human Fip1 is a subunit of CPSF that binds to U-rich RNA elements and stimulates poly(A) polymerase. Embo J. 2004;23:616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Clerici M, Faini M, Aebersold R, et al. Structural insights into the assembly and polyA signal recognition mechanism of the human CPSF complex. eLife. 2017;6:e33111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nemeroff ME, Barabino SML, Li Y, et al. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol Cell. 1998;1:991–1000. [DOI] [PubMed] [Google Scholar]
- [46].Das K, Ma LC, Xiao R, et al. Structural basis for suppression of a host antiviral response by influenza virus. Proc Natl Acad Sci USA. 2008;105:13093–13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hamilton K, Sun Y, Tong L. Biophysical characterizations of the recognition of the AAUAAA polyadenylation signal. RNA. 2019;25:1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yang W, Hsu PL, Yang F, et al. Reconstitution of the CstF complex unveils a regulatory role for CstF-50 in recognition of 3′-end processing signals. Nucleic Acid Res. 2018;46:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kennedy SA, Frazier ML, Steiniger M, et al. Crystal structure of the HEAT domain from the pre-mRNA processing factor symplekin. J Mol Biol. 2009;392:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ghazy MA, He X, Singh BN, et al. The essential N terminus of the Pta1 scaffold protein is required for snoRNA transcription termination and Ssu72 function but is dispensible for pre-mRNA 3′-end processing. Mol Cell Biol. 2009;29:2296–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Xiang K, Nagaike T, Xiang S, et al. Crystal structure of the human symplekin-Ssu72-CTD phosphopeptide complex. Nature. 2010;467:729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Xiang K, Manley JL, Tong L. An unexpected binding mode for a Pol II CTD peptide phosphorylated at Ser7 in the active site of the CTD phosphatase Ssu72. Genes Dev. 2012;26:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ruepp MD, Schweingruber C, Kleinschmidt N, et al. Interactions of CstF-64, CstF-77, and symplekin: implications on localisation and function. Mol Biol Cell. 2011;22:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Krishnamurthy S, He X, Reyes-Reyes M, et al. Ssu72 is an RNA polymerase II CTD phosphatase. Mol Cell. 2004;14:387–394. [DOI] [PubMed] [Google Scholar]
- [55].Hausmann S, Koiwa H, Krishnamurthy S, et al. Different strategies for carboxyl-terminal domain (CTD) recognition by serine 5-specific CTD phosphatases. J Biol Chem. 2005;280:37681–37688. [DOI] [PubMed] [Google Scholar]
- [56].Werner-Allen JW, Lee C-J, Liu P, et al. cis-Proline-mediated Ser(P)5 dephosphorylation by the RNA polymerase II C-terminal domain phosphatase Ssu72. J Biol Chem. 2011;286:5717–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bataille AR, Jeronimo C, Jacques P-E, Laramee L, Fortin M-E, Forest A, et al . A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Mol Cell. 2012;45:158–170. [DOI] [PubMed] [Google Scholar]
- [58].Zhang DW, Mosley AL, Ramisetty SR, et al. Ssu72 phosphatase dependent erasure of phospho-Ser7 marks on the RNA polymerase II C-terminal domain is essential for viability and transcription termination. J Biol Chem. 2012;287:8541–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Goddard TD, Huang CC, Ferrin TE. Visualizing density maps with UCSF Chimera. J Struct Biol. 2007;157:281–287. [DOI] [PubMed] [Google Scholar]
- [60].Mandel CR, Gebauer D, Zhang H, et al. A serendipitous discovery that in situ proteolysis is required for the crystallization of yeast CPSF-100 (Ydh1p). Acta Cryst. 2006;F62:1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Preker PJ, Keller W. The HAT helix, a repetitive motif implicated in RNA processing. Trends Biochem Sci. 1998;23:15–16. [DOI] [PubMed] [Google Scholar]
- [62].Bai Y, Auperin TC, Chou C-Y, et al. Crystal structure of murine CstF-77: dimeric association and implications for polyadenylation of mRNA precursors. Mol Cell. 2007;25:863–875. [DOI] [PubMed] [Google Scholar]
- [63].Legrand P, Pinaud N, Minvielle-Sebastia L, et al. The structure of CstF-77 homodimer provides insights into CstF assembly. Nucleic Acid Res. 2007;35:4515–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Moreno-Morcillo M, Minvielle-Sebastia L, Mackereth C, et al. Hexameric architecture of CstF supported by CstF-50 homodimerization domain structure. RNA. 2011;17:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Grozdanov PN, Masoumzadeh E, Latham MP, et al. The structural basis of CstF-77 modulation of cleavage and polyadenylation through stimulation of CstF-64 activity. Nucleic Acids Res. 2018;46:12022–12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Paulson AR, Tong L. Crystal structure of the Rna14-Rna15 complex. RNA. 2012;18:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Moreno-Morcillo M, Minvielle-Sebastia L, Fribourg S, et al. Locked tether formation by cooperative folding of Rna14p monkeytail and Rna15p hinge domains in the yeast CFIA complex. Structure. 2011;19:534–545. [DOI] [PubMed] [Google Scholar]
- [68].Schafer P, Tuting C, Schonemann L, et al. Reconstitution of mammalian cleavage factor II involved in 3′ processing of mRNA precursors. RNA. 2018;24:1721–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Di Giammartino DC, Nishida Y, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell. 2011;43:853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Scrima A, Konickova R, Czyzewski BK, et al. Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell. 2008;135:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fischer ES, Scrima A, Bohm K, et al. The molecular basis of CRL4(DDB2/CSA) ubiquitin ligase architecture, targeting, and activation. Cell. 2011;147:1024–1039. [DOI] [PubMed] [Google Scholar]
- [72].Matsumoto S, Cavadini S, Bunker RD, et al. DNA damage detection in nucleosomes involves DNA register shifting. Nature. 2019;571:79–84. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li T, Chen X, Garbutt KC, et al. Structure of DDB1 in complex with a paramyxovirus V protein: viral hijack of a propeller cluster in ubiquitin ligase. Cell. 2006;124:105–117. [DOI] [PubMed] [Google Scholar]
- [74].Angers S, Li T, Yi X, et al. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. [DOI] [PubMed] [Google Scholar]
- [75].Fischer ES, Bohm K, Lydeard JR, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Faust TB, Yoon H, Nowak RP, et al. Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Nat Chem Biol. 2020;16:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bussiere DE, Xie L, Srinivas H, et al. Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex. Nat Chem Biol. 2020;16:15–23. [DOI] [PubMed] [Google Scholar]
- [78].Tian B, Manley JL. Alternative polyadenylation of mRNA precursors. Nat Rev Mol Cell Biol. 2017;18:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gruber AJ, Zavolan M. Alternative cleavage and polyadenylation in health and disease. Nat Rev Genet. 2019;20:599–614. [DOI] [PubMed] [Google Scholar]
- [80].Yang SW, Li L, Connelly JP, et al. A cancer-specific ubiquitin ligase drives mRNA alternative polyadenylation by ubiquitinating the mRNA 3′ end processing complex. Mol Cell. 2020;77:1206–1221.e7. [DOI] [PubMed] [Google Scholar]
- [81].Xiang Y, Ye Y, Lou Y, et al. Comprehensive characterization of alternative polyadenylation in human cancer. J Natl Cancer Inst. 2018;110:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Masamha CP, Xia Z, Yang J, et al. CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature. 2014;510:412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhu Y, Wang X, Forouzmand E, et al. Molecular mechanisms for CFIm-mediated regulation of mRNA alternative polyadenylation. Mol Cell. 2018;69:62–74.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Brumbaugh J, Di Stefano B, Wang X, et al. Nudt21 controls cell fate by connecting alternative polyadenylation to chromatin signaling. Cell. 2018;172:106–120.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sommerkamp P, Altamura S, Renders S, et al. Differential alternative polyadenylation landscapes mediate hematopoietic stem cell activation and regulate glutamine metabolism. Cell Stem Cell. 2020;26:722–738.e7. [DOI] [PubMed] [Google Scholar]
- [86].Takagaki Y, Manley JL. Complex protein interactions within the human polyadenylation machinery identify a novel component. Mol Cell Biol. 2000;20:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dass B, McDaniel L, Schultz RA, et al. The Gene CSTF2T, encoding the human variant CstF-64 polyadenylation protein tCstF-64, lacks introns and may be associated with male sterility. Genomics. 2002;80:509–514. [PubMed] [Google Scholar]
- [88].Dass B, Tardif S, Park JY, et al. Loss of polyadenylation protein tauCstF-64 causes spermatogenic defects and male infertility. Proc Natl Acad Sci USA. 2007;104:20374–20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yao C, Choi EA, Weng L, et al. Overlapping and distinct functions of CstF64 and CstF64tau in mammalian mRNA 3′ processing. RNA. 2013;19:1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kargapolova Y, Levin M, Lackner K, et al. sCLIP-an integrated platform to study RNA-protein interactomes in biomedical research: identification of CSTF2tau in alternative processing of small nuclear RNAs. Nucleic Acids Res. 2017;45:6074–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rehfeld A, Plass M, Krogh A, et al. Alterations in polyadenylation and its implications for endocrine disease. Front Endocrinol (Lausanne). 2013;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Curinha A, Oliveira Braz S, Pereira-Castro I, et al. Implications of polyadenylation in health and disease. Nucleus. 2014;5:508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ouyang J, Sun W, Xiao X, et al. CPSF1 mutations are associated with early-onset high myopia and involved in retinal ganglion cell axon projection. Hum Mol Genet. 2019;28:1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kakegawa J, Sakane N, Suzuki K, et al. JTE-607, a multiple cytokine production inhibitor, targets CPSF3 and inhibits pre-mRNA processing. Biochem Biophys Res Commun. 2019;518:32–37. [DOI] [PubMed] [Google Scholar]
- [95].Ross NT, Lohmann F, Carbonneau S, et al. CPSF3-dependent pre-mRNA processing as a druggable node in AML and Ewing’s sarcoma. Nat Chem Biol. 2020;16:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kakutani M, Takeuchi K, Waga I, et al. JTE-607, a novel inflammatory cytokine synthesis inhibitor without immunosuppression, protects from endotoxin shock in mice. Inflamm Res. 1999;48:461–468. [DOI] [PubMed] [Google Scholar]
- [97].Desterro J, Bak-Gordon P, Carmo-Fonseca M. Targeting mRNA processing as an anticancer strategy. Nat Rev Drug Discov. 2020;19:112–129. [DOI] [PubMed] [Google Scholar]
- [98].Sonoiki E, Ng CL, Lee MC, et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat Commun. 2017;8:14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Palencia A, Bougdour A, Brenier-Pinchart MP, et al. Targeting Toxoplasma gondii CPSF3 as a new approach to control toxoplasmosis. EMBO Mol Med. 2017;9:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Jacobs RT, Nare B, Wring SA, et al. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl Trop Dis. 2011;5:e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jacobs RT, Plattner JJ, Keenan M. Boron-based drugs as antiprotozoals. Curr Opin Infect Dis. 2011;24:586–592. [DOI] [PubMed] [Google Scholar]
- [102].Swale C, Bougdour A, Gnahoui-David A, et al. Metal-captured inhibition of pre-mRNA processing activity by CPSF3 controls Cryptosporidium infection. Sci Transl Med. 2019;11:eaax7161. [DOI] [PubMed] [Google Scholar]


