Abstract
Hidradenitis suppurativa (HS) is a chronic, recurrent inflammatory skin condition resulting in the formation of nodules, sinus tracts, and abscesses typically in intertriginous regions. HS management is often difficult and involves a multimodal approach, evaluating the benefit of both medical and surgical treatment options, along with treating associated pain and medical comorbidities that present concomitantly with the disease. In this article, we synthesize for the nondermatology clinician the evidence for various HS treatments, along with the diagnostic and therapeutic guidelines for HS published by the British Association of Dermatologists, US and Canadian HS Foundations, HS ALLIANCE, Canadian Dermatology Association, and Brazilian Society of Dermatology. Management of HS requires an individualized, patient-centered approach due to the lack of rigorous evidence for many interventions.
Keywords: Acne inversa, diagnostic guidelines, hidradenitis suppurativa, treatment, Verneuil’s disease
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition presenting as painful, inflamed nodules or abscesses in apocrine gland-bearing areas such as the axillae, groin, and inframammary regions. It can progress to form deeper abscesses, fistulae, sinus tracts, and extensive scarring which cause pain, drainage, and limited range of motion. Typically, onset is postpubertal, between ages 30 and 40, with an estimated prevalence of 1% to 4% worldwide. HS is more common in women, African Americans, and patients of lower socioeconomic status. 1 One-third of patients with HS report a positive family history. Risk factors include tobacco use and obesity. Associated conditions include polycystic ovarian syndrome, metabolic syndrome, severe acne, inflammatory bowel disease, cardiovascular disease, thyroid disease, and arthropathy, among others. 1 HS patients show increased rates of social isolation, depression, anxiety, and suicide. 2 The exact pathogenesis of HS remains to be elucidated. This review aims to summarize HS classification systems and synthesize the most recent treatment guidelines for HS.
DIAGNOSIS
The diagnosis of HS is made clinically. No biopsy or laboratory, genetic, or biomarker testing is routinely indicated. 3 However, certain tests may be performed, if necessary, to exclude other diagnoses or to narrow a differential. The differential diagnosis for HS includes folliculitis, furunculosis, carbuncles, abscesses, infected Bartholin’s gland, cutaneous Crohn’s disease, and inflamed epidermal cysts.
According to the 2016 European guidelines, the primary diagnostic criteria for HS include a history of recurrent tender or suppurating lesions occurring more than twice in the past 6 months in typical sites, such as the axillae, genitofemoral area, perineum, gluteal area, and inframammary area. Nodules, abscesses, scarring, or sinus tracts may also be present. A family history of HS may be a secondary diagnostic criterion for HS. 4
The most commonly used system to assess HS severity in clinical trials and clinical practice is the Hurley staging system. The Hurley staging method is not favorable for monitoring treatment response, as scarring does not regress, even after effective treatment. Additional classification systems have been proposed and validated (Table 1). 5–7 Other methods to document disease severity include assessing pain via a visual analogue scale or numeric rating scale (0–10) or assessing quality of life with the Dermatology Life Quality Index and Skindex scale. 8
Table 1.
Classification systems for hidradenitis suppurativa
| Scoring system | Description |
|---|---|
| Hurley staging | Hurley stage I = single or multiple nodules and/or abscesses without permanent lesions or scarring; Hurley stage II = widely separated recurrent nodules and/or abscesses with sinus tracts and scarring; Hurley stage III = multiple interconnected sinus tracts, abscesses, and scarring affecting an entire anatomical area |
| IHS47 | International Hidradenitis Suppurativa Severity Score System: (Number of nodules × 1) + (number of abscesses × 2) + (number of draining tunnels × 4) ≤3 = mild 4–10 = moderate ≥11 = severe |
| Sartorius score 6 | Three points per anatomic region involved: axilla, groin, gluteal (left/right), or other region. Count total number of different lesions: Nodule = 2 points Fistula = 4 points Scar = 1 point “Other” = 1 point. The longest distance between two relevant lesions (or if a single lesion, size of lesion) in each region: <5 cm = 2 points 5–10 cm = 4 points >10 cm = 8 points. Are all lesions separated by normal skin? Yes = 0 points No = 6 points |
| HiSCR 7 | Hidradenitis Suppurativa Clinical Response: Defined as at least a 50% reduction in the total inflammatory nodule count (abscesses + inflammatory nodules) and no increase in abscess count or draining fistula count relative to baseline. (This is an outcome measure and a meaningful clinical endpoint for treatment. It is not a true classification system.) |
SCREENING FOR COMORBIDITIES
HS patients should be screened for comorbidities including metabolic syndrome, diabetes, smoking, depression, anxiety, follicular occlusion tetrad, inflammatory bowel disease, and squamous cell carcinoma of the skin affected by HS. Recommended screening for comorbidities should occur yearly and more frequently if the clinical situation indicates. 9 Screening should include blood pressure, body mass index, complete blood count, complete metabolic profile with cholesterol panel and fasting blood glucose, and glycosylated hemoglobin type A1. Obtaining a smoking history and performing periodic skin cancer screening are also recommended. Menstrual irregularities or signs of androgen excess should prompt screening for polycystic ovarian syndrome.
TREATMENT
The management of HS involves a multimodal approach, incorporating lifestyle modifications, such as weight loss and smoking cessation, topical therapies, systemic antibiotics, hormonal therapies, retinoids, immunomodulators, and biologic agents.
Topical treatments for HS include skin cleansers, keratolytic agents, and antibiotics. 10 There is evidence to support their use in Hurley stage I and mild stage II HS, as monotherapy, or in conjunction with other treatments. 11–18 Benzoyl peroxide, chlorhexidine, and zinc pyrithione may be used in conjunction with other HS treatments. While all are lacking in formal evidence, these compounds are recommended by anecdotal evidence and expert opinion. Topical cleanser selection should be made with patient and clinician preference in mind and may be driven by cost and availability. 13 Chlorhexidine should only be used on actively draining areas. Side effects of zinc pyrithione include skin irritation. Side effects of benzoyl peroxide and chlorhexidine include itching or burning, stinging or redness, swelling, peeling, and dryness. In the North American guidelines, zinc pyrithione and benzoyl peroxide are formally recommended, while chlorhexidine is presented as expert opinion.
Of all topical antibiotics, only twice-daily clindamycin has formal evidence supporting its use in HS. 12 Clindamycin 1% lotion applied daily for 3 months or longer can be used for Hurley stages I and II. It is also used as a topical adjunct to systemic therapy and in conjunction with benzoyl peroxide, as it may rapidly induce resistance when used as monotherapy. 15 , 17 Side effects of topical clindamycin include burning sensation and skin irritation. Topical clindamycin is recommended by the British, North American, HS ALLIANCE, Canadian Dermatology Association (second-line), and Brazilian Society of Dermatology guidelines. Topical dapsone is listed as a potential treatment by expert opinion only. 19
Twice daily 15% resorcinol cream, a keratolytic and antiseptic agent, has been tested in a small number of patients for the treatment of HS. 14 Although a few early stage studies with resorcinol suggest efficacy in patients with Hurley stages I and II, there is insufficient evidence to suggest it at this time. Resorcinol is recommended as a potential treatment in the North American and Canadian guidelines (second line) as well as the Brazilian Society of Dermatology guidelines (to shorten the duration of painful outbreaks).
Intralesional corticosteroids have evidence to support their use in providing relief from acute lesions. 16 , 20 , 21 Intralesional triamcinolone (10 mg/mL) injected into inflamed HS lesions is commonly used as an adjunctive measure, leading to significant reduction in erythema, edema, suppuration, and nodule size. 16 If suspicion for bacterial infection exists, corticosteroid use is not recommended. Side effects of intralesional corticosteroids include telangiectasias, dyspigmentation, and atrophy in the treated area. Intralesional corticosteroids, such as triamcinolone, are recommended for acute, short-term control by the British, North American, HS ALLIANCE, Canadian Dermatology Association, and Brazilian Society of Dermatology guidelines.
Systemic antibiotics are indicated for all stages of HS. Although HS is not inherently an infection, bacteria do play a role, and secondary infections may occur. Certain antibiotics also have immunomodulatory properties, which can decrease inflammation associated with HS. Monotherapy is utilized for mild recalcitrant disease, but for severe disease, systemic antibiotics are used along with other treatments. Most antibiotics are recommended based on clinical experience or case studies; only combination therapies have been systematically evaluated.
Oral tetracyclines are used as a first-line treatment option for widespread Hurley stage I or mild stage II disease. One study reported a 30% reduction in disease severity with tetracycline use. 4 Another study showed that oral doxycycline 200 mg/day was the preferred first-line therapy in Hurley stage I and II, with 60% Hidradenitis Suppurativa Clinical Response. 22 Although no significant difference was noted in one double-blind, randomized controlled trial comparing twice-daily tetracycline 500 mg to topical clindamycin, the use of tetracycline 500 mg twice daily, doxycycline 100 mg daily, or minocycline 50 to 100 mg twice daily is widely accepted. 19 Common side effects include nausea, vomiting, sun sensitivity, and yeast infections. Tetracyclines are contraindicated in pregnancy due to teratogenicity. They should not be taken with dairy products, as calcium impedes the absorption of the medication. The use of tetracyclines is supported by all HS guidelines.
Systemic combination therapy with oral clindamycin and rifampin is the best studied antibiotic regimen and is used first line for Hurley stage II or III disease. Several case series have shown combination twice-daily clindamycin 300 mg and rifampin 300 mg for up to 10 weeks to be effective. In one study, 47% of patients treated for 10 weeks reported total remission and 35% had at least some improvement in their HS. 23 Nearly 70% of 116 patients in one survey reported significant improvement after 10 weeks of treatment. 24 Common side effects of clindamycin include Clostridium difficile–associated diarrhea, rash, and hepatotoxicity. 25 Rifampin causes orange-tinged bodily fluids and CYP450 interactions. The use of clindamycin and rifampin up to 12 weeks is supported by all HS guidelines.
Other systemic antibiotics include oral dapsone as well as a combination of twice-daily rifampin 300 mg, moxifloxacin 400 mg once daily, and metronidazole 500 mg three times daily. Oral dapsone at 25 to 200 mg for 4 to 12 weeks is recommended for those unresponsive to other antibiotic therapies. A retrospective review of patients with Hurley stage I and II disease showed a response rate of 38% to oral dapsone. 26 Dapsone may cause hemolytic anemia, methemoglobinemia, cyanosis of the lips, and neuropathy. The use of dapsone as third-line therapy is supported by all guidelines. A retrospective review of 28 patients taking rifampin-moxifloxacin-metronidazole combination therapy for 1 to 12 months showed that 6 of 6 patients with Hurley stage I disease, 8 of 10 with Hurley stage II disease, and 2 of 12 with Hurley stage III disease had complete remission. 27 Longer-term use of this combination, however, may lead to increased risk of hepatotoxicity. The use of rifampin-moxifloxacin-metronidazole is supported by the North American and HS ALLIANCE guidelines as second- or third-line therapy in Hurley stage II/III disease.
One gram of intravenous ertapenem daily for 6 weeks can be used as third-line therapy for Hurley stage I, II, or III HS. It is most often used as rescue therapy for severe inflammatory variants of HS. 19 It can also be used as a bridge to surgery. As relapse rates are high after discontinuation, patients must continue on alternative therapies after discontinuing ertapenem. Although it is highly efficacious, there are concerns of antibiotic resistance and practical difficulties with home infusions of ertapenem that make this treatment option unfavorable. The use of intravenous ertapenem for select, severe disease is supported by the North American and HS ALLIANCE guidelines.
Hormonal therapies may be considered, especially in women, for Hurley stage I/II disease and as adjunctive treatment for Hurley stage III. Patients who experience flares around menses, as well as those with concomitant polycystic ovarian syndrome may benefit most. Oral contraceptives studied in HS include cyproterone acetate (CPA) 100 mg, Ogestrel (50 µg ethinyl estradiol/500 µg norgestrel), and combination ethinyl estradiol/50 mg CPA. CPA 100 mg controlled disease in all 4 patients in one small study. 28 Ogestrel and combination 50 µg ethinyl estradiol/50 mg CPA showed similar efficacy (about half of patients experienced improvement with either therapy) at any Hurley stage. 29 Of these options, only Ogestrel is currently commercially available in the USA. In Canada, CPA is available as a stand-alone drug or as a combination CPA/ethinyl estradiol pill, although not at the doses studied in HS. Side effects of oral contraceptives include nausea/vomiting, headache, and dizziness. Oral contraceptive use is supported by both the North American and Brazilian Society of Dermatology guidelines.
Spironolactone, an aldosterone receptor antagonist and antiandrogen, is effective (improvement in 17 of 20 patients in one study) for Hurley stage I and II disease within 3 to 6 months. 30 Because of its diuretic effect, baseline blood pressure should be recorded and considered before prescribing to prevent symptomatic hypotension. Other side effects include gynecomastia, cramping, hyperkalemia, and feminization of male fetuses (avoid with pregnancy). The use of spironolactone is recommended by the North American and Brazilian Society of Dermatology guidelines.
Metformin should be considered in patients with concomitant diabetes mellitus or polycystic ovarian syndrome. Standard dosing is 500 mg two to three times daily. 31 Side effects include diarrhea, nausea, flatulence, and lactic acidosis. Metformin use is supported by the British Association of Dermatologists, North American, and Brazilian guidelines for appropriate patients.
Finasteride and dutasteride have also shown efficacy in men and women in a few cases. 32–34 Both are teratogenic. Finasteride use is supported by the North American guidelines for female patients and by the Brazilian guidelines for children under 12 years old.
Oral immunomodulators may be utilized in Hurley stage I and II. Systemic steroids may be given for acute flares, while the use of colchicine, cyclosporine, methotrexate, and azathioprine are not routinely recommended for HS due to limited efficacy data. Systemic steroids, including hydrocortisone, dexamethasone, and prednisolone, are used in all Hurley stages. High doses of systemic steroids for short courses can be especially useful for decreasing pain and inflammation of an acute HS outbreak. 35 Short-term hydrocortisone monotherapy is recommended at doses of 60 to 80 mg daily. 36 Dexamethasone, when used in conjunction with a gonadotropin-releasing hormone agonist, leuprolide acetate, has shown some efficacy as a treatment. 37 Prednisolone should be dosed in pulses or multiweek tapers at 0.5 to 1 mg/kg daily as rescue therapy for flares or as a bridge to long-term therapy. 38 Although limited by their side effect profile, systemic corticosteroids are recommended by all guidelines.
Retinoids, additional immunomodulators, and biologics for use in Hurley stage II and III patients are reviewed in Table 2. Patients requiring these therapies should be referred to a dermatologist for management.
Table 2.
Treatments for hidradenitis suppurativa with strength of recommendation
| Drug | Strength of recommendation(ABCDE) | Guidelines that support | Notes |
|---|---|---|---|
| Topical/intralesional | |||
| Benzoyl peroxide | E | NA | |
| Chlorhexidine | E | NA | Expert opinion |
| Zinc pyrithione | E | NA | |
| Clindamycin | A | NA, Bri, HS, Can, Bra | C = second line |
| Resorcinol | C | NA, Bra, Can | Br = shorten outbreak duration; C = second line |
| Dapsone | E | NA | Expert opinion |
| Intralesional triamcinolone | A | NA, Bri, HS, Can, Bra | For acute use |
| Systemic antibiotics | |||
| Tetracyclines | A | NA, Bri, HS, Can, Bra | |
| Clindamycin + rifampin | A | NA, Bri, HS, Can, Bra | |
| Rifampin + moxifloxacin + metronidazole | D | NA, HS | Second or third line in Hurley stage II/III disease |
| Dapsone | A | NA, Bri, HS, Can, Bra | Third line |
| Intravenous ertapenem | D | NA, HS | |
| Hormonal | |||
| Oral contraceptives | D | NA, Bra | |
| Spironolactone | D | NA, Bra | |
| Metformin | C | NA, Bra, Bri | |
| Finasteride | D | NA, Bra | Bra, if <12 years; NA, if female |
| Retinoids | |||
| Acitretin | A | NA, Bri, HS, Can, Bra | Weak recommendation from British guideline |
| Isotretinoin | B | NA, Bri, Can, Bra | For concomitant acne vulgaris |
| Systemic immunomodulators | |||
| Systemic steroids | A | NA, Bri, HS, Can, Bra | Short course for acute flares only |
| Methotrexate | Not recommended by NA, E | ||
| Azathioprine | Not recommended by NA, E | ||
| Colchicine | E (in combination with minocycline only) | NA | |
| Cyclosporine | E | Bra | Third line |
| Biologics | |||
| Adalimumab | A | NA, Bri, HS, Can, Bra | |
| Infliximab | A | NA, Bri, HS, Can, Bra | Second line |
| Anakinra | D | NA, HS | |
| Ustekinumab | C | NA, HS, Bra |
A indicates supported by all five guidelines; B, supported by four guidelines; C, supported by three guidelines; D, supported by two guidelines; E, supported by one guideline; NA [North American], US and Canadian HS Foundations’ guideline; Bri, British Association of Dermatologists’ guideline; Can, Canadian Dermatology Association’s guideline; Bra, Brazilian Society of Dermatology’s guideline; HS, HS ALLIANCE’s guideline.
PAIN CONTROL
Pain can be severely debilitating for HS patients and can lead to more distress than the presence of lesions alone. As such, management of both acute and chronic pain is important. No formal evidence exists regarding pain management in HS. Management of pain relies on general pain management guidelines as well as patient and provider preference. Topical analgesics, nonsteroidal anti-inflammatory drugs, or opioids are often used to alleviate pain and inflammation in HS. Some guidelines recommend nonsteroidal anti-inflammatory drugs for acute pain management. Contraindications to nonsteroidal anti-inflammatory drug use include liver or renal impairment, gastrointestinal bleeding, peptic ulcers, inflammatory bowel disease, and heart failure. Opioids are used for refractory pain management and are contraindicated in patients with liver, renal, and pulmonary impairment. Codeine and hydrocodone should be used only for acute pain management and for a limited amount of time and dosage. 4 Consulting a pain management specialist may also be beneficial. The key to treating HS-related pain and discomfort is to control underlying inflammation by decreasing the amount and severity of flares. Most importantly, providers and patients must acknowledge that treatment of the inflammatory disease state is key to controlling underlying pain. 39 The use of opiates is mentioned as a possible adjunctive treatment for severe cases of HS in very few guidelines.
CONCLUSION
HS is a debilitating condition that greatly impacts a patient’s quality of life. The disease is multifactorial, with interplay between multiple genetic, immunological, behavioral, and endocrine factors playing a role in its development. Further studies are needed to ascertain whether certain genetic, clinical, or phenotypic factors may predict or guide treatment outcomes. On average, HS patients may wait 7.2 years from the onset of first symptoms to obtain a proper diagnosis. 40 HS should be considered in the differential diagnosis of nodular lesions, abscesses, or sinus tracts present in the axillae, groin, perineal, and inframammary fold regions. Early clinical diagnosis and prompt treatment are essential to reduce HS burden and to improve a patient’s quality of life.
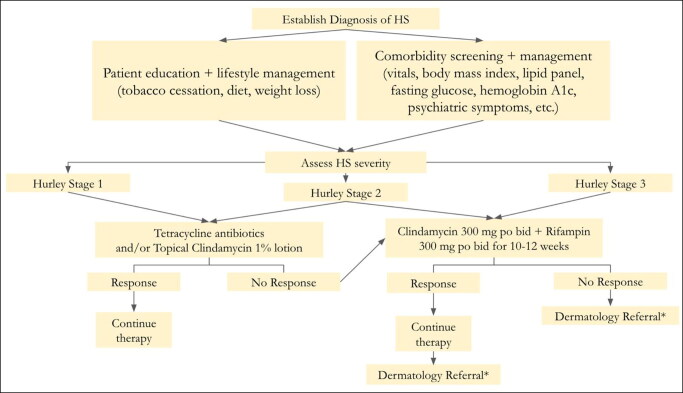
Most therapeutic options are utilized off-label with little formal evidence. Physicians should strongly consider treatment of HS with options that have been consistently supported by evidence and expert guidelines (Table 2). 41 This review is meant to serve as an aid for nondermatology physicians in making optimal clinical decisions to improve outcomes for patients with HS (Figure 1).
Figure 1.
A simple initial treatment management guideline for hidradenitis suppurativa. *For any significant, extensive disease or stubborn, refractory cases, dermatology referral is warranted.
References
- 1. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian hidradenitis suppurativa foundations: Part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81(1):76–90. doi: 10.1016/j.jaad.2019.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vinkel C, Thomsen SF.. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11(10):17–23. [PMC free article] [PubMed] [Google Scholar]
- 3. Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105–115. doi: 10.2147/CCID.S111019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 5. Scuderi N, Monfrecola A, Dessy LA, et al. Medical and surgical treatment of hidradenitis suppurativa: a review. Skin Appendage Disord. 2017;3(2):95–110. doi: 10.1159/000462979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019;180(5):1009–1017. doi: 10.1111/bjd.17537. [DOI] [PubMed] [Google Scholar]
- 7. Zouboulis CC, Tzellos T, Kyrgidis A, et al. ; European Hidradenitis Suppurativa Foundation Investigator Group . Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–1409. doi: 10.1111/bjd.15748. [DOI] [PubMed] [Google Scholar]
- 8. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831–839. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- 9. Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30(6):989–994. doi: 10.1111/jdv.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dauden E, Lazaro P, Aguilar MD, et al. Recommendations for the management of comorbidity in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2018;32(1):129–144. doi: 10.1111/jdv.14517. [DOI] [PubMed] [Google Scholar]
- 11. Jemec GB, Wendelboe P.. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39(6):971–974. doi: 10.1016/s0190-9622(98)70272-5. [DOI] [PubMed] [Google Scholar]
- 12. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22(5):325–328. doi: 10.1111/j.1365-4362.1983.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 13. Danesh MJ, Kimball AB.. Pyrithione zinc as a general management strategy for hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5):e175doi: 10.1016/j.jaad.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 14. Boer J, Jemec GB.. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35(1):36–40. doi: 10.1111/j.1365-2230.2009.03377. [DOI] [PubMed] [Google Scholar]
- 15. Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the longpulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62(4):637–645. doi: 10.1016/j.jaad.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 16. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75(6):1151–1155. doi: 10.1016/j.jaad.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 17. Fischer AH, Haskin A, Okoye GA.. Patterns of antimicrobial resistance in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(2):309–313.e302. doi: 10.1016/j.jaad.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 18. Alavi A, Kirsner RS.. Local wound care and topical management of hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 Suppl 1):S55–S61. doi: 10.1016/j.jaad.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 19. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91–101. doi: 10.1016/j.jaad.2019.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jemec GBE, Revuz J, Leyden J.. Hidradenitis Suppurativa. Berlin, Germany: Springer; 2006:138–140. [Google Scholar]
- 21. Firooz A, Tehranchi-Nia Z, Ahmed AR.. Benefits and risks of intralesional corticosteroid injection in the treatment of dermatological diseases. Clin Exp Dermatol. 1995;20(5):363–370. doi: 10.1111/j.1365-2230.1995.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 22. Vural S, Gündoğdu M, Akay BN, et al. Hidradenitis suppurativa: clinical characteristics and determinants of treatment efficacy. Dermatol Ther. 2019;32(5):e13003. doi: 10.1111/dth.13003. [DOI] [PubMed] [Google Scholar]
- 23. van der Zee HH, Boer J, Prens EP, et al. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology (Basel). 2009;219(2):143–147. doi: 10.1159/000228337. [DOI] [PubMed] [Google Scholar]
- 24. Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219(2):148–154. doi: 10.1159/000228334. [DOI] [PubMed] [Google Scholar]
- 25. Smieja M. Current indications for the use of clindamycin: a critical review. Can J Infect Dis. 1998;9(1):22–28. doi: 10.1155/1998/538090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yazdanyar S, Boer J, Ingvarsson G, et al. Dapsone therapy for hidradenitis suppurativa: a series of 24 patients. Dermatology (Basel). 2011;222(4):342–346. doi: 10.1159/000329023. [DOI] [PubMed] [Google Scholar]
- 27. Join-Lambert O, Coignard H, Jais JP, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology (Basel). 2011;222(1):49–58. doi: 10.1159/000321716. [DOI] [PubMed] [Google Scholar]
- 28. Mortimer PS, Dawber RP, Gales MA, Moore RA.. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115(3):263–268. doi: 10.1111/j.1365-2133.1986.tb05740. [DOI] [PubMed] [Google Scholar]
- 29. Sawers RS, Randall VA, Ebling FJ.. Control of hidradenitis suppurativa in women using combined antiandrogen (cyproterone acetate) and oestrogen therapy. Br J Dermatol. 1986;115(3):269–274. doi: 10.1111/j.1365-2133.1986.tb05741. [DOI] [PubMed] [Google Scholar]
- 30. Lee A, Fischer G.. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56(3):192–196. doi: 10.1111/ajd.12362. [DOI] [PubMed] [Google Scholar]
- 31. Verdolini R, Clayton N, Smith A, Alwash N, Mannello B.. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27(9):1101–1108. doi: 10.1111/j.1468-3083.2012.04668. [DOI] [PubMed] [Google Scholar]
- 32. Khandalavala BN, Do MV.. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9(6):44–50. [PMC free article] [PubMed] [Google Scholar]
- 33. Randhawa HK, Hamilton J, Pope E.. Finasteride for the treatment of hidradenitis suppurativa in children and adolescents. JAMA Dermatol. 2013;149(6):732–735. doi: 10.1001/jamadermatol.2013.2874. [DOI] [PubMed] [Google Scholar]
- 34. Joseph MA, Jayaseelan E, Ganapathi B, Stephen J.. Hidradenitis suppurativa treated with finasteride. J Dermatolog Treat. 2005;16(2):75–78. doi: 10.1080/09546630510031403. [DOI] [PubMed] [Google Scholar]
- 35. Wiseman MC. Hidradenitis suppurativa: a review. Dermatol Ther. 2004;17(1):50–54. doi: 10.1111/j.1396-0296.2004.04007. [DOI] [PubMed] [Google Scholar]
- 36. Danto JL. Preliminary studies of the effect of hydrocortisone on hidradenitis suppurativa. J Invest Dermatol. 1958;31(6):299–300. doi: 10.1038/jid.1958.124. [DOI] [PubMed] [Google Scholar]
- 37. Camisa C, Sexton C, Friedman C.. Treatment of hidradenitis suppurativa with combination hypothalamic-pituitary-ovarian and adrenal suppression. A case report. J Reprod Med. 1989;34(8):543–546. [PubMed] [Google Scholar]
- 38. Wong D, Walsh S, Alhusayen R.. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016;75(5):1059–1062. doi: 10.1016/j.jaad.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 39. Reddy S, Orenstein LAV, Strunk A, Garg A.. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155(11):1284–1290. doi: 10.1001/jamadermatol.2019.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173(6):1546–1549. doi: 10.1111/bjd.14038. [DOI] [PubMed] [Google Scholar]
- 41. Hendricks AJ, Hsiao JL, Lowes MA, et al. A comparison of international management guidelines for hidradenitis suppurativa. Dermatology (Basel). 2019;:1–16. [DOI] [PubMed] [Google Scholar]