Abstract
Alopecia universalis, the complete loss of body hair, during anti–tumor necrosis factor-alpha (TNF-α) biologic therapy is a rare occurrence that has infrequently been reported in the literature. In this case, a 50-year-old man with psoriatic arthritis exhibited alopecia universalis with concomitant onychodystrophy 3 months after initiation with adalimumab. Given the role of TNF-α in the pathogenesis of alopecia areata, it would seem unlikely for anti-TNF-α drugs to induce hair loss; however, it is hypothesized that alopecia areata and its variants may not be dependent on TNF-α and that other factors must be involved. It is important to be aware of such associated adverse effects given that many patients undergo therapy with TNF-α-blocking agents.
Keywords: Adalimumab, alopecia areata, alopecia universalis, onychodystrophy
Alopecia areata (AA) is an autoimmune disease directed at the hair follicle that results in nonscarring hair loss. 1 , 2 AA can be divided into alopecia focalis (patchy hair loss), alopecia totalis (total loss of scalp hair), and alopecia universalis (complete loss of scalp and body hair). 1 The incidence of AA is estimated to be 2%, with a prevalence ranging from 0.1% to 0.2%. In patients with AA, the risk of progression to more severe forms is approximately 5%. 3 The etiology of AA is complicated and still an area of investigation; however, AA is postulated to be due to loss of immune privilege of the hair follicle, autoimmune destruction, and up-regulation of inflammatory pathways. 3 These mechanisms are supported by the presence of T-lymphocyte infiltration around the hair follicle on histology and the involvement of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1-alpha, and interferon gamma. 2 , 4
CASE DESCRIPTION
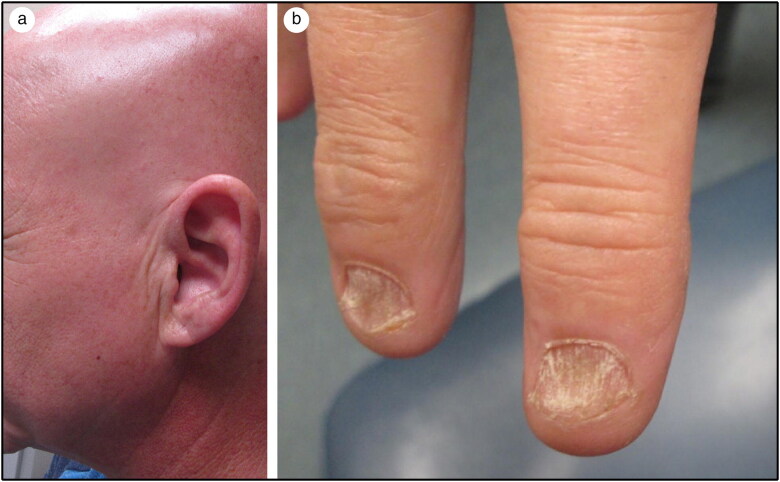
A 50-year-old man with a past medical history of polyarticular psoriatic arthritis, hypothyroidism, and type 2 diabetes mellitus presented to the dermatology clinic for hair loss. The patient started therapy with adalimumab for his psoriatic arthritis in February 2019 and stopped the medication 3 months later after noticing hair loss that started with his facial hair and then subsequently involved the rest of his body. He also observed changes to his nails and claimed that some had begun to fall off. Despite the effect on his hair and nails, he did experience significant improvement of his arthritis after starting adalimumab. His psoriatic arthritis had previously been diagnosed by his rheumatologist and was treated with methotrexate; however, the patient stopped the methotrexate due to adverse effects. He denied a history of psoriatic skin lesions, lichen planus, or hair disorders. He endorsed past pitting of a toenail, but he never experienced the extent of dystrophy as in his current presentation. At the time of our encounter, the only additional medications he was taking were levothyroxine and metformin for his hypothyroidism and diabetes, respectively. A thorough review of systems was negative except for cervical, wrist, and elbow joint pain. On exam, he was found to have diffuse loss of terminal hairs with sparse terminal hairs on the anterior medial thighs (Figure 1a). Additionally, he exhibited trachyonychia characterized by longitudinal ridging, crumbling, brittleness, and roughness of all the digits and toes (Figure 1b). No active psoriatic lesions were evident. He was diagnosed with alopecia universalis with concomitant onychodystrophy. Multiple therapies were offered including systemic, intralesional, and topical biologics; immune modulators; and steroids; however, he was averse to try any other medications and ultimately elected for no therapy at the time.
Figure 1.
(a) Total loss of scalp and facial hair. (b) Onychodystrophy of the digits showing rough textured opalescent nails with longitudinal ridging and crumbling.
DISCUSSION
Patients with psoriatic arthritis, rheumatoid arthritis, inflammatory bowel disease, hidradenitis suppurativa, and other conditions often undergo therapy with TNF-α-blocking agents such as adalimumab, infliximab, and etanercept. 5 Given the role of TNF-α in the pathogenesis of AA, it would seem unlikely that these drugs induce hair loss. Interestingly, a paradoxical effect has been observed with reports of alopecia occurring after initiation of TNF-α inhibitors, with the onset of AA noted to occur within 1 to 89 months after exposure to TNF-α inhibitors. 2 , 4–8 In a review of the available literature, this was determined to be a rare occurrence. These cases support the hypothesis that AA and its variants may not be dependent on TNF-α and that other factors must be involved. It is also postulated that TNF-α blockade may actually cause a dysregulation of cytokines that subsequently triggers hair loss. 6 , 7
Additionally, our patient experienced onychodystrophy. Nail change is an associated abnormality of AA and includes, but is not limited to, nail pitting, longitudinal striations, brittleness, red lunula, trachyonychia, and Beau’s lines. The frequency of nail involvement ranges from 10% to 20% and is typically associated with more extensive hair loss. 3 , 9 , 10
For patients who experience drug-induced alopecia, different therapies can be offered. Initially, discontinuation of the offending agent(s) should be considered. 2 Topical and intralesional glucocorticoids and topical immunotherapy are typically first-line agents. Systemic glucocorticoids, minoxidil, anthralin, phototherapy, prostaglandin analogues, cyclosporine, sulfasalazine, methotrexate, and azathioprine are second-line options. 1 , 11 Tofacitinib, a Janus kinase inhibitor, has also been shown to be successful in stimulating hair regrowth and is currently in clinical trials for AA. Not only does tofacitinib affect hair regrowth but it can also normalize dystrophic nails associated with AA. 12 Furthermore, a recent study by Wambier et al demonstrated a more efficacious approach with the combination of oral tofacitinib and oral minoxidil. 13 Of note, no therapy is an option, and spontaneous hair regrowth is possible. 2
Alopecia universalis with concomitant onychodystrophy is a rare complication of TNF-α inhibitors. Few reports depicting this occurrence exist in the literature. Our goal for this case report is to raise awareness of such associated adverse effects given that many patients undergo therapy with TNF-α blocking agents. Additional reporting of adverse effects of anti-TNF-α drugs is encouraged to optimize treatment modalities and to provide further insight into the pathophysiology of AA.
References
- 1. Kassira S, Korta DZ, Chapman LW, Dann F.. Review of treatment for alopecia totalis and alopecia universalis. Int J Dermatol. 2017;56:801–810. doi: 10.1111/ijd.13612. [DOI] [PubMed] [Google Scholar]
- 2. Lazzarini R, Capareli GC, Buense R, Lellis RF.. Alopecia universalis during treatment with leflunomide and adalimumab—case report. An Bras Dermatol. 2014;89:320–322. doi: 10.1590/abd1806-4841.20142944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strazzulla LC, Wang EHC, Avila L, et al. . Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1–12. doi: 10.1016/j.jaad.2017.04.1141. [DOI] [PubMed] [Google Scholar]
- 4. Garcia Bartels N, Lee H-H, Worm M, Burmester G-R, Sterry W, Blume-Peytavi U.. Development of alopecia areata universalis in a patient receiving adalimumab. Arch Dermatol. 2006;142:1654–1655. doi: 10.1001/archderm.142.12.1654. [DOI] [PubMed] [Google Scholar]
- 5. Ostojic P, Pavlov-Dolijanovic S.. Alopecia universalis in a patient with rheumatoid arthritis developed during treatment with adalimumab. Z Rheumatol. 2018;77:412–415. doi: 10.1007/s00393-018-0464-z. [DOI] [PubMed] [Google Scholar]
- 6. Chaves Y, Duarte G, Ben-Said B, Tebib J, Berard F, Nicolas JF.. Alopecia areata universalis during treatment of rheumatoid arthritis with anti-TNF-alpha antibody (adalimumab). Dermatology (Basel). 2008;217:380. doi: 10.1159/000162180. [DOI] [PubMed] [Google Scholar]
- 7. Ettefagh L, Nedorost S, Mirmirani P.. Alopecia areata in a patient using infliximab: new insights into the role of tumor necrosis factor on human hair follicles. Arch Dermatol. 2004;140:1012. doi: 10.1001/archderm.140.8.1012-a. [DOI] [PubMed] [Google Scholar]
- 8. Tauber M, Buche S, Reygagne P, et al. ; Groupe d’études thérapeutiques des affections inflammatoires du tube digestif (GETAID) . Alopecia areata occurring during anti-TNF therapy: a national multicenter prospective study. J Am Acad Dermatol. 2014;70:1146–1149. doi: 10.1016/j.jaad.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 9. Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J.. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177–188; quiz 189–190. doi: 10.1016/j.jaad.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 10. Gandhi V, Baruah MC, Bhattacharaya SN.. Nail changes in alopecia areata: incidence and pattern. Indian J Dermatol Venereol Leprol. 2003;69:114–115. [PubMed] [Google Scholar]
- 11. Gorcey L, Gordon Spratt EA, Leger MC.. Alopecia universalis successfully treated with adalimumab. JAMA Dermatol. 2014;150:1341–1344. doi: 10.1001/jamadermatol.2014.1544. [DOI] [PubMed] [Google Scholar]
- 12. Dhayalan A, King BA.. Tofacitinib citrate for the treatment of nail dystrophy associated with alopecia universalis. JAMA Dermatol. 2016;152:492–493. doi: 10.1001/jamadermatol.2015.3772. [DOI] [PubMed] [Google Scholar]
- 13. Wambier CG, Craiglow BG, King BA.. Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. J Am Acad Dermatol. 2019. [Epub ahead of print]. doi: 10.1016/j.jaad.2019.08.080. [DOI] [PubMed] [Google Scholar]