Abstract
In the COVID-19 era, the heart failure community has witnessed an unprecedented reduction in heart failure–related patient visits and hospitalizations. Social distancing measures present a dilemma for patients with heart failure who require frequent surveillance of volume status and vital signs to minimize heart failure–related symptoms and hospitalizations. With the rise of telemedicine comes an increased focus on remote monitoring technologies. This report describes use of a multisensor device algorithm in implantable cardioverter defibrillator devices by Boston Scientific, called HeartLogic. We present 2 cases of patients with advanced heart failure who were actively surveilled by the HeartLogic device algorithm to guide care. (Level of Difficulty: Beginner.)
Key Words: heart failure, HeartLogic, remote monitoring
Abbreviations and Acronyms: COVID-19, coronavirus diseae-2019; CRT-D, cardiac resynchronization therapy–defibrillator; HF, heart failure; ICD, implantable cardioverter defibrillator devices; IV, intravenous; LVAD, left ventricular assist device
Graphical abstract
The coronavirus diseae-2019 (COVID-19) pandemic has had a significant impact on the way we care for patients, particularly patients with heart failure (HF) who are at higher risk for morbidity and mortality (1). The adoption of telehealth has been instrumental, although there are inherent limitations (2). A series of remote monitoring technologies are available that can enhance the surveillance of HF signs and assist clinicians with therapeutic interventions (3). Remote monitoring for patients with HF is available in several forms: 1) structured telephone support service with frequent review of HF symptoms, weight, medication compliance, and medical plan adjusted based on the patient’s report; 2) invasive devices implanted to solely monitor surrogates of left ventricular filling pressures (e.g., CardioMEMS sensor implanted in the pulmonary artery), results of which are transmitted electronically and medical therapy is adjusted based on the trend in the recordings; and 3) monitoring via parameters derived from cardiovascular implantable electronic devices (implantable cardioverter-defibrillator [ICD] devices and cardiac resynchronization therapy–defibrillators [CRT-D]), such as for Medtronic (Minneapolis, Minnesota) OptiVol monitoring system that evaluates lung impedance as a surrogate of lung water and volume status (3). Another device that falls into the latter category is the Boston Scientific (Boston, Massachusetts) HeartLogic HF algorithm (4). The algorithm encompasses multiple sensors that are embedded within certain Boston Scientific ICD and CRT-D. The algorithm generates a daily HF index score, which is a proprietary numeric composite of the following physiologic parameters taken together: 1) heart sounds (S1 and S3); 2) thoracic impedance; 3) respiration rate (and its ratio to tidal volume); 4) heart rate; and 5) patient activity (Table 1) (4). The device computes every day the deviation of individual sensors from their baseline and calculates a composite index. An alert is triggered when the modular threshold is exceeded (usually set at 16, but can be adjusted by the clinician) signaling a high risk for a HF event (hospitalization or outpatient intravenous [IV] diuretics). When the patient enters alert status (>16) the index threshold is automatically dropped to a recovery value (nominal value 6).
Learning Objectives
-
•
To recognize the change in methods used to deliver medical care and that avoiding medical care during the COVID-19 pandemic is common among patients.
-
•
To appreciate the role of heart failure remote monitoring devices, such as the HeartLogic, in providing care for heart failure patients during the COVID-19 pandemic.
Table 1.
Review of the HeartLogic Heart Failure Index Physiologic Parameters
| HeartLogic Physiological Variable | Clinical Significance | Directional Change Concerning for Worsening Heart Failure |
|---|---|---|
| S1 | Surrogate for ventricular contraction. | Decrease |
| S3 | Surrogate for early ventricular diastolic filling. Suggesting decreased LV contractility. | Increase |
| Respiratory rate | Ratio of respiration to tidal volume. Marker of rapid shallow breathing (i.e., dyspnea). | Increase |
| Thoracic impedance | Measure of lung fluid accumulation. | Decrease |
| Activity level | Gauge of the functional status and how sedentary the patient is. | Decrease |
The sensitivity for detection was reported at 70%, whereas the median time from the alert onset to the HF event occurrence was 34 days indicating the potential for a very early warning, signaling worsening HF (4). Patients can be contacted and adjustments in the care plan may be instituted thus potentially avoiding hospitalization or need for outpatient IV diuretics particularly during the COVID-19 pandemic.
Case 1
Past medical history
A 78-year-old man with a history of chronic systolic and diastolic HF (left ventricular ejection fraction, 40% to 45%), atrial fibrillation status post ablation × 2, ventricular tachycardia, status post CRT-D, severe tricuspid regurgitation, and chronic kidney disease. During a prior work-up for infiltrative cardiomyopathy, he was found to have wild-type transthyretin cardiac amyloidosis and was subsequently initiated on tafamidis.
History of presentation
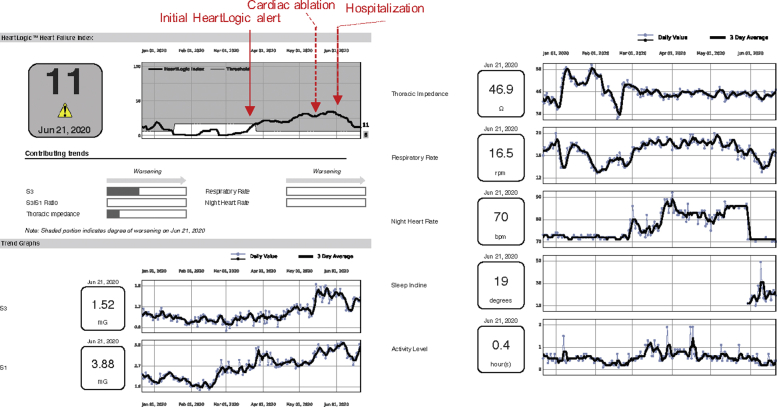
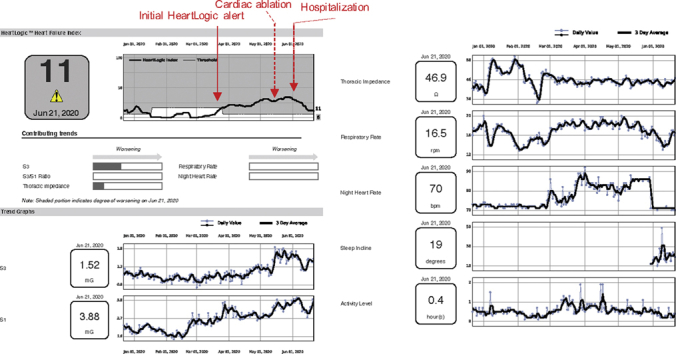
He was last seen in cardiology clinic in March 2020. He underwent an atrioventricular junction ablation in mid-May for nonoptimized biventricular pacing in setting of refractory atrial arrhythmias. Within 1 week following ablation the patient went into HeartLogic status. The HeartLogic report indicated an increase in S3, nighttime heart rate, and respiratory rate without a change in thoracic impedance (Figure 1).
Figure 1.
Patient With Cardiac Amyloid
Patient with cardiac amyloid with heart failure decompensation after elective procedure.
Management
The patient was called by the HF clinic and reported weight gain and swelling. Oral diuretics were increased. He was then seen in clinic at the end of May and reported a weight gain of 7 lbs since discharge with recurrent signs of right-sided congestion (lower extremity edema and ascites) on examination and exertional dyspnea since the cardiac ablation procedure despite diuretic increase. N-terminal pro–B-type natriuretic peptide was ∼1,700 pg/dl (up from 890 last month). Oral diuretics were further escalated; however, the patient required admission 2 weeks later for IV diuresis. Inpatient IV diuresis corresponded to a gradual improvement in the HeartLogic score (Figure 1).
Case 2
Past medical history
A 35-year-old man with a history of pulmonary embolism, idiopathic dilated cardiomyopathy status post-HeartMate 3 left ventricular assist device (LVAD) implant as bridge to decision, and ventricular tachycardia status post-ICD.
History of presentation
He presented probing via video telehealth visit for evaluation related to dyspnea on exertion, orthopnea, cough, and fatigue for 5 days duration. The patient was concerned about worsening HF. He denied any fevers, chills, or sick contacts. There was no bleeding or dark urine.
Investigations
On video, he was in no apparent distress. Jugular venous pressure was not elevated. Abdomen was nondistended. There was no visible lower extremity edema. Driveline site was well healed. No studies were obtained because the patient declined a face-to-face visit citing concerns about COVID-19 exposure within the clinical setting.
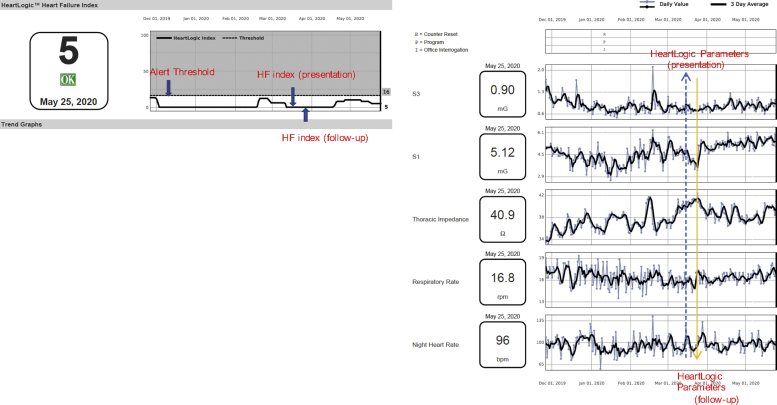
The differential diagnosis included decompensated HF, device malfunction, arrhythmia, or acute bronchitis. His LVAD parameters were within normal limits without alarms (speed: 5,500 rpm; pump power: 4 W; pulsatility index: 5; flow: 4 l), hence device malfunction or pump thrombosis were unlikely. Review of the transmitted ICD interrogation showed no arrhythmia. The HF index score was 0 and all prior scores were below the alert threshold signifying a low likelihood of a HF event (Figure 2). There was no change in the respiratory rate parameter and his thoracic impedance was trending upward relative to baseline, suggesting no pulmonary edema.
Figure 2.
Patient With Left Ventricular Assist Device
Patient with left ventricular assist device, review of the patient’s HeartLogic data. HF = heart failure.
Management
Sacubitril/valsartan, spironolactone, metoprolol succinate, and furosemide (as needed) were continued without changes. The patient was reassured. On telehealth follow-up 1 week later, he was asymptomatic and had safely avoided any direct health care contacts. His HF index continues to remain below threshold.
Discussion
The European Society of Heart Failure guidelines support the use of remote monitoring devices in improving clinical outcomes (5). Remote monitoring can take many forms as described in the introduction section. Here we reviewed 2 cases in which the HeartLogic algorithm provided critical data that expedited care delivery, allowed for appropriate triage of patients, with reduction in unnecessary clinic visits. In the first case HeartLogic was used to surveil a challenging patient with amyloid cardiomyopathy prone to fluid retention. Although the patient experienced clinical signs and symptoms of HF at the time of the initial alert the patient did not seek medical care. Delay in seeking care was in large part caused by an anxiety of contracting SARS-CoV-2. The second patient highlighted the other end of the utility of remote monitoring, that is, safely managed at home with good clinical outcome without requiring admission or an outpatient visit.
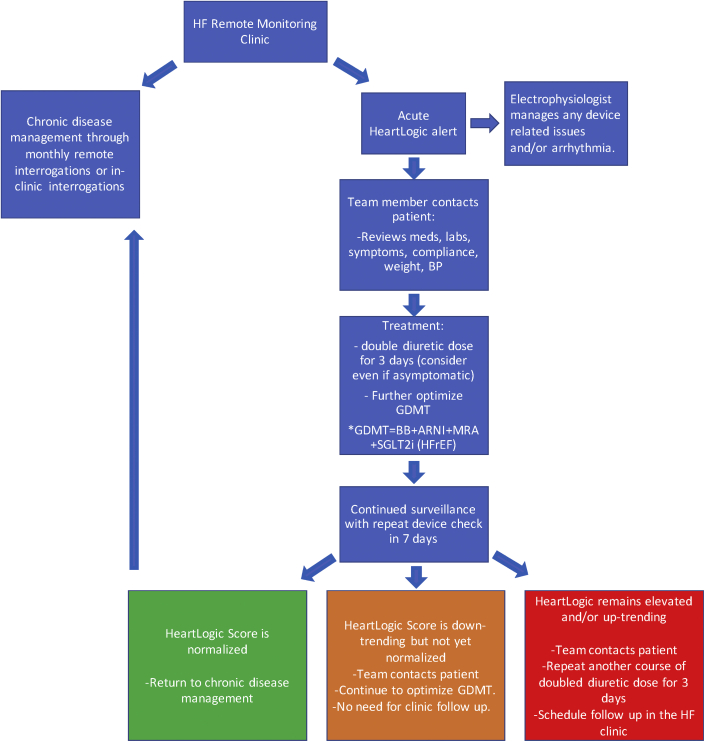
Here we provide a review of our approach to a HeartLogic index alert (Figure 3). In brief, this includes intensifying the diuretic regimen and optimizing guideline-directed medical therapy after obtaining a detailed history from the patient. Any detected device-related issues (e.g., <92% CRT pacing or arrhythmia) is evaluated and managed by the care team electrophysiologist. In addition to treatment, the HF algorithm allows for evaluation of other differential diagnosis as noted in the case review. The HeartLogic data can be used for more than diuretic medication optimization, such as adjusting beta-blockers in response to the recorded heart rate. The activity parameter can facilitate monitoring of how sedentary the patient is, prompting encouragement for more activity. Essentially all the monitored parameters can be used in a manner to promote improved HF care and may lead to a reduction in admissions for HF and reduce the need for unnecessary cardiology visits and/or evaluations. Additionally, we demonstrated that the HeartLogic can be applied to LVAD patients. Initially there were concerns that the S3 and S1 sounds may not be appreciated; however, the algorithm senses the “sounds” as distinct vibrations and in our experience the presence of an LVAD has not affected the functionality. This presents yet another group of patients that may benefit from HF monitoring given the inherent limitations of the physical examination in these patients (i.e., auscultation of the lung and heart sounds is obscured by the LVAD mechanical hum).
Figure 3.
Proposed Management Protocol
Review of a proposed management protocol based on HeartLogic data. ARNI = angiotensin receptor inhibitor and neprilysin inhibitor; BB = beta-blockers; BP = blood pressure; GDMT = guideline-directed medical therapy; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid inhibitor.
Conclusions
The COVID-19 pandemic has changed how and where we care for patients. Remote HF monitoring and telehealth can allow for optimization of care during this time. The HeartLogic algorithm can be an important tool in the remote monitoring armamentarium. Two prospective studies are currently evaluating the impact of the HeartLogic algorithm on clinical outcomes as part of a registry (Precision Event Monitoring for Patients With Heart Failure Using HeartLogic [PREEMPT-HF]; NCT03579641]) and as part of a randomized clinical trial (Multiple Cardiac Sensors for the Management of Heart Failure [MANAGE-HF]; NCT03237858]).
Author Disclosures
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wosik J., Fudim M., Cameron B. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27:957–962. doi: 10.1093/jamia/ocaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planinc I., Milicic D., Cikes M. Telemonitoring in heart failure management. Card Fail Rev. 2020;6:e06. doi: 10.15420/cfr.2019.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehmer J.P., Hariharan R., Devecchi F.G. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE Study. J Am Coll Cardiol HF. 2017;5:216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Seferovic P.M., Ponikowski P., Anker S.D. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]