Abstract
Coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has led to a world-wide pandemic since its onset in December of 2019. Although, a primary respiratory pathogen, over the ensuing period, its extra-pulmonary effects have come to the forefront. The virus, having multi-organ tropism, has been shown to affect a host of other organs beyond the lung, including the pancreas. The data on pancreatic involvement by COVID-19, however, have been limited. Moreover, whether the effects on the pancreas are due to the direct effects of the virus or is just an epi-phenomenon is debatable. The prevalence of pancreatic injury and degree of injury are the other issues that need to be addressed. Pancreatic cancer has a dismal prognosis and the management of the same in the COVID era needs to be tailored assessing the risk-benefit ratio for the same. Additionally, pancreatic surgery increases not only the morbidity of the patient, but also the risk of the operator and burden on the health care system. Hence, the decision for such major procedures needs to be rationalized for optimum benefit during this pandemic. Similarly, for the endoscopist, pancreatic endoscopy needs to be carefully regulated to reduce risk to both the patient and the physician and yet deliver optimum patient care. This review gives a concise summary of various aspects of pancreatic involvement and pancreatic disease management during this pandemic.
Keywords: Coronavirus, COVID-19, Pancreatitis, Pancreatic carcinoma, Pancreatic transplant
Introduction
The world has been shaken by the worst pandemic since the Spanish flu of 1918, by a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Since its first detection in Wuhan City of China in December 2019, it has ripped through more than 200 countries affecting more than 26 million people and claiming more than 800,000 lives as on 6th September, 2020 [1]. A part of the coronavirus family, coronavirus disease 2019 (COVID-19) is primarily associated with features of pneumonia, respiratory failure and death. However, with passage of time, increasing reports of its multi-system involvement have come forth. COVID-19 has been reported to involve the gastrointestinal (GI) tract, liver, pancreas, cardiovascular system, nervous system, ocular system and so on [2,3]. Since the report of the first case of COVID-19 having gastrointestinal (GI) complaints [4], additional reports have explored extra-pulmonary effects of the virus. Involvement of the pancreas secondary to viral infections is not uncommon. Commonly reported viruses affecting the pancreas include hepatotropic viruses such as hepatitis A, B, E, Epstein Barr virus, coxsackie virus, cytomegalovirus, herpes zoster virus, HIV, and viruses ceasing mumps, measles, and varicella-zoster. The mechanism of a viral infection causing pancreatic injury depends on the type of virus [5]. SARS-CoV-2 has also been found to affect the pancreas to varying degrees.
We here give a concise review of the relevant published articles and have summarized the overall incidence rates for pancreatic involvement in COVID-19. Moreover, strategies to be adopted for the management of patients with pancreatic cancer and those requiring pancreatic surgery have been outlined. The effect of COVID-19 infection on pancreas transplant recipients have also been reviewed. Finally, the recent guidance on the pancreatic endo-therapy has been briefed for the practicing gastroenterologists and pancreatologists.
Information sources and literature search
A literature review was carried out through PubMed, Medline and Google Scholar search engines for all relevant English-language articles/abstracts using a search query constructed with the following medical subject heading (MeSH) terms: (“severe acute respiratory syndrome coronavirus 2” OR “COVID-19” OR “coronavirus 2019” OR “SARS-CoV-2”) AND (“pancreas” OR “pancreatitis” OR “hyperamylasaemia” OR “pancreatic cancer” OR “ pancreatic surgery” OR “pancreatic transplant”). The reference list of the papers, webpages of major gastroenterology and hepatology journals and websites of World Health Organisation (WHO), and Centre for Disease Control (CDC) publications were reviewed and all relevant data extracted. Additional published and unpublished studies from other platforms such as medRxiv and Social Science Research Network (SSRN) were also searched. For the recommendations, all the latest guidelines of the major gastrointestinal and endoscopy societies were reviewed (including online suggestions and pre-proofs) by the authors and were framed into a narrative review.
SARS-CoV-2: The pathogen
Corona viruses are a family of viruses that is responsible for a significant fraction of the human common cold. They are enveloped, positive, single stranded RNA viruses that belong to the family Coronaviridae and the order Nidovirales [6]. The most frequently encountered ones causing mild upper respiratory infections are 229E, NL63, OC43 and HKU1. However, the ones which are zoonotic in origin, can infect humans and lead to more fatal disease [7], among which are Severe Acute Respiratory Syndrome-related Coronavirus (SARS-CoV), Middle East Respiratory Syndrome-related Coronavirus (MERS-CoV) and the recently detected novel coronavirus 2019 (SARS-CoV-2). The genome sequence of SAR-CoV-2 is about 89% identical to bat SARS-like-CoVZXC21 and 82% identical to human SARS-CoV [8]. The world has already witnessed pandemics of the former 2 strains, SARS (2002–2003) and MERS (2012). The third coronavirus was designated as SARS-CoV-2 by the International Committee on Taxonomy of Viruses and later rechristened as COVID-19 by WHO [9].
Electron micrograph images reveal its diameter to be 60–140 nm and appearance of a solar corona (crown like) due to the presence of spikes (diameter of 9–12 nm). A spike glycoprotein (S Glycoprotein) attaches the virus to the host cell membrane and is a key factor for host range restriction [10]. The virus been identified to have three subtypes: “A”, “B”. and “C” [11]. While “A” and “C” subtypes have been reported from USA and Europe, type “B” is found predominantly in East Asia.
Pathophysiology of pancreatic injury
Hypothesized to have originated from bats or through pangolins [12], COVID-19 uses angiotensin converting enzyme 2 (ACE 2) as a receptor akin to SARS-CoV. Pathogenesis for lung injury encompasses attachment of the virus to ACE2 receptor on the lung type 2 alveolar cells. Once the spike protein (S) attaches to the alveolar cells, it leads to a cytokine storm which results in alveolar flooding and denudation of the lining epithelium, hampering oxygen exchange and manifesting clinically as ARDS [13]. This ACE 2 receptor expression is not only present in the lungs, but is also in abundance in the esophageal epithelial cells, absorptive enterocytes of the ileum and colon, cardiovascular and renal tissues, and the pancreas [14]. In fact, messenger RNA levels of ACE2 were found to be higher in pancreas than the lung [15]. This explains the multi-organ tropism of the virus.
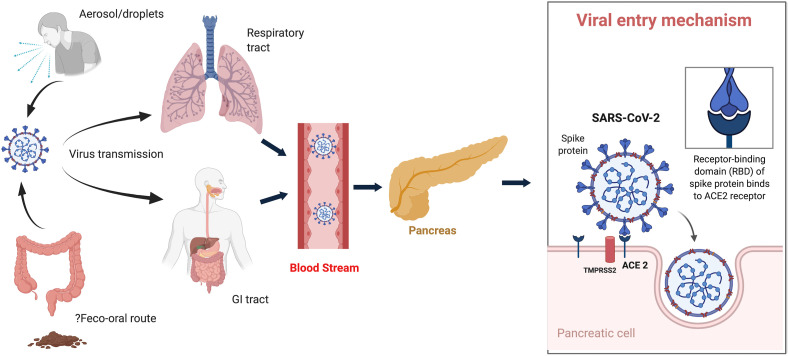
ACE 2 expression is noted both in the exocrine pancreatic glands as well as in the islets. The spike protein (S) engages ACE 2 as the entry receptor [16]. Additionally, the cell entry is facilitated by priming of the S protein by transmembrane protease serine 2 (TMPRSS2) or other proteases [17] (Fig. 1 ). Combined expression of both ACE 2 and TMPRSS2 is needed for the successful virus entry into the cell. In fact, single-cell RNA-sequencing studies have demonstrated that this co-expression is noted not only in the lung alveolar epithelial type 2 cells, but also in various organs including the pancreatic ß cells [18]. Moreover, the receptor affinity of SARS-CoV-2 to ACE2 is greater compared to SARS-CoV, which can explain the higher efficiency and enhanced transmissibility of SARS-CoV-2 [19]. Binding of the virus to ACE 2 receptor mediates pancreatic injury. Dysregulated immune response and cytokine burst have been seen in severe COVID-19 infection with higher levels of interleukin-6 (IL-6) found to be associated with worse prognosis [20,21]. Moreover, it has been noted that pancreatic injury was higher in severe disease as compared to milder COVID-19 infection probably as a fallout of the cytokine burst and immune dysregulation [15].
Fig. 1.
Schematic diagram showing transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to the pancreas and mechanism of the virus entry. TMPRSS, transmembrane serine protease; ACE2, angiotensin receptor 2.
Obesity is known to be a poor prognostic marker for various critical illness including AP, because of the increased risk for development of organ failure [22,23]. Obesity entails increased visceral adipose tissue [24], including intra-pancreatic fat (IPF). On release of pancreatic enzymes during an episode of AP, unsaturated fatty acids (UFAs) of the triglycerides in the adipocytes get released by hydrolysis and perpetuates fat necrosis in AP. The unbuffered portion of the released UFAs leads to cytokine burst, organ failure and end-organ damage. A similar pathogenetic mechanism of cell injury by UFAs has been recently described in COVID-19 infection and has been found to be linked to mortality [25]. Additionally, obesity has been shown to be a risk factor for poor prognosis in COVID-19 infection [26]. Moreover, as mentioned earlier, ACE2, expressed both in adipose tissue and pancreas [15,27], is a key receptor for the virus. Thus, this pathway of virus induced lipotoxicity and cytokine storm [28] can explain the pancreatic injury. In fact, the cytokine profile of both AP and COVID-19 infection has been found to be similar, with increased levels of IL-6, IL-8 and IL-10 [29].
In the earlier studies, SARS-CoV had been shown to cause higher fasting plasma glucose levels [30] and the damage of the pancreatic beta cells was incriminated as a cause for development of “acute diabetes” [31]. The virus can also affect the glucose regulation through the Na+/H+ exchanger (NHE) and lactate pathways [32]. When ACE2 is blocked, angiotensin II levels increase which activate NHE. This can lead to cellular hypoxia and generation of reactive oxygen species resulting in endothelial damage and insulin resistance. Additionally, infection of the exocrine pancreas can lead to collateral damage to beta cells via inflammatory cytokines [33].
Thus, damage to the pancreas, both exocrine and endocrine, is multifactorial: i) direct virus mediated injury of the exocrine pancreas through the ACE2 receptors; ii) severe COVID-19 infection can lead to systemic inflammation and pancreatic injury; iii) virus mediated injury to the islet cells; iv) damage by pro-inflammatory milieu through high levels of interleukin-1β, Monocyte Chemoattractant Protein (MCP)-1 etc and immune dysregulation; v) virus-induced lipotoxicity from UFAs causing hyperlipasemia; v) drug-induced pancreatic injury (due to non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids).
Real world data of COVID-19 and pancreatic injury
Although mechanistically, there is a reason to believe that SARS-CoV-2 can cause significant pancreatic injury, the real-world data are quite contrasting. There are only a few studies looking into evidence of pancreatic injury in a cohort of proven COVID-19 patients while the remaining are case reports of evident pancreatitis in COVID-19 patients.
-
a)
Observational case-control studies on pancreatic injury
Limited studies have looked into the prevalence of pancreatic injury in COVID-19 patients (Table 1 ). Various studies have defined pancreatic injury differently. Most of the authors have defined the pancreatic abnormality as elevation of serum amylase/lipase levels above the normal limit. Imaging findings in the form of computed tomography (CT) abnormalities have been reported by only some studies.
Table 1.
Studies showing incidence of pancreatic injury in COVID-19 patients.
| Study (country) | No. of patients with pancreatic injury/Total no. of patients | Raised amylase | Raised Lipase | Pain abdomen (pancreatic) | CT/imaging findings | Severe disease with injury | Remarks |
|---|---|---|---|---|---|---|---|
| Liu et al.[15](China) | 13/121 (10.74%) | 13/121 (10.74%) | 12/121 (9.92%) | 3/13 | Normal - 8 (3.62%); Enlargement - 5 (4.13%); Necrosis - 0 | 12/67 | Non-severe cases: 1.85%; Severe - 17.9%; NSAIDs - 2, Corticosteroids - 4 |
| Bruno et al.[34](Italy) | 6/70 (8.5%) | 6/70 (8.5%) | 6/70 (8.5%) | None | Normal CT in one case | 5/6 | Pancreatic abnormality in one case at admission; Associated GI symptoms in all; No definite clinical AP |
| McNabb-Baltar et al.[35](USA) | 9/71 (12.1%) | .. | 9/71 (12.1%); 2/71 (2.8%) had levels >3∗ULN | None | No evidence of pancreatitis | 4/9 (44.4%) | Associated GI symptoms; No clinical AP |
| Wang et al.[36](China) | 9/52 (17.3%) | 7/52 (13.5%) | 5/52 (9.6%) | None | .. | 4/9 (44.4%) | 7/9 patients - corticosteroids; No definite clinical AP |
| Barlass et al. [37](USA) | 14/83 (16.8%) | .. | 14/83 (16.8%) | None | .. | 13/14 (92.9%) | Elevated lipase associated with increased ICU admission and intubation |
COVID-19 Coronavirus disease 2019; CT Computed tomography; NSAIDs Non-steroidal anti-inflammatory drugs; GI Gastrointestinal; ULN Upper limit of normal; AP Acute pancreatitis; ICU Intensive care unit.
Laboratory abnormalities suggestive of pancreatic injury have been noted in 8.5%–17.3% of cases [15,[34], [35], [36], [37]] in various studies. Overall 51 (12.8%) of the 397 reported patients had pancreatic injury. Acute pancreatitis (AP) is defined when two of the following three features are met: i) pancreatic type abdominal pain; ii) serum lipase/amylase more than three times the upper limit of the normal (ULN); iii) characteristic imaging findings of AP on CT/MRI/ultrasonography [38]. However, using these criteria, only 3/397 (0.76%) patients had features of clinical AP. In fact, McNabb-Baltar et al. [35] pointed out that although 12.1% cases had raised lipase, only 2.8% (2/71) had levels more than 3 times the ULN. Moreover, 6/13 patients in the study by Liu et al. [15] and 7/9 patients in the report by Wang et al. [36] had concomitant drug history (corticosteroids and NSAIDs). Liu et al. [15] reported that only 5/13 cases (overall 4.13%) with pancreatic injury had abnormal CT findings in the form pancreatic enlargement and none of them had pancreatic necrosis. McNabb-Baltar et al. [35] had pointed out that none of the cases, where imaging was done, showed any feature characteristic of AP.
Interestingly, pancreatic abnormalities have been more frequently noted in the sub-group of patients having severe COVID disease (Table 1). Liu et al. [15] showed that 17.9% of cases had raised amylase in the severe group compared to only 1.85% among non-severe cases. Looking at data from three studies, 20 (22%) of the 91 severe cases had pancreatic abnormalities [15,35,36]. Barlass et al. [37], on multivariate analysis, showed that elevated lipase level was associated with a significantly increased need for ICU admission and intubation. Additionally, most of the studies have found that a majority of patients with reported pancreatic abnormalities had GI symptoms such as nausea, anorexia, abdominal discomfort (not pain) and diarrhea [[34], [35], [36]].
-
b)
Case reports/series of acute pancreatitis with COVID-19
Multiple case reports and a few case series have reported clinical AP in the setting of COVID-19 infection (Table 2 ). Most of these cases had severe COVID-19 infection at baseline. However, a few of these cases had no respiratory symptoms to start with [[39], [40], [41]]. One of the initial reports was that of development of AP in 2 out of 3 COVID-19 positive hospitalized members of a family in Denmark [42]. Alloway et al. [39] reported a case of a 7-yr-old girl with AP without any respiratory symptoms at onset. On evaluation, she was found to be COVID-19 positive. Stevens et al. reported a 10-yr-old female child presenting with AP who progressed to develop multisystem inflammatory syndrome of children, secondary to COVID-19 infection [43].
Table 2.
Case reports/series of acute pancreatitis with COVID-19 infection.
| Study (country) | Type of study | Raised amylase/lipase | pain abdomen | CT/imaging findings | Remarks |
|---|---|---|---|---|---|
| Mierles et al. [51](Portugal) | Case report | ✚ | ✚ | Normal | Clinical AP |
| Hadi et al. [42](Denmark) | Case series | ✚ | ✚ (1/2) | 1 had voluminous pancreas on USG | 2 out of 3 members of the family had AP |
| Aloysius et al. [52](USA) | Case report | ✚ | ✚ | Normal | Clinical AP |
| Alloway et al. [39] | Case report | ✚ | ✚ | suggestive of necrotising pancreatitis | 7-yr-old girl with AP; No respiratory symptoms to begin with |
| Miao et al. [41](France) | Case report | ✚ | ✚ | Enlarged pancreas | No respiratory symptom with clinical AP |
| Anand et al. [53](UK) | Case report | — | ✚ | Edematous pancreas | Clinical AP |
| Pinte et al. [54](Romania) | Case report | ✚ | ✚ | Edematous pancreas | Clinical AP |
| Schepis et al. [46](Italy) | Case report | — | ✚ | Pseudocyst of pancreas | Cyst fluid positive for SARS-CoV-2 |
| karimzadeh et al. [40](Iran) | Case report | ✚ | ✚ | Normal | No respiratory symptoms |
| Rabice et al. [44](USA) | Case report | ✚ | ✚ | Not done | Pregnant female with Clinical AP |
| Morrison et al. [55](USA) | Case report | ✚ | — | Not done | Tocilizumab induced hypertriglyceridemia; 1 case had AP |
| Szatmary et al. [47](UK) | Case series | — | ✚ | Mild edematous pancreas | 5 cases attributed to COVID in a cohort of 35 proven AP |
| Elhence et al. [48] (India) | Case series | ✚ | ✚ | Necrotising pancreatitis | 3 cases had nosocomial COVID infection in proven AP while 2 had AP with COVID infection at diagnosis |
| Cheung et al. [45] | Case report | ✚ | ✚ | Suggestive of AP | Reported case of recurrent AP secondary to COVID-19 |
| Stevens et al. [43] (USA) | Case report | ✚ | ✚ | Suggestive of AP | 10-yr-old female with AP, later developing MIS-C |
| Bokhari et al. [56] (Pakistan) | Case report | ✚ | ✚ | Bulky pancreas with peri-pancreatic inflammatory fluid | Clinical AP |
| Gonzalo-Voltas et al. [57] (Spain) | Case report | ✚ | ✚ | Consistent with AP | Clinical AP |
| Mazrouei et al. [58] (UAE) | Case report | ✚ | ✚ | Edema of pancreas with fluid collection | Clinical AP |
| Brikman et al. [59] (Israel) | Case report | ✚ | ✚ | Evidence of pancreatitis | Clinical AP; patient received antecedent tocilizumab, dexamethasone. |
| Kataria et al. [60] (USA) | Case report | ✚ | ✚ | Edematous pancreas | Clinical AP |
| Gadiparthi et al. [61] (USA) | Case report | ✚ | ✚ | Peripancreatic stranding with fluid | Clinical AP with antecedent hypertriglyceridemia and type 2 diabetes |
AP acute pancreatitis; COVID Coronavirus disease; SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2; MIS-C Multisystem inflammatory syndrome of children.
There are reports of development of AP in patients of COVID-19 infection having respiratory symptoms as well as reports of patients with AP developing hospital acquired COVID-19. Rabice et al. [44] reported a case of a 36-yr-old pregnant female with respiratory symptoms followed by development of clinical AP and she was found to be COVID-19 positive. Cheung et al. [45] reported an interesting case of recurrent AP, probably secondary to COVID-19 infection. Schepis et al. [46] described an intriguing finding of SARS-CoV-2 positivity in pancreatic pseudocyst sample of a follow-up case of AP presenting with pain abdomen and chest infiltrates. This finding is interesting as SARS-CoV-2 has already been detected in various body fluids such as urine and blood besides respiratory aerosols. The finding of the virus in pseudocyst fluid hints to the fact that the virus might be detected in other body fluids as well, and hence to chances of transmission.
In a cohort of 35 proven AP patients, Szatmary et al. [47] found 10 cases with SARS-CoV-2 positivity of whom 5 had no definite etiology and were attributed to COVID-19 infection. In a study by Elhence et al. [48], 3 cases of hospitalized AP patients developed nosocomial COVID-19 infection, but none had worsening of the disease. However, a recent study, that has compared AP patients with and without COVID-19 infection, revealed contrasting results. COVID-19 patients with AP had significantly higher requirement of mechanical ventilation and length of hospital stay compared to the COVID-19 negative ones. Interestingly, 69% of the COVID positive AP cases had idiopathic pancreatitis compared to 21% of the COVID-19 negative AP [49]. Similar findings were voiced by Dirweesh et al. [50], with higher rates of organ failure and mortality in the cohort of COVID-19 positive AP cases when compared to COVID-19 negative patients.
-
c)
COVID-19 infection with past history of pancreatitis
Pancreatitis, acute or chronic, is known to increase morbidity and often have nutritional deficiencies which might lead to compromised immune systems. Gubatan et al. [62], in an interesting study, found that the prevalence of COVID-19 infection in patients with prior pancreatitis was 7.8% compared to 2.8% of the general population. In multi-variate analysis, idiopathic pancreatitis was found to be an independent risk factor for COVID-19 infection. Interestingly, none of the patients with past history of pancreatitis developed AP during the COVID-19 infection [62].
-
d)
Autopsy data of pancreatic injury
While majority of the autopsy data in COVID-19 have described lung findings, data on pancreatic injury is limited. In an autopsy series of 3 COVID-19 cases by Yao et al. [63] it was noted that only a few islet cells were degenerated while the exocrine pancreas had no abnormality and was negative for SARS-CoV on immunohistochemistry. On the contrary, using a murine monoclonal antibody, Ding et al. [64] demonstrated that SARS-CoV-2 could be detected in multiple organs including the pancreas in 4 autopsy cases of COVID-19 related death. Lax et al. [65] in another autopsy study, pointed out that 5 out of 11 cases (45.5%) had focal pancreatitis, although none had clinical suspicion of pancreatitis. This high proportion of focal pancreatitis could be part of end-organ damage rather than the actual virus inflicted injury. In another autopsy series, 2 (25%) of the 8 cases had pancreatic abnormalities. One 22-yr-old man had haemorrhagic, necrotic changes while another 97-yr-old man had microscopic evidence of pancreatitis [66].
Clinical implications of the available data
Since viral infections are known to cause pancreatitis [5] and pain abdomen has been a well reported GI symptom among COVID-19 patients [67], the association of COVID-19 infection with pancreatic injury/inflammation seems logical. However, a critical analysis of the available data cast doubts on this probable association. As pointed out earlier, most of the cohort studies have used mere elevation of serum amylase/lipase levels as surrogate markers of pancreatic injury. Moreover, only a fraction (2.8% in one study) of these patients had levels >3 times ULN and practically none had robust evidence of clinical AP as per the diagnostic criteria [38]. Raised serum amylase levels are not specific for AP and can have different sources including the healthy and diseased lung [68]. Lipase is more specific for diagnosing AP but is not exclusive. Lipase levels can be elevated because of various other conditions such as underlying diabetes or renal dysfunction [69], critical illness [70] or drugs such as corticosteroids [71]. All of these factors are commonly encountered in COVID-19 positive patients.
Since GI involvement in COVID-19 is well documented, increased intestinal permeability secondary to SARS-CoV-2 infection can lead to increased pancreatic enzyme levels [72]. Gastroenteritis is also known to cause hyperamylasaemia [73]. Interestingly, COVID-19 patients with pancreatic abnormalities, as mentioned above, had higher prevalence of GI symptoms. Thus, GI involvement, by itself, can explain the isolated rise in the amylase/lipase without associated pancreatitis in this group of patients.
Elevated pancreatic enzymes or AP can also be secondary to cytokine storm and multi-organ dysfunction leading to pancreatic injury rather than the direct cytopathic effects of the virus. Mechanical ventilation or shock, too, can lead to hypoxic pancreatic damage [74]. This corroborates with the evidence that pancreatic injury was higher among patients with more severe disease.
Multiple drugs such as corticosteroids and NSAIDs used during management of COVID-19 can precipitate pancreatitis [75]. Many of the case series and reports highlighted above had antecedent history of these drugs which may account for the rise of pancreatic enzymes rather than it being the effects of the virus. Additionally, drugs such as tocilizumab (TCZ) and lopinavir/ritonavir can lead to hypertriglyceridemia. This in turn can result in pancreatitis as well. Morrison et al. have reported one case of TCZ-induced hypertriglyceridemia related AP [55]. Similarly, Baricitinib, a drug used for COVID-19 treatment can lead to AP as well [76]. Thus, drugs are an important contributor for pancreatic injury among COVID-19 patients. Conversely, high dose steroids used for conditions like autoimmune pancreatitis (AIP) can theoretically pose high a risk for poor outcome in COVID-19 infection. However, Liaquat et al. [77] have reported favourable outcome of COVID-19 infection in a case of AIP treated with steroids. Data on actual risk is lacking, but it would be advisable to shorten the induction schedule for steroids and reduce azathioprine in case of lymphopenia for AIP [78].
As very few cohort studies exist on the prevalence of pancreatic injury/inflammation among COVID-19 patients, a possibility of under reporting may also need consideration. A lot of additional questions remain unanswered for COVID-19 related AP such as: i) timing of onset of AP from the time of infection; ii) disease course; iii) assessment of disease severity in the background of COVID-19 related symptoms; iv) overall outcome of concomitant AP with COVID-19 infection. Thus, more robust data and national/international registries are required for better understanding of this conundrum of COVID-19 infection and pancreatic injury.
Pancreatic endocrine system and COVID-19
The interaction of COVID-19 with pancreatic endocrine system complicates the scenario for patients with diabetes mellitus (DM) infected with the virus. DM with associated hyperglycemia has been found to have poor prognosis with increased morbidity and mortality [79,80]. This can be explained to be secondary to depressed innate immune response and exaggerated cytokine response in DM [81]. Moreover, the severity of the infection is related to the concentration of glycosylated ACE2 receptors in pulmonary epithelium. Thus, increased receptors in lung of individuals with uncontrolled DM can perpetuate binding of the virus and in turn cause severe disease [80]. Conversely, COVID-19 infection can worsen the glycaemic control through damage to the pancreatic beta cells either directly by SARS-CoV-2 or via cytokine mediated damage. The complex interplay of DM and COVID-19 is beyond the scope of this article and hence not discussed in much details here.
Pancreatic cancer and COVID-19
Presence of comorbidities not only pre-disposes to increased likelihood of being infected with COVID-19 but also leads to a more severe disease course [79]. Cancer has been found to be a risk factor for increased likelihood of infection and also severe disease [82], more so with lung and colorectal cancer [83]. In one study, pan-cancer analysis showed pancreatic adenocarcinoma to have increased ACE2 expression [84], and deep deletions for TMPRSS2 [85]. This may hint towards increased baseline risk in this group of patients. Moreover, chemotherapeutic drugs, when used for management of pancreatic cancer, cause immunosuppression leading to added risk. A very high rate (40%) of adverse events and complications has been reported with chemotherapeutic regimens in COVID-19 patients [86].
Pancreatic cancer, as such, has a very dismal prognosis. The added risk of this pandemic warrants more careful assessment of the risk-benefit ratio. While major pancreatic onco-surgeries have a high morbidity and complications rate, chemotherapy treatment can compound the risk. Thus, the decision for medical and surgical therapy needs to be tailor made after a multi-disciplinary team consultation [87,88]. A tiered approach has been advocated to prioritise various interventions for optimum benefit: i) tier 1 (high priority) includes life threatening conditions or where there is significant improvement in the survival; ii) tier 2 (medium priority) encompasses condition where a 6–8 weeks delay would not hamper the overall outcome; iii) tier 3 (low priority) entails stable patient conditions wherein services can be delayed for the pandemic to be over or situation where there will be no survival gain. European Society for Medical Oncology (ESMO) [89] and other societies including National Cancer Institute of Milan [90] have come up with recommendations for management during this pandemic. A synopsis of the recommendations has been outlined in panel 1 .
Panel 1. Key recommendations for the management of pancreatic cancer during COVID-19 pandemic.
-
•Outpatient visit priorities
-
•Patients with newly diagnosed resectable cancer to be consulted on high priority, preferably through telemedicine, along with multi-disciplinary assessment for plan for surgery.
-
•Unstable patients or those requiring interventions such as jaundice, cholangitis, post-surgical complications etc to be given priority
-
•Patients with established diagnosis with minor problems should be directed towards telemedicine.
-
•Post-operative patients with no complications or with no issues should be discouraged from OPD visits.
-
•
-
•Imaging priorities
-
•Symptomatic patients, such as jaundice or intestinal occlusion should be given priority
-
•For diagnosis of pancreatic cancer, CT followed by EUS, if resectable, need to be considered
-
•Routine follow up for recurrence or restaging after surgery needs to be kept on low priority
-
•
-
•Surgical oncology and endoscopic intervention procedures
-
•Resectable malignancy including cystic lesions with suspicion of malignancy to be kept high priority
-
•Endoscopic stenting for biliary obstruction, cholangitis, or for those planned for neoadjuvant/palliative treatment should be carried out on high priority
-
•Post-surgical complications to be managed promptly
-
•For non-resectable diseases/metastatic diseases with life expectancy >3 months, biliary or duodenal obstruction can be managed with hepatojejunostomy/hepatogastrojejunostomy/endoscopic stenting can be done with lower priority
-
•
-
•Medical oncology priorities
-
•For localised or locally advanced disease
-
oInitiation or completion of neoadjuvant/adjuvant treatment with high priority
-
oAdjuvant treatment to be initiated with ECOG PS 0, <70 yrs old, based on pathologic state and individual risk assessment
-
oModified FOLFIRINOX regimen to be considered, and delaying treatment up to 12 weeks after surgery
-
oFollow up imaging or restaging in asymptomatic cases to be deferred
-
o
-
•For advanced/metastatic disease
-
oFirst line chemotherapy likely to improve survival in patients who can tolerate combined regimen
-
oMonotherapy regimen for asymptomatic/oligosymptomatic cases to be assessed based on the risk-benefit ratio
-
oFollow-up imaging/restaging studies to be deferred
-
oAnti-resorptive therapy such as zolendronate to be deferred
-
o
-
•Prophylactic use of G-CSF for minimising neutropenia-associated risks is debatable
-
•Adequate venous thromboprophylaxis to be provided
-
•
COVID-19 Coronavirus disease 2019; OPD outpatient department; EUS endoscopic ultrasonography; ECOG Eastern Cooperative Oncology Group; PS Performance Status; G-CSF Granulocyte colonystimulating factor
Alt-text: Panel 1
Pancreatic surgery and COVID-19
Surgery, specifically pancreatic, uses up an immense amount of hospital resources in the form of logistics, health care workers, time and adnexal support requirements. American College of Surgeons (ACS) guidelines have defined the type of surgery that can be performed depending on the phase of the pandemic [91]. As most countries are now in the phase II/III of the pandemic, patients requiring surgery within hours to within a few days are the ones that can be taken up for surgery, keeping the rest at bay. Worldwide surveys have shown a reduction in the rates of pancreatic surgeries. In a survey of 267 centres spanning 37 countries, Oba et al. showed that while in most centres, the weekly pancreatic resections decreased from a median of 3 to 1, the remaining centres (30.7%) did not perform any pancreatic surgery at all [92]. A European-African Hepato-Pancreato-Biliary Association (E-AHPBA) survey revealed that most centres had reduction in the number of surgeries including cancer surgery due to inadequate ICU beds and the fear of post-operative COVID infection [93]. Non-essential surgeries were not carried out by 83% of the respondents. In fact, in COVID-high countries, chemotherapy was preferably used over surgery even for resectable pancreatic cancer.
Surgery for potentially curable pancreatic adenocarcinoma and pancreatic cystic lesions with high grade dysplasia are advocated (Panel 1). Pre-operative testing for COVID-19 has been advocated. All suspected and confirmed cases are to be transported by dedicated medical staff with adequate precautions by the shortest possible route. For confirmed cases, dedicated operating theatres with negative pressure facilities or an air exchange rate of ≥25cycles/hour are recommended. A Joint statement from ACS recommended that resumption of elective surgeries can be done only when there is sustained reduction in the new COVID-19 cases in that geographical location for at least 14 days and there is adequate logistic back-up to cater to non-elective cases [94].
Pancreatic transplant recipient and COVID-19
Limited data exist on the effects of COVID-19 infection on pancreatic transplant recipients. Dube et al. [95] reported the first case series of 4 simultaneous kidney-pancreas transplant (SPK) recipients, of whom 1 died while the remaining cases improved. Suwanwongse et al. [96] described a case of SPK on immunosuppressants developing COVID-19 infection and respiratory failure with fatal outcome. A Swiss Transplant Cohort study [97] of 21 cases of solid organ transplant recipients included 1 pancreatic and 2 SPK recipients. Most of them presented with classical COVID-19 symptoms, of which 1 SPK recipient required ICU admission, but without any mortality. Transplant recipients are on long standing immunosuppressants and theoretically are at an increased risk for COVID-19 infection and possible severe disease course. More data is warranted before the actual risk or the course of COVID-19 infection in these sub-group of patients can be optimally assessed.
Pancreatic endoscopy during the COVID-19 pandemic
Pancreatic endoscopy, in the wake of the COVID-19 pandemic, is a debatable option that the endoscopist needs to assess considering all the pros and cons. Endoscopy, being an aerosol generating procedure, poses a high risk for the operating endoscopist and assisting technical staff [98]. The possible routes of transmission during an endoscopic procedure can be person to person, droplet mode, aerosols generated in the positive pressure room, and contact with contaminated bodily fluids and faecal matter [99]. The risk of transmission enhances manifold due to multitude of factors: i) aerosols from the patient’s respiratory secretions contaminates the endoscopy room, more so when the viral load is high; ii) endoscopic procedures are high aerosol generating procedures, because of coughing and retching during upper GI endoscopy and passage of flatus during colonoscopy; iii) Suctioning and exchange of accessories during endoscopy pose further risk by splashing and spreading of infective material [100,101]. Pancreatic endoscopy entails endoscopic retrograde cholangiopancreatographies (ERCPs) and endoscopic ultrasounds (EUS). Both these procedures may pose still higher risk due to larger scope diameter, longer procedure time and higher need for exchange of accessories during the procedure. Thus, for all interventions, a triaging system is essential to optimise patient care and minimise health care worker risk.
Endoscopists need to assess the following: i) need for the procedure – risk benefit ratio; b) if the procedure is time sensitive, then whether it is urgent (<2weeks) or semi-urgent (2–8 weeks); iii) if the procedure can be deferred beyond 8 weeks [102]. Important parameters that need to be reviewed include threat to patient’s life, risk of disease progression/worsening or risk of metastasis. Thus, ERCP for obstructive jaundice or cholangitis, EUS guided drainage for infected/symptomatic pancreatic fluid collection or for fine needle aspiration/biopsy for pancreatic lesion/cyst without metastatic lesions may be carried out on a priority basis [103]. Endotherapy for chronic pancreatitis, celiac plexus block, evaluation for idiopathic/recurrent pancreatitis etc. can be deferred. The precautions to be taken during the procedure have been outlined in various society guidelines.
Conclusion
Although mechanistically SARS-CoV-2 is expected to cause pancreatic injury, robust real-world data on its actual impact is lacking. The elevation of serum levels of pancreatic enzymes, amylase and lipase, has been considered as a marker of pancreatic injury and attributed to the effect of COVID-19 infection. However, actual evidence of clinical pancreatitis secondary to SARS-CoV-2 is dubious. Whether this reported pancreatic enzyme elevation in COVID-19 positive patients, more for severe cases, has a cause-effect relationship or just an epiphenomenon needs to be established. Larger data and international registries focused on this aspect can possibly give an answer. Pancreatic cancer management and pancreatic surgeries in this COVID era have to be tailor made to individual cases after multi-disciplinary assessment of the available options and the risk-benefit ratio. Interventions including pancreatic endotherapy not only burdens the over-stretched health system in this COVID era, but also increases risk for health care workers. Thus, triaging of cases and catering to only the essential ones are recommended. Adequate precaution, avoiding elective cases, and “lying low” in terms of interventions are the key strategies that will enable the gastroenterologists and pancreatologists to tide over this COVID-19 crisis.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
We acknowledge BioRender for design support for Fig. 1.
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int September 6 [Available from:
- 2.Samanta J., Dhar J., Khaliq A., Kochhar R. Novel coronavirus infection: gastrointestinal manifestations. J Dig Endosc. 2019;11:13–18. 2020. [Google Scholar]
- 3.Sultan S., Altayar O., Siddique S.M., Davitkov P., Feuerstein J.D., Lim J.K., et al. AGA Institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawla P., Bandaru S.S., Vellipuram A.R. Review of infectious etiology of acute pancreatitis. Gastroenterol Res. 2017;10:153. doi: 10.14740/gr858w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richman D.D.W.R., Hayden F.G. John Wiley & Sons; 2016. Clinical virology. [Google Scholar]
- 7.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci Unit States Am. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam T.T., Jia N., Zhang Y.W., Shum M.H., Jiang J.F., Zhu H.C., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muus C., Luecken M.D., Eraslan G., Waghray A., Heimberg G., Sikkema L., et al. BioRxiv; 2020. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. [Google Scholar]
- 17.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904 e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papachristou G.I., Papachristou D.J., Avula H., Slivka A., Whitcomb D.C. Obesity increases the severity of acute pancreatitis: performance of Apache-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 23.Navina S., Acharya C., DeLany J.P., Orlichenko L.S., Baty C.J., Shiva S.S., et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002573. 107ra110- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatua B., El-Kurdi B., Singh V.P. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33:374–382. doi: 10.1097/MOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Kurdi B., Khatua B., Rood C., Snozek C., Cartin-Ceba R., Singh V.P., et al. Mortality from coronavirus disease 2019 increases with unsaturated fat and may Be reduced by early calcium and albumin supplementation. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Oliveira C., Khatua B., Noel P., Kostenko S., Bag A., Balakrishnan B., et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest. 2020;130:1931–1947. doi: 10.1172/JCI132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegyi P., Szakacs Z., Sahin-Toth M. Lipotoxicity and cytokine storm in severe acute pancreatitis and COVID-19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 31.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cure E., Cumhur Cure M. COVID-19 may affect the endocrine pancreas by activating Na(+)/H(+) exchanger 2 and increasing lactate levels. J Endocrinol Invest. 2020 doi: 10.1007/s40618-020-01307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeckel E., Manns M., Von Herrath M. Viruses and diabetes. Ann N Y Acad Sci. 2002;958:7–25. doi: 10.1111/j.1749-6632.2002.tb02943.x. [DOI] [PubMed] [Google Scholar]
- 34.Bruno G., Fabrizio C., Santoro C.R., Buccoliero G.B. Pancreatic injury in the course of coronavirus disease 2019 (COVID-19): a not-so-rare occurrence. J Med Virol. 2020 doi: 10.1002/jmv.26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNabb-Baltar J., Jin D.X., Grover A.S., Redd W.D., Zhou J.C., Hathorn K.E., et al. Lipase elevation in patients with COVID-19. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barlass U., Wiliams B., Dhana K., Adnan D., Khan S.R., Mahdavinia M., et al. Marked elevation of lipase in COVID-19 disease: a cohort study. Clin Transl Gastroenterol. 2020;11 doi: 10.14309/ctg.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 39.Alloway B.C., Yaeger S.K., Mazzaccaro R.J., Villalobos T., Hardy S.G. Suspected case of COVID-19-associated pancreatitis in a child. Radiol Case Rep. 2020;15:1309–1312. doi: 10.1016/j.radcr.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karimzadeh S., Manzuri A., Ebrahimi M., Huy N.T. COVID-19 presenting as acute pancreatitis: lessons from a patient in Iran. Pancreatology. 2020 doi: 10.1016/j.pan.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao Y., Lidove O., Mauhin W. First case of acute pancreatitis related to SARS-CoV-2 infection. Br J Surg. 2020;107 doi: 10.1002/bjs.11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadi A., Werge M., Kristiansen K.T., Pedersen U.G., Karstensen J.G., Novovic S., et al. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology. 2020;20:665–667. doi: 10.1016/j.pan.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens J.P., Brownell J.N., Freeman A.J., Bashaw H. COVID-19-Associated multisystem inflammatory syndrome in children presenting as acute pancreatitis. J Pediatr Gastroenterol Nutr. 2020 doi: 10.1097/mpg.0000000000002860. [DOI] [PubMed] [Google Scholar]
- 44.Rabice S.R., Altshuler P.C., Bovet C., Sullivan C., Gagnon A.J. COVID-19 infection presenting as pancreatitis in a pregnant woman: a case report. Case Rep Womens Health. 2020;27 doi: 10.1016/j.crwh.2020.e00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung S., Delgado Fuentes A., Fetterman A.D. Recurrent acute pancreatitis in a patient with COVID-19 infection. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.927076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schepis T., Larghi A., Papa A., Miele L., Panzuto F., De Biase L., et al. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology. 2020 doi: 10.1016/j.pan.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szatmary P., Arora A., Raraty M.G.T., Dunne D.F.J., Baron R.D., Halloran C.M. Emerging phenotype of SARS-CoV2 associated pancreatitis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elhence A., Mahapatra S.J., Vajpai T., Garg P.K. Acute pancreatitis and nosocomial COVID-19: cause specific host responses may determine lung injury. Pancreatology. 2020 doi: 10.1016/j.pan.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inamdar S., Benias P.C., Liu Y., Sejpal D.V., Satapathy S.K., Trindade A.J., et al. Prevalence, risk factors, and outcomes of hospitalized patients with COVID-19 presenting as acute pancreatitis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dirweesh A., Li Y., Trikudanathan G., Mallery J.S., Freeman M.L., Amateau S.K. Clinical outcomes of acute pancreatitis in patients with COVID-19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meireles P.A., Bessa F., Gaspar P., Parreira I., Silva V.D., Mota C., et al. Acalculous acute pancreatitis in a COVID-19 patient. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aloysius M.M., Thatti A., Gupta A., Sharma N., Bansal P., Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020 doi: 10.1016/j.pan.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anand E.R., Major C., Pickering O., Nelson M. Acute pancreatitis in a COVID-19 patient. Br J Surg. 2020;107:e182. doi: 10.1002/bjs.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinte L., Baicus C. Pancreatic involvement in SARS-CoV-2: case report and living review. J Gastrointestin Liver Dis. 2020;29:275–276. doi: 10.15403/jgld-2618. [DOI] [PubMed] [Google Scholar]
- 55.Morrison A.R., Johnson J.M., Ramesh M., Bradley P., Jennings J., Smith Z.R. Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J Med Virol. 2020 doi: 10.1002/jmv.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bokhari S., Mahmood F. Case report: novel coronavirus-A potential cause of acute pancreatitis? Am J Trop Med Hyg. 2020;103:1154–1155. doi: 10.4269/ajtmh.20-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalo-Voltas A., Uxia Fernández-Pérez-Torres C., Baena-Díez J.M. Acute pancreatitis in a patient with COVID-19 infection. Med Clin. 2020;155:183–184. doi: 10.1016/j.medcle.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazrouei S.S.A., Saeed G.A., Al Helali A.A. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep. 2020;15:1601–1603. doi: 10.1016/j.radcr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brikman S., Denysova V., Menzal H., Dori G. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ (Can Med Assoc J) 2020;192:E858–E859. doi: 10.1503/cmaj.201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kataria S., Sharif A., Rehman A.U., Ahmed Z., Hanan A. COVID-19 induced acute pancreatitis: a case report and literature review. Cureus. 2020;12 doi: 10.7759/cureus.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gadiparthi C., Bassi M., Yegneswaran B., Ho S., Pitchumoni C.S. Hyperglycemia, hypertriglyceridemia, and acute pancreatitis in COVID-19 infection: clinical implications. Pancreas. 2020;49:e62–e63. doi: 10.1097/MPA.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gubatan J., Levitte S., Patel A., Balabanis T., Sharma A., Jones E., et al. Prevalence, risk factors and clinical outcomes of COVID-19 in patients with a history of pancreatitis in Northern California. Gut. 2020 doi: 10.1136/gutjnl-2020-321772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao X., Li T., He Z., Ping Y., Liu H., Yu S., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi Chin J Pathol. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 64.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020 doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo S., Zhang X., Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18:1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berk J.E., Shimamura J., Fridhandler L. Amylase changes in disorders of the lung. Gastroenterology. 1978;74:1313–1317. [PubMed] [Google Scholar]
- 69.Steinberg W.M., Nauck M.A., Zinman B., Daniels G.H., Bergenstal R.M., Mann J.F., et al. Leader 3—lipase and amylase activity in subjects with type 2 diabetes: baseline data from over 9000 subjects in the LEADER Trial. Pancreas. 2014;43:1223. doi: 10.1097/MPA.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manjuck J., Zein J., Carpati C., Astiz M. Clinical significance of increased lipase levels on admission to the ICU. Chest. 2005;127:246–250. doi: 10.1378/chest.127.1.246. [DOI] [PubMed] [Google Scholar]
- 71.Völzke H., Lüdemann J., Mayerle J., Kraft M., John U., Lerch M.M. Prevalence and determinants of increased serum lipase levels in a general population. Pancreas. 2008;37:411–417. doi: 10.1097/MPA.0b013e31817f527d. [DOI] [PubMed] [Google Scholar]
- 72.Pieper-Bigelow C., Strocchi A., Levitt M.D. Where does serum amylase come from and where does it go? Gastroenterol Clin N Am. 1990;19:793–810. [PubMed] [Google Scholar]
- 73.Tositti G., Fabris P., Barnes E., Furlan F., Franzetti M., Stecca C., et al. Pancreatic hyperamylasemia during acute gastroenteritis: incidence and clinical relevance. BMC Infect Dis. 2001;1:18. doi: 10.1186/1471-2334-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muniraj T., Dang S., Pitchumoni C.S. PANCREATITIS OR NOT?--Elevated lipase and amylase in ICU patients. J Crit Care. 2015;30:1370–1375. doi: 10.1016/j.jcrc.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 75.Nitsche C.J., Jamieson N., Lerch M.M., Mayerle J.V. Drug induced pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24:143–155. doi: 10.1016/j.bpg.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Cerda-Contreras C., Nuzzolo-Shihadeh L., Camacho-Ortiz A., Perez-Alba E. Baricitinib as treatment for COVID-19: friend or foe of the pancreas? Clin Infect Dis. 2020 Aug 14 doi: 10.1093/cid/ciaa1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liaquat H., Shupp B., Kapoor S., Matin A. High-dose prednisone for treatment of autoimmune pancreatitis in a patient with coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Am J Case Rep. 2020;21 doi: 10.12659/AJCR.926475. e926475-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miranda-Zazueta G., González-Regueiro J.A., García-Juárez I., Moctezuma-Velázquez C., López-Díaz F.J., Pérez-González B., et al. Pharmacologic management of patients with hepatic and pancreatic diseases that involve immunosuppressive therapies. Position statement within the framework of the SARS-CoV-2 (COVID-19) pandemic. Rev Gastroenterol México. 2020;85:312–320. doi: 10.1016/j.rgmx.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guan W-j, Liang W-h, Zhao Y., Liang H-r, Chen Z-s, Li Y-m, et al. Comorbidity and its impact on 1590 patients with covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J.F., Xiong X.F., Xiao Y., Wei L., Li L., Yang M., et al. The single nucleotide polymorphism rs11643718 in SLC12A3 is associated with the development of diabetic kidney disease in Chinese people with type 2 diabetes. Diabet Med. 2020 doi: 10.1111/dme.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pal R., Bhadada S.K. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr. 2020;14:513–517. doi: 10.1016/j.dsx.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang B., Huang Y. Which type of cancer patients are more susceptible to the SARS-COX-2: evidence from a meta-analysis and bioinformatics analysis. Crit Rev Oncol Hematol. 2020;153:103032. doi: 10.1016/j.critrevonc.2020.103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chai P., Yu J., Ge S., Jia R., Fan X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: a pan-cancer analysis. J Hematol Oncol. 2020;13:43. doi: 10.1186/s13045-020-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katopodis P., Anikin V., Randeva H.S., Spandidos D.A., Chatha K., Kyrou I., et al. Pancancer analysis of transmembrane protease serine 2 and cathepsin L that mediate cellular SARSCoV2 infection leading to COVID-19. Int J Oncol. 2020;57:533–539. doi: 10.3892/ijo.2020.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel R., Saif M.W. Management of pancreatic cancer during COVID-19 pandemic: to treat or not to treat? JOP. 2020;21:27–28. [PMC free article] [PubMed] [Google Scholar]
- 87.Burki T.K. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21:629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17:268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Catanese S., Pentheroudakis G., Douillard J.Y., Lordick F. ESMO Management and treatment adapted recommendations in the COVID-19 era: pancreatic Cancer. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pietrantonio F., Morano F., Niger M., Corallo S., Antista M., Raimondi A., et al. Systemic treatment of patients with gastrointestinal cancers during the COVID-19 outbreak: COVID-19-adapted recommendations of the National Cancer Institute of Milan. Clin Colorectal Canc. 2020 May 23;19:156–164. doi: 10.1016/j.clcc.2020.05.004. S1533-0028:30077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ACS ACoS COVID-19: guidance for triage of non-emergent surgical procedures. 2020. https://www.facs.org/covid-19/clinical-guidance/triage March 17 [Available from:
- 92.Oba A., Stoop T.F., Lohr M., Hackert T., Zyromski N., Nealon W.H., et al. Global survey on pancreatic surgery during the COVID-19 pandemic. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balakrishnan A., Lesurtel M., Siriwardena A.K., Heinrich S., Serrablo A., Besselink M.G., et al. HPB; 2020. Delivery of hepato-pancreato-biliary surgery during the COVID-19 pandemic: an European-African Hepato-Pancreato-Biliary Association (E-AHPBA) cross-sectional survey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.American College of Surgeons ASoA, Association of periOperative Registered Nurses, Association tAH Joint statement: roadmap for resuming elective surgery after COVID-19 pandemic. Am Soc Anesthesiol. 2020 https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/joint-statement-on-elective-surgery-after-covid-19-pandemic [Available at: [Google Scholar]
- 95.Dube G.K., Husain S.A., McCune K.R., Sandoval P.R., Ratner L.E., Cohen D.J. COVID-19 infection in pancreas transplant recipients. Transpl Infect Dis. 2020 Jun 9 doi: 10.1111/tid.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suwanwongse K.S.N. Fatal outcome in a kidney-pancreas transplant recipient with COVID-19. Cureus. 2020, June 18;12 doi: 10.7759/cureus.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tschopp J., L’Huillier A., Mombelli M., Mueller N.J., Khanna N., Garzoni C., et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020 May 15 doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiu P.W.Y., Ng S.C., Inoue H., Reddy D.N., Hu E.L., Cho J.Y., et al. Practice of endoscopy during COVID-19 pandemic: position statements of the asian pacific society for digestive endoscopy (APSDE-COVID statements) Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soetikno R., Teoh A.Y., Kaltenbach T., Lau J.Y., Asokkumar R., Cabral-Prodigalidad P., et al. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:176–183. doi: 10.1016/j.gie.2020.03.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vavricka S.R., Tutuian R., Imhof A., Wildi S., Gubler C., Fruehauf H., et al. Air suctioning during colon biopsy forceps removal reduces bacterial air contamination in the endoscopy suite. Endoscopy. 2010;42:736–741. doi: 10.1055/s-0030-1255615. [DOI] [PubMed] [Google Scholar]
- 101.Johnston E.R., Habib-Bein N., Dueker J.M., Quiroz B., Corsaro E., Ambrogio M., et al. Risk of bacterial exposure to the endoscopist’s face during endoscopy. Gastrointest Endosc. 2019;89:818–824. doi: 10.1016/j.gie.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 102.AGA. Joint GI society message: COVID-19 clinical insights for our community of gastroenterologists and gastroenterology care providers. 2020. https://gastro.org/press-releases/joint-gi-society-message-covid-19-clinical-insights-for-our-community-of-gastroenterologists-and-gastroenterology-care-providers/ March 16 [Available from:
- 103.Machicado J.D., Papachristou G.I., Cote G.A., Wani S., Groce J.R., Conwell D.L., et al. Pancreaticobiliary endoscopy in the COVID-19 pandemic era. Pancreas. 2020;49:729–732. doi: 10.1097/MPA.0000000000001580. [DOI] [PMC free article] [PubMed] [Google Scholar]