Abstract
Cerebral palsy (CP) is a neurodevelopmental movement disorder that affects coordination and balance. Therapeutic treatments for balance deficiencies in this population primarily focus on the musculoskeletal system, while the neural basis of balance impairment is often overlooked. Magnetic resonance elastography (MRE) is an emerging technique which has the ability to sensitively assess microstructural brain health through in vivo measurements of neural tissue stiffness. Using MRE, we have previously measured significantly softer grey matter in children with CP as compared with typically developing children. To further allow MRE to be a clinically useful tool in rehabilitation, we aim to understand how brain stiffness in children with CP is related to dynamic balance reaction performance as measured through anterior and posterior single-stepping thresholds, defined as the standing perturbation magnitudes that elicit anterior or posterior recovery steps. We found that global brain stiffness is significantly correlated with posterior stepping thresholds (p=0.024) such that higher brain stiffness was related to better balance recovery. We further identified specific regions of the brain where stiffness was correlated with stepping thresholds, including the precentral and postcentral gyri, the precuneus and cuneus, and the superior temporal gyrus. Identifying brain regions affected in CP and related to balance impairment can help inform rehabilitation strategies targeting neuroplasticity to improve motor function.
Keywords: cerebral palsy, magnetic resonance elastography, stiffness, viscoelasticity, brain, pediatric, balance, single-stepping threshold
1. Introduction
Cerebral palsy (CP) is a neurodevelopmental disorder caused by brain trauma during fetal development or shortly after birth1. CP can cause significant movement impairment in children, including decreased motor function and muscle coordination2. Children with CP also exhibit impaired balance that further suppresses gait ability and physical activity3,4. Therefore, efforts at understanding balance impairment and improving balance function are essential for therapy in pediatric CP5. Balance training in children with CP has primarily focused on addressing muscular coordination deficiencies6–9; however, neural damage is a primary source of balance impairment in CP. Therefore, identifying strong relationships between brain health and balance function in children with CP may be useful for determining the neuroplastic benefits of interventions in this population.
Balance and postural equilibrium are affected by the cerebral cortex both directly, via corticospinal loops, and indirectly, via communication with the brainstem centers10. Specific regions of the cortex are essential for directly maintaining postural control, including the prefrontal, premotor, supplementary motor, and parietal cortical areas11,12, as well as numerous indirect neural pathways affecting locomotion13. Understanding neural deficiencies underlying balance impairments in this population is likely valuable. However, identifying specific deficiencies is challenging as many children with CP do not display the characteristic lesions in their structural MRI scans14, and thus these structural images are not always well correlated with functional ability15. Alternatively, quantitative neuroimaging measures sensitive to microstructural brain health may be able to identify relationships with functional impairment in CP16.
Brain mechanical properties, as measured through magnetic resonance elastography (MRE)17, have emerged as sensitive measures of neural tissue microstructural health18. Previous MRE studies have shown that brain mechanical properties such as stiffness are affected by several neurological disorders in adults19,20 including Alzheimer’s disease21,22, multiple sclerosis23–25, and Parkinson’s disease26,27. However, brain mechanics of neurodevelopmental disorders are far less characterized28, and only a limited number of studies have reported healthy pediatric brain mechanical properties29,30. We previously performed the first study of brain stiffness in children with CP using MRE and found that global gray matter stiffness, as well as local regions of gray and white matter, were significantly less stiff in children with CP than in typically-developing (TD) children31. This study provided the first evidence that brain mechanical properties are affected in CP, indicating reduced structural integrity of neural tissue in this population. Our next goal is to determine how these altered brain properties may relate to neuromuscular function.
Impaired balance function in children with CP is evident when observing their rapid, standing balance reactions to an external perturbation. Compared to TD counterparts, children with CP have a delayed neuromuscular response to the perturbation, with the response characterized by co-contraction at each joint and an altered coordination between lower-extremity joints32–35. This altered neural control in children with CP likely underlies impaired balance-reaction performance, as evident by smaller anterior single-stepping thresholds, or the perturbation magnitudes that elicit a forward step, as compared to TD children33,36. We have established single-stepping thresholds as repeatable, precise, and objective measures of balance reaction performance37. Given that CP is associated with impaired balance reactions, the underlying mechanisms of which appear to be related to the neural response to the perturbation, it is reasonable to investigate the extent to which brain health corresponds to this motor function.
In this study, we have built on our previous work to examine relationships between brain stiffness and balance function in children with CP. Brain MRE measures have recently been shown to correlate with performance on assessments of cognitive function such as memory and fluid intelligence38–41; however, relationships with assessments of motor functions, such as balance reaction, have not yet been examined with MRE. Improved information on the relationships between brain health and balance reaction in children with CP may allow for the development of rehabilitation strategies that target therapeutic neuroplasticity.
2. Methods
2.1. Participants
The MRE data in this analysis is the same as previously collected by our group to compare brain viscoelasticity of children with CP, ages 5-12, with typically-developing children31. Of the 12 children with CP who participated in that study, 10 (6 females, 4 males; mean age = 8.6 ± 1.9 years; mean BMI = 16.7 ± 1.5 kg/m2) additionally completed a set of functional assessments as part of a separate study. Participants were recruited through the orthopedic surgery clinic at Nemours/A.I. duPont Hospital for Children and had medically-diagnosed spastic cerebral palsy. Participants were classified on the Gross Motor Function Classification System (GMFCS) as level 1 (N = 8) or level 2 (N = 2), representing the highest levels of functionality in children with CP. This study was approved by the University of Delaware Institutional Review Board and informed written consent was obtained from all participants and their guardians prior to testing.
2.2. Magnetic Resonance Elastography
MRI scans were completed at the University of Delaware using a Siemens 3T Prisma scanner and 20-channel head RF-receive coil. MRE data was acquired using a single-shot EPI sequence with a pneumatic driver system (Resoundant, Inc.; Rochester, MN) which delivered vibrations to the head at 50 Hz. Four phase offsets were acquired in a total scan time of 3 minutes 15 seconds. The following imaging parameters were used: FOV = 240 × 240 mm2; matrix = 96 × 96 mm2; 48 slices; final resolution = 2.5 × 2.5 × 2.5 mm3; TR/TE = 6720/69 ms; GRAPPA R = 3. Complex displacement fields were created from MRE phase images using FSL PRELUDE (Jenkinson, 2003) and temporal Fourier filtering. These displacement fields were used to calculate maps of viscoelastic shear stiffness and damping ratio using the nonlinear inversion (NLI) algorithm42. NLI returns property maps of the complex viscoelastic shear modulus (G = G’+iG’’), where G’ is the storage modulus and G” is the loss modulus. Viscoelastic shear stiffness, μ, was calculated as μ=2|G|2/(G’+|G|) 43 and damping ratio, ξ, was calculated as ξ = G’’/2G’ 44. Figure 1 illustrates the MRE technique.
Figure 1:
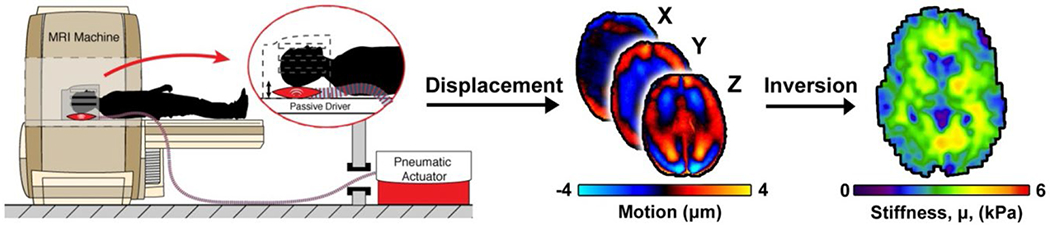
Illustration of the magnetic resonance elastography (MRE) technique: the participant’s head is vibrated in the MRI scanner with a pneumatic actuator, displacement wave fields are acquired, and the inversion algorithm calculates viscoelastic shear stiffness.
2.3. Dynamic Balance Reactions
In a separate experimental session on the same day, each participant completed a perturbation-based balance recovery assessment—a reliable assessment of stepping thresholds described in detail in a previous publication37. The participant was secured with a safety harness instrumented with a force transducer (Dillon, Fairmont, MN). The child stood on a computer-controlled treadmill (ActiveStep®, Simbex, Lebanon, NH) and was instructed to “try not to step” in response to treadmill-delivered, 400 ms surface translations either anteriorly or posteriorly with a fixed acceleration. The participant did not know the direction or timing of the upcoming perturbation prior to the trial. Anterior or posterior perturbation accelerations were increased by one-half meter per second squared if the participant successfully recovered with no step. A response was considered as a failure if the participant took one or more steps to recover or exerted a force of more than 20% of their body weight onto the harness. The perturbation acceleration that elicited four consecutive failures was identified as the single-stepping threshold (SST) for anterior or posterior perturbations (Figure 2). We have previously reported that the group of subjects in this study performed 22.5% and 40% worse than TD children for both the anterior and posterior SST tasks, respectively45.
Figure 2:

Illustration of the balance reaction task. Participants stood on the treadmill, and they received anterior and posterior treadmill-belt accelerations that perturbed standing balance. Accelerations were incrementally increased until thresholds that consistently elicited a step were established. These single-stepping thresholds (SST) were determined in the anterior and posterior directions (i.e. the direction of the induced fall).
2.4. Analyses
MRE magnitude images were nonlinearly registered to a T2-weighted MNI standard-space template at 2.0 mm resolution using FSL FNIRT46,47. This subject-specific transform was applied to the corresponding stiffness and damping ratio maps to transform them to standard-space. Gray matter and white matter were segmented from the MNI template using FSL FAST48 and constrained to the cerebrum using the WFU PickAtlas49 to create a final region-of-interest of global cerebral gray and white matter. Multivariate analyses of covariance (MANCOVAs) were conducted to test for relationships between global mechanical properties and balance reaction tasks. Two tests were performed with either stiffness and damping ratio as independent variable, and with both anterior and poster SST included together as dependent variables. Significance was determined at p < 0.025 following Bonferroni correction for the two separate tests performed. Post hoc correlations tested relationships for each individual balance reaction measure (anterior and posterior SST) with MRE measure with significance determined at p < 0.05. Participant age was included as a covariate in all analyses. Statistical analyses were performed using JMP Pro 14.1.0 (SAS Institute, Inc.; Cary, NC).
Voxel-wise analyses of the relationships between brain stiffness and balance reaction measures were also conducted using the property maps registered to MNI space. Spearman rank correlations were performed comparing stiffness at each voxel with anterior or posterior single-stepping threshold, separately, with participant age as a covariate. An estimation of the false discovery rate (FDR)50, which is a common technique used for multiple hypothesis testing, was found and all voxels with FDR > 0.05 were excluded so that each voxel has at most a 5% chance of being a false positive. This pipeline is similar to that of the voxel-wise comparison between CP and TD brain stiffness used by Chaze et. al. (2019). The anatomical locations of the centers of these regions were identified by the MNI coordinates. The 3dClusterSim command-line tool from the NIH AFNI toolbox (Analysis of Functional NeuroImages, http://afni.nimh.nih.gov/afni/) was used to set a statistical threshold for cluster size from the MNI space stiffness maps. This Monte Carlo simulation included the following parameters: single voxel significance at 0.05, 1-sided thresholding, third-nearest neighbor clustering, and alpha = probability(cluster>=given size) of 0.05. From this we obtained an a-priori threshold that significant voxels clusters must be of at minimum size of 53 voxels. For conciseness of reporting, and to further reduce the chance of false-positive significance, clusters with less than 200 significant adjacent voxels were removed from our analysis. This large cluster size was chosen to account for the inherent smoothing in MRE property maps and still be larger than the expected point spread function of the NLI processing51. All voxel-wise analyses were performed in Matlab (MathWorks, Inc.; Natick, MA).
3. Results
Figure 3 shows the relationships between global stiffness of the cerebrum and dynamic balance reactions in children with CP. Age of the participants was significantly correlated with their cerebral brain stiffness (r = 0.50; p = 0.022), but age was not significantly correlated with either anterior (r = 0.01; p = 0.790) or posterior (r = 0.13; p = 0.304) single-stepping threshold. MANCOVA tested the relationships between stiffness and balance reactions, and the entire model was significant (p = 0.022). Post hoc correlations, accounting for age, found cerebral stiffness significantly correlated with posterior single-stepping threshold (r = 0.74; p = 0.024), but no significant correlation was found between cerebral stiffness and anterior SST (r = 0.46; p = 0.214). Greater brain stiffness was associated with larger SST accelerations, indicative of better balance performance. Cerebral damping ratio was not significantly correlated with balance reactions in the MANCOVA model (p = 0.71) and therefore cannot be separately tested for either anterior or posterior SST and was not considered further.
Figure 3:
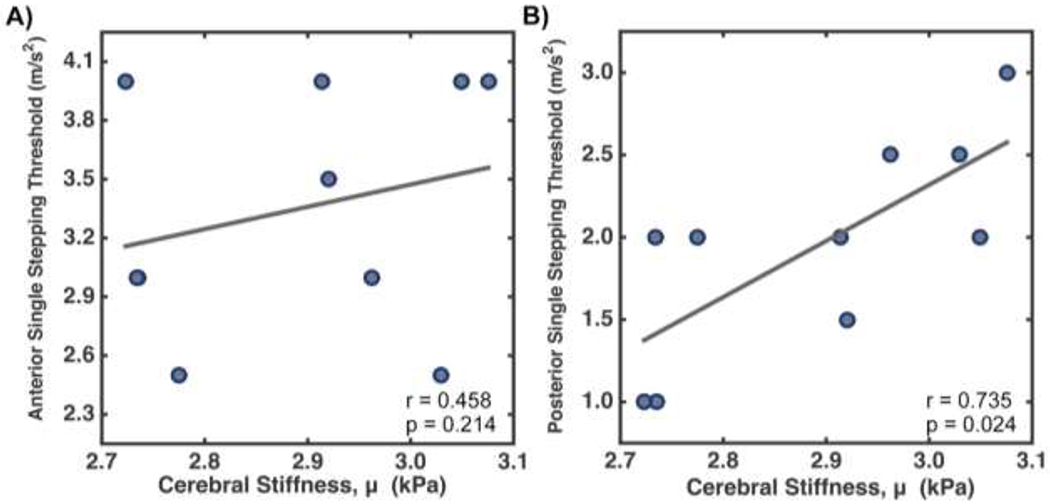
Spearman correlations between cerebral stiffness and (A) anterior and (B) posterior single-step threshold in children with cerebral palsy.
Voxel-wise correlations between stiffness and anterior and posterior SST are shown in Figure 4. Representative slices in MNI standard-space are included with correlation coefficient, rs, represented by color and associated F-statistics represented by opacity. Clusters of voxels with significant correlations (p < 0.05) are fully opaque and outlined in black.
Figure 4:
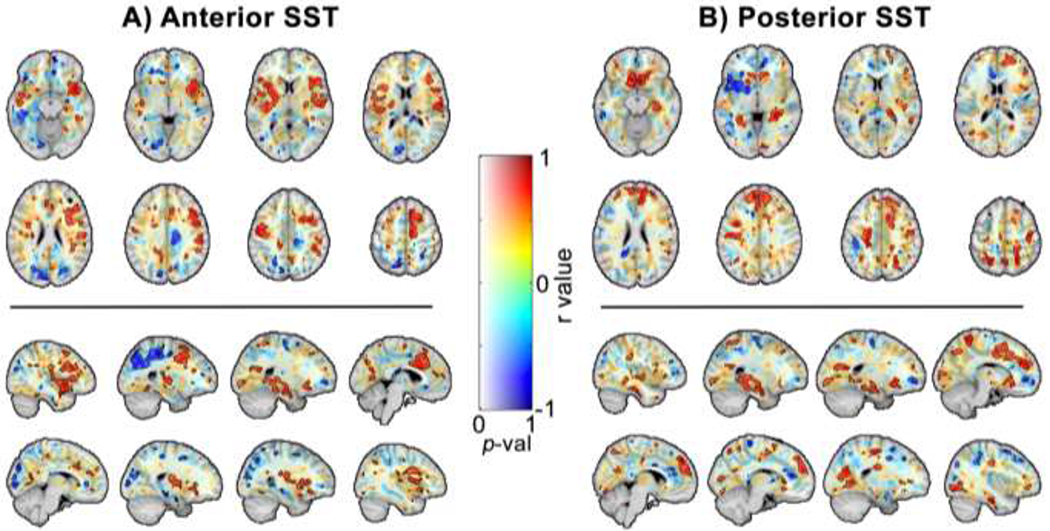
Maps of voxel-wise correlation coefficient, rs, reveals clusters of regions in which brain stiffness significantly correlate with (A) anterior and (B) single-step threshold balance reaction task.
We identified clusters of more than 200 contiguous voxels exhibiting significant correlation between stiffness and anterior or posterior single-stepping threshold (Tables 1 and 2). These clusters exhibited average rs values ranging from −0.78 to 0.78 for anterior SST, as we found both positively and negatively correlated clusters, and 0.73 to 0.79 for posterior SST, as there were only postively correlated clusters of more than 200 voxels. The largest clusters of voxels with stiffness that correlated with anterior SST include the right superior temporal gyrus and the left middle temporal gyrus. The largest clusters that correlated with posterior SST included the right parahippocammpal gyrus, the medial frontal gyrus, and the caudate. Other notable structures are the cuneus and precuneus, where stiffness correlated with anterior SST, and the precentral and postcentral gyrus, which each correlated with both anterior and posterior SST.
Table 1:
Anatomical regions of clusters of voxels exhibiting signficant correlations between stiffness and anterior single-stepping threshold, limited to clusters larger than 200 voxels.
| Cluster | # of Voxels | MNI Coordinates | Anatomical Location |
|---|---|---|---|
| 1 | 4091 | (39,15,−33) | // Right Temporal Lobe // Superior Temporal Gyrus |
| 2 | 1449 | (−63,−55,−4) | // Left Temporal Lobe // Middle Temporal Gyrus |
| 3 | 1359 | (41,−32,31) | // Right Parietal Lobe // Inferior Parietal Lobule |
| 4 | 613 | (47,15,10) | // Right Frontal Lobe // Precentral Gyrus |
| 5 | 550 | (−22,−82,22) | // Left Occipital Lobe // Cuneus |
| 6 | 307 | (−2,−28,80) | // Left Parietal Lobe // Postcentral Gyrus |
| 7 | 245 | (23,−82,42) | // Right Parietal Lobe // Precuneus |
Table 2:
Anatomical regions of clusters of voxels exhibiting signficant correlations between stiffness and posterior single-stepping threshold, limited to clusters larger than 200 voxels.
| Cluster | # of Voxels | MNI Coordinates | Anatomical Location |
|---|---|---|---|
| 1 | 1181 | (28,−22,−13) | // Right Limbic Lobe // Parahippocampal Gyrus |
| 2 | 1083 | (−1,52,30) | // Left Frontal Lobe // Medial Frontal Gyrus |
| 3 | 615 | (−34,−68,−2) | // Left Occipital Lobe // Sub-Gyral |
| 4 | 331 | (11,18,−5) | // Right Sub-lobar // Basal Ganglia // Caudate |
| 5 | 313 | (−24,−67,19) | // Left Temporal Lobe // Sub-Gyral |
| 6 | 284 | (−24,−14,54) | // Left Frontal Lobe // Precentral Gyrus |
| 7 | 256 | (−50,−43,45) | // Left Parietal Lobe // Inferior Parietal Lobule |
| 8 | 256 | (12,−47,65) | // Right Parietal Lobe // Postcentral Gyrus |
4. Discussion
We observed, for the first time, a significant relationship between brain stiffness as measured with MRE and dynamic balance reactions. In general, a stiffer brain was related to better balance, as seen through both global and voxel-wise stiffness, where we expected significant relationships with known motor regions in the brain. Our study identified twelve distinct regions of 200 or more contiguous voxels where brain stiffness correlates with balance in children with CP; ten of these regions have been previously identified as important for balance and locomotion12,13. It is notable that this was a highly functional group of children with cerebral palsy – all were GMFCS level 1 or 2. Children at this low level of impairment may frequently have little or no discernable motor impairment while doing daily activities. However, we have used MRE previously to observe significant differences in brain stiffness between the same group of children with CP at GMFCS level 1 and 2 compared with TD children31, indicating sensitivity of stiffness to brain health in this population, and significant correlations between brain stiffness and balance in children with CP are again reported here.
The two largest clusters where stiffness correlated with anterior single-stepping threshold were centered on the right superior temporal gyrus and the left middle temporal gyrus, though these clusters are large and span 4091 voxels and 1449 2 mm isotropic voxels, respectively. The superior and middle temporal gyri have been cited as sensory processing centers that are engaged during active balance conditions52,53. Notably these regions were shown to be activated during balance simulation in a previous fMRI study54, and are key structures for multisensory integration particularly between the visual and vestibular feedback systems54. Brain stiffness is a measure of structural integrity of the tissue, including myelin content and glial matrix composition, and the stiffness of the superior and middle temporal gyri may reflect the health of tissue in these regions that influences dynamic balance performance.
The largest cluster where stiffness correlated with posterior SST is centered on the parahippocampal gyrus, which plays a role in encoding and recognition of environmental situations55 and is located on the direct locomotion pathway13. The caudate, another region where stiffness was associated with posterior SST, is part of the basal ganglia and contributes to speed of adaptability and control of balance responses56. Interestingly, caudate deficiencies have been theorized to play a significant role in severity of motor function in people with Parkinson’s disease57. People with Parkinson’s disease and with CP show many similar locomotive functional and physiological symptoms, such as balance impairment and decreased coordination58. Damage to the basal ganglia is a defining feature of dyskinetic CP59; while our participants were classified as having spastic CP, it is likely that secondary damage to the basal ganglia, observed by MRE, is partially responsible for impaired balance.
Interestingly, there were very few regions where stiffness correlated with both anterior and posterior SST. This disparity between perturbation directions may be due to fundamentally different response-loop mechanisms of balance recovery depending on perturbation direction and how motor, sensory, and visual responses are engaged60, and may further be differentiated in this population due to the asymmetrical effects cerebral palsy has on the brain. Brain regions are either part of the longer-latency response or indirectly modify shorter-latency responses; the posterior SST reaction shares a common pathway with the startle response, while the anterior reaction does not, therefore, it is not surprising that the two stepping thresholds directions are associated with different areas of the brain61. It may be that, given different characteristics of the base of support, joint range of motion, and force-producing capabilities of the agonists and antagonists, compensation for neural impairment is more available in response to an anterior perturbation compared to that of the posterior perturbation response. Specifically, the anterior response benefits from larger plantar flexors relative to the dorsiflexors, a larger base of support relative to the ankle joint, and more hip flexion range of motion than hip extension62. Therefore, posterior thresholds may be more influenced by the rapid, startle-like response, while anterior thresholds are influenced by longer-latency responses characterized by multi-joint coordination.
Two regions where stiffness was related to both anterior and posterior SST are the precentral and postcentral gyrus, which are the sites of the primary motor cortex and somatosensory cortex, and are thought to control voluntary movements of skeletal muscles63–65. Other studies examining the neural correlates of balance reactions have found relationships with functional activity in this region observed with both fMRI54,66 and fNIRS11. In this study, we found a similar relationship with brain stiffness, which is instead a measure of brain tissue microstructure, and changes in this measure likely reflect damage that affects balance function. Indeed, in this population we previously observed differences in the stiffness of the precentral gyrus between CP and TD children31.
The cuneus and precuneus each reside at the center of clusters associated with anterior SST. Interestingly, these are two regions where stiffness is inversely related to balance performance. These regions have been reported as involved in motor imagery and motor coordination by directing attention in space when an individual moves67–69. It has been suggested that children with CP have a difficult time using proprioception for locomotion as compared to TD children, and thus people with CP may rely more on visual feedback for balance recovery70,71. The compensation of increased need for visual feedback as compared with proprioceptive feedback is consistent with the observed inverse relation between cuneus and precuneus stiffness and balance reactions.
This study has several limitations. First, the measurements were limited to a cross-sectional assessment of a CP population of only ten participants. Future work with longitudinal measures along development in a larger population, as well as stiffness and balance measurements before and after training interventions, would allow for more conclusive identification of the structure-function relationships observed in this work. Additionally, balance reactions are only a small part of a comprehensive assessment of balance, and potentially provide only a limited understanding of the neural structures supporting balance performance in CP. However, given the short duration of the task, it is less likely that children are able to compensate for motor impairment during this task, thus potentially improving the strong structure-function relationships we have observed. Alternative balance tasks may provide a less sensitive assessment of function in this population, altering the link between function and MRE findings. The MRE spatial resolution used in this study, 2.5 mm isotropic, was chosen to minimize scan time and reduce the potential for motion errors in this potentially uncooperative population. While this resolution is comparable to other MRE studies, it potentially limits the ability to analyze small anatomical structures and better localize the brain regions implicated in impaired balance. Finally, although the effects of CSF contamination into the tissue region were reduced, they were not entirely eliminated, and thus may still be contaminating the results. MRE imaging and inversion approaches with higher spatial resolution should be considered for use in future studies.
5. Conclusion
Brain mechanical properties, as measured through MRE, have emerged as sensitive indicators of structural health and relationships with function. Here we find that brain stiffness in children with CP is related to dynamic balance performance measured through the single-stepping threshold balance recovery task. We have found that greater brain stiffness is indicative of a higher balance recovery threshold in many primary locomotion regions. We have identified the brain regions where this correlation is the strongest, meaning greater microstructural brain health in these regions related to superior balance recovery. We have also identified smaller areas, primarily in regions responsible for visual feedback in balance, where the relationship between brain stiffness and balance is inversely related. We expect that this is a compensation mechanism, as children with CP are particularly poor at using proprioceptive feedback and therefore must rely on an additional source of information, such as visual feedback. Understanding relationships between brain health and balance performance may allow for the development of neurorehabilitation strategies targeting improvement in motor function in children with CP.
Acknowledgements
This work is supported by the Delaware INBRE program with a grant from the National Institutes of General Medical Sciences (P20-GM103446) from the National Institutes of Health and the State of Delaware.
Footnotes
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical statement
This research was conducted in accordance with The Code of Ethics of the World Medical Association. This study was approved by the University of Delaware Institutional Review Board and written informed consent was obtained from all persons before participation.
References
- 1.Burtner PA, Qualls C, Woollacott MH. Muscle activation characteristics of stance balance control in children with spastic cerebral palsy. Gait Posture. 1998;8:163–174. [DOI] [PubMed] [Google Scholar]
- 2.O’Shea TM. Diagnosis, Treatment, and Prevention of Cerebral Palsy in Near- Term/Term Infants. Clin Obstet Gynecol. 2011;51(4):816–828. doi: 10.1097/GRF.0b013e3181870ba7.Diagnosis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shumway-Cook A, Hutchinson S, Kartin D, Price R, Woollacott M. Effect of balance training on recovery of stability in children with cerebral palsy. Dev Med Child Neurol. 2010;45(9):591–602. doi: 10.1111/j.1469-8749.2003.tb00963.x [DOI] [PubMed] [Google Scholar]
- 4.Carlberg EB, Mijna H- A. Postural dysfunction in children with cerebral palsy: Some implications for therapeutic guidance. Neural Plast. 2005;12(2–3):221–228. http://www.hindawi.com/journals/np/%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed7&NEWS=N&AN=2005362074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler PB. A preliminary report on the effectiveness of trunk targeting in achieving independent sitting balance in children with cerebral palsy. Clin Rehabil. 1998;12(4):281–293. http://ezproxy.bangor.ac.uk/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=c8h&AN=107171190&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 6.Woollacott MH, Burtner P. Neural and musculoskeletal contributions to the development of stance balance control in typical children and in children with cerebral palsy. Acta Paediatr. 1996;416(Oct):58–62. http://www.ncbi.nlm.nih.gov/pubmed/8997450. [DOI] [PubMed] [Google Scholar]
- 7.Aisen ML, Kerkovich D, Mast J, et al. Cerebral palsy: Clinical care and neurological rehabilitation. Lancet Neurol. 2011;10(9):844–852. doi: 10.1016/S1474-4422(11)70176-4 [DOI] [PubMed] [Google Scholar]
- 8.Dewar R, Love S, Johnston LM. Exercise interventions improve postural control in children with cerebral palsy: A systematic review. Dev Med Child Neurol. 2014;57(6):504–520. doi: 10.1111/dmcn.12660 [DOI] [PubMed] [Google Scholar]
- 9.Tatla SK, Sauve K, Virji-Babul N, Holsti L, Butler C, Van Der Loos HFM. Evidence for outcomes of motivational rehabilitation interventions for children and adolescents with cerebral palsy: An american academy for cerebral palsy and developmental medicine systematic review. Dev Med Child Neurol. 2013;55(7):593–601. doi: 10.1111/dmcn.12147 [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0.Cortical [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihara M, Miyai I, Hattori N, Hatakenaka M. Cortical control of postural balance in patients with hemiplegic stroke. Mot Syst. 2012;23:314–319. doi: 10.1097/WNR.0b013e328351757b [DOI] [PubMed] [Google Scholar]
- 12.Wittenberg E, Thompson J, Nam CS, Franz JR. Neuroimaging of Human Balance Control: A Systematic Review. Front Hum Neurosci. 2017;11(April):1–25. doi: 10.3389/fnhum.2017.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.la Fougère C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50(4):1589–1598. doi: 10.1016/j.neuroimage.2009.12.060 [DOI] [PubMed] [Google Scholar]
- 14.Himmelmann K, Horber V, De La Cruz J, et al. MRI classification system (MRICS) for children with cerebral palsy: development, reliability, and recommendations. Dev Med Child Neurol. 2017;59(1):57–64. doi: 10.1111/dmcn.13166 [DOI] [PubMed] [Google Scholar]
- 15.Robinson MN, Peake LJ, Ditchfield MR, Reid SM, Lanigan A, Reddihough DS. Magnetic resonance imaging findings in a population-based cohort of children with cerebral palsy. Dev Cogn Neurosci. 2009;51(1):39–45. doi: 10.1111/j.1469-8749.2008.03127.x [DOI] [PubMed] [Google Scholar]
- 16.Thomas B, Eyssen M, Peeters R, et al. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain. 2005;128(11):2562–2577. doi: 10.1093/brain/awh600 [DOI] [PubMed] [Google Scholar]
- 17.Muthupillai AR, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman L. Magnetic Resonance Elastography by Direct Visualization of Propagating Acoustic Strain Waves. Science (80- ). 1995;269(5232):1854–1857. [DOI] [PubMed] [Google Scholar]
- 18.Sack I, Jöhrens K, Würfel J, Braun J. Structure-sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter. 2013;9(24):5672–5680. doi: 10.1039/c3sm50552a [DOI] [Google Scholar]
- 19.Hiscox LV, Johnson CL, Barnhill E, et al. Magnetic resonance elastography (MRE) of the human brain : technique , findings and clinical applications. Phys Med Biol. 2016;61:R401–R437. doi: 10.1088/0031-9155/61/24/R401 [DOI] [PubMed] [Google Scholar]
- 20.Murphy MC, Iii JH, Ehman RL. MR elastography of the brain and its application in neurological diseases. Neuroimage. 2019;187:176–183. doi: 10.1016/j.neuroimage.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy MC, Jones DT, Jack CR, et al. Regional brain stiffness changes across the Alzheimer’s disease spectrum. NeuroImage Clin. 2016;10:283–290. doi: 10.1016/j.nicl.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy MC, Huston J, Jack CR, et al. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. J Magn Reson Imaging. 2011;34(3):494–498. doi: 10.1002/jmri.22707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehlner A, Behrens JR, Streitberger K, et al. Higher-Resolution MR Elastography Reveals Early Mechanical Signatures of Neuroinflammation in Patients with Clinically Isolated Syndrome. J Magn Reson Imaging. 2016;44:51–58. doi: 10.1002/jmri.25129 [DOI] [PubMed] [Google Scholar]
- 24.Streitberger KJ, Sack I, Krefting D, et al. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS One. 2012;7(1):e29888. doi: 10.1371/journal.pone.0029888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuerfel J, Paul F, Beierbach B, et al. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage. 2010;49(3):2520–2525. doi: 10.1016/j.neuroimage.2009.06.018 [DOI] [PubMed] [Google Scholar]
- 26.Lipp A, Trbojevic R, Paul F, et al. Clinical Cerebral magnetic resonance elastography in supranuclear palsy and idiopathic Parkinson’s disease. NeuroImage Clin. 2013;3:381–387. doi: 10.1016/j.nicl.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipp A, Skowronek C, Fehlner A, Streitberger K- J, Braun J, Sack I. Progressive supranuclear palsy and idiopathic Parkinson’s disease are associated with local reduction of in vivo brain viscoelasticity. Eur Radiol. 2018;28(8):3347–3354. doi: 10.1007/s00330-017-5269-y [DOI] [PubMed] [Google Scholar]
- 28.Johnson CL, Telzer EH. Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain. Dev Cogn Neurosci. 2018;33:176–181. doi: 10.1016/j.dcn.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIlvain G, Schwarb H, Cohen NJ, Telzer EH, Johnson CL. Mechanical properties of the in vivo adolescent human brain. Dev Cogn Neurosci. 2018;34(June):27–33. doi: 10.1016/j.dcn.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung J, Jugé L, Hatt A, Bilston LE. Paediatric brain tissue properties measured with magnetic resonance elastography. Biomech Model Mechanobiol. 2019;(0123456789). doi: 10.1007/s10237-019-01157-x [DOI] [PubMed] [Google Scholar]
- 31.Chaze CA, McIlvain G, Smith DR, et al. Altered brain tissue stiffness in pediatric cerebral palsy measured by magnetic resonance elastography. NeuroImage Clin. 2019;22. doi: 10.1016/j.nicl.2019.101750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nashner LM, Shumway-Cook A, Marin O. Stance posture control in select groups of children with cerebral palsy: Deficits in sensory organization and muscular coordination. Exp Brain Res. 1983;49(3):393–409. doi: 10.1007/BF00238781 [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Woollacott MH. Lower extremity kinetics for balance control in children with cerebral palsy. J Mot Behav. 2007;39(4):306–316. doi: 10.3200/JMBR.39.4.306-316 [DOI] [PubMed] [Google Scholar]
- 34.Roncesvalles MN, Woollacott MH, Burtner PA. Neural factors underlying reduced postural adaptability in children with cerebral palsy. Neuroreport. 2002;13:2407–2410. doi: 10.1097/00001756-200212200-00006 [DOI] [PubMed] [Google Scholar]
- 35.Woollacott MH, Shumway-cook A. Postural Dysfunction During Standing and Walking in Children with Cerebral Palsy : What Are the Underlying Problems and What New Therapies Might Improve Balance ? Neural Plast. 2005;12(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burtner PA, Woollacott MH, Craft GL, et al. The capacity to adapt to changing balance threats : A comparison of children with cerebral palsy and typically developing children. Dev Neurorehabil. 2007;8423:249–260. doi: 10.1080/17518420701303066 [DOI] [PubMed] [Google Scholar]
- 37.Crenshaw JR, Kaufman KR. The intra-rater reliability and agreement of compensatory stepping thresholds of healthy subjects. Gait Posture. 2014;39(2):810–815. doi: 10.1016/j.gaitpost.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson CL, Schwarb H, Horecka KM, et al. Double dissociation of structure-function relationships in memory and fluid intelligence observed with magnetic resonance elastography. Neuroimage. 2018;171(December 2017):99–106. doi: 10.1016/j.neuroimage.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarb H, Johnson CL, Daugherty AM, et al. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage. 2017;153(December 2016):179–188. doi: 10.1016/j.neuroimage.2017.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarb H, Johnson CL, McGarry MDJ, Cohen NJ. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage. 2016;132:534–541. doi: 10.1016/j.neuroimage.2016.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiscox L, Johnson CL, McGarry MDJ, et al. Hippocampal viscoelasticity and episodic memory performance in healthy older adults examined with magnetic resonance elastography. Brain Imaging Behav. 2018. doi: 10.1007/s11682-018-9988-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGarry MDJ, Houten EEW Van, Johnson CL, et al. Multiresolution MR elastography using nonlinear inversion. Med Phys. 2012;39(6388):6388–6396. doi: 10.1118/1.4754649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5(4):237–254. doi: 10.1016/S1361-8415(00)00039-6 [DOI] [PubMed] [Google Scholar]
- 44.McGarry MDJ, Van Houten EEW. Use of a Rayleigh damping model in elastography. Med Biol Eng Comput. 2008;46(8):759–766. doi: 10.1007/s11517-008-0356-5 [DOI] [PubMed] [Google Scholar]
- 45.Petersen D, Conner B, Pigman J, et al. Anteroposterior stepping thresholds in children with and without cerebral palsy. In: ; 2018. https://app.oxfordabstracts.com/stages/123/programme-builder/submission/20503?backHref=/events/123/sessions/622&view=published. [Google Scholar]
- 46.Andersson JLR, Smith SM, Jenkinson M. FNIRT - FMRIB; non-linear image registration tool. Fourteenth Annu Meet Organ Hum Brain Mapping, Melbourne, Aust. 2008:496. [Google Scholar]
- 47.Jenkinson M, Beckmann CF, Behrens TEJJ, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- 49.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- 50.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;Series B(1):289–300. [Google Scholar]
- 51.Solamen LM, Mcgarry MD, Tan L, Weaver JB, Paulsen KD. Phantom evaluations of nonlinear inversion MR elastography. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouchi Y, Okada H, Yoshikawa E, Nobezawa S, Futatsubashi M. Brain activation during maintenance of standing postures in humans. Brain. 1999;122(2):329–338. doi: 10.1093/brain/122.2.329 [DOI] [PubMed] [Google Scholar]
- 53.Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2012;33(6):1073–1084. doi: 10.1016/j.neurobiolaging.2010.09.022 [DOI] [PubMed] [Google Scholar]
- 54.Karim HT, Sparto PJ, Aizenstein HJ, et al. Functional MR imaging of a simulated balance task. Brain Res. 2014;1555:20–27. doi: 10.1016/j.brainres.2014.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. [DOI] [PubMed] [Google Scholar]
- 56.Visser JE, Bloem BR. Role of the basal ganglia in balance control. Neural Plast. 2005;12(2–3):161–174. doi: 10.1155/NP.2005.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broussolle E, Dentresangle C, Landais P, et al. The relation of putamen and caudate nucleus 18F-Dopa uptake to motor and cognitive performances in Parkinson’s disease. J Neurol Sci. 1999;166(2):141–151. doi: 10.1016/S0022-510X(99)00127-6 [DOI] [PubMed] [Google Scholar]
- 58.Livneh H Review of Research on Psychosocial Adaptation to Neuromuscular Disorders: I. Cerebral Palsy, Muscular Dystrophy, and Parkinson’s Disease. J Soc Behav Pers. 1994;9(5):201. [Google Scholar]
- 59.Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: A systematic review. Dev Med Child Neurol. 2007;49(2):144–151. doi: 10.1111/j.1469-8749.2007.00144.x [DOI] [PubMed] [Google Scholar]
- 60.Nonnekes J, Scotti A, Oude Nijhuis LB, et al. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience. 2013;245:109–120. doi: 10.1016/j.neuroscience.2013.04.036 [DOI] [PubMed] [Google Scholar]
- 61.Jacobs JV, Horak FB. Cortical control of postrual response. J Neural Transm. 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0.Cortical [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Runge CF, Shupert CL, Horak FB, Zajac FE. Ankle and hip postural strategies defined by joint torques. Gait Posture. 1999;10:161–170. [DOI] [PubMed] [Google Scholar]
- 63.Hauk O, Johnsrude I, Pulvermu F. Somatotopic Representation of Action Words in Human Motor and Premotor Cortex. Neuron. 2004;41:301–307. [DOI] [PubMed] [Google Scholar]
- 64.Lotze M, Halsband U. Motor imagery. J Physiol Paris. 2006;99(4–6):386–395. doi: 10.1016/j.jphysparis.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 65.Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86(5):2125–2143. doi: 10.1152/jn.2001.86.5.2125 [DOI] [PubMed] [Google Scholar]
- 66.Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron J. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task ? An fMRI study. Neuroimage. 2006;32:1250–1256. doi: 10.1016/j.neuroimage.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 67.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- 68.Kawashima R, Roland P, O’Sullivan B. Functional anatomy of reaching and visuomotor learning: a positron emission tomography study. Cereb Cortex. 1995;Mar/April:111–122. papers2://publication/uuid/2BB5ED45-09D8-4CFF-BFC3-897D28C02A0B. [DOI] [PubMed] [Google Scholar]
- 69.Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci. 2005;22(1):235–246. doi: 10.1111/j.1460-9568.2005.04176.x [DOI] [PubMed] [Google Scholar]
- 70.Goble DJ, Hurvitz EA, Brown SH. Deficits in the ability to use proprioceptive feedback in children with hemiplegic cerebral palsy. Int J Rehabil Res. 2009;32(3):267–269. doi: 10.1097/MRR.0b013e32832a62d5 [DOI] [PubMed] [Google Scholar]
- 71.Wann JP. The integrity of visual-proprioceptive mapping in cerebral palsy. Neuropsychologia. 1991;29(11):1095–1106. doi: 10.1016/0028-3932(91)90079-N [DOI] [PubMed] [Google Scholar]


