Abstract
Background
Pulmonary embolism (PE)-related mortality is decreasing in Europe. However, time trends in the USA and Canada remain uncertain because the most recent analyses of PE-related mortality were published in the early 2000s.
Methods
For this retrospective epidemiological study, we accessed medically certified vital registration data from the WHO Mortality Database (USA and Canada, 2000–17) and the Multiple Cause of Death database produced by the Division of Vital Statistics of the US Centers for Disease Control and Prevention (CDC; US, 2000–18). We investigated contemporary time trends in PE-related mortality in the USA and Canada and the prevalence of conditions contributing to PE-related mortality reported on the death certificates. We also estimated PE-related mortality by age group and sex. A subgroup analysis by race was performed for the USA.
Findings
In the USA, the age-standardised annual mortality rate (PE as the underlying cause) decreased from 6·0 deaths per 100 000 population (95% CI 5·9–6·1) in 2000 to 4·4 deaths per 100 000 population (4·3–4·5) in 2006. Thereafter, it continued to decrease to 4·1 deaths per 100 000 population (4·0–4·2) in women in 2017 and plateaued at 4·5 deaths per 100 000 population (4·4–4·7) in men in 2017. Among adults aged 25–64 years, it increased after 2006. The median age at death from PE decreased from 73 years to 68 years (2000–18). The prevalence of cancer, respiratory diseases, and infections as a contributing cause of PE-related death increased in all age categories from 2000 to 2018. The annual age-standardised PE-related mortality was consistently higher by up to 50% in Black individuals than in White individuals; these rates were approximately 50% higher in White individuals than in those of other races. In Canada, the annual age-standardised mortality rate from PE as the underlying cause of death decreased from 4·7 deaths per 100 000 population (4·4–5·0) in 2000 to 2·6 deaths per 100 000 population (2·4–2·8) in 2017; this decline slowed after 2006 across age groups and sexes.
Interpretation
After 2006, the initially decreasing PE-related mortality rates in North America progressively reached a plateau in Canada, while a rebound increase was observed among young and middle-aged adults in the USA. These findings parallel recent upward trends in mortality from other cardiovascular diseases and might reflect increasing inequalities in the exposure to risk factors and access to health care.
Funding
None.
Introduction
The annual incidence of acute pulmonary embolism (PE), estimated at 115 cases per 100 000 people in the USA,1 has been increasing globally over the past decades because of a longer life expectancy and an increase in sensitive diagnostic tests.2, 3, 4, 5 Nevertheless, global awareness of PE and deep vein thrombosis remains deficient.6
PE can be fatal, in particular if it leads to right ventricular failure and haemodynamic instability.7, 8 In analyses of global mortality, PE is not yet recognised as a separate clinical entity and a potential underlying cause of death,1, 9 although at least half of cases present in the absence of a provoking factor.10, 11, 12 Understanding temporal trends in PE-related mortality is necessary to implement preventive and management strategies and assess their utility. Furthermore, PE has been identified as a major clinical challenge since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, possibly contributing to COVID-19-related morbidity.13, 14, 15, 16, 17 Therefore, the relevance of acute PE in terms of disease burden will rise even further in the coming years.
In Europe, PE-related mortality declined from 2000 to 2015,18 as did other acute cardiovascular diseases.19, 20 In the USA, the latest analysis on PE-related mortality covered the years 1979–98,21 and the only recent information available is on hospitalisation-related case fatality rates.22 Data on trends in incidence of venous thromboembolism and case fatality rates are available for the most recent years for only two Canadian regions,23, 24 whereas information on PE-related mortality in Canada has not been reported after 2009.25
Research in context.
Evidence before this study
We searched PubMed, Web of Science, and Google Scholar for epidemiological studies and reviews published in English from Jan 1, 1995, to Jan 1, 2020, using the search terms “mortality”, “deaths”, “fatality”, “pulmonary embolism”, “venous thromboembolism”, “epidemiology”, “systematic review”, “incidence”, “United States”, and “Canada”. Over the past 5 years, the World Thrombosis Day Steering Committee has published a series of systematic and narrative reviews of the literature summarising available data on the global burden of thrombosis. The Global Burden of Diseases, Injuries, and Risk Factors (GBD) study had provided online material to retrieve information on global mortality related to cardiopulmonary diseases.
The latest estimates of time trends in PE-related mortality dated back to the period of 2000–09 in Canada and to 1979–98 in the USA. In both countries, mortality due to PE both as an underlying cause of death and as a contributing condition had slowly declined, paralleling other cardiovascular and cardiometabolic diseases. More recently, however, evidence of substantial breaks in these trends had been accumulating in both countries. Life expectancy had been decreasing from 2011 in the USA and plateaued after 2016 in Canada. Mortality from cardiovascular and cardiometabolic causes had been increasing in young and middle-aged adults in the USA. PE was not included as an independent cause of death in the analyses of the GBD study. This choice was primarily based on doubts on the accuracy of its attribution as cause of death in the absence of autopsy and on whether PE represented an underlying, or rather intermediate, cause of death. However, acute PE can be the primary cause of death in patients with no comorbidities or identifiable risk factors; PE represents a preventable cause of death in some contexts, such as pregnancy, oral contraceptive treatment, or elective surgical procedures.
Added value of this study
Our study, based on medically certified vital registration data from the WHO Mortality Database and the Multiple Cause of Death database from the US Centers for Disease Control and Prevention, provides a comprehensive analysis of the disease burden from PE and time trends in PE-related mortality in Canada and the USA for a period of 19 years. Our results reveal a current age-standardised PE-related mortality close to 4 deaths per 100 000 population per year.
In the USA, between 2000 and 2006, PE-related mortality continued its previous decreasing trend. Thereafter, it plateaued in both sexes but increased among young and middle-aged adults. The prevalence of cancer, respiratory diseases, and infections as a contributing cause of PE-related death increased in all age categories. In Canada, the rate of decrease of PE-related mortality slowed down, with a consistent trend across age groups and sexes. These trends parallel the recent concerning data on mortality from other cardiovascular diseases.
Implications of all the available evidence
Additional research is needed to understand the root causes of the increase in PE-related mortality among young and middle-aged adults in the USA and the plateau in its decline in Canada. The overall decrease might reflect improvements in prophylaxis and treatment of PE or overdiagnosis of non-fatal PE, whereas the differences across age groups might reflect increasing inequities in the exposure to behavioural risk factors and barriers to access to health care among young and middle-aged adults, differences in health-care policies, or a selective decline in autopsy and hence underdiagnosis of PE in patients aged 65 years or older and, particularly, in those aged 80 years or older. PE should be included in estimates of global mortality to support continued efforts to ensure optimal prevention, early detection, and treatment.
In this study, we investigate contemporary time trends in age-standardised PE-related mortality and changes in the prevalence of contributing conditions to PE-related mortality in the USA and Canada.
Methods
Data sources
We examined time trends in PE-related mortality (deaths per 100 000) in the USA and Canada based on the WHO Mortality Database, years 2000–17.26 We additionally described the prevalence of conditions contributing to PE-related mortality in the USA by accessing the data from the US Centers for Disease Control and Prevention (CDC) Multiple Cause of Death database (years 2000–18).27
The WHO Mortality Database contains aggregate mortality data, classified by age and sex, on the underlying causes of death, as reported in the civil registries of individual countries. Medically certified deaths registered at local civil registries are compiled by national authorities in civil registration systems and regularly transmitted to the WHO, together with population data,28 within 12–18 months after the closure of the latest national record.29 Since 1999, the four-digit ICD-10 codes have been used in the USA and Canada to classify and report causes of death. The population estimates reported in the WHO Mortality Database are taken from the UN Population Division.
The Multiple Cause of Death database contains information from death certificates of all US residents. It also includes demographic, geographical, and diagnostic data such as age, sex, race, Hispanic origin, urbanisation category, county, place of death, and whether an autopsy was performed. For each death, up to 20 causes or conditions are listed as reported by physicians (“entity axis” field). Of these causes, only one, the condition that directly led to death, is chosen as underlying cause; all others are considered contributing causes. The data of the entity axis are converted to a record axis after processing, translation of codes, elimination of repetitions, and automatic reassignment to best describe the medical certification section of the death certificate and the underlying cause of death. Quality checks are regularly performed by nosologists at the state level and at the CDC National Center for Health Statistics. We used the record axis for the present analysis in line with previous studies21 and the research objectives of the Multiple Cause of Death database.30
In the WHO Mortality Database, we defined PE-related deaths as those in which codes for PE or venous thromboembolism were listed as the underlying cause of death.18, 31 In the Multiple Cause of Death database, we extracted and analysed records listing a PE-related code in any position of the death certificate, namely cited as either underlying cause or contributing cause; we did separate analyses for underlying causes of death. An overview of the codes used to define PE showing agreement between the two databases is provided in the appendix (pp 2–3).
Using the Multiple Cause of Death database, we studied the contribution of other conditions to mortality as underlying or contributing causes in patients with PE in the USA, including cancer, cardiovascular diseases, external causes (eg, trauma, poisoning, and other injuries from environmental causes), haemorrhage, infectious diseases, and respiratory diseases. “Other causes” were defined as all causes of death not included in the above categories. These causes are listed and defined in the appendix (pp 4–6).
For race-specific and ethnicity-specific estimates, we used annual national population totals for sex, age group, race, and Hispanic origin obtained by the US Census Bureau.32, 33 In the USA, race and Hispanic origin are two distinct attributes, and they are reported separately on death certificates as supplied by an informant, usually the next of kin. We used the recoding of primary race in the three categories “White”, “Black”, and “Other” (also including Asians and American Indians)34 for comparison with previous studies.21 We classified “ethnicity” into four categories (“non-Hispanic white”, “non-Hispanic black”, “non-Hispanic other” and “Hispanic”) using a combination of the two attributes for the non-Hispanic population, consistent with previous reports from the US CDC35 and studies in the scientific literature.36 We then calculated the annual crude and age-standardised PE-related mortality rates by race and ethnicity recorded in the certificates of the Multiple Cause of Death database, using the annual national population totals from the US Census Bureau.
Data extraction and validation were performed separately by two independent investigators (LV, SHM), and the final statistical analysis was performed by LV. We followed the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) standards.37 The present study did not require approval by an institutional review board.
Statistical analysis
We calculated crude PE-related mortality rates for individual years by dividing the number of PE-related deaths by the total population (deaths per 100 000 population). We further provided age-standardised PE-related mortality rates based on 5-year age groups using the 2013 European standard population38 to allow comparison with a previous analysis of the European Region.18 We depicted the proportion of PE-related deaths out of total deaths and time trends in age-standardised mortality rates with smoothed lines generated by locally estimated scatterplot smoothing.39 We used Joinpoint regression (Joinpoint, version 4.6.0.0, National Cancer Institute, USA) to identify changes in trends and timepoints with significant inflections and calculated the average annual percentage change with corresponding 95% CIs.40
A recent analysis of life expectancy and overall mortality rates in the USA indicated that all-cause mortality rates steadily declined from 1959 to 2000, reached a nadir in 2010, and increased thereafter, especially among young and middle-aged adults and across all racial groups.36 Therefore, we performed a similar analysis of time trends in age-standardised PE-related mortality for patients according to the following age groups: 25–64 years, 65–79 years, and 80 years or older. We calculated age-standardised mortality rates within each age group using weights from the European standard population.38 R version 3.6.2 (tidyverse, metafor) served for data analysis.41
Role of the funding source
The authors are entirely responsible for the contents of this work. SB, LV, and SHM had access to the raw data. The corresponding author had full access to all the data and the final responsibility to submit for publication.
Results
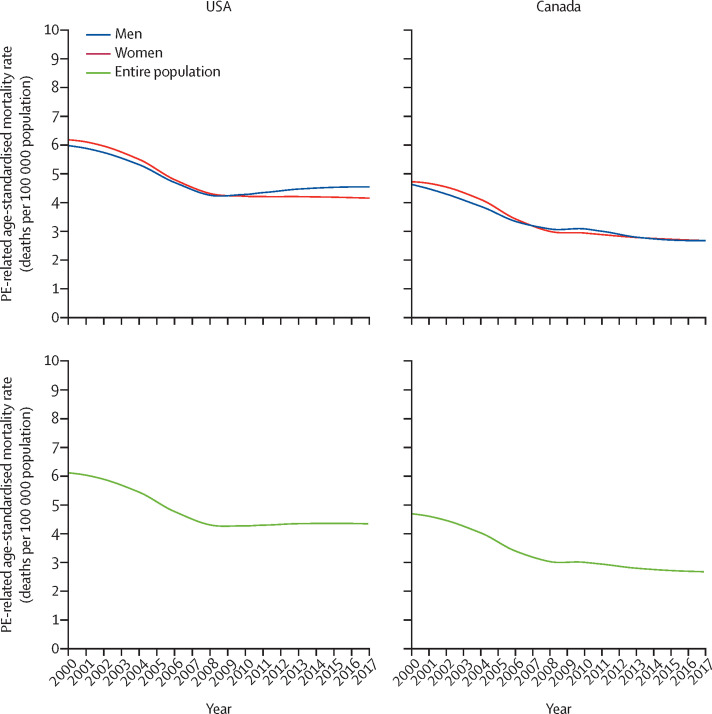
We first looked at the time trends in age-standardised mortality with PE as the underlying cause of death in the USA and Canada using data from the WHO Mortality Database. In the USA, PE was recorded as the underlying cause of death in 11 761 patients in 2000 and in 11 954 patients in 2017. In the setting of a constant population growth (appendix p 7),28 the decrease in age-standardised PE-related mortality rate from 2000 to 2017 was not linear (figure 1 ; appendix pp 8–9). This rate decreased from 6·0 deaths per 100 000 population (95% CI 5·9–6·1) in 2000 to 4·4 deaths per 100 000 population (4·3–4·5) in 2006; thereafter, it slowed down its decrease to 4·1 deaths per 100 000 population (4·0–4·2) in women in 2017, and plateaued at 4·5 deaths per 100 000 population (4·4–4·7) in men in 2017. These data were partly explained by differences in sex-specific trends across age groups (appendix p 17). Between 2000 and 2006, the decrease in PE-related age-standardised mortality was rapid and did not differ between men and women across all age groups. However, after 2006, PE-related age-standardised mortality increased in men (25–64 years and 65–79 years) and in young and middle-aged women (25–64 years), whereas it continued decreasing, but at a slower rate, in older women (80 years or older; appendix, p 17).
Figure 1.
Trends in PE-related age-standardised mortality in women and men in the USA and Canada, 2000–17
Data from the WHO Mortality Database. Locally weighted scatterplot smoother lines were used to depict the annual PE-related age-standardised mortality rate (number of deaths with PE as the underlying cause per 100 000 population). PE=pulmonary embolism.
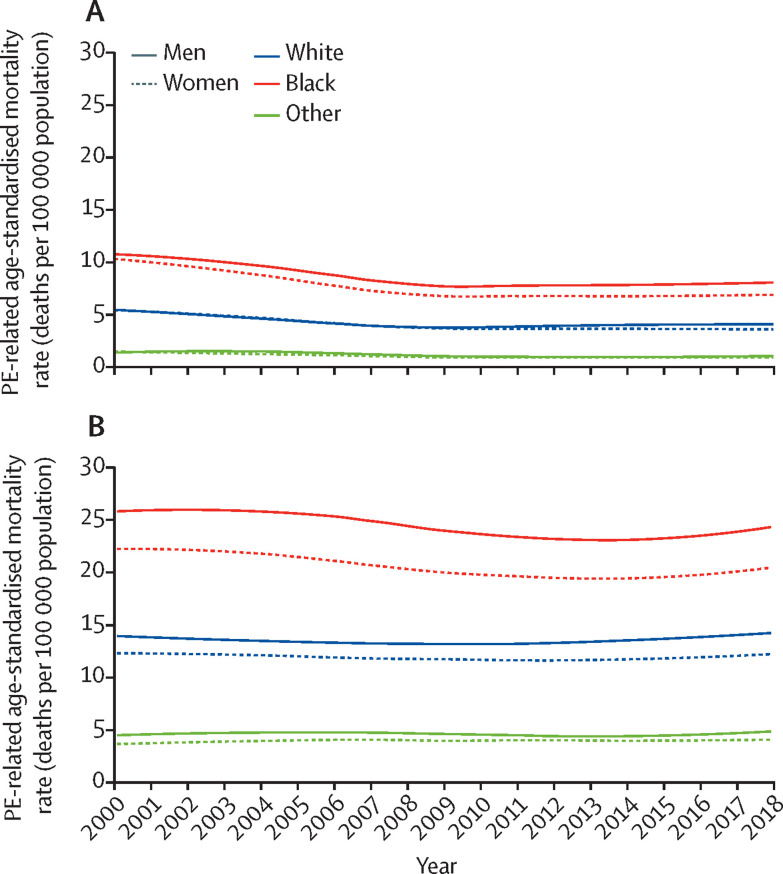
The age-standardised annual mortality rates related to PE, calculated using the Multiple Cause of Death database, were consistently higher (by up to 50%) in Black individuals than in White individuals (figure 2A ). These rates were approximately 50% higher in White individuals than in those recorded as “Other” for primary race category. These findings were independent of the position of PE codes in death certificates (figure 2B) and were consistent with age-standardised mortality rates across ethnicities (appendix p 18).
Figure 2.
Trends in PE-related age-standardised mortality by race and sex in the USA, 2000–18.
Data from the Multiple Cause of Death Database. Locally weighted scatterplot smoother) lines for the age-standardised mortality rate (number of deaths per 100 000 population) related to PE listed as the underlying cause of death (A) or in any position of the death certificate (B). PE=pulmonary embolism.
In Canada, the WHO Mortality Database showed that PE was listed as the underlying cause of death in 955 patients in 2000 and in 863 in 2017, corresponding to an annual age-standardised mortality rate of 4·7 deaths per 100 000 population (95% CI 4·4–5·0) in 2000 and of 2·6 deaths per 100 000 (2·4–2·8) in 2017. The rate of decrease in age-standardised PE-related mortality rate slowed after 2006, in both men and women (figure 1) and across age groups (appendix pp 8–9).
We then looked at the contribution of PE-related deaths to the total mortality, using the WHO Mortality Database. In the USA, PE-related deaths decreased from 4·9 deaths per 1000 total deaths (95% CI 4·8–5·0) in 2000 to 4·2 deaths per 1000 total deaths (4·2–4·3) in 2017. In Canada, PE-related deaths decreased from 4·4 deaths per 1000 total deaths (4·1–4·7) in 2000 to 3·1 deaths per 1000 total deaths (2·9–3·3) in 2017.
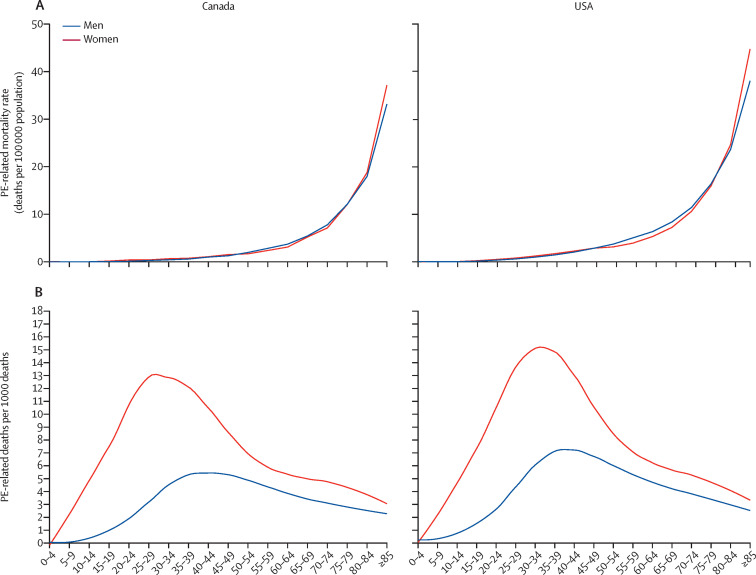
In the setting of the WHO Mortality Database, PE-related mortality was low in childhood and adolescence and increased with age with a seemingly exponential distribution, in line with European data18 (figure 3 ). In both countries, PE was an especially relevant contributor to overall mortality among women aged 20–50 years. Within this group, PE was recorded as the underlying cause of death in up to 16 deaths per 1000 total deaths in the USA and up to 13 deaths per 1000 total deaths in Canada (figure 3; appendix p 19).
Figure 3.
PE-related mortality across age groups and contribution of PE-related mortality to total mortality in the USA and Canada, 2000–17
Data from the WHO Mortality Database. Mortality rate by age group (observed; A) and average annual number of deaths with PE as the underlying cause per 1000 total deaths (proportionate mortality [B]; showed by locally weighted scatterplot smoother lines). PE=pulmonary embolism.
Using the Multiple Cause of Death Database, we calculated the US time trends in age-standardised mortality rate on the basis of number of records with PE listed in any position of the death certificate. Mortality decreased in men from 14·7 deaths per 100 000 population (95% CI 14·4–15·0) in 2000 to 13·4 deaths per 100 000 population (13·2–13·7) in 2009, and it increased again to 14·8 deaths per 100 000 population (14·5–15·0) in 2017 (appendix, p 20). In women, it followed a similar pattern, decreasing from 12·9 deaths per 100 000 population (12·7–13·1) in 2000 to 12·2 deaths per 100 000 population (12·0–12·4) in 2009, and then increasing back to 12·9 deaths per 100 000 population (12·7–13·1) in 2018.
In the year 2000, PE (or limb vein thrombosis with PE) was the underlying cause of death in 11 113 (41·9%) of 26 540 death records with PE codes, decreasing to 11 547 (30·2%) of 38 215 death records in 2018 (appendix pp 10–11). The median age at death decreased from 73 to 71 years for deaths with PE in any position of the death certificates and from 73 to 68 years for deaths with PE as the underlying cause (appendix p 21). The counts of deaths attributed to PE in the Multiple Cause of Death Database matched those reported in the WHO Mortality Database for the USA in the same period.
In the setting of the CDC Multiple Cause of Death database, young and middle-aged adults aged 25–64 years exhibited an increase in PE-related age-standardised mortality rate after 2006. This increase occurred irrespective of whether the codes for PE were listed as the underlying cause of death (alone or preceded by codes for limb vein thrombosis) or in any position of the death certificate (figure 4 ).
Figure 4.
Trends in PE-related age-standardised mortality in the USA, 2000–18
Data from the Multiple Cause of Death Database. Locally weighted scatterplot smoother lines for the age-standardised mortality rate (number of deaths per 100 000 population) related to PE listed as the underlying cause of death or in any position of the death certificate. To account for differences in age distribution within each age group, age-standardised mortality rates were calculated within each class using weights from the European standard population. PE=pulmonary embolism.
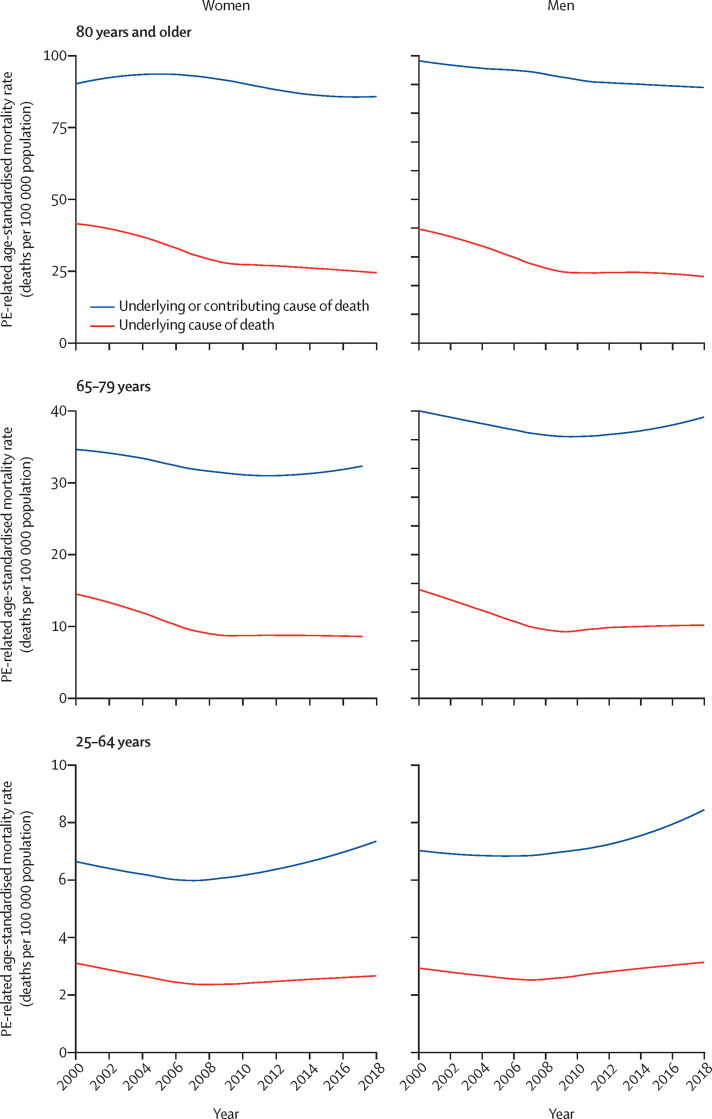
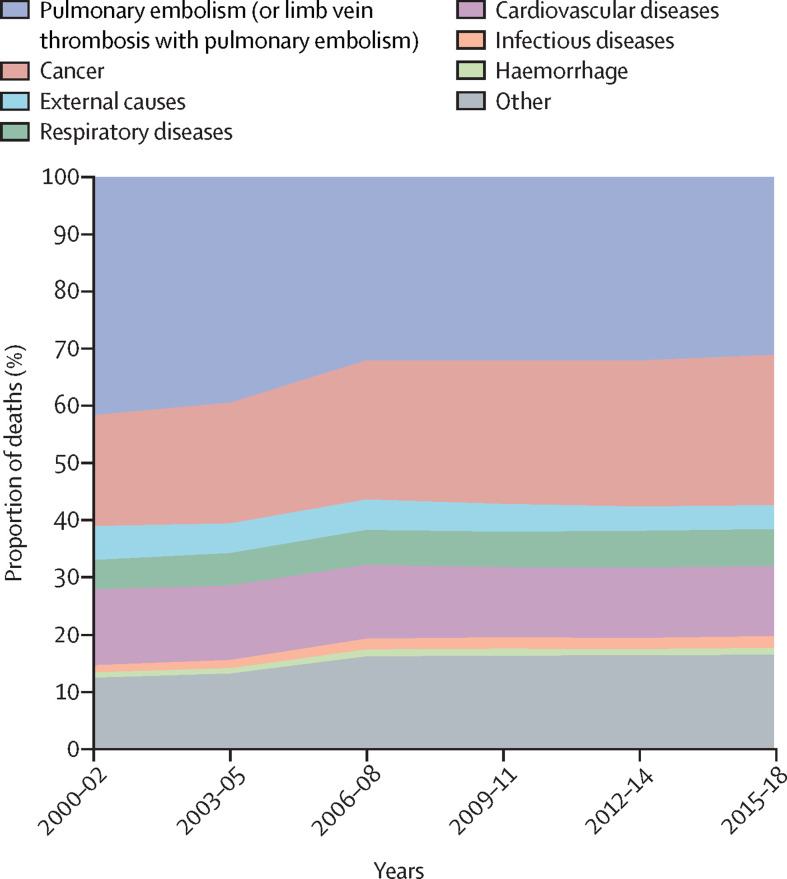
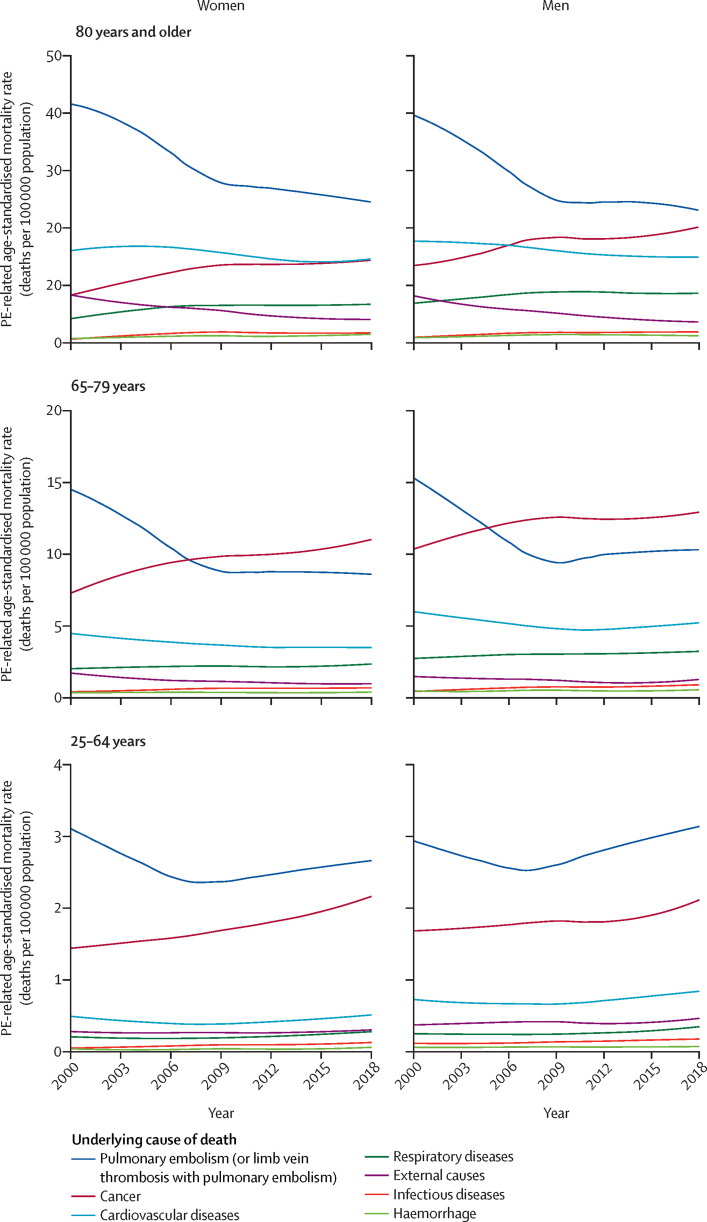
We then looked at the prevalence of other conditions listed as the underlying causes of death in records with PE in the Multiple Cause of Death certificate across 2000–18 (figure 5 ; appendix pp 10–11). Cancer was listed as the underlying cause of death in 4972 (18·7%) of all 26 540 death records with PE codes in 2000, increasing to 10 316 (27·0%) of 38 215 death records in 2018 (appendix pp 10–11). An increase was also observed for respiratory diseases (from 5·1% in 2000 to 6·7% in 2018). Cardiovascular diseases and risk factors were listed as the underlying cause of death in 13·5% of cases in 2000 and in 12·1% of cases in 2018. Codes for venous thrombosis in patients with PE or with limb vein thrombosis with PE were listed as the underlying cause of death in 2498 (22·5%) of 11 113 cases in 2000 and 2426 (21·0%) of 11 547 cases in 2018. The cause-specific age-standardised mortality rate due to several conditions increased between 2000 and 2018 (appendix pp 13–14): for cancer, it increased from 2·57 to 3·70 deaths per 100 000; for respiratory diseases, it increased from 0·73 to 0·96 deaths per 100 000 population; for infectious diseases, it increased from 0·15 to 0·28 deaths per 100 000 population; and for haemorrhage, it increased from 0·14 to 0·17 deaths per 100 000 population (appendix pp 13–14). Cause-specific age-standardised mortality rate due to cardiovascular diseases or external causes decreased between 2000 and 2018 (appendix pp 13–14). Figure 6 depicts age-specific and sex-specific differences in cause-specific age-standardised mortality rate in patients with PE.
Figure 5.
Prevalence of conditions listed as the underlying cause of death on death records of patients with PE in the USA, 2000–18
Data from the Multiple Cause of Death Database. Number of deaths by the underlying cause and proportion of cases among those listing PE codes. PE=pulmonary embolism.
Figure 6.
Trends in age-standardised mortality according to the underlying cause of death on records listing PE among the causes of death in the USA, 2000–18
Data from the Multiple Cause of Death Database. Locally weighted scatterplot smoother lines for the annual age-standardised pulmonary embolism-related mortality rate (number of deaths per 100 000 population). PE=pulmonary embolism.
The appendix (p 12) shows the prevalence of conditions listed in any position of the death certificate of patients with PE. The cause-specific age-standardised mortality rates were increased by accounting for these cause-specific codes listed in any position of the death certificate: for cancer, it increased from 3·27 to 4·10 deaths per 100 000 population; for respiratory diseases, it increased from 3·28 to 4·66 deaths per 100 000 population; for infectious diseases, it increased from 0·68 to 1·20 deaths per 100 000 population; and for haemorrhage from 0·58 to 0·86 deaths per 100 000 population (appendix pp 15–16).
Information on the use of autopsy for the diagnosis of PE in the USA is provided in the appendix (p 22).
Discussion
This study provides information on the mortality due to PE in the USA and Canada, based on official vital registration data from the past two decades. The decreasing trend of PE-related mortality observed in the USA between 2000 and 2006 is of similar magnitude to that recorded between 1979 and 1998, and to recent reports for Europe.18, 21 After 2006, however, PE-related mortality increased in the USA in young and middle-aged adults, whereas the rate of decrease slowed and plateaued among those aged 65 years and older. As a consequence, the median age at death from PE decreased between 2000 and 2018. In contrast, the age-standardised PE-related mortality in Canada decreased from 2000 to 2017; the decline slowed after 2006 across age groups.
In the USA and Canada, the relative contribution of PE to total mortality was higher in younger and middle-aged women, peaking at 1·6% in the USA and 1·3% in Canada. In the USA, several conditions were increasingly reported in association with PE-related deaths over time: cancer alone was listed as the underlying cause of death in 27% of deaths with PE in 2018, whereas PE itself was listed as the underlying cause of death in 23·9% of death certificates that year.
The most recent estimates for age-standardised mortality, with PE as the underlying cause, in the USA and Canada are similar to those of other high-income countries in 2014 and 2015.18, 42 As previously illustrated, mortality estimates are two-to-three times higher if PE codes listed in any position of the death certificate are considered.5, 43 As in previous analyses of vital registration data,18, 21 estimates are lower than indirect calculations and epidemiological models relying on incidence and fatality assumptions accounting for potential PE underdiagnosis.44, 45
Our findings should be read in the context of a decrease in PE-related mortality in the USA across sexes and races, which started in the 1980s and continued until 1998.21 This improvement could be partly explained by advances in the prevention of venous thromboembolism, especially the current focus on prevention of hospital-associated thromboembolism, and the implementation of novel diagnostic pathways and treatment strategies.46, 47 Cohort studies reported a similar improvement in early fatality after PE.3, 5, 22 This downward trend in PE-related mortality is similar to that of cardiovascular and cardiometabolic diseases, which showed a similar rapid decrease until the 2000s, followed by a deceleration over the past few years.48, 49, 50 A similar plateau or deceleration in the decrease of PE-related mortality has been observed in some western European countries during the same time period.18
A reduction of life expectancy in the USA has been reported after 2011, mainly attributed to substance abuse, suicide, and organ system diseases among young and middle-aged adults.36 Canada is also witnessing a decrease in life expectancy after 2016, primarily among younger men and attributed in part to the so-called opioids epidemic.51 In the USA, the increase of PE-related mortality observed among young and middle-aged adults reflects that of overall mortality and appears to be discordant with other high-income countries.36, 52 A similar increase has also been described for other cardiovascular and pulmonary diseases, diabetes, and disability.36 Potential socio-economic causes include exposure to behavioural risk factors,53, 54 social inequalities and barriers to the access to health care,55, 56 and US medical care price inflation.36, 49, 50, 57 However, we cannot exclude the possibility that the decreasing trends in PE-related mortality in the older population are due to the presence of competing causes of death, including other cardiopulmonary diseases or cancer. In this population, these deaths might be occurring at an earlier stage than PE usually occurs or be less successfully prevented (or treated) than PE.
In agreement with previous reports, we showed that trends in PE-related mortality in the USA were consistent across races and ethnicities, but that Black individuals had a higher mortality due to PE than did White individuals and those of other races.21 These data are in agreement also with historical incidence data in the USA demonstrating the highest annual venous thromboembolism rate among Black individuals, followed by other groups in a descending order paralleling that of PE-related mortality.58 Furthermore, recent reports reported similar disparities among Black individuals in terms of all-cause mortality and life expectancy.36
Misclassification and underdiagnosis of PE cases because of decreasing autopsy rates might be partly responsible for the trends observed in our study.59 It is well recognised that sudden death is often arbitrarily classified in patients with an underlying cardiovascular disease or chest pain, and that in several cases PE is diagnosed only at autopsy.60, 61, 62 While the percentage of deaths attributed to PE diagnosed by autopsy remained stable over time, we showed that the autopsy rate in the general US population decreased from 2000 to 2007.59 A selective decline in autopsy and hence underdiagnosis of PE in older patients might partly explain the observed differences in trends across age groups.
PE remains a frequent cause of death in women aged 20–50 years. Younger women are characterised by an overall low mortality and present with specific risk factors for venous thromboembolism, such as pregnancy63 and the use of oestrogen-containing oral agents, such as the combined oral contraceptive pill. In the USA, this peak appeared to extend to older women between 2000 and 2017, possibly reflecting the increase in the mean age of mothers for all birth orders observed during the same period.
The attribution of death to a single identifiable underlying cause is obvious in patients without severe comorbidities. This scenario occurs in a large proportion of patients with unprovoked PE or following autopsy for sudden death. In the USA, approximately a third of the deaths for which a PE code was reported had PE (or limb vein thrombosis associated with PE) listed as the underlying cause of death. This proportion appears to be consistent with data indicating that venous thromboembolism is not associated with recognisable provoking risk factors in half of patients.12 Our estimates of PE-related mortality are in line with epidemiological data demonstrating an incidence rate of 115 cases per 100 000 population1 and an early fatality not exceeding 10%.22
The difficulty in classifying PE as the underlying or contributing cause of death underlines that it is essential to recognise venous thromboembolism as a distinct clinical entity and not only as a (fatal) complication of other diseases.6 The progressive rise of the reporting of cancer and other venous thromboembolism-provoking factors, such as respiratory diseases and infections, and the parallel decline of PE itself among the underlying causes of death in deaths with PE must be interpreted in this context. While this trend might be due to the increasing sensitivity of diagnostic techniques that identify cases of PE of low clinical severity that are unlikely to be fatal,3 it might also reflect an improvement in prophylaxis and treatment of PE.
We acknowledge several limitations. First, death certificates are not always entirely reliable. This issue might be even more relevant for PE-related deaths, for which there is still no unanimously accepted definition and which might be subject to misclassification.31, 64, 65 Considering PE codes listed as a contributing condition might, in contrast, inflate the rates because of the potential overdiagnosis of incidental PE as a result of increased use of high-quality imaging tests.3 We cannot exclude that changes in the ICD version used for death certification have had an impact. However, this change is unlikely to have affected the observed trends in the period we investigated. Whereas the ICD-10-CM/PCS (Clinical Modification and Procedure Coding System) used for morbidity documentation was only introduced in 2015 in the USA,66 the ICD-10 for death certification replaced the ICD-9 from 1999 in the USA and 2000 in Canada with no transition period. All major ICD-10 codes for PE or deep vein thrombosis had a correspondent ICD-9 code with high comparability ratios;67 indeed, a limited impact of the coding switch on reported mortality trends was only observed in the first year of implementation and in diseases with significant changes in coding.68 Moreover, change in coding practices due to the fear of malpractice litigation cannot be excluded. This aspect might reflect geographical heterogeneity in addition to other differences between the USA and Canada in terms of access to health care, policies, and population. Finally, we were unable to examine the influence of highly prevalent underlying risk factors such as obesity (body-mass index >30 kg/m2), which, although it only doubles the risk of venous thromboembolism, is now so prevalent, particularly in the USA population, that it must be considered a major risk factor.69 Recent figures have shown that the prevalence of obesity has increased considerably in the USA from 28·8% in 2000 to 42% in 2020.70
In conclusion, our findings highlight the need for continued efforts to ensure optimal prevention, early detection, and treatment of PE on a population level, as well as within age-specific, sex-specific, and race-specific groups characterised by substantial PE-related mortality. PE should be included in estimates of global mortality to support these efforts and evaluate the effectiveness of interventions.
Data sharing
Statistical source code used to generate estimates can be obtained from the study statistician (Dr. Luca Valerio, luca.valerio@uni-mainz.de). Source data can be downloaded from the WHO website (WHO Mortality data) and the US Centers for Disease Control and Prevention (CDC) website (CDC, National Center for Health Statistics: Mortality Multiple Cause Files).
Acknowledgments
This work was not externally funded. The work of SB, LV, and SVK was supported by the German Federal Ministry of Education and Research (grant numbers BMBF 01EO1003 and 01EO1503).
Contributors
SB contributed to the concept and design of the study, interpretation of the results, writing of the manuscript, and final approval of the Article. LV contributed to the design of the study, statistical analysis, interpretation of the results, critical revision of the manuscript, and writing and final approval of the Article. SHM contributed to statistical analysis, interpretation of the results, critical revision of the manuscript, and final approval. WA, ATC, SZG, BJH, AI, DJ, FAK, NK, SM, TM, VT, AMW, and SVK contributed to the interpretation of the results, critical revision of the manuscript, and final approval of the Article.
Declaration of interests
SB received lecture and consultant fees from Bayer HealthCare, BTG Pharmaceuticals, and LeoPharma; economical support for travel and congress costs from Daiichi Sankyo and Bayer HealthCare; and institutional grants from Sanofi. ATC reports grants and personal fees from Bristol Myers Squibb, Daiichi Sankyo Europe, and Pfizer, and personal fees from AbbVie, Bayer, Boehringer Ingelheim, Janssen, ONO Pharmaceuticals and Portola, outside of the submitted work. FAK reports research grants from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, MSD and Actelion, the Dutch Heart foundation, the Netherlands Organisation for Health Research and Development, and the Dutch Thrombosis association. WA has received grant support from Bayer and honoraria from Bayer, Boehringer Ingelheim, BMS/Pfizer, Daiichi Sankyo, Janssen, Portola, Sanofi, Aspen. SM reports grants and fees paid to her institution from GlaxoSmithKline, BMS/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. SVK reports grants, personal fees and nonfinancial support from Bayer AG; grants and personal fees from Boehringer Ingelheim, Actelion, Daiichy Sankyo, Biocompatibles. SHM, LV, BH, DJ, NK, TM, VT, AMW, and AI declare no competing interests.
Supplementary Material
References
- 1.Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118:1340–1347. doi: 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ., 3rd Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–837. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehnert P, Lange T, Møller CH, Olsen PS, Carlsen J. Acute pulmonary embolism in a national Danish cohort: increasing incidence and decreasing mortality. Thromb Haemost. 2018;118:539–546. doi: 10.1160/TH17-08-0531. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides SV, Barco S, Lankeit M, Meyer G. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016;67:976–990. doi: 10.1016/j.jacc.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 6.Wendelboe AM, McCumber M, Hylek EM, Buller H, Weitz JI, Raskob G. Global public awareness of venous thromboembolism. J Thromb Haemost. 2015;13:1365–1371. doi: 10.1111/jth.13031. [DOI] [PubMed] [Google Scholar]
- 7.Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018;4 doi: 10.1038/nrdp.2018.28. [DOI] [PubMed] [Google Scholar]
- 8.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 9.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3:3898–3944. doi: 10.1182/bloodadvances.2019000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ageno W, Haas S, Weitz JI, et al. Characteristics and management of patients with venous thromboembolism: the GARFIELD-VTE registry. Thromb Haemost. 2019;119:319–327. doi: 10.1055/s-0038-1676611. [DOI] [PubMed] [Google Scholar]
- 13.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 16.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barco S, Mahmoudpour SH, Valerio L, et al. Trends in mortality related to pulmonary embolism in the European Region, 2000–15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med. 2020;8:277–287. doi: 10.1016/S2213-2600(19)30354-6. [DOI] [PubMed] [Google Scholar]
- 19.Shah R, Wilkins E, Nichols M, et al. Epidemiology report: trends in sex-specific cerebrovascular disease mortality in Europe based on WHO mortality data. Eur Heart J. 2019;40:755–764. doi: 10.1093/eurheartj/ehy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37:3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 21.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 22.Bikdeli B, Wang Y, Jimenez D, et al. Pulmonary Embolism Hospitalization, Readmission, and Mortality Rates in US Older Adults, 1999–2015. JAMA. 2019;322:574–576. doi: 10.1001/jama.2019.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular trends in incidence and mortality of acute venous thromboembolism: the AB-VTE population-based study. Am J Med. 2016;129:879.e19–879.e25. doi: 10.1016/j.amjmed.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13–832.e21. doi: 10.1016/j.amjmed.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Laribi S, Aouba A, Resche-Rigon M, et al. Trends in death attributed to myocardial infarction, heart failure and pulmonary embolism in Europe and Canada over the last decade. QJM. 2014;107:813–820. doi: 10.1093/qjmed/hcu083. [DOI] [PubMed] [Google Scholar]
- 26.WHO . World Health Organization; Geneva: 2019. WHO Mortality Database. [Google Scholar]
- 27.CDC . National Center for Health Statistics; Hyattsville, Md: 2019. Mortality Multiple Cause-of-Death Public Use Record. Centers for Disease Control and Prevention. [Google Scholar]
- 28.United Nations. Department of Economic and Social Affairs. Population Division World Population Prospects. 2019. https://population.un.org/wpp/DownloadWPP2019_PopulationByAgeSex_MeWdium.csv
- 29.WHO WHO Mortality Database. About the database: updating and querying. 2019. https://www.who.int/healthinfo/statistics/mortdatabase/en/
- 30.Giuntini C, Di Ricco G, Marini C, Melillo E, Palla A. Pulmonary embolism: epidemiology. Chest. 1995;107(suppl):3S–9S. doi: 10.1378/chest.107.1_supplement.3s. [DOI] [PubMed] [Google Scholar]
- 31.Tritschler T, Kraaijpoel N, Langlois N, et al. Development of a standardized definition of pulmonary embolism-related death: a cross-sectional survey of international thrombosis experts. J Thromb Haemost. 2020;18:1415–1420. doi: 10.1111/jth.14775. [DOI] [PubMed] [Google Scholar]
- 32.US Census Bureau Population Division. Monthly population estimates by age, sex, race, and hispanic origin for the United States: April 1, 2010 to July 1, 2019 (with short-term projections to December 2020) June 2020. https://www.census.gov/data/datasets/time-series/demo/popest/intercensal-2000-2010-national.html
- 33.US Census Bureau Population Division. Intercensal estimates of the resident population by five-year age groups, sex, race, and hispanic origin for the United States: April 1, 2000 to July 1, 2010. September 2011. https://www.census.gov/data/datasets/time-series/demo/popest/intercensal-2000-2010-national.html
- 34.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat. 2003;2:1–55. [PubMed] [Google Scholar]
- 35.Curtin SC, Arias E. National Center for Health Statistics; Hyattsville, MD: 2019. Mortality trends by race and ethnicity among adults aged 25 and over, 2000–2017. NCHS Data Brief, no 342. [PubMed] [Google Scholar]
- 36.Woolf SH, Schoomaker H. Life Expectancy and Mortality Rates in the United States, 1959–2017. JAMA. 2019;322:1996–2016. doi: 10.1001/jama.2019.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 38.Revision of the European Standard Population . Publications Office of the European Union; Luxembourg: 2013. Report of Eurostat's task force. [Google Scholar]
- 39.Ebmeier S, Thayabaran D, Braithwaite I, Bénamara C, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012) Lancet. 2017;390:935–945. doi: 10.1016/S0140-6736(17)31448-4. [DOI] [PubMed] [Google Scholar]
- 40.Douketis JD, Crowther MA, Stanton EB, Ginsberg JS. Elevated cardiac troponin levels in patients with submassive pulmonary embolism. Arch Intern Med. 2002;162:79–81. doi: 10.1001/archinte.162.1.79. [DOI] [PubMed] [Google Scholar]
- 41.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: a language and environment for statistical computing. [Google Scholar]
- 42.Shiraev TP, Omari A, Rushworth RL. Trends in pulmonary embolism morbidity and mortality in Australia. Thromb Res. 2013;132:19–25. doi: 10.1016/j.thromres.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 43.Roberts LN, Whyte MB, Arya R. Pulmonary embolism mortality trends in the European region-too good to be true? Lancet Respir Med. 2020;8:e2. doi: 10.1016/S2213-2600(19)30448-5. [DOI] [PubMed] [Google Scholar]
- 44.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl):S495–S501. doi: 10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Heit JA, Cohen AT, Anderson FA., Jr Group oBotVIA. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910. [Google Scholar]
- 46.Bikdeli B, Monreal M, Jiménez D. Pulmonary embolism in Europe remains a cause of concern despite declining deaths. Lancet Respir Med. 2020;8:222–224. doi: 10.1016/S2213-2600(19)30360-1. [DOI] [PubMed] [Google Scholar]
- 47.Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320:1583–1594. doi: 10.1001/jama.2018.14346. [DOI] [PubMed] [Google Scholar]
- 48.Shah NS, Lloyd-Jones DM, O'Flaherty M, et al. Trends in cardiometabolic mortality in the United States, 1999–2017. JAMA. 2019;322:780–782. doi: 10.1001/jama.2019.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, et al. Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation. 2016;133:967–978. doi: 10.1161/CIRCULATIONAHA.115.019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sidney S, Quesenberry CP, Jr, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–599. doi: 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- 51.Statistics Canada Life tables, Canada, provinces and territories, 2016/2018. Jan 28, 2020. https://www150.statcan.gc.ca/n1/daily-quotidien/200128/dq200128a-eng.htm
- 52.Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: findings from a National Research Council/Institute of Medicine report. JAMA. 2013;309:771–772. doi: 10.1001/jama.2013.91. [DOI] [PubMed] [Google Scholar]
- 53.Fenelon A, Preston SH. Estimating smoking-attributable mortality in the United States. Demography. 2012;49:797–818. doi: 10.1007/s13524-012-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamel E, Pacouret G, Vincentelli D, et al. Thrombolysis or heparin therapy in massive pulmonary embolism with right ventricular dilation: results from a 128-patient monocenter registry. Chest. 2001;120:120–125. doi: 10.1378/chest.120.1.120. [DOI] [PubMed] [Google Scholar]
- 55.Daniel H, Bornstein SS, Kane GC. Addressing social determinants to improve patient care and promote health equity: an American College of Physicians position paper. Ann Intern Med. 2018;168:577–578. doi: 10.7326/M17-2441. [DOI] [PubMed] [Google Scholar]
- 56.Access GBDH, Quality C. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236–2271. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med. 2002;136:691–700. doi: 10.7326/0003-4819-136-9-200205070-00012. [DOI] [PubMed] [Google Scholar]
- 58.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(suppl 1):I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 59.Hoyert DL. The changing profile of autopsied deaths in the United States, 1972–2007. NCHS Data Brief. No. 67. August, 2011. https://www.cdc.gov/nchs/data/databriefs/db67.pdf [PubMed]
- 60.Issa VS, Dinardi LF, Pereira TV, et al. Diagnostic discrepancies in clinical practice: an autopsy study in patients with heart failure. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tejerina E, Esteban A, Fernández-Segoviano P, et al. Clinical diagnoses and autopsy findings: discrepancies in critically ill patients*. Crit Care Med. 2012;40:842–846. doi: 10.1097/CCM.0b013e318236f64f. [DOI] [PubMed] [Google Scholar]
- 62.Marshall HS, Milikowski C. Comparison of clinical diagnoses and autopsy findings: six-year retrospective study. Arch Pathol Lab Med. 2017;141:1262–1266. doi: 10.5858/arpa.2016-0488-OA. [DOI] [PubMed] [Google Scholar]
- 63.Hobohm L, Keller K, Valerio L, et al. Fatality rates and use of systemic thrombolysis in pregnant women with pulmonary embolism. ESC Heart Fail. 2020 doi: 10.1002/ehf2.12775. ehf2.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraaijpoel N, Tritschler T, Guillo E, Girard P, Le Gal G. Definitions, adjudication, and reporting of pulmonary embolism-related death in clinical studies: a systematic review. J Thromb Haemost. 2019;17:1590–1607. doi: 10.1111/jth.14570. [DOI] [PubMed] [Google Scholar]
- 65.Tritschler T, Kraaijpoel N, Girard P, et al. Definition of pulmonary embolism-related death and classification of the cause of death in venous thromboembolism studies: communication from the SSC of the ISTH. J Thromb Haemost. 2020;18:1495–1500. doi: 10.1111/jth.14769. [DOI] [PubMed] [Google Scholar]
- 66.Kusnoor SV, Blasingame MN, Williams AM, DesAutels SJ, Su J, Giuse NB. A narrative review of the impact of the transition to ICD-10 and ICD-10-CM/PCS. JAMIA Open. 2019;3:126–131. doi: 10.1093/jamiaopen/ooz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Public Health Surveillance and Environmental Health Branch Public Health Division Alberta Health & Wellness. ICD-9 to ICD-10 coding with reference to causes of death grouping in Alberta. 2006 Jul.. https://open.alberta.ca/dataset/5bbd50c0-ed15-4dc8-b51f-affa45b82a17
- 68.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- 69.Hunt BJ. Hemostasis at extremes of body weight. Semin Thromb Hemost. 2018;44:632–639. doi: 10.1055/s-0038-1661385. [DOI] [PubMed] [Google Scholar]
- 70.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Statistical source code used to generate estimates can be obtained from the study statistician (Dr. Luca Valerio, luca.valerio@uni-mainz.de). Source data can be downloaded from the WHO website (WHO Mortality data) and the US Centers for Disease Control and Prevention (CDC) website (CDC, National Center for Health Statistics: Mortality Multiple Cause Files).