Abstract
A 62-year-old woman with coronavirus disease 2019 developed acute respiratory failure and cardiogenic shock in the setting of a systemic hyperinflammatory state and apparent ST-elevation myocardial infarction. Cardiac magnetic resonance imaging showed fulminant acute myocarditis with severe left ventricular dysfunction. Treatment with the recombinant interleukin-1 receptor antagonist anakinra and dexamethasone resulted in rapid clinical improvement, reduction in serum inflammatory markers, and a marked recovery in cardiac magnetic resonance–-based markers of inflammation and contractile dysfunction. The patient was subsequently discharged from the hospital. Emerging evidence supports use of anti-inflammatory therapies, including anakinra and dexamethasone, in severe cases of coronavirus disease 2019.
Résumé
Une femme de 62 ans atteinte de la COVID-19 a développé une insuffisance respiratoire aiguë et un choc cardiogène dans le contexte d’un état hyperinflammatoire général et d’un infarctus du myocarde avec élévation du segment ST apparent. L’imagerie par résonance magnétique cardiaque a révélé une myocardite aiguë fulminante accompagnée d’une dysfonction ventriculaire gauche sévère. Le traitement par l’anakinra, un antagoniste des récepteurs de l’interleukine 1 recombinant, et la dexaméthasone, a entraîné une amélioration clinique rapide, une diminution des marqueurs inflammatoires sériques et un rétablissement marqué selon les marqueurs de l’inflammation et de la dysfonction contractile à la résonance magnétique cardiaque. La patiente a par la suite reçu son congé de l’hôpital. De nouvelles données probantes militent en faveur de l’emploi de traitements anti-inflammatoires, comme l’anakinra et la dexaméthasone, dans les cas sévères de COVID-19.
Since the onset of the COVID-19 pandemic in late December 2019 in Wuhan, China, over 35 million cases have been described worldwide, leading to more than 1 million deaths.1 Observational data have shown myocardial injury, as detected by serum marker elevation, to be a strong independent predictor of adverse outcomes. Although several mechanisms of myocardial injury are postulated, acute myocarditis due to cytokine storm–like syndrome is of interest given the potential for it to be mitigated by therapies directly targeting the inflammasome.2 We report a case of fulminant myocarditis in a patient with severe COVID-19 complicated by hyperinflammation successfully treated with the recombinant human interleukin (IL)-1 receptor antagonist anakinra and dexamethasone.
Case
A 62-year-old woman with a prior medical history of primary progressive multiple sclerosis presented with an acute altered level of consciousness, hypoxemia, and shock. One week earlier she had tested positive by nasopharyngeal swab polymerase chain reaction for severe acute respiratory syndrome coronavirus-2. Initial blood pressure was 55/32 mm Hg, with a heart rate of 120 beats/min, respiratory rate of 32 breaths/min, and oxygen saturation of 95% on 100% oxygen by a non-rebreather face mask. A chest radiograph revealed dense airspace opacities in the right lung, with ground-glass opacity in the left lung, and a 12-lead electrocardiogram showed sinus tachycardia with diffuse anterolateral ST-elevation (Supplemental Figure S1). High-sensitivity cardiac Troponin T was 4986 ng/L, and N-terminal pro-B-type natriuretic peptide was 51,439 ng/L. Other results included ferritin 3067 U/L, C-reactive protein 68.2 mg/L, D-dimer 6920 ng/mL, lactate dehydrogenase 1094 U/L, lactate 2.4 mmol/L, and creatinine 252 umol/L. Blood and sputum cultures drawn at admission were negative. The patient was resuscitated with titrated boluses of intravenous fluids and received one dose of intravenous dexamethasone, in addition to a full course of ceftriaxone and azithromycin. Based on advanced directives related to the patient’s underlying multiple sclerosis, no intubation was carried out, and no inotropes were administered.
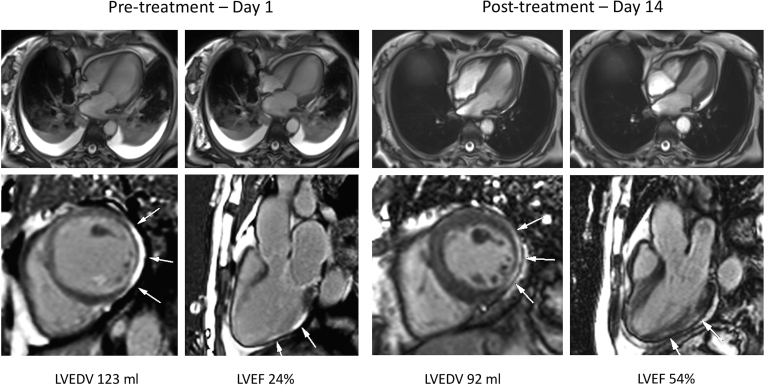
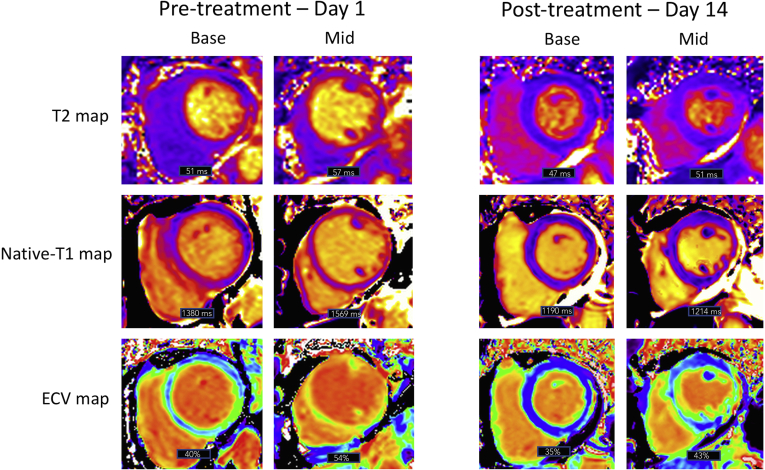
Point-of-care ultrasound demonstrated severe left ventricular dysfunction, with lung ultrasound findings consistent with pneumonia. Urgent cardiac magnetic resonance (CMR) imaging was performed to discriminate between ST-elevation myocardial infarction, sepsis-related cardiomyopathy, Takotsubo cardiomyopathy, and COVID-19 myocarditis. Imaging was performed on high-flow nasal cannular supplemental oxygen (placed below an N95 respiratory mask), leveraging free-breathing techniques as available (ie, multiple averages for cine imaging, single shot techniques for late gadolinium enhancement (LGE) fibrosis imaging). CMR findings were highly consistent with acute myocarditis (Fig. 1). Global hypokinesia with relative sparing of the basal segments was observed. There was extensive sub-epicardial LGE in the anterolateral and inferolateral left ventricular walls accompanied by elevation in tissue mapping-based markers of inflammatory injury. Both native T1 and T2 values were elevated, with marked expansion of the extracellular volume (Fig. 2). The left ventricular ejection fraction was 24%. Based on these findings, anakinra was administered intravenously at a dose of 100 mg twice daily.
Figure 1.
Cardiac magnetic resonance imaging 24 hours post admission and day 14 following treatment with anakinra. Top: Cine imaging presented in 4-chamber view at diastole and systole, demonstrating marked improvement in systolic function and complete resolution of lung consolidation and pleural effusions. Bottom: Late gadolinium enhancement imaging in short-axis and 3-chamber views, illustrating reduction in sub-epicardial injury (white arrows) over the treatment period. LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction.
Figure 2.
Tissue mapping performed 24 hours post admission and day 14 following treatment with anakinra. Imaging performed at 3-Tesla using T2-prep GRE and MOLLI sequences and is shown in basal and mid short-axis views. Mid global T2 value reduced from 57 ms to 51 ms (lab normal range 38-48 ms). Mid global T1 value reduced from 1569 ms to 1214 ms (lab normal range1103-1263 ms). Mid global extracellular volume (ECV) fraction reduced from 54% to 43% (lab normal range 23%-31%).
The patient experienced rapid clinical improvement during the following 72 hours, with reduced oxygen requirements, improved blood pressure, and a reduction in heart rate. Anakinra treatment was continued for 5 days in total. Renal function improved, and a progressive reduction in inflammatory markers was observed (Supplemental Table S1). At this time, the patient was alert, hemodynamically stable, and required only 1 liter of supplemental oxygen. On day 14 (12 days following the initiation of anakinra), repeat CMR imaging demonstrated marked improvement in left ventricular ejection fraction, from 24% to 54%, with a reduction in LGE signal intensity and global reductions in myocardial T1and T2 values and extracellular volume (Figs. 1 and 2). Although no baseline pericardial changes were observed, convalescent imaging demonstrated a small pericardial effusion with enhancement of the parietal pericardium along the basal to mid inferolateral wall (Fig. 1), consistent with pericarditis. Resolution of pulmonary consolidation and pleural effusions was observed. The patient was discharged from the hospital several days later.
Discussion
Myocardial injury is commonly observed in cases of severe COVID-19 and is strongly predictive of adverse outcomes.3 The incidence of myocarditis is unknown, and exceptional cases in which CMR imaging was performed have shown varying degrees of inflammatory myocardial injury.4 A cytokine-storm syndrome, resembling secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome, increasingly is being seen in patients with severe COVID-19.2 Characterized by excess activation of proinflammatory cytokines from both the IL and tumour necrosis factor (TNF) families, cytokine activation may participate in both the initiation and propagation of myocardial injury. Proinflammatory cytokines, including IL-1, IL-6, and TNF-alpha, lead to activation of proinflammatory T-lymphocytes, invoking myocyte injury while provoking secondary cytokine release that further catalyzes the autoinflammatory response. Interrupting this feedback loop through immunomodulatory therapy has become a common therapeutic strategy in the battle against serious COVID-19-related complications.
Immunomodulatory options for the treatment of cytokine-storm syndromes include corticosteroids in addition to bespoke therapies targeting IL-6 inhibition, TNF-alpha antagonism, and IL-1 blockade.2 Of these, anakinra is a recombinant human competitive IL-1 receptor antagonist with favorable safety and tolerance profiles. Initially approved for treatment of rheumatoid arthritis, small studies have suggested it has benefit for the suppression of cardiac inflammation in the setting of immune-mediated pericarditis and myocarditis. Specifically, prior case reports have described its use in fulminant myocarditis in the setting of a systemic hyperinflammatory syndrome.5,6 Based on such promising findings, a randomized clinical trial assessing the use of anakinra in acute myocarditis is currently enrolling participants (Anakinra Versus Placebo for the Treatment of Acute Myocarditis [ARAMIS] trial; https://clinicaltrials.gov/ct2/show/NCT03018834)).
This report represents the first documented case of COVID-19-related fulminant myocarditis successfully treated with anakinra and dexamethasone. The marked clinical improvement observed in this patient complements those benefits recently appreciated for COVID-19-related respiratory failure treated with anakinra, described by 2 observational cohort studies. The Ana-COVID study recruited 52 patients with bilateral COVID-19 pneumonia and hypoxemia, demonstrating markedly lower rates of death or intensive care unit admission compared to 44 historical patients (25% vs 73%).7 A similar intervention tested by Cavalli et al. showed significantly lower mortality (10% vs 56% at 21 days) compared to historic control patients when therapy was initiated in patients not yet requiring mechanical ventilatory support.8 In this exemplary case, and in those collectively summarized by these 2 recent cohort studies, anakinra demonstrated rapid and robust suppression of systemic hyperinflammation and improvement in clinical status. The use of dexamethasone may also have contributed to the favorable outcome in this case. Overall, rapidly emerging evidence supports the use of anti-inflammatory therapy, including IL-1 blockade by anakinra, in the management of severe COVID-19.
Conclusions
We describe the first case of fulminant COVID-19 myocarditis successfully treated by off-label use of anakinra, a recombinant IL-1 receptor antagonist, and dexamethasone. This treatment was associated with rapid and marked improvement in inflammatory markers, improvement in CMR imaging–based markers of tissue injury, and marked improvement in left ventricular systolic function.
Novel Teaching Points.
-
•
COVID-19 myocarditis may present as acute ST-segment elevation myocardial infarction.
-
•
COVID-19 myocarditis can be associated with systemic hyperinflammation.
-
•
Emerging evidence supports use of anti-inflammatory therapies, including anakinra and dexamethasone, in severe COVID-19.
Funding Sources
Dr James White has received research funding from Siemens Healthineers. All the other authors have no funding sources to declare.
Disclosures
Dr James White is a shareholder of Cohesic Inc, and has received research funding from Siemens Healthineers. All the other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported has adhered to the relevant ethical guidelines.
See page 213 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.10.003.
Supplementary Material
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirzada A., Mokhtar A.T., Moeller A.D. COVID-19 and myocarditis: What do we know so far? CJC Open. 2020;2:278–285. doi: 10.1016/j.cjco.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalli G., Foppoli M., Cabrini L. Interleukin-1 receptor blockade rescues myocarditis-associated end-stage heart failure. Front Immunol. 2017;8:131. doi: 10.3389/fimmu.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalli G., Pappalardo F., Mangieri A. Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med. 2016;44:e751–e754. doi: 10.1097/CCM.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 7.Huet T., Beaussier H., Voisin O. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli G., De Luca G., Campochiaro C. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.