Abstract
Almost from all organs, both mesenchymal stromal cells and fibroblasts can be isolated. Mesenchymal stromal cells (MSCs) are the most preferred cellular therapeutic agents with the regenerative potential, and fibroblasts are one of the most abundant cell types with the ability to maintain homeostasis. Because of the promising properties of MSCs, they have been well studied and their differentiation potentials, immunomodulatory potentials, gene expression profiles are identified. It has been observed that fibroblasts and mesenchymal stromal cells have similar morphology, gene expression patterns, surface markers, proliferation, differentiation, and immunomodulatory capacities. Thus, it is hard to distinguish these two cell types. Epigenetic signatures, i.e., methylation patterns of cells, are the only usable promising difference between them. Such significant similarities show that these two cells may be related to each other.
Abbreviations: BMP2, bone morphogenetic protein 2; CD, cluster of differentiation; DES, desmin; FN, fibronectin; GFAP, glial fibrillary acidic protein; HNK, human natural killer-1; ICAM1, intercellular adhesion molecule 1; LDHA, lactate dehydrogenase A; NES, nestin; OPN, osteopontin; POU5F1, POU class 5 homeobox 1; RUNX2, runt-related transcription factor 2; SOX2, SRY-box transcription factor 2; STAT3, signal transducer and activator of transcription 3; TERT, telomerase reverse transcriptase; THY1, Thy-1 cell surface antigen; TPM, tropomyosin; TRO, troponin; VCAM1, vascular cell adhesion molecule 1; VIM, vimentin; ZFP42, zinc finger protein 42; αSMA, alpha-smooth muscle actin
Keywords: Mesenchymal stromal cells, Fibroblasts, Proliferation, Differentiation, Cell surface markers, Gene expression
1. Introduction
In the 1960s, a small subpopulation in the non-hematopoietic cells of bone marrow has been identified with the rapid adherence and fibroblast-like morphology and they are called firstly as “stromal stem cells” (Friedenstein et al., 1966; Owen and Friedenstein, 2007). These cells with self-renewal, multilineage potential have been called “mesenchymal stem cells” by Caplan in 1991 (Caplan, 1991). Then, at the early of the 2000s, “mesenchymal stromal cells” started to be used instead of “mesenchymal stem cells” (Dominici et al., 2006). Mesenchymal stromal cells can be obtained from many other sources than bone marrow such as Wharton’s Jelly (Sarugaser et al., 2005), peripheral blood (Li et al., 2015), umbilical cord blood (Secco et al., 2008), menstrual blood (Hida et al., 2008), dental pulp (Jo et al., 2007), adipose tissue (Zannettino et al., 2008), amnion (Hauser et al., 2010), heart (Oldershaw et al., 2019), etc. Each MSC population can have different gene expressions according to their sources. However; the minimum criteria that MSCs must meet have been determined regardless of source: (i) under standard conditions, MSCs must be adherent; (ii) MSCs must express CD105, CD73 and CD90 whereas do not express CD14, CD19, CD34, CD45, and CD79α; (iii) MSCs can differentiate into three cell lineages: osteogenic, adipogenic, chondrogenic (Dominici et al., 2006). Characteristics of MSCs are not limited to differentiation; they also have self-renewal property, and cross-talking with other cells by their secretions resulting in immunomodulation or angiogenesis properties (Ichim et al., 2018; Soundararajan and Kannan, 2018). Starting from the end of the 1990s, MSCs have been used for lots of clinical studies as a cellular pharmaceutical with both animal models and humans (Galipeau and Sensébé, 2018). MSCs are mostly used for autoimmune diseases, cardiovascular diseases, and neurodegenerative diseases (Ullah et al., 2015).
Friendenstein likened clonogenic stromal cells to fibroblasts because of the adherence and colony-forming potentials (Friedenstein, 2015). Fibroblasts constitute the majority of the cell of connective tissue and they are found in almost all organs. They produce extracellular matrix components such as collagen fibers; therefore, they have a role in tissue maintenance and repair. Fibroblasts can be isolated from several tissue types (Denu et al., 2016; Desjardins-Park et al., 2018). According to the source of the fibroblasts, gene expressions and produced extracellular matrix component types can change (Fries et al., 1994). Although it was thought that fibroblasts are nearly terminally differentiated cells and they can differentiate only into myoblasts for wound healing in the past, it is now known that they differentiate into different cell types such as adipose, osteoblast, or chondroblast (Blasi et al., 2011). Since fibroblasts have such characteristics, they have been used for clinical studies mostly for wound healing treatments (Buechler and Turley, 2018; Ichim et al., 2018).
According to the literature, there are several standard features of MSCs and fibroblasts to show fibroblasts as an alternative of MSCs. Because of such similarity, it is quite difficult to distinguish these cells. In this review, two cell types are compared in detail.
2. Comparison of proliferation capacities
Because the telomeres are shortening with each cell division, cells can divide in a limited number, approximately 50 doublings, until they reach the senescence (Kim and Hong, 2014; Signer and Morrison, 2013; Watts, 2011). It has been reported that besides the morphologic similarities of MSCs and fibroblasts, they also have similar proliferation capacities (Alt et al., 2011; Blasi et al., 2011). Different studies have found various proliferation capacities for MSCs and fibroblasts, 40−50 doublings and approximately 75 doublings, respectively, according to Ning et al. (2003) and 34 doublings and 52 doublings, respectively, according to Lysy et al. (2007). Sources of cells, experimental conditions, and source age can affect the proliferation capacities of the cells that give rise to different doubling numbers (Soundararajan and Kannan, 2018).
The presence of telomerase activity can increase population doublings; however, mesenchymal stromal cells have very few or no detectable telomerase activity, which is related with not only proliferation capacity but also differentiation capacity, like fibroblasts (Laroye et al., 2019; Simonsen et al., 2002; Steinert et al., 2000; Trachana et al., 2017; Zimmermann et al., 2003). Similarly, according to our unpublished data, both adipose tissue derived mesenchymal stromal cells (AT-MSCs) and fibroblasts do not have detectable levels of telomerase activity (data not shown).
3. Comparison of differentiation capacities
Several studies have been used the differentiation capacity of the MSCs because of being multipotent cells. According to the International Society for Cellular Therapy, one of the three minimum MSC criteria is the ability to differentiate into chondrocyte, adipocytes, and osteoblasts in vitro (Dominici et al., 2006). Besides, there are in vitro studies to show that, at the presence of the appropriate conditions and inductors, MSCs can differentiate into neural cells (Wilkins et al., 2009), pancreatic island cells (Tang et al., 2012), hepatocytes (Stock et al., 2014), melanocytes (Seifrtová et al., 2012), cardiomyoblasts (Choi et al., 2010), myocytes (Bartsch et al., 2005), etc.
Differentiation capacity of the fibroblast has been shown with several in vitro studies for adipogenic, chondrogenic, and osteogenic (Blasi et al., 2011; Brohem et al., 2013) lineages, which are also essential criteria for MSCs, and for hepatocytes (Lysy et al., 2007), corneal epithelial cells (Katikireddy et al., 2014), neural cells (Chen et al., 2007), pancreatic island cells (Bi et al., 2010), muscle cells (Sabatini et al., 2005), epidermal cells (Crigler et al., 2007) when there are appropriate conditions and stimulations. Even though the mentioned studies showed the fibroblast differentiation capacities, some of them suggest that the differentiation is due to a heterogeneous population containing precursor cells (Chen et al., 2007). To show heterogeneous population of the fibroblasts, there are several clonal analyses (Bi et al., 2010; Chen et al., 2007). Differentiation assays were performed for the single-clone-derived cell populations in order to see whether all fibroblasts have differentiation capacity. For example, for the mouse dermis, it has been found that not all fibroblasts but precursor fibroblasts can differentiate into arrector pilli muscle, reticular cells, dermal papilla, and adipocytes (Lynch and Watt, 2018). So, there are also controversial results for the differentiation capacity of the fibroblast.
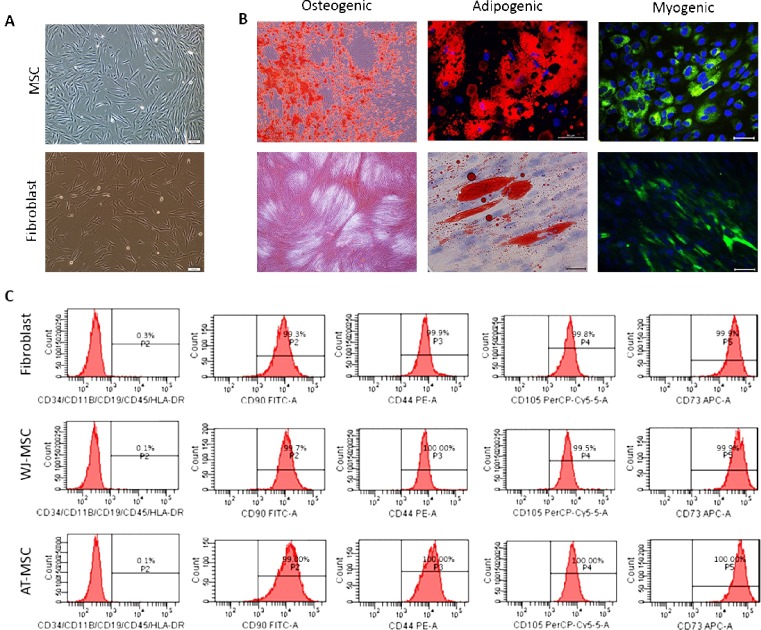
In addition, according to our unpublished data, fibroblast osteogenic, adipogenic, and myogenic differentiation has been observed; however, any clonal analysis was not performed (Fig. 1 B).
Fig. 1.
(A) Morphologies of mesenchymal stromal cell and human dermal fibroblast. (B) Ostegenic, adipogenic and myogenic differentiation of WJ-MSC and fibroblast. Alizarin Red (AR) staining has been used for osteogenic, Oil Red O (ORO) staining has been used for adipogenic, and Myoblast Determination Protein 1 (MyoD) and Desmin (Des) IF stainings have been used for myogenic differentiation. (C) Flow cytometry data of WJ-MSC, AT-MSC, and fibroblast for MSC markers.
4. Comparison of surface markers
The global opinion on mesenchymal stromal cell surface markers has been declared by The International Society for Cellular Therapy, and a standard has been created. According to this criterion, MSCs should highly express (≥ 95 %) CD105, CD73, and CD90 markers and should not express (≤ 2 %) CD45, CD34, CD14 or CD11b, CD79α or CD19, and Human Leukocyte Antigen-DR isotype (HLA-DR) (Dominici et al., 2006). On the other hand, studies showed that all fibroblasts express CD44, CD90, CD105, fibroblast surface protein (FSP), collagen (COLL), vimentin (VIM), alpha-smooth muscle actin (αSMA) and do not express CD14 and CD34, CD19 and HLA-DR (Alt et al., 2011; Denu et al., 2016; Sudo et al., 2007). We checked the fibroblast expression for MSC surface markers and found that fibroblasts express essential surface markers of both Wharton’s Jelly derived mesenchymal stromal cells (WJ-MSC) and adipose tissue derived mesenchymal stromal cells (unpublished data, Fig. 1C).
CD105 as a marker is a controversial topic. Although CD105 expression, which is related to proliferation and colony-forming, is specified as a criterion, studies have been carried out showing that there is CD105-negative MSC subpopulation (Anderson et al., 2013; Pham et al., 2019). However, CD105+ cells shown higher immunomodulation properties (Pham et al., 2019). Also, fibroblasts have CD105 expression at high levels and when expressions of MSC and fibroblast compared, it is found that they both have high expression levels; however, different colony forming potentials (Alt et al., 2011). Besides, markers of collagen, vimentin, FSP, heat shock protein 47 (HSP47), αSMA have been defined as fibroblast surface markers; however, their expressions have also been found in MSCs (Alt et al., 2011; Denu et al., 2016).
In order to distinguish MSCs and fibroblasts from each other, it is required to have specifically expressed markers. Even the CD44 has been used to identify fibroblasts in induced pluripotent cultures (Quintanilla et al., 2014), MSCs can express CD44 also (L. Ramos et al., 2016) (Fig. 2 ). A candidate marker can be Stage-Specific Embryonic Antigen-4 (SSEA4), which is a stemness marker, its expression positive at MSCs and negative at fibroblasts, according to some studies (Gang et al., 2007; Halfon et al., 2011). However, both SSEA4+ and SSEA4− subpopulations have been observed in the limbal fibroblast population (Katikireddy et al., 2014). Also, in some pathological cases such as idiopathic pulmonary fibrosis and oral submucous fibrosis, fibroblasts express SSEA4 and show both MSC and fibroblast characteristics (Xia et al., 2014; Yu et al., 2016). According to Halfon et al., other introduced identification markers can be CD166, CD146, CD106, CD9, integrin α1, insulin-like growth factor 2 (IGF2) because their expression levels are different for MSCs and fibroblast. As the expression phenomenon is not stable not only for cell types and subtypes but also for a cell lineage, instead of specifically positive-negative markers, it is required to compare expression levels of markers. For example, CD166, CD106, IGF2, and integrin alpha have higher expression levels at MSCs, but CD9 has lower expression than fibroblasts. However, these expressions can change with the passaging (Cappellesso-Fleury et al., 2010; Halfon et al., 2011).
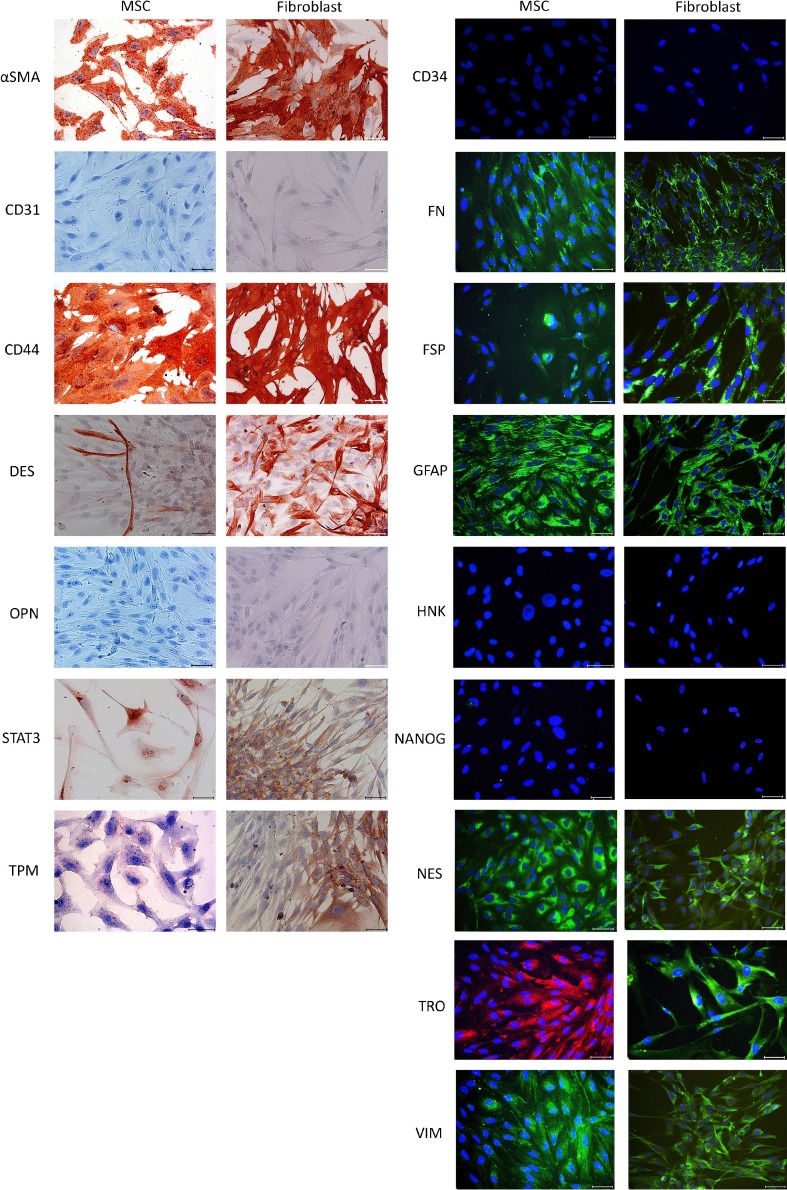
Fig. 2.
Immunocytochemistry (ICC) and immunofluorescence (IF) staining of MSC and fibroblast for some markers.
5. Comparison of gene expressions and epigenetic patterns
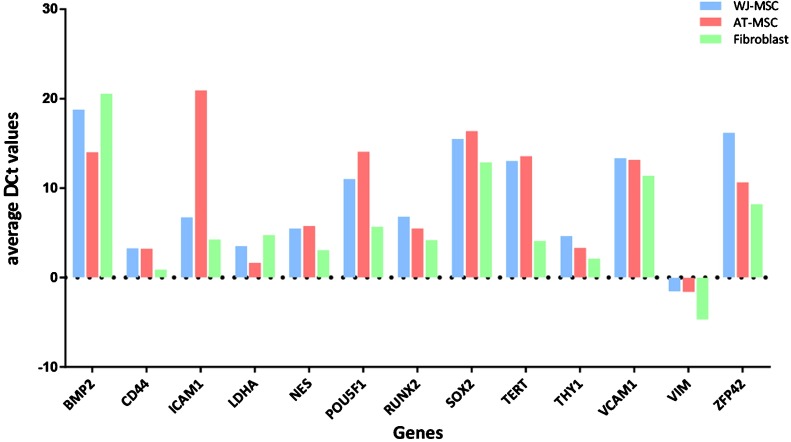
One of the discriminating identification methods is gene expression profiling of cell types. Expression patterns of specific genes may be used as a selective tool to differentiate or identify similar cells (Abdolhosseini et al., 2019). Gene expressions of mesenchymal stromal cells and fibroblasts have been investigated by looking at various numbers of genes. According to these studies, MSCs and fibroblasts have very similar gene profiles with some differences (Bae et al., 2009; Brendel et al., 2004; Brohem et al., 2013). According to Brendel et al. (2004), the differences arise from expressions of development and stromal cell function-related genes; according to Bae et al. (2009), the differences are based on transmembrane genes and tumor-associated genes, and according to Brohem et al. (2013), 20 differently expressed genes exist, but the difference of 16 of 20 is statistically significant and expression levels changes according to sources of MSCs. Also, we performed RT-PCR for MSC and fibroblast for some marker genes and we also performed immunocytochemistry (ICC) and immunofluorescence (IF) stainings to compare cells. Both cell types showed similar positive and negative results (unpublished data, Fig. 2). According to our RT-PCR results, there were expression differences for the cell types; however, such variations also observed between different sources of mesenchymal stromal cells (unpublished data, Fig. 3 ).
Fig. 3.
Comparison of some markers for WJ-MSC, AT-MSC and fibroblast by RT-PCR. Average ΔCt values have been shown.
Another important point to identify the difference between MSCs and fibroblasts is epigenetic pattern differences because even the gene profiles are similar, differences can be obtained by considering epigenetic patterns. Several studies compared fibroblast and mesenchymal stromal cell methylation patterns and show some signature patterns with certain similarities. Koch et al. (2011) have found differences containing hyper-methylated 766 CpG sites and hypo-methylated 752 CpG sites at fibroblasts that give rise to the inability to differentiate. According to de Almeida et al. (2016), it is possible to use epigenetic signatures of cells to identify a score to distinguish from fibroblasts and different MSC sources because it has been found that there are 346 different CpG sites at MSCs and 152 different CpG sites at fibroblasts.
Replicative senescence can be moderated by methylation modifications; therefore, methylation patterns can change with aging. Hyper-methylation increases in MSCs with passaging or aging; on the contrary, total methylation decreases in fibroblasts with passaging or aging (Koch et al., 2011; Schellenberg et al., 2011).
6. Comparison of immunomodulation properties
One of the characteristic features of the mesenchymal stromal cells is immunomodulation capacity. Firstly, MSCs have major histocompatibility complex class I (MHC class I) expression which results in not being recognized from the natural killer (NK) cells, but no MHC class II expression which results in not being recognized by alloreactive CD4 + T cells (Cappellesso-Fleury et al., 2010; Li and Hua, 2017; Regmi et al., 2019; Ryan et al., 2005). Like other surface markers, MHC class protein expression can be affected by some unknown conditions. Some studies showed that MSCs have high MHC class I expressions that cause no immune stimulation effect (Jacobs et al., 2013), whereas others indicated that MSCs have low MHC class I expression levels, but they still do not cause immune stimulation effect because of alternative pathways (Marti et al., 2011; Salami et al., 2018).
Secondly, mesenchymal stromal cells have effects on T-lymphocytes, they can alleviate CD4+ and CD8 + T cell proliferation via producing cytokines, suppress cytotoxic T-cell production, and induce regulatory T cell proliferation not only by cell-to-cell contact but also by chemical induction (Duffy et al., 2011; Jiang and Xu, 2020; Karaöz et al., 2017; Marti et al., 2011; Nicola et al., 2002; Tan et al., 2017). MSCs also have effects on B lymphocytes; they can inhibit B-cell differentiation into plasma cells and dendritic cells by suppressing their antigen production (Jiang and Xu, 2020; Li and Hua, 2017; Marti et al., 2011). A study by Traggiai et al. (2008) contrary showed that MSCs can induce B-cell differentiation into plasma cells and dendritic cells. In addition, MSCs can activate macrophages by changing their immunophenotype by increasing expressions of some surface markers such as CD206 (Denu et al., 2016) to enhance tissue regeneration in the inflammatory site (Chaturvedi et al., 2014; Jiang and Xu, 2020; Liu et al., 2015). Otherwise, by secreting some anti-inflammatory cytokines or soluble mediators, MSCs can inhibit macrophage and monocyte activation and expansion (Djouad et al., 2007; Nauta et al., 2006; Spaggiari et al., 2009). These features of MSCs have made inroads into use in clinical applications such as Graft versus Host Disease (GvHD) (Bozkurt et al., 2019; Elgaz et al., 2019; Sato et al., 2010).
On the other hand, fibroblasts, which have a lot of similarities with MSCs, also share similar immunomodulation properties with MSCs. They can suppress T-cell proliferation and alter macrophage immunophenotype (Denu et al., 2016; Ichim et al., 2018). Both foreskin and dermal fibroblasts can suppress T-cell proliferation to a similar extent to MSCs. Also, MHC class II does not be expressed by fibroblasts that give advantage to not recognized by T cells (Cappellesso-Fleury et al., 2010). If fibroblasts or MSCs are induced by using IFN gamma, both start to express the MHC class II antigen (Hematti, 2012).
Like MSCs, fibroblasts also can alter the immunophenotype of macrophages by increasing their CD206 expression (Denu et al., 2016). Even though there are opposite views about the mediators of immunomodulation mechanisms of fibroblasts, dependent or independent from soluble immunomodulatory mediators, such as indolamine 2,3-deoxygenase (IDO), nitric oxide (NO), prostaglandin 2 (PGE2), etc. (Cappellesso-Fleury et al., 2010; Jacobs et al., 2013; Wada et al., 2011), it has been known that interferon-alpha is one of the major immunomodulator mediators expressing by both fibroblasts and MSCs (Wada et al., 2011). Thus, fibroblasts and MSCs can use similar mechanisms for immunomodulation and that makes fibroblast as an alternative source for regenerative medicine.
Fibroblasts do not merely have immunosuppressant features, but also some subtypes of fibroblast can induce immune response for some pathological cases. For example, for arthritis, there are two subtypes of fibroblasts; one is FAP (Fibroblast Activation Protein)-α-1+ THY+ (Thymus Cell Antigen 1) fibroblasts which have immune effector features and the other one FAP-α-1+ THY− fibroblasts which cause destruction in the tissue (Croft et al., 2019).
7. Clinical uses of mesenchymal stromal cells and fibroblasts
Mesenchymal stromal cells are one of the promising regenerative medicine tools because of their differentiation capacity, regenerative, immunomodulatory, angiogenic, anti-apoptotic, and anti-inflammatory features (Fitzsimmons et al., 2018; Zhao et al., 2015). There are over 1200 MSC-based clinical studies for an extensive range of diseases such as Lupus nephritis, diabetic nephropathy, diabetes mellitus type I, epilepsy, chronic renal failure, osteogenesis, Multiple Sclerosis, and even today’s pandemic disease Covid-19, etc. (ClinicalTrials.gov, 2020).
Because of the immunomodulatory feature of mesenchymal stromal cells, they have been used for immunity-related diseases such as graft-versus-host disease (GvHD), Diabetes Mellitus, Multiple Sclerosis, Lupus, etc. For instance, GvHD is a severe disease that can occur after bone marrow transplantation due to donor T cell immune attack (Zhao et al., 2015). Therefore, the immune system has been tried to modulate with MSCs. Sánchez-Guijo et al. (2014) injected allogeneic bone marrow-derived mesenchymal stromal cells (BM-MSC; 1.1 × 106 cells/ kg) expanded with platelet lysate to 25 patients as a second-line treatment. After 60 days after the first intravenous injection of four sequential injections, 11 patients had complete responses whereas 6 has partial responses. Even the response rate was 71 %, the survival rate was 44 % after 12 mounts. They also did not observe any response rate differences between GvHD grade II, III, and IV. However, Introna et al. (2014) found that GvHD grade II had a higher rate of complete response, and also children had a higher rate of complete response. They also injected allogeneic BM-MSCs (1.5 × 106 cells/kg) intravenously and sequentially to 40 patients. They observed that 30 % of 40 patients are completely recovered, whereas 40 % of patients were alive. Both groups did not observe any acute toxicity; however, MSCs suppress the immune system, and patients may die due to infections (Introna et al., 2014; Sánchez-Guijo et al., 2014).
Both immunomodulatory effects and the differentiation capacity of MSCs can be useful for Diabetes Mellitus (DM). Because DM is an autoimmune disease, the immunomodulatory effect can reduce immune attacks to protect β-cells, and the differentiation feature can be used for producing insulin-secreting cells (ISC) (Dave et al., 2013). Hu et al. (2016) conducted a double-blinded randomized study to test whether WJ-MSC can be used for type II DM therapy. 31 of the 61 patients using oral hypoglycemic drug and insulin therapy had WJ-MSC infusions twice, whereas the other 30 patients had regular saline infusions. After 36-mount follow up, they concluded that WJ-MSC injections eventuate improvement of β-cell function, reduction of requirement of insulin or other hypoglycemic drugs, and no incidents of complications. They also did not report an adverse effect. Dave et al. (2013) differentiate AT-MSCs into ISCs for the treatment of insulin-dependent DM. They injected autologous AT-MSC derived ISCs along with autologous bone marrow-derived hematopoietic stem cells (HSC) to 10 patients. They concluded that all the patients had reduced the requirement of insulin, and they have a stable decline in blood sugar levels.
Other conditions in which MSC transplantation has been tried are neurological diseases such as Frederich’s Ataxia, Parkinson’s Disease, Alzheimer Disease, Cerebral Palsy, Duchenne Muscular Dystrophy, Hypoxic-Ischemic Encephalopathy, Degenerative Disc Disease, etc. not only with the immunomodulatory feature but also their paracrine effects (Dong et al., 2018). Huang et al. (2018) conducted a randomized study with 54 cerebral palsy (CP) patients. Half of the patients had intravenous umbilical cord-derived mesenchymal stromal cell (UC-MSC; 5 × 107 cells/dose) injections 4 times with normal rehabilitation, while the control group patients have 0.9 % normal saline injections with normal rehabilitation. Patients were evaluated at 3, 6, 9, 12, and 24 months and the study group had functional improvement at all time points. Besides the paracrine effect of MSCs, differentiation capacity had also been used by Chen et al. (2013). They differentiate BM-MSCs into neural stem cell-like cells (NSCs) and transplant 1−6 × 107 NSCs into subarachnoid cavity. After a 6-month follow up, they found that NSC transplants cause an improvement in motor functions; however, there was no significant change in language quotients. Similarly, other studies like Boruczkowski and Zdolińska-Malinowska (2019); Gu et al. (2020) and Okur et al. (2018) observed improvements of their motor and comprehensive function, quality of life, and self-sufficiency levels.
In other respects, in recent years, not only mesenchymal stromal cells are being used for clinical purposes but also their exosomes (Lou et al., 2017) and mitochondria (Caicedo et al., 2017) are being used. MSC mitochondria are being used to improve oocyte quality (Labarta et al., 2019) and to restore ischemic myocardial tissue (McCully et al., 2016) and also can be used for mitochondrial diseases (Caicedo et al., 2017). When mesenchymal stromal cell mitochondria have been injected into ischemic myocardial tissue, healthy MSC mitochondria have taken the place of damaged mitochondria and have rescued the cellular function without an immune response (McCully et al., 2016). By maintaining cell homeostasis, MSCs have regenerative potential via mitochondrial transfer (Paliwal et al., 2018). Another MSC-derived acellular therapeutic tool is exosomes because of their no tumor formation risk and no immune reaction potential (Lou et al., 2017). Exosomes are small extracellular vesicles that contain small portions of cytoplasm containing functional proteins, phospholipids, RNA and small DNA molecules. Thus, they can also help to maintain cellular homeostasis (Maumus et al., 2013; Qiu et al., 2019). There are clinical trials which have been used allogeneic MSC derived exosome were used for cerebrovascular disorders, healing of macular holes and Diabetes Mellitus type I (Yin et al., 2019).
Mesenchymal stromal cells are used according to their promising features. Similarly, fibroblasts can also be considered as a therapeutic tool because of their similarities with MSCs such as immunomodulatory properties. Fibroblasts are the connective tissue cells that can be obtained from each organ and they can produce collagen fibers to maintain the extracellular matrix. Therefore, they have been used for clinical studies of mostly dermal diseases, wound healing, inflammation suppression (Ichim et al., 2018). For the burn healing studies, fetal fibroblasts with collagen scaffolds were used as grafts and accelerated healing has been observed (Momeni et al., 2019). Fibroblasts and keratinocytes have also been tried with collagen scaffolds for the wide range full-thickness burn treatments and reduction of mortality rates have been observed by Boyce et al. (2017). Martínez et al. (2016) showed that the wound healing rate of chronic leg ulcers can be increased by using hairy punch grafts. Autologous fibroblasts have been used as filler material for the nasojugal groove to correct wrinkles (Moon et al., 2018) and with keratinocytes have been used for neck wrinkles (Wang et al., 2018). For the gingival recession studies, collagen scaffolds with autologous fibroblast could not promote a statistically significant change (Milinkovic et al., 2015). However, promising results have been obtained for Recessive Dystrophic Epidermolysis Bullosa (RDEB) by Moravvej et al. (2018) by using allogeneic fibroblasts. They compare 2 different fibroblast applications, i.e. injections of allogeneic fibroblasts and fibroblast seeded amniotic membrane scaffolds (FAMS) for the RDEB wounds. 3 wounds were treated with each application and 1 wound was used as a control. Both applications cause a significant decrease in wound area than the control wound. When the applications were compared, it is concluded that fibroblasts resulted in a significant decrease compared with the results of FAMS. Similarly, Venugopal et al. (2013) conducted a randomized vehicle-controlled study with allogeneic fibroblasts. Both fibroblasts and only-vehicle injection cause rapid wound healing independently from collagen type VII expressions.
To sum up, mesenchymal stromal cells are mostly used for their “stem cell” properties; however, fibroblasts are mostly used because of their origin; nevertheless, fibroblasts are useful tools as MSCs.
8. Conclusion
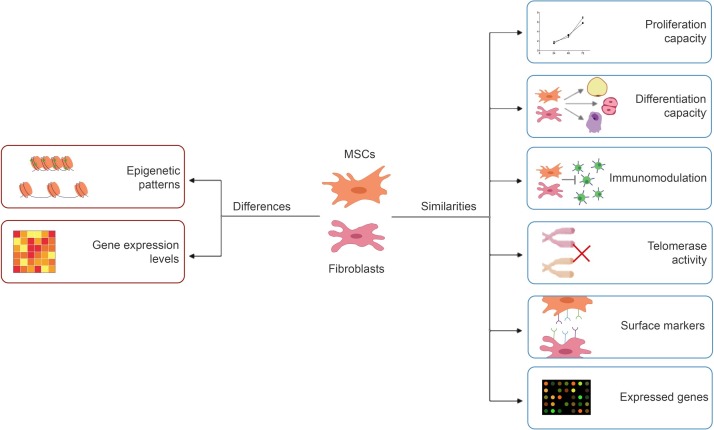
In this review, MSCs and fibroblasts have been compared according to their promising features for clinical uses (Table 1 , Fig. 4 ). Firstly, even the contradictory results of studies, it is concluded that MSCs and fibroblasts have similar proliferation capacities. Also, in recent years it has been showed that fibroblasts also have differentiation capacity as MSCs and can differentiate into several cell types such as osteoblasts, chondrocytes, adipocytes, hepatocytes, neuron-like cells, etc. No significant difference was found even in surface markers, one of the most useful description elements. The only difference between surface markers is their expression levels like other gene expression patterns. Even between subtypes of cell-lines, slightly different gene expression patterns can be observed. Similarly, immunomodulation properties have been observed for both cell types. Since each cell have specific epigenetic patterns, even they have similar gene expression patterns, like fingerprints, they can be identified. MSCs and fibroblasts may have similar properties; however, scientists prefer MSCs more for clinical studies.
Table 1.
Summary table for comparison of MSC and fibroblast characteristics.
| Feature | MSC | Fibroblast |
|---|---|---|
| Proliferation capacity |
|
|
| Telomerase activity |
|
|
| Differentiation capacity |
|
|
| Immunomodulation |
|
|
| Clinical Use |
|
|
Fig. 4.
Comparison diagram of MSCs and fibroblasts.
CRediT authorship contribution statement
Burcu Ugurlu: Investigation, Writing - original draft, Visualization, Writing - review & editing. Erdal Karaoz: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
No conflict of interest.
Acknowledgments
We thank Alihan Doğan for his help in collecting data and technical assistance.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.acthis.2020.151634.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abdolhosseini F., Azarkhalili B., Maazallahi A., Kamal A., Motahari A., Sharifi-Zarchi A., Chitsaz H. Cell identity codes: understanding cell identity from gene expression profiles using deep neural networks. Sci. Rep. 2019;9:2342. doi: 10.1038/s41598-019-38798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt E., Yan Y., Gehmert Sebastian, Song Y.-H., Altman A., Gehmert Sanga, Vykoukal D., Bai X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol. Cell. 2011;103:197–208. doi: 10.1042/bc20100117. [DOI] [PubMed] [Google Scholar]
- Anderson P., Carrillo-Gálvez A.B., García-Pérez A., Cobo M., Martín F. CD105 (Endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., Ahn J.H., Park C.W., Son H.K., Kim K.S., Lim N.K., Jeon C.J., Kim H. Gene and microRNA expression signatures of human mesenchymal stromal cells in comparison to fibroblasts. Cell Tissue Res. 2009;335:565–573. doi: 10.1007/s00441-008-0729-y. [DOI] [PubMed] [Google Scholar]
- Bartsch G., Yoo J.J., De Coppi P., Siddiqui M.M., Schuch G., Pohl H.G., Fuhr J., Perin L., Soker S., Atala A. Propagation, expansion, and multilineage differentiation of human somatic stem cells from dermal progenitors. Stem Cells Dev. 2005;14:337–348. doi: 10.1089/scd.2005.14.337. [DOI] [PubMed] [Google Scholar]
- Bi D., Chen F.G., Zhang W.J., Zhou G.D., Cui L., Liu W., Cao Y. Differentiation of human multipotent dermal fibroblasts into islet-like cell clusters. BMC Cell Biol. 2010;11:46. doi: 10.1186/1471-2121-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A., Martino C., Balducci L., Saldarelli M., Soleti A., Navone S., Canzi L., Cristini S., Invernici G., Parati E., Alessandri G. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc. Cell. 2011;3:5. doi: 10.1186/2045-824X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruczkowski D., Zdolińska-Malinowska I. Mesenchymal stem cell administration improves quality of life and self-sufficiency in children with cerebral palsy: results from a retrospective study. Stem Cells Int. 2019;2019 doi: 10.1155/2019/7402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S.T., Simpson P.S., Rieman M.T., Warner P.M., Yakuboff K.P., Bailey J.K., Nelson J.K., Fowler L.A., Kagan R.J. Randomized, paired-site comparison of autologous engineered skin substitutes and split-thickness skin graft for closure of extensive, full-thickness BurnsBrendel, C., Kuklick, L., Hartmann, O., Kim, T.D., Boudriot, U., Schwell, D., Neubauer, A., 2004. Dis. J. Burn Care Res. 2017;38:61–70. doi: 10.1097/BCR.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt C., Karaöz E., Aksoy B.A., Aydoğdu S., Fışgın T. The use of allogeneic mesenchymal stem cells in childhood steroid-resistant acute graft-versus-host disease: a retrospective study of a single-center experience. Turkish J. Hematol. 2019;36:186–192. doi: 10.4274/tjh.galenos.2019.2019.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel C., Kuklick L., Hartmann O., Kim T.D., Boudriot U., Schwell D., Neubauer A. Distinct gene expression profile of human mesenchymal stem cells in comparison to skin fibroblasts employing cDNA microarray analysis of 9600 genes. Gene Expr. 2004;12:245–257. doi: 10.3727/000000005783992043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohem C.A., De Carvalho C.M., Radoski C.L., Santi F.C., Baptista M.C., Swinka B.B., De C., De Araujo L.R.R., Graf R.M., Feferman I.H.S., Lorencini M. Comparison between fibroblasts and mesenchymal stem cells derived from dermal and adipose tissue. Int. J. Cosmet. Sci. 2013;35:448–457. doi: 10.1111/ics.12064. [DOI] [PubMed] [Google Scholar]
- Buechler M.B., Turley S.J. A short field guide to fibroblast function in immunity. Semin. Immunol. 2018;35:48–58. doi: 10.1016/j.smim.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Caicedo A., Aponte P.M., Cabrera F., Hidalgo C., Khoury M. Review article artificial mitochondria transfer: current challenges, advances, and future applications. Stem Cells Int. 2017;2017 doi: 10.1155/2017/7610414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Cappellesso-Fleury S., Puissant-Lubrano B., Apoil P.A., Titeux M., Winterton P., Casteilla L., Bourin P., Blancher A. Human fibroblasts share immunosuppressive properties with bone marrow mesenchymal stem cells. J. Clin. Immunol. 2010;30:607–619. doi: 10.1007/s10875-010-9415-4. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P., Gilkes D.M., Takano N., Semenza G.L. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2120. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.G., Zhang W.J., Bi D., Liu W., Wei X., Chen F.F., Zhu L., Cui L., Cao Y. Clonal analysis of nestin- vimentin+ multipotent fibroblasts isolated from human dermis. J. Cell. Sci. 2007;120:2875–2883. doi: 10.1242/jcs.03478. [DOI] [PubMed] [Google Scholar]
- Chen G., Wang Yali, Xu Z., Fang F., Xu R., Wang Yue, Hu X., Fan L., Liu H. Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy. J. Transl. Med. 2013:11. doi: 10.1186/1479-5876-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.S., Dusting G.J., Stubbs S., Arunothayaraj S., Han X.L., Collas P., Morrison W.A., Dilley R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010;14:878–889. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov, 2020. [WWW Document], URL https://clinicaltrials.gov/ (accessed 6.28.20).
- Crigler L., Kazhanie A., Yoon T.-J., Zakhari J., Anders J., Taylor B., Virador V.M. Isolation of a mesenchymal cell population from murine dermis that contains progenitors of multiple cell lineages. FASEB J. 2007;21:2050–2063. doi: 10.1096/fj.06-5880com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft A.P., Campos J., Jansen K., Turner J.D., Marshall J., Attar M., Savary L., Wehmeyer C., Naylor A.J., Kemble S., Begum J., Dürholz K., Perlman H., Barone F., McGettrick H.M., Fearon D.T., Wei K., Raychaudhuri S., Korsunsky I., Brenner M.B., Coles M., Sansom S.N., Filer A., Buckley C.D. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570:246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave S.D., Vanikar A.V., Trivedi H.L., Thakkar U.G., Gopal S.C., Chandra T. Novel therapy for insulin-dependent diabetes mellitus: infusion of in vitro-generated insulin-secreting cells. Clin. Exp. Med. 2013;15:41–45. doi: 10.1007/s10238-013-0266-1. [DOI] [PubMed] [Google Scholar]
- De Almeida D.C., Ferreira M.R.P., Franzen J., Weidner C.I., Frobel J., Zenke M., Costa I.G., Wagner W. Epigenetic classification of human mesenchymal stromal cells. Stem Cell Reports. 2016;6:168–175. doi: 10.1016/j.stemcr.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu R.A., Nemcek S., Bloom D.D., Goodrich A.D., Kim J., Mosher D.F., Hematti P. Fibroblasts and mesenchymal Stromal/Stem cells are phenotypically indistinguishable. Acta Haematol. 2016;136:85–97. doi: 10.1159/000445096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins-Park H.E., Foster D.S., Longaker M.T. Fibroblasts and wound healing: an update. Regen. Med. 2018;13:491–495. doi: 10.2217/rme-2018-0073. [DOI] [PubMed] [Google Scholar]
- Djouad F., Charbonnier L.-M., Bouffi C., Louis-Plence P., Bony C., Apparailly F., Cantos C., Jorgensen C., Noël D. Mesenchymal Stem Cells Inhibit the Differentiation of Dendritic Cells Through an Interleukin-6-Dependent Mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Dong H., Li G., Shang C., Yin H., Luo Y., Meng H., Li X., Wang Y., Lin L., Zhao M. Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am. J. Transl. Res. 2018;10:901–906. [PMC free article] [PubMed] [Google Scholar]
- Duffy M.M., Ritter T., Ceredig R., Griffin M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgaz S., Kuçi Z., Kuçi S., Bönig H., Bader P. Clinical use of mesenchymal stromal cells in the treatment of acute graft-versus-Host disease. Transf. Med. Hemother. 2019;46:27–34. doi: 10.1159/000496809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons R.E.B., Mazurek M.S., Soos A., Simmons C.A. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018;2018 doi: 10.1155/2018/8031718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein A.J. Marrow stromal fibroblasts. Calcif. Tissue Int. 2015;56:S17. doi: 10.1007/BF03354643. [DOI] [Google Scholar]
- Friedenstein A.J., Piatetzky-Shapiro I., Petrakova, K. V Osteogenesis in transplants of bone marrow cells. Embryol. Exp. Morph. 1966;16 581–390. [PubMed] [Google Scholar]
- Fries K.M., Blieden T., Looney R.J., Sempowski G.D., Silvera M.R., Willis R.A., Phipps R.P. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin. Immunol. Immunopathol. 1994;72:283–292. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang E.J., Bosnakovski D., Figueiredo C.A., Visser J.W., Perlingeiro R.C.R. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- Gu J., Huang L., Zhang C., Wang Y., Zhang R., Tu Z., Wang H., Zhou X., Xiao Z., Liu Z., Hu X., Ke Z., Wang D., Liu L. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: a randomized, controlled trial. Stem Cell Res. Ther. 2020;11:43. doi: 10.1186/s13287-019-1545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon S., Abramov N., Grinblat B., Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- Hauser P.V., De Fazio R., Bruno S., Sdei S., Grange C., Bussolati B., Benedetto C., Camussi G. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am. J. Pathol. 2010;177:2011–2021. doi: 10.2353/ajpath.2010.091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hematti P. Mesenchymal stromal cells and fibroblasts: A case of mistaken identity? Cytotherapy. 2012;14:516–521. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- Hida N., Nishiyama N., Miyoshi S., Kira S., Segawa K., Uyama T., Mori T., Miyado K., Ikegami Y., Cui C., Kiyono T., Kyo S., Shimizu T., Okano T., Sakamoto M., Ogawa S., Umezawa A. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- Hu J., Wang Y., Gong H., Yu C., Guo C., Wang F., Yan S., Xu H. Long term effect and safety of wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes. Exp. Ther. Med. 2016;12:1857–1866. doi: 10.3892/etm.2016.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Zhang C., Gu J., Wu W., Shen Z., Zhou X., Lu H. A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transpl. 2018;27:325–334. doi: 10.1177/0963689717729379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichim T.E., O’Heeron P., Kesari S. Fibroblasts as a practical alternative to mesenchymal stem cells. J. Transl. Med. 2018;16:1–9. doi: 10.1186/s12967-018-1536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Lucchini G., Dander E., Galimberti S., Rovelli A., Balduzzi A., Longoni D., Pavan F., Masciocchi F., Algarotti A., Mico C., Grassi A., Deola S., Cavattoni I., Gaipa G., Belotti D., Perseghin P., Parma M., Pogliani E., Golay J., Pedrini O., Capelli C., Cortelazzo S., D’Amico G., Biondi A., Rambaldi A., Biagi E. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol. Blood Marrow Transpl. 2014;20:375–381. doi: 10.1016/j.bbmt.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Jacobs S.A., Roobrouck V.D., Verfaillie C.M., Van Gool S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013;91:32–39. doi: 10.1038/icb.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53 doi: 10.1111/cpr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y.Y., Lee H.J., Kook S.Y., Choung H.W., Park J.Y., Chung J.H., Choung Y.H., Kim E.S., Yang H.C., Choung P.H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–773. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- Karaöz E., Demircan P.Ç., Erman G., Güngörürler E., Sarıboyacı A.E. Kemik İliği, Wharton Jölesi ve İnsan Yağ Doku-Kaynaklı Mezenkimal Kök Hücrelerin İmmünsüpresif Özelliklerinin Karşılaştırmalı Olarak İncelenmesi. Turk. J. Hematol. 2017;34:213–225. doi: 10.4274/tjh.2016.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katikireddy K.R., Dana R., Jurkunas U.V. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells. 2014;32:717–729. doi: 10.1002/stem.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-S., Hong J.-S. Telomere length and telomerase activity are related with immortalization frequency but not with replicative senescence in mammalian embryonic fibroblasts, except human embryonic fibroblasts. Animal Cells Syst. (Seoul) 2014;18:387–393. doi: 10.1080/19768354.2014.987815. [DOI] [Google Scholar]
- Koch C.M., Suschek C.V., Lin Q., Bork S., Goergens M., Joussen S., Pallua N., Ho A.D., Zenke M., Wagner W. Specific age-associated DNA methylation changes in human dermal fibroblasts. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarta E., de los Santos M.J., Herraiz S., Escribá M.J., Marzal A., Buigues A., Pellicer A. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization—a randomized pilot study. Fertil. Steril. 2019;111:86–96. doi: 10.1016/j.fertnstert.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Laroye C., Gauthier M., Antonot H., Decot V., Reppel L., Bensoussan D. Mesenchymal Stem/Stromal cell production compliant with good manufacturing practice: comparison between bone marrow, the gold standard adult source, and Wharton’s jelly, an extraembryonic source. J. Clin. Med. 2019;8:2207. doi: 10.3390/jcm8122207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Hua J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017;74:2345–2360. doi: 10.1007/s00018-017-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Huang K.-J., Wu J.-C., Hu M.S., Sanyal M., Hu M., Longaker M.T., Lorenz H.P. Peripheral blood-derived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl. Med. 2015;4:359–368. doi: 10.5966/sctm.2014-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zhang S., Gu S., Sang L., Dai C. Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFβ1. Cell. Physiol. Biochem. 2015;35:858–865. doi: 10.1159/000369743. [DOI] [PubMed] [Google Scholar]
- Lorenz K., Sicker M., Schmelzer E., Rupf T., Salvetter J., Schulz-Siegmund M., Bader A. Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp. Dermatol. 2008;17:925–932. doi: 10.1111/j.1600-0625.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- Lou G., Chen Z., Zheng M., Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017;49 doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M.D., Watt F.M. Fibroblast heterogeneity: implications for human disease. J. Clin. Invest. 2018;128:26–35. doi: 10.1172/JCI93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysy P.A., Smets F., Sibille C., Najimi M., Sokal E.M. Human skin fibroblasts: from mesodermal to hepatocyte-like differentiation. Hepatology. 2007;46:1574–1585. doi: 10.1002/hep.21839. [DOI] [PubMed] [Google Scholar]
- Marti L.C., Ribeiro A.A.F., Hamerschlak N. Immunomodulatory effect of mesenchymal stem cells. Einstein (São Paulo) 2011;9:224–228. doi: 10.1590/s1679-45082011rw1843. [DOI] [PubMed] [Google Scholar]
- Martínez M.L., Escario E., Poblet E., Sánchez D., Buchón F.F., Izeta A., Jimenez F. Hair follicle–containing punch grafts accelerate chronic ulcer healing: a randomized controlled trial. J. Am. Acad. Dermatol. 2016;75:1007–1014. doi: 10.1016/j.jaad.2016.02.1161. [DOI] [PubMed] [Google Scholar]
- Maumus M., Jorgensen C., Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95:2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- McCully J.D., Levitsky S., del Nido P.J., Cowan D.B. Mitochondrial transplantation for therapeutic use. Clin. Transl. Med. 2016;5:16. doi: 10.1186/s40169-016-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinkovic I., Aleksic Z., Jankovic S., Popovic O., Bajic M., Cakic S., Lekovic V. Clinical application of autologous fibroblast cell culture in gingival recession treatment. J. Periodontal Res. 2015;50:363–370. doi: 10.1111/jre.12215. [DOI] [PubMed] [Google Scholar]
- Momeni M., Fallah N., Bajouri A., Bagheri T., Orouji Z., Pahlevanpour P., Shafieyan S., Sodeifi N., Alizadeh A., Aghdami N., Fatemi M.J. A randomized, double-blind, phase I clinical trial of fetal cell-based skin substitutes on healing of donor sites in burn patients. Burns. 2019;45:914–922. doi: 10.1016/j.burns.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Moon K.C., Lee H.S., Han S.K., Chung H.Y. Correcting nasojugal groove with autologous cultured fibroblast injection: a pilot study. Aesthetic Plast. Surg. 2018;42:815–824. doi: 10.1007/s00266-017-1044-3. [DOI] [PubMed] [Google Scholar]
- Moravvej H., Abdollahimajd F., Naseh M.H., Piravar Z., Abolhasani E., Mozafari N., Niknejad H. Cultured allogeneic fibroblast injection vs. Fibroblasts cultured on amniotic membrane scaffold for dystrophic epidermolysis bullosa treatment. Br. J. Dermatol. 2018;179:72–79. doi: 10.1111/bjd.16338. [DOI] [PubMed] [Google Scholar]
- Nauta A.J., Kruisselbrink A.B., Lurvink E., Willemze R., Fibbe W.E. Mesenchymal stem cells inhibit generation and function of both CD34 + -Derived and monocyte-derived dendritic cells. J. Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- Nicola M..Di, Carlo-Stella C., Magni M., Milanesi M., Longoni P.D., Matteucci P., Grisanti S., Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- Ning Y., Xu J.-F., Li Y., Chavez L., Riethman H.C., Lansdorp P.M., Weng N.-P. Telomere length and the expression of natural telomeric genes in human fibroblasts. Hum. Mol. Genet. 2003;12:1329–1336. doi: 10.1093/hmg/ddg139. [DOI] [PubMed] [Google Scholar]
- Okur S.Ç., Erdoğan S., Demir C.S., Günel G., Karaöz E. The effect of umbilical cord-derived mesenchymal stem cell transplantation in a patient with cerebral palsy: a case report. Int. J. Stem Cells. 2018;11:141–147. doi: 10.15283/ijsc17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw R., Owens W.A., Sutherland R., Linney M., Liddle R., Magana L., Lash G.E., Gill J.H., Richardson G., Meeson A. Human cardiac-mesenchymal stem cell-like cells, a novel cell population with therapeutic potential. Stem Cells Dev. 2019;28:593–607. doi: 10.1089/scd.2018.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M., Friedenstein A.J. Stromal stem cells: marrow-derived osteogenic precursors. In: Evered D., Harnett S., editors. Cell and Molecular Biology of Vertebrate Hard Tissues. Ciba Found Symp; 2007. pp. 42–60. [DOI] [PubMed] [Google Scholar]
- Paliwal S., Chaudhuri R., Agrawal A., Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018;25:31. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham L.H., Vu N.B., Van Pham P. The subpopulation of CD105 negative mesenchymal stem cells show strong immunomodulation capacity compared to CD105 positive mesenchymal stem cells. Biomed. Res. Ther. 2019;6:3131–3140. doi: 10.15419/bmrat.v6i4.538. [DOI] [Google Scholar]
- Qiu G., Zheng G., Ge M., Wang J., Huang R., Shu Q., Xu J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019;10:359. doi: 10.1186/s13287-019-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla R.H., Asprer J.S.T., Vaz C., Tanavde V., Lakshmipathy U. CD44 is a negative cell surface marker for pluripotent stem cell identification during human fibroblast reprogramming. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L.T., Sánchez-Abarca L.I., Muntión S., Preciado S., Puig N., López-Ruano G., Hernández-Hernández Á., Redondo A., Ortega R., Rodríguez C., Sánchez-Guijo F., del Cañizo C. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi S., Pathak S., Kim J.O., Yong C.S., Jeong J.H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019;98 doi: 10.1016/j.ejcb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Ryan J.M., Barry F.P., Murphy J.M., Mahon B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005:2. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini F., Petecchia L., Tavian M., De Villeroché V.J., Rossi G.A., Brouty-Boyé D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab. Investig. 2005;85:962–971. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- Salami F., Tavassoli A., Mehrzad J., Parham A. Immunomodulatory effects of mesenchymal stem cells on leukocytes with emphasis on neutrophils. Immunobiology. 2018;223:786–791. doi: 10.1016/j.imbio.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Sánchez-Guijo F., Caballero-Velázquez T., López-Villar O., Redondo A., Parody R., Martínez C., Olavarría E., Andreu E., Prósper F., Díez-Campelo M., Regidor C., Villaron E., López-Corral L., Caballero D., Cañizo M.Cdel, Pérez-Simon J.A. Sequential third-party mesenchymal stromal cell therapy for refractory acute graft-versus-host disease. Biol. Blood Marrow Transpl. 2014;20:1580–1585. doi: 10.1016/j.bbmt.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Sarugaser R., Lickorish D., Baksh D., Hosseini M.M., Davies J.E. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- Sato K., Ozaki K., Mori M., Muroi K., Ozawa K. Mesenchymal stromal cells for graft-versus-host disease : basic aspects and clinical outcomes. J. Clin. Exp. Hematop. 2010;50:79–89. doi: 10.3960/jslrt.50.79. [DOI] [PubMed] [Google Scholar]
- Schellenberg A., Lin Q., Schüler H., Koch C.M., Joussen S., Denecke B., Walenda G., Pallua N., Suschek C.V., Zenke M., Wagner W. Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging (Albany. NY) 2011;3:873–888. doi: 10.18632/aging.100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco M., Zucconi E., Vieira N.M., Fogaça L.L.Q., Cerqueira A., Carvalho M.D.F., Jazedje T., Okamoto O.K., Muotri A.R., Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- Seifrtová M., Havelek R., Ćmielová J., Jiroutová A., Soukup T., Brůčková L., Mokrý J., English D., Řezáčová M. The response of human ectomesenchymal dental pulp stem cells to cisplatin treatment. Int. Endod. J. 2012;45:401–412. doi: 10.1111/j.1365-2591.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- Signer R.A.J., Morrison S.J. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen J.L., Rosada C., Serakinci N., Justesen J., Stenderup K., Rattan S.I.S., Jensen T.G., Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- Soundararajan M., Kannan S. Fibroblasts and mesenchymal stem cells: two sides of the same coin? J. Cell. Physiol. 2018;233:9099–9109. doi: 10.1002/jcp.26860. [DOI] [PubMed] [Google Scholar]
- Spaggiari G.M., Abdelrazik H., Becchetti F., Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- Steinert S., Shay J.W., Wright W.E. Transient expression of human telomerase extends the life span of normal human fibroblasts. Biochem. Biophys. Res. Commun. 2000;273:1095–1098. doi: 10.1006/bbrc.2000.3080. [DOI] [PubMed] [Google Scholar]
- Stock P., Brückner S., Winkler S., Dollinger M.M., Christ B. Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury. Int. J. Mol. Sci. 2014;15:7004–7028. doi: 10.3390/ijms15047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K., Kanno M., Miharada K., Ogawa S., Hiroyama T., Saijo K., Nakamura Y. Mesenchymal progenitors able to differentiate into osteogenic, Chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells. 2007;25:1610–1617. doi: 10.1634/stemcells.2006-0504. [DOI] [PubMed] [Google Scholar]
- Tan K., Zheng K., Li D., Lu H., Wang S., Sun X. Impact of adipose tissue or umbilical cord derived mesenchymal stem cells on the immunogenicity of human cord blood derived endothelial progenitor cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D.Q., Wang Q., Burkhardt B.R., Litherland S.A., Atkinson M.A., Yang L.J. In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation. Am. J. Stem Cells. 2012;1:114–127. [PMC free article] [PubMed] [Google Scholar]
- Trachana V., Petrakis S., Fotiadis Z., Siska E.K., Balis V., Gonos E.S., Kaloyianni M., Koliakos G. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy. 2017;19:808–820. doi: 10.1016/j.jcyt.2017.03.078. [DOI] [PubMed] [Google Scholar]
- Traggiai E., Volpi S., Schena F., Gattorno M., Ferlito F., Moretta L., Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells - Current trends and future prospective. Biosci. Rep. 2015;35 doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal S.S., Yan W., Frew J.W., Cohn H.I., Rhodes L.M., Tran K., Melbourne W., Nelson J.A., Sturm M., Fogarty J., Marinkovich M.P., Igawa S., Ishida-Yamamoto A., Murrell D.F. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. J. Am. Acad. Dermatol. 2013:69. doi: 10.1016/j.jaad.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Wada N., Bartold P.M., Gronthos S. Human foreskin fibroblasts exert immunomodulatory properties by a different mechanism to bone marrow stromal/stem cells. Stem Cells Dev. 2011;20:647–659. doi: 10.1089/scd.2010.0246. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang B., Zhang Q., Ma J. New soft tissue filler derived from autologous keratin and fibroblast for neck wrinkles. J. Cosmet. Dermatol. 2018;17:600–605. doi: 10.1111/jocd.12438. [DOI] [PubMed] [Google Scholar]
- Watts G. Leonard Hayflick and the limits of ageing. Lancet. 2011;377:2075. doi: 10.1016/S0140-6736(11)60908-2. [DOI] [PubMed] [Google Scholar]
- Wilkins A., Kemp K., Ginty M., Hares K., Mallam E., Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Xia H., Bodempudi V., Benyumov A., Hergert P., Tank D., Herrera J., Braziunas J., Larsson O., Parker M., Rossi D., Smith K., Peterson M., Limper A., Jessurun J., Connett J., Ingbar D., Phan S., Bitterman P.B., Henke C.A. Identification of a cell-of-origin for fibroblasts comprising the fibrotic reticulum in idiopathic pulmonary fibrosis. Am. J. Pathol. 2014;184:1369–1383. doi: 10.1016/j.ajpath.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark. Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.C., Yu C.H., Chang Y.C. Aberrant SSEA-4 upregulation mediates myofibroblast activity to promote pre-cancerous oral submucous fibrosis. Sci. Rep. 2016;6:37004. doi: 10.1038/srep37004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannettino A.C.W., Paton S., Arthur A., Khor F., Itescu S., Gimble J.M., Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J. Cell. Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- Zhao K., Lou R., Huang F., Peng Y., Jiang Z., Huang K., Wu X., Zhang Y., Fan Z., Zhou H., Liu C., Xiao Y., Sun J., Li Y., Xiang P., Liu Q. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2015;21:97–104. doi: 10.1016/j.bbmt.2014.09.030. [DOI] [PubMed] [Google Scholar]
- Zimmermann S., Voss M., Kaiser S., Kapp U., Waller C.F., Martens U.M. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003;17:1146–1149. doi: 10.1038/sj.leu.2402962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.