Abstract
Influenza in pregnancy is a common condition that is associated with an increased risk of hospital admission. Women with comorbidities are at a greater risk of severe outcomes. There are substantial gaps in our knowledge of the impact of severe influenza on perinatal outcomes, particularly in low- and middle-income countries, but preterm birth, fetal death, infant respiratory infection and hospital admission may be increased. Thus, influenza is a major burden on health services. Immunisation is cost-effective, safe and effective in preventing influenza in pregnant women and their infants but policies and uptake vary worldwide. Operational challenges and concern over the safety, efficacy and necessity of immunisation are common, and there is a lack of evidence on how to overcome these barriers. This review identifies learning points that are relevant to the current coronavirus disease-2019 pandemic through describing the epidemiology and impact of seasonal and A(H1N1)pdm09 influenza in pregnancy, alongside the effectiveness and use of immunisation.
Keywords: Influenza, Immunisation, Pregnancy, Vaccine, Pandemic
Introduction
Influenza is a highly contagious acute viral infection, which affects the respiratory tract. In the majority of the population, influenza causes mild symptoms that are self-limiting. However, influenza can cause severe illness and even death, with 290,000 to 650,000 respiratory deaths being attributed to influenza annually [1]. It is mainly caused by influenza, types A and B, which occur in seasonal patterns with epidemics in winter in the northern hemisphere [1]. In 2009, a novel strain of the influenza A virus, termed A(H1N1)pdm09 (H1N1 for brevity in this review), led to a global pandemic estimated to cause between 100,000 and 400,000 deaths globally in the first year.
Influenza pandemics are impossible to predict and can cause a spectrum of mild to severe disease. Whilst the majority of severe cases are in vulnerable groups such as those with chronic medical conditions, healthy persons are more likely to experience serious disease from the pandemic than seasonal influenza. This places significant sustained strain on health services and may result in significant economic loss [2]. The aim of this review is to describe the current evidence around the epidemiology and impact of seasonal and H1N1 influenza in pregnancy, the effectiveness and use of influenza immunisation with the aim to identify learning points from the past pandemic relevant to the current coronavirus disease-2019 (COVID-19) pandemic.
Epidmiology of influenza in pregnancy
Incidence of influenza in pregnancy
Seasonal influenza has been reported to affect between 483 and 1097 pregnant women per 10,000 [3]. These estimates are based on serological testing in women with and without symptoms of infection, and it is estimated that approximately 25% develop symptoms [4,5]. Studies that reported the incidence of confirmed influenza in pregnant women during the 2009 H1N1 pandemic included only those that were symptomatic of infection and/or hospitalised and reported much lower rates of 0.10–6.6 per 10,000 pregnancies.
Impact of influenza in pregnancy on maternal outcomes
Pregnant women have been widely considered to be at a greater risk of severe illness from influenza as compared to outside of pregnancy [6,7]. In a recent systematic review, over half of observational studies reported significantly higher mortality rates from influenza in pregnancy as compared to the non-pregnant population, alongside increased risk of hospitalisation and ICU admission [8]. During the pandemic period in the United States (US), it was reported that 4.3%–5.8% of influenza deaths were in pregnant women, despite this group representing only 1% of the population [6,7]. However, more recent systematic analyses of observational studies [8] and individual patient data, which take the account of important confounders such as prior vaccine exposure, age, underlying co-morbidities and antiviral treatments [9], have challenged this association. They have demonstrated that whilst pregnant women were at a significantly increased risk of hospital admission with influenza as compared to non-pregnant patients (OR 6.80 and 95% CI: 6.02–7.68) [9], their risk of death was not increased (OR 1.00 and 95% CI: 0.75–1.34) and the risk of ICU admission was significantly reduced (OR 0.57 and 95% CI: 0.48–0.69) [9].
Although the risk of death or severe outcome may not be increased in pregnancy as compared to the general population, preventable deaths as a result of influenza in pregnancy continue to occur in high-income countries today. Between 2009 and 2012, which includes the pandemic period, 36 pregnant or recently pregnant women died from influenza in the UK, 32 of which were due to H1N1 [10]. More than half of these deaths (62%) were after a vaccine was available and were therefore considered preventable. A minority of these women had refused vaccination (n = 3), but it is not clear how many others were offered vaccination [10].
In nearly every instance (94%), there were delays in appropriate referral, testing and treatment as influenza was not considered a possible diagnosis when pregnant women presented with respiratory illness. This report, therefore, recommended the promotion of influenza vaccination to pregnant women at any stage of pregnancy, and that influenza should be considered early on presentation to health care facilities to test and initiate treatment [10].
There are two antivirals used to treat influenza in pregnancy, oseltamivir (Tamiflu) and zanamivir; both are neuraminidase inhibitors. A 2014 Cochrane systematic review reported that, aside from reduced time to alleviate symptoms, there was little evidence of benefit of treatment in the general adult population [11]. However, pregnant women were specifically excluded from most trials included in the review. Evidence from observational studies suggests that as compared to those that did not receive treatment, treatment with neuraminidase inhibitors in pregnancy reduces the length of hospital stay [12] and the risk of ICU admission [6,13], and early treatment reduced the risk of death as compared to those who received late treatment [6]. Therefore, UK guidelines recommend that antiviral treatment should be commenced as early as possible in pregnant women with signs of influenza [14]. More recently, the 2017 confidential enquiry into maternal deaths and morbidity in the UK, described a further death of a pregnant woman from influenza following delay of diagnosis and initiation of treatment. This highlights the necessity for these recommendations to remain on the international agenda even outside of pandemic periods [15].
‘A previously healthy woman in the early third trimester of pregnancy was admitted with a respiratory illness during the peak of the influenza season. Neither her GP nor the hospital team considered the diagnosis of influenza. It was not considered until a week into her admission by which time she was receiving intensive respiratory support on the critical care unit. Antiviral medication was not commenced empirically but only following a positive tracheal aspirate which confirmed H1N1, almost two weeks after the start of her illness. She continued to deteriorate despite ECMO and died. She had not been immunised. It was unclear whether immunisation had been offered’. Reproduced with permission from ‘Saving Lives, Improving Mothers’ Care: Lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012’.
Impact of influenza in pregnancy on perinatal outcomes
In 2015, the WHO Taskforce for influenza in pregnancy undertook a systematic review of the impact of influenza on adverse birth outcomes [16]. This review identified 21 low and very low-quality comparative studies, 20 of which were undertaken in high-income countries, again showing the limited evidence about the impact of influenza in other contexts such as in the presence of maternal malnutrition [17]. The two highest quality studies identified that severe H1N1 influenza increased the risk of preterm birth (OR 2.39, 95% CI 1.64–3.49 [18] and OR 4.0, 95% CI 2.71–5.90 [19]), whereas, a further study reported no association when women with mild to moderate H1N1 were included [16]. More recently, two studies reported no association between predominantly mild H1N1 influenza and the risk of preterm birth (OR 0.77, 95% CI 0.32–1.88 [20] and aHR 1.0, 95% CI 0.98–1.1(21)), although the risk was increased among women with pre-existing medical conditions (aHR 1.5, 95% CI 1.1–2.2) including for spontaneous preterm birth (aHR 1.7, 95% CI 1.1–2.6) [21]. Furthermore, a national study of women hospitalised with seasonal influenza found that whilst a higher proportion gave birth at <37 weeks, the risk was not significantly increased as compared to women without influenza [22].
The WHO systematic review reported, from five studies, that maternal influenza did not significantly affect the risk of the infant being small for gestational age (OR 1.24, 95% CI 0.96–1.59) [16]. However, the two of the studies based on seasonal influenza reported an increased risk, including the only study of women with severe influenza (aOR 1.66, 95% CI 1.11–2.49) [23], whereas the three studies that reported pandemic influenza found no association [16]. Similarly, a recent prospective cohort study found no association between predominantly mild H1N1 influenza and the risk of the infant being small for gestational age (OR 1.30, 95% CI 0.74–2.81) [20].
A number of studies have reported the impact of maternal influenza on fetal death, but these are generally very low quality with variable definitions and low case numbers [16]. The two highest quality studies identified by the 2015 systematic review were undertaken during the 2009 pandemic. Both reported an increased risk of fetal death following severe (aOR 4.2, 95% CI 1.4–12.4) [19] and mild to moderate maternal influenza (aHR 1.91, 95% CI 1.07–3.41) [24]. However, a recent national study of women hospitalised with seasonal influenza in the UK found no increased risk of fetal death, but an increased risk of admission to neonatal ICU (aOR 1.86, 95% CI 1.1–3.42) [22]. Similarly, a recent Norwegian registry cohort reported that seasonal influenza was not associated with an increased risk of fetal death, whereas pandemic influenza significantly increased the risk (aHR 0.90, 95% CI 0.64–1.27 and 1.75, 1.21–2.54, respectively). This study also explored the impact of gestation at the time of influenza and found that the risk of fetal death was highest following influenza-like illness in the first trimester (aHR 2.28, 95% CI 1.45–3.59) [25]. The risk of congenital anomalies has also been reported to be increased (aOR 2.00, 95% CI 1.62–2.48), including neural tube defects, hydrocephaly and congenital heart defects [26].
As infants have no prior exposure or immunity to influenza, they are highly susceptible to influenza illness, which results in the significant utilisation of health services [27]. A 2017 systematic review undertaken by the WHO influenza working group reported that there was limited evidence reported about influenza morbidity and mortality outcomes in infants, particularly in LMIC [28]. The evidence that is available suggests that infant influenza under 6 months of age is associated with increased rates of hospitalisation, severe acute lower respiratory infection [28] and higher death rates [28,29].
Characteristics associated with severe outcomes from influenza
Several large prospective cohort studies, predominantly undertaken in the pandemic period, have reported that within the pregnant population there are factors which increase the risk of hospitalisation or severe outcomes from influenza [13,22,30,31]. Pregnant women with a high BMI are more likely to be admitted to hospital with influenza (aOR 1.9, 95% CI 1.15–1.93) and more likely to need intensive care (aOR 2.93, 95% CI 1.99–4.31) [30]; however, this association was not seen in a recent population cohort with seasonal influenza [22]. Whilst not conclusive across all studies, women from black or other minority ethnic groups have also been reported to be at increased risk of hospital admission with H1N1 influenza (aOR 1.6, 95% CI 1.1–2.3) [30]. This is in keeping with other studies, which reported that indigenous women were at a greater risk of ICU admission from H1N1 in Australia and New Zealand (aOR 2.3, 95% CI 1.4–3.7) [31], and pregnant women admitted to hospital with H1N1 in California were more likely to be Hispanic than non-pregnant women [13].
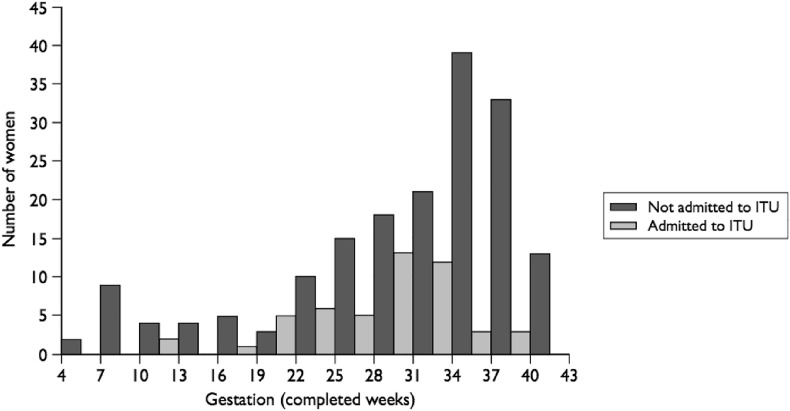
A high proportion of pregnant women with severe outcomes from influenza are reported to have underlying medical conditions (range: from 33.8% to 55.3%) [6,13]. The risk of hospital admission with pandemic influenza in pregnancy has also been reported to be increased in multiparous women, those with multiple pregnancies and in women below the age of 25 years who smoked [30]. There is some evidence that the risk of complications from influenza may be greater in the second and third trimesters than the first trimester of pregnancy. Women with a gestation of less than 20 weeks have been reported to have double the risk of ICU admission with H1N1 as compared to non-pregnant women (RR 2.4, 95% CI 1.3–4.6), whereas those with a gestation of 20 weeks or more had a 13-fold greater risk of admission (RR 13.2, 95% CI 9.6–18.3) [31]. Similarly, in the UK, 86% of pregnant women admitted to the ICU with H1N1 were in their third trimester as shown in Fig. 1 [30], and in the US, 49% of ICU admissions and 64% of deaths from H1N1 were in their third trimester [6].
Fig. 1.
Gestation at admission for pregnant women with confirmed H1N1 influenza admitted to an ITU and those admitted to hospital but not to an ITU [30].
Vaccination in pregnancy
In 2012, the World Health Organisation released a position statement recommending that pregnant women should be prioritised in vaccination programmes for seasonal influenza and that they should be vaccinated at any stage of pregnancy. This was made on the basis of the risk of severe disease, safety and efficacy of the vaccine in the prevention of influenza in women and their infants and operational feasibility [32]. Prior to this, there has been international effort to increase the availability and capacity to deliver influenza vaccines, particularly in low- and middle-income countries in the event of a pandemic [33]. Global production capacity increased substantially from 2006 to 2016, and the WHO continues to work to increase access in low-resource countries through the Pandemic Influenzas Preparedness framework [34].
Vaccine safety and efficacy
Influenza vaccines have been used in pregnant women in the US since the 1950s, but the uptake increased dramatically following the 2009 pandemic. It is widely accepted as safe throughout pregnancy [35] and effective in preventing seasonal influenza in pregnant women and their infants [[36], [37], [38], [39], [40]]. A pooled analysis of three randomised controlled trials (RCT) undertaken in Nepal, Mali and South Africa demonstrated that maternal immunisation prevents 50% (95% CI 32%–63%) of confirmed influenza in pregnancy and up to six months post-partum. The benefit was greater when immunisation was given at or after 29 weeks of gestation (71%, 95% CI 50–83% as compared to 30%, 95% CI 2%–52% before 29 weeks’) [41]. However, the mechanism behind this response was unclear as previous studies have shown that antibody response to influenza immunisation might decline as pregnancy progresses; therefore, the authors reported that the study may be underpowered for this outcome.
Some studies have reported potential benefit in a reduced risk of adverse pregnancy outcomes following maternal immunisation, including in preterm birth [42,43], low birth weight [43,44] and stillbirth [45]. However, the findings differ between countries, for example, an RCT undertaken in Nepal showed a 15% reduction in low birth weight infants in immunised mothers (95% CI 3%–25%) [38], whereas two studies undertaken in Africa showed no difference [37,40]. Overall, a pooled analysis found that maternal immunisation did not significantly affect the risk of preterm birth, low birthweight or still birth [41]. This apparent lack of effect may be expected given the heterogeneous nature of studies on birth outcomes.
Currently, influenza vaccines are not licensed for infants under 6 months of age [46]. Therefore, maternal immunisation offers the sole route of protection for infants through transplacental transfer of antibodies from the mother to fetus [28]. Recent systematic reviews have found that maternal immunisation reduces the risk of laboratory-confirmed influenza in infants from 34% (95% CI 15%–50%) [47] to 48% (95% CI 33%–59%) and associated hospital admissions by 72% (95% CI 39–87%) [46], with the greatest benefit in infants in the first two months of age [41,46]. A pooled analysis of three RCTs undertaken in LMIC reported that maternal influenza immunisation is also 20% effective at protecting young infants against severe pneumonia [48] and reduces the risk of hospital admission with all cause acute lower respiratory illness by 44% (95% CI 1%–68%) [46].
Cost effectiveness
Influenza immunisation in pregnancy has been demonstrated to be cost-effective for both seasonal and pandemic influenza [[49], [50], [51]]. A study in the UK found that to immunise pregnant women against seasonal influenza was associated with an incremental cost-effectiveness ratio of around £23,000. This assumed that the vaccine is well-matched to the circulating strain and is delivered largely by nurses in primary care settings during routine antenatal care. Delivery by midwives or in separate appointments would be associated with additional cost [52]. Further studies have suggested that immunisation targeted on women with co-morbidities would be cost-saving [53].
One study in Mali estimated that maternal influenza immunisation could be highly cost-effective in low-income countries if programmatic costs are kept low, for example, through delivery in combination with existing vaccine programmes [54]. However, overall there is little evidence on the economic burden of influenza and thus the value of seasonal influenza immunisation in pregnancy in LMIC [55]. The limited availability of incidence and impact data in this setting, with differing access to care and underlying comorbidities such as HIV and malnutrition, undoubtedly underlies this problem. Indeed, in 2013, the Global Alliance for Vaccines and Immunisation considered and rejected an investment in maternal influenza immunisation programmes in LMIC due to limited data about the local burden of disease and anticipated impact [17], which was also reflected in a report by Bill and Melinda Gates in 2015 [56]. More recent studies that demonstrated the impact on infants in LMIC, may go some way towards meeting this challenge in the future [46,48].
Global variation in influenza immunisation policy
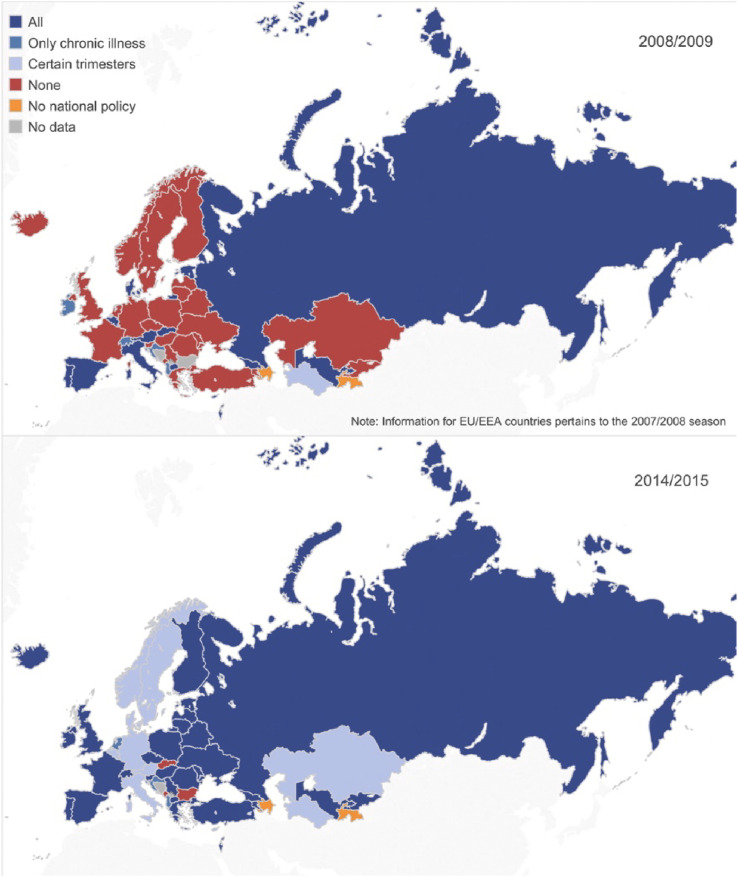
A 2014 report identified that worldwide, 42% of WHO member states had influenza immunisation policies that targeted pregnant women. This varied widely by region from 6% in Africa to 64% in Europe [57]. Countries with national policies were more likely to be high or upper-middle income classification [57]. Since the 2009 pandemic, immunisation is increasingly recommended for all pregnant women regardless of trimester or underlying comorbidities as shown in Fig. 2 , but still in 2014, a few countries did not have a policy for influenza immunisation in pregnancy (Bulgaria, Malta and Slovakia) [58]. This likely reflects the ambiguity around the effectiveness and cost-effectiveness of influenza immunisation in different contexts.
Fig. 2.
Vaccine recommendations for pregnant women in the WHO European Region between 2008/2009 and 2014/2015 [58].
Uptake of influenza immunisation
During the pandemic, the majority of countries rapidly accepted public health advice and recommended that all pregnant women were immunised [59]. However, a survey led by the UK Department of Health, which included over 90% of GP practices, found that only 14.9% of pregnant women received H1N1 immunisation (range: 2.1%–24.7%) [60]. Across Europe, the uptake varied with Spain, Hungary, Estonia and Slovenia reporting that <10% of pregnant women were immunised and the Netherlands reporting the highest rates of 58% [61]. Prior to the 2009 pandemic, few countries had policies to immunise all pregnant women against influenza. In the UK, the uptake varied by gestation, with the lowest probability in the first trimester. Women with underlying health conditions had much higher uptake [62]. This suggests further emphasis is needed on the safety and benefits of immunisation in pregnancy.
For seasonal influenza, only a small number of countries meet the World Health Assembly resolution goal to immunise >75% of vulnerable persons [57]. Of the 11 EU member states for which data are available, immunisation coverage varies considerably, from <1% in Armenia, the former Yugoslav Republic of Macedonia, Lithuania and Ukraine to 86.5% of women in the second and third trimester targeted in Kazakhstan [58]. Globally, Argentina reports one of the highest coverage rates, reaching over 95% of pregnant women annually from 2012 to 2014 [63]. The uptake of influenza immunisation in pregnant women in the UK is relatively low, with only 45% of all pregnant women receiving immunisation in 2018–2019 [64].
A 2013 systematic review of the uptake of influenza vaccination in pregnant women identified a number of characteristics that increased the likelihood of immunisation. These included women that were of white ethnicity and non-smokers [65], which have been confirmed in more recent studies [22,66]. Several studies have reported an association with higher socio-economic status such as professional employment or higher education [65] but not all studies are conclusive [22]. The characteristics of pregnant women associated with influenza vaccination uptake during seasonal campaigns and the H1N1 pandemic have been reported to be similar [65].
The reasons for differing coverage are complex, interrelated and specific to different contexts. In lower-resourced countries, limited vaccine procurement impedes coverage [58]. Where there is adequate policy and procurement in place, a number of other barriers have been cited. The circulating influenza strain is constantly changing; therefore, the strain of vaccine has been changing annually in both the Northern and Southern Hemisphere to match the circulating strain. This, along with diminishing immunity over time, means that vaccination in every pregnancy is recommended. In temperate climates, there are clear seasonal patterns of influenza transmission, so the vaccine is targeted in annual campaigns at the start of these periods. This has considerable operational implications, for example, in procurement, cold chain storage and health system capacity [56].
It is also recommended that vaccine delivery occurs through pre-existing healthcare contacts, for example, through routine antenatal care [67] as easy access to vaccination services improve the uptake [65]. In many LMIC, the coverage of antenatal care means that the opportunities for vaccine provision may be fewer. Argentina's success has been attributed to joint effort between operational and central health teams with the support of scientific societies and opinion leaders, and a committed campaign for community awareness, including media communications [63]. In the UK, vaccines can be provided by primary care, hospital-based maternity care or community pharmacies. Whilst this provides multiple opportunities for provision, it means accurate data capture is challenging and local policies differ, with immunisations rarely being provided in the same context as antenatal care. In the light of the SARS-CoV-2 pandemic, in the 2020/21 influenza season, all NHS Hospitals will be asked to offer vaccinations to pregnant women attending maternity appointments with the aim of improving coverage [68].
In the UK, there is variation in the proportion of women immunised against influenza based on their estimated due date. Women in their first or third trimester during the main influenza immunisation programme in November are less likely to be immunised [22]. Whilst in the first trimester, this may represent the delay in recognising or booking pregnancy, women in their third trimester have substantial contact with HCP and opportunity for immunisation. The most commonly cited reasons for not being immunised include: the perception that influenza is a mild disease and that the risk of infection was low and the lack of accurate knowledge that the vaccine is effective and safe [65,66,69]. Data from Public Health England show that only a small proportion of pregnant women in the UK refuse or decline immunisation (5·7%) [64]. Similarly, a systematic review identified that often women were not aware that they should receive the vaccine [65]. The provision of information on immunisation by HCP has consistently been shown to increase immunisation uptake by as much as 100-fold [[70], [71], [72]]. Pregnant women who were recommended immunisation by HCP were also more likely to believe it to be safe and effective [65]. However, evidence from surveys of HCP found that only 62% of midwives had received training on immunisation in pregnancy and 60% were confident in giving advice. Only 9% gave immunisations, despite the majority being happy to do so [73].
Whilst there is considerable evidence about the barriers to immunisation in pregnancy, there is little evidence to support interventions to overcome them. The National Institute for Health and Care Excellence (NICE) undertook a systematic review in 2018, which identified few studies in pregnancy. A number of interventions, including education resources/information by healthcare professionals, message reminder services and multi-component interventions, including vaccine champions and interactive educational materials have been studied, with predominantly negative findings [74].
Lessons learned
We have described a number of characteristics that increase the risk of severe outcomes with influenza in pregnancy. It is important to note that the similarities with those hospitalised with SARS-CoV-2, where over half of pregnant women admitted to hospital with SARS-CoV-2 infection in pregnancy were from black or other ethnic minority groups (56%), two-thirds were overweight or obese (69%) and a third had pre-existing co-morbidities (34%) [75]. This suggests that these groups are at a greater risk of future viral pandemic illness, and pandemic preparedness plans should take this into account. There may also be a role in optimising health pre-pregnancy to reduce the risks associated with seasonal influenza, alongside the promotion of immunisation and a lower threshold for seeking health care and admitting women from these risk groups, as has been recommended for women of BAME groups by the Royal College of Obstetricians and Gynaecologists (RCOG) in the SARS-CoV-2 pandemic [76].
Despite proven safety and efficacy, influenza immunisation policy and uptake vary globally. For policymakers to make informed decisions about immunisation programmes, reliable data are essential. It is, therefore, vital that pregnant women are included in immunisation trials, yet they are often systematically excluded [77]. A recent media report suggested that the first two COVID-19 vaccines to enter large-scale testing in the US will not include pregnant women [78]; similarly, pregnant women or those planning to become pregnant are currently excluded from both national vaccine trials in the UK [79]. Maternal immunisation has the potential to protect not just the mother but also the baby before delivery, and in the first few months of life, as shown with the influenza vaccine. Research should, therefore, also aim to assess the impact of immunisation throughout this vulnerable period to fully inform investment decisions.
Should a COVID-19 vaccine become available for use in pregnancy, implementation programmes will need to take into account the challenges faced in delivery of other immunisations. However, there is a lack of evidence to identify ways to overcome barriers to the vaccination of pregnant women. Strategies could consider the ease of access, ideally within routine antenatal care with training of maternity care providers and dissemination of accessible information on the risks and benefits to the mother, fetus and infant. Communication strategies would need to remain flexible as information arises and may differ as risk groups are identified, for example, targeting language barriers [80].
Summary
Influenza in pregnancy is a relatively common condition that can cause increased risk of hospital admission, particularly in the later stages of pregnancy. Whilst the risk of severe morbidity and mortality from influenza in pregnancy is not increased as compared to the general population, it still causes excess hospital admissions and preventable deaths. Some studies suggest that women with a raised BMI, of black or other minority ethnic group, or with underlying medical conditions are at a greater risk of severe outcomes. Data on the perinatal impact are very heterogeneous but there is evidence that severe influenza may increase the risk of preterm birth and fetal death or intensive care admission. In infants under 6 months, limited evidence suggests that influenza increases the risk of hospitalisation and severe lower respiratory infections. There are substantial gaps in our knowledge of the impact of influenza, particularly in LMIC. Future pandemic strategies in pregnancy should consider mechanisms for collecting and communicating data.
Influenza vaccines are cost-effective, safe and effective to help prevent influenza in pregnant women and their infants. However, the majority of evidence relates to high-income settings. Pregnant women should be included in vaccine trials, which should also examine the impact on infants. Policies on immunisation in pregnancy vary worldwide and many countries have poor uptake. There are many interrelated reasons for this, but operational challenges and concern over the safety, efficacy and necessity of immunisation are common. There is insufficient evidence on how to overcome these barriers.
Practice points.
-
•
Influenza immunisation should be promoted to all pregnant women at any stage of pregnancy.
-
•
Antiviral treatment should be commenced as early as possible in pregnant women with signs of influenza.
-
•
Pandemic preparedness plans should consider mechanisms to collect and communicate data and how to deliver essential maternity care safely.
Research agenda.
-
•
There is a lack of epidemiological, and therefore cost-effectiveness, studies of influenza in pregnancy undertaken in low- and middle-income countries. Current surrogates of hospital and ICU admission may inadequately describe morbidity in this setting.
-
•
Vaccine trials should include pregnant women and should explore the longer-term impact of maternal immunisation on infant outcomes.
-
•
Future research should explore how to overcome barriers to immunisation in pregnancy.
Declaration of competing interest
The authors have no conflict of interests to declare.
Acknowledgements
Professor Knight is a National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bpobgyn.2020.08.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organisation . 2018. Influenza (seasonal) factsheet.https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) [06/08/2020]. Available from: [Google Scholar]
- 2.World Health Organisation . 2018. Pandemic influenza.https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/pandemic-influenza Available from: [Google Scholar]
- 3.Katz M.A., Gessner B.D., Johnson J., Skidmore B., Knight M., Bhat N., et al. Incidence of influenza virus infection among pregnant women: a systematic review. BMC Pregnancy Childbirth. 2017;17(1):155. doi: 10.1186/s12884-017-1333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horby P.W. Community studies of influenza: new knowledge, new questions. Lancet Respir Med. 2014;2(6):430–431. doi: 10.1016/S2213-2600(14)70053-0. [DOI] [PubMed] [Google Scholar]
- 5.Hayward A.C., Fragaszy E.B., Bermingham A., Wang L., Copas A., Edmunds W.J., et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2(6):445–454. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siston A.M., Rasmussen S.A., Honein M.A., Fry A.M., Seib K., Callaghan W.M., et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. J Am Med Assoc. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creanga A.A., Johnson T.F., Graitcer S.B., Hartman L.K., Al-Samarrai T., Schwarz A.G., et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115(4):717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 8.Mertz D., Geraci J., Winkup J., Gessner B.D., Ortiz J.R., Loeb M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and meta-analysis of observational studies. Vaccine. 2017;35(4):521–528. doi: 10.1016/j.vaccine.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertz D., Lo C.K.-F., Lytvyn L., Ortiz J.R., Loeb M., Ang L.W., et al. Pregnancy as a risk factor for severe influenza infection: an individual participant data meta-analysis. BMC Infect Dis. 2019;19(1):683. doi: 10.1186/s12879-019-4318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight M.K.S., Brocklehurst P., Neilson J., Shakespeare J., Kurinczuk J., On behalf of MBRRACE-UK . National Perinatal Epidemiology Unit; Oxford: 2014. Saving Lives, Improving Mothers' Care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and Morbidity 2009–2012. [Google Scholar]
- 11.Jefferson T., Jones M.A., Doshi P., Del Mar C.B., Hama R., Thompson M.J., et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;2014(4) [Google Scholar]
- 12.Oboho I.K., Reed C., Gargiullo P., Leon M., Aragon D., Meek J., et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis. 2016;214(4):507–515. doi: 10.1093/infdis/jiw033. [DOI] [PubMed] [Google Scholar]
- 13.Louie J.K., Acosta M., Jamieson D.J., Honein M.A. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health and Royal College of Obstetricians and Gynaecologists . 2009. Pandemic H1N1 2009 influenza: clnical managament guidelines for pregnancy. [Google Scholar]
- 15.Knight M.N.M., Tuffnell D., Shakespeare J., Kenyon S., Kurinczuk J.J., on behalf of MBRRACE-UK . 2013. Saving Lives, improving mother's care- Lessons learned to inform maternity care from the UK and Ireland confidential Enquiries into maternal deaths and morbidity; p. 152017. [Google Scholar]
- 16.Fell D.B., Savitz D.A., Kramer M.S., Gessner B.D., Katz M.A., Knight M., et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG An Int J Obstet Gynaecol. 2017;124(1):48–59. doi: 10.1111/1471-0528.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fell D.B., Azziz-Baumgartner E., Baker M.G., Batra M., Beauté J., Beutels P., et al. Influenza epidemiology and immunization during pregnancy: final report of a World Health Organization working group. Vaccine. 2017;35(43):5738–5750. doi: 10.1016/j.vaccine.2017.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle T.J., Goodin K., Hamilton J.J. Maternal and neonatal outcomes among pregnant women with 2009 pandemic influenza A(H1N1) illness in Florida, 2009-2010: a population-based cohort study. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0079040. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce M., Kurinczuk J.J., Spark P., Brocklehurst P., Knight M. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ. 2011;342:d3214. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laake I., Tunheim G., Robertson A.H., Hungnes O., Waalen K., Haberg S.E., et al. Risk of pregnancy complications and adverse birth outcomes after maternal A(H1N1)pdm09 influenza: a Norwegian population-based cohort study. BMC Infect Dis. 2018;18(1):525. doi: 10.1186/s12879-018-3435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fell D.B., Platt R.W., Basso O., Wilson K., Kaufman J.S., Buckeridge D.L., et al. The relationship between 2009 pandemic H1N1 influenza during pregnancy and preterm birth: a population-based cohort study. Epidemiology. 2018;29(1):107–116. doi: 10.1097/EDE.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 22.Vousden N., Bunch K., Knight M., the UKOSS Influenza Co-Investigators Group . 2020. Incidence, risk factors and impact of seasonal influenza in pregnancy: a population-basede case control study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeil S.A., Dodds L.A., Fell D.B., Allen V.M., Halperin B.A., Steinhoff M.C., et al. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S54–S57. doi: 10.1016/j.ajog.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Håberg S.E., Trogstad L., Gunnes N., Wilcox A.J., Gjessing H.K., Samuelsen S.O., et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368(4):333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunnes N., Gjessing H.K., Bakken I.J., Ghaderi S., Gran J.M., Hungnes O., et al. Seasonal and pandemic influenza during pregnancy and risk of fetal death: a Norwegian registry-based cohort study. Eur J Epidemiol. 2020;35(4):371–379. doi: 10.1007/s10654-020-00600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luteijn J.M., Brown M.J., Dolk H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum Reprod. 2014;29(4):809–823. doi: 10.1093/humrep/det455. [DOI] [PubMed] [Google Scholar]
- 27.Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A., et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 28.Fell D.B., Johnson J., Mor Z., Katz M.A., Skidmore B., Neuzil K.M., et al. Incidence of laboratory-confirmed influenza disease among infants under 6 months of age: a systematic review. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-016526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhat N., Wright J.G., Broder K.R., Murray E.L., Greenberg M.E., Glover M.J., et al. Influenza-associated deaths among children in the United States, 2003-2004. N Engl J Med. 2005;353(24):2559–2567. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 30.Yates L.M., Pierce M., Stephens S., Mill A.C., Spark P., Kurinczuk J.J., et al. Influenza A/H1N1v in pregnancy: aninvestigation of the characteristics of affected women and the relationship to pregnancy outcomes for mother and infant. Health Technol Assess. 2010;14(34):109–182. doi: 10.3310/hta14340-02. [DOI] [PubMed] [Google Scholar]
- 31.The ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillace System Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organisation . World Health Organisation; Geneva, Switzerland: 2012. WHO position paper on influenza vaccines - November 2012. [Google Scholar]
- 33.McLean K.A., Goldin S., Nannei C., Sparrow E., Torelli G. The 2015 global production capacity of seasonal and pandemic influenza vaccine. Vaccine. 2016;34(45):5410–5413. doi: 10.1016/j.vaccine.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organisation . 2011. Pandemic influenza preparedness framework. Geneva, Switzerland. [Google Scholar]
- 35.Munoz F.M. Safety of influenza vaccines in pregnant women. Am J Obstet Gynecol. 2012;207(3):S33–S37. doi: 10.1016/j.ajog.2012.06.072. [DOI] [PubMed] [Google Scholar]
- 36.Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E., et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 37.Tapia M.D., Sow S.O., Tamboura B., Tégueté I., Pasetti M.F., Kodio M., et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16(9):1026–1035. doi: 10.1016/S1473-3099(16)30054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinhoff M.C., Katz J., Englund J.A., Khatry S.K., Shrestha L., Kuypers J., et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17(9):981–989. doi: 10.1016/S1473-3099(17)30252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson M.G., Kwong J.C., Regan A.K., Katz M.A., Drews S.J., Azziz-Baumgartner E., et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: a multi-country retrospective test negative design study, 2010-2016. Clin Infect Dis: Off Public Infect Dis Soc Am. 2019;68(9):1444–1453. doi: 10.1093/cid/ciy737. [DOI] [PubMed] [Google Scholar]
- 40.Madhi S.A., Cutland C.L., Kuwanda L., Weinberg A., Hugo A., Jones S., et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 41.Omer S.B., Clark D.R., Madhi S.A., Tapia M.D., Nunes M.C., Cutland C.L., et al. Efficacy, duration of protection, birth outcomes, and infant growth associated with influenza vaccination in pregnancy: a pooled analysis of three randomised controlled trials. Lancet Respir Med. 2020;8(6):597–608. doi: 10.1016/S2213-2600(19)30479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fell D.B., Platt R.W., Lanes A., Wilson K., Kaufman J.S., Basso O., et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG An Int J Obstet Gynaecol. 2015;122(1):17–26. doi: 10.1111/1471-0528.12977. [DOI] [PubMed] [Google Scholar]
- 43.Nunes M.C., Aqil A.R., Omer S.B., Madhi S.A. The effects of influenza vaccination during pregnancy on birth outcomes: a systematic review and meta-analysis. Am J Perinatol. 2016;33(11):1104–1114. doi: 10.1055/s-0036-1586101. [DOI] [PubMed] [Google Scholar]
- 44.Giles M.L., Krishnaswamy S., Macartney K., Cheng A. The safety of inactivated influenza vaccines in pregnancy for birth outcomes: a systematic review. Hum Vaccines Immunother. 2019;15(3):687–699. doi: 10.1080/21645515.2018.1540807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bratton K.N., Wardle M.T., Orenstein W.A., Omer S.B. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Infect Dis : Off Public Infect Dis Soc Am. 2015;60(5):e11–e19. doi: 10.1093/cid/ciu915. [DOI] [PubMed] [Google Scholar]
- 46.Nunes M.C., Madhi S.A. Influenza vaccination during pregnancy for prevention of influenza confirmed illness in the infants: a systematic review and meta-analysis. Hum Vaccines Immunother. 2018;14(3):758–766. doi: 10.1080/21645515.2017.1345385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jarvis J.R., Dorey R.B., Warricker F.D.M., Alwan N.A., Jones C.E. The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: systematic review and meta-analysis. Vaccine. 2020;38(7):1601–1613. doi: 10.1016/j.vaccine.2019.12.056. [DOI] [PubMed] [Google Scholar]
- 48.Omer S.B., Clark D.R., Aqil A.R., Tapia M.D., Nunes M.C., Kozuki N., et al. Maternal influenza immunization and prevention of severe clinical pneumonia in young infants: analysis of randomized controlled trials conducted in Nepal, Mali and South Africa. Pediatr Infect Dis J. 2018;37(5):436–440. doi: 10.1097/INF.0000000000001914. [DOI] [PubMed] [Google Scholar]
- 49.Beigi R.H., Wiringa A.E., Bailey R.R., Assi T.-M., Lee B.Y. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis: Off Public Infect Dis Soc Am. 2009;49(12):1784–1792. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baguelin M., Hoek A.J.V., Jit M., Flasche S., White P.J., Edmunds W.J. Vaccination against pandemic influenza A/H1N1v in England: a real-time economic evaluation. Vaccine. 2010;28(12):2370–2384. doi: 10.1016/j.vaccine.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Xu J., Zhou F., Reed C., Chaves S.S., Messonnier M., Kim I.K. Cost-effectiveness of seasonal inactivated influenza vaccination among pregnant women. Vaccine. 2016;34(27):3149–3155. doi: 10.1016/j.vaccine.2016.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jit M., Cromer D., Baguelin M., Stowe J., Andrews N., Miller E. The cost-effectiveness of vaccinating pregnant women against seasonal influenza in England and Wales. Vaccine. 2010;29(1):115–122. doi: 10.1016/j.vaccine.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 53.Skedgel C., Langley J.M., MacDonald N.E., Scott J., McNeil S. An incremental economic evaluation of targeted and universal influenza vaccination in pregnant women. Can J Public Health. 2011;102(6):445–450. doi: 10.1007/BF03404197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orenstein E.W., Orenstein L.A.V., Diarra K., Djiteye M., Sidibé D., Haidara F.C., et al. Cost-effectiveness of maternal influenza immunization in Bamako, Mali: a decision analysis. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0171499. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Francisco N., Donadel M., Jit M., Hutubessy R. A systematic review of the social and economic burden of influenza in low- and middle-income countries. Vaccine. 2015;33(48):6537–6544. doi: 10.1016/j.vaccine.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 56.Sobanjo-ter Meulen A., Abramson J., Mason E., Rees H., Schwalbe N., Bergquist S., et al. Path to impact: a report from the Bill and Melinda Gates Foundation convening on maternal immunization in resource-limited settings; Berlin – January 29–30, 2015. Vaccine. 2015;33(47):6388–6395. doi: 10.1016/j.vaccine.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 57.Ortiz J.R., Perut M., Dumolard L., Wijesinghe P.R., Jorgensen P., Ropero A.M., et al. A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine. 2016;34(45):5400–5405. doi: 10.1016/j.vaccine.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jorgensen P., Mereckiene J., Cotter S., Johansen K., Tsolova S., Brown C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018;36(4):442–452. doi: 10.1016/j.vaccine.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng S., Wu P., Nishiura H., Ip D.K., Lee E.S., Cowling B.J. An analysis of national target groups for monovalent 2009 pandemic influenza vaccine and trivalent seasonal influenza vaccines in 2009-10 and 2010-11. BMC Infect Dis. 2011;11:230. doi: 10.1186/1471-2334-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sethi MP R. Department of Health, Health Protection Authority; 2010. Pandemic H1N1 (Swine) influenza vaccine uptake amongst patient groups in primary care in England. [Google Scholar]
- 61.O'Flanagan D., Cotter S., Mereckiene J. 2011. Pandemic A(H1N1) 2009 influenza vaccination survey, influenza season 2009/2010. [Google Scholar]
- 62.Sammon C.J., McGrogan A., Snowball J., de Vries C.S. Pandemic influenza vaccination during pregnancy: an investigation of vaccine uptake during the 2009/10 pandemic vaccination campaign in Great Britain. Hum Vaccines Immunother. 2013;9(4):917–923. doi: 10.4161/hv.23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vizzotti C., Neyro S., Katz N., Juárez M.V., Perez Carrega M.E., Aquino A., et al. Maternal immunization in Argentina: a storyline from the prospective of a middle income country. Vaccine. 2015;33(47):6413–6419. doi: 10.1016/j.vaccine.2015.07.109. [DOI] [PubMed] [Google Scholar]
- 64.Public Health England . Public Health England; Wellington House, London: 2019. Seasonal influenza vaccine uptake in GP patients: winter season 2018 to 2019. [Google Scholar]
- 65.Yuen C.Y.S., Tarrant M. Determinants of uptake of influenza vaccination among pregnant women – a systematic review. Vaccine. 2014;32(36):4602–4613. doi: 10.1016/j.vaccine.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 66.Wilcox C.R., Calvert A., Metz J., Kilich E., MacLeod R., Beadon K., et al. Determinants of influenza and pertussis vaccination uptake in pregnancy: a multicenter questionnaire study of pregnant women and healthcare professionals. Pediatr Infect Dis J. 2019;38(6):625–630. doi: 10.1097/INF.0000000000002242. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organisation . Department of immunization, vaccines and biologicals, World Health Organisation; Geneva: 2017. How to implement influenza vaccination of pregnant women. [Google Scholar]
- 68.Department of Health & Social Care and Public Health England . 2020. The national flu immunisation programme 2020 to 2021 - update.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907149/Letter_annualflu_2020_to_2021_update.pdf 06/08/20. [Google Scholar]
- 69.Bodeker B., Walter D., Reiter S., Wichmann O. Cross-sectional study on factors associated with influenza vaccine uptake and pertussis vaccination status among pregnant women in Germany. Vaccine. 2014;32(33):4131–4139. doi: 10.1016/j.vaccine.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Healy C.M., Rench M.A., Montesinos D.P., Ng N., Swaim L.S. Knowledge and attitiudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine. 2015;33(41):5445–5451. doi: 10.1016/j.vaccine.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 71.Ishola D.A., Jr., Permalloo N., Cordery R.J., Anderson S.R. Midwives' influenza vaccine uptake and their views on vaccination of pregnant women. J Publ Health. 2013;35(4):570–577. doi: 10.1093/pubmed/fds109. [DOI] [PubMed] [Google Scholar]
- 72.Collins J., Alona I., Tooher R., Marshall H. Increased awareness and health care provider endorsement is required to encourage pregnant women to be vaccinated. Hum Vaccines Immunother. 2014;10(10):2922–2929. doi: 10.4161/21645515.2014.971606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vishram B., Letley L., Jan Van Hoek A., Silverton L., Donovan H., Adams C., et al. Vaccination in pregnancy: attitudes of nurses, midwives and health visitors in England. Hum Vaccines Immunother. 2018;14(1):179–188. doi: 10.1080/21645515.2017.1382789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; 2018. Flu vaccination: increasing uptake, Evidence reviews for increasing uptake in clinical risk groups. [Google Scholar]
- 75.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Royal College of Obstetricians and Gynaecologists and The Royal College of Midwives . 2020. Coronavirus (COVID-19) infection in pregnancy: information for healthcare professionals. London. [Google Scholar]
- 77.Whitehead C.L., Walker S.P. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet. 2020;395(10237):e92. doi: 10.1016/S0140-6736(20)31029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steenhuysen J. Reuters Chicago; 2020. Large U.S. COVID-19 vaccine trials will exclude pregnant women for now. [Google Scholar]
- 79.University of Oxford . 2020. COVID-19 vaccine study (COV002) Oxford, United Kingdom.https://covid19vaccinetrial.co.uk/participate-oxford Available from: [Google Scholar]
- 80.Rasmussen S.A., Jamieson D.J., Macfarlane K., Cragan J.D., Williams J., Henderson Z., et al. Pandemic influenza and pregnant women: summary of a meeting of experts. Am J Publ Health. 2009;99(Suppl 2):S248–S254. doi: 10.2105/AJPH.2008.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.