Abstract
Molecular tension sensors measure piconewton forces experienced by individual proteins in the context of the cellular microenvironment. Current genetically-encoded tension sensors use FRET to report on extension of a deformable peptide encoded in a cellular protein of interest. Here we present the development and characterization of a new type of molecular tension sensor based on bioluminescence resonance energy transfer (BRET) which exhibits more desirable spectral properties and an enhanced dynamic range compared to other molecular tension sensors. Moreover, it avoids many disadvantages of FRET measurements in cells, including autofluorescence, photobleaching, and corrections of direct acceptor excitation. We benchmark the sensor by inserting it into the canonical mechanosensing focal adhesion protein vinculin, observing highly resolved gradients of tensional changes across focal adhesions. We anticipate that the BRET-TS will expand the toolkit available to study mechanotransduction at a molecular level and allow potential extension to an in vivo context.
Keywords: molecular tension sensor, TSMod, bioluminescence, BRET, NanoLuc
Graphical abstract
The mechanical microenvironment of a cell guides cellular processes such as migration, differentiation, division, and signaling1, and is thus often altered in disease2,3. At a molecular level, mechanical stimuli alter conformations of mechanosensing proteins to communicate the stimulus to the cell interior and effect a downstream cellular response in a process called mechanotransduction4,5. Measuring tensions sensed by cellular proteins and how tensions are altered by cellular context will lead to better understanding of the molecular mechanisms used in mechanosensing in disease-related processes such as tumor migration.
Tools to measure tensions at a cell and tissue level such as traction force microscopy and atomic force microscopy are well-established. Tools to measure molecular level tensions have only emerged more recently to characterize the pN forces present across mechanosensitive proteins6 and have been used to measure tensions sensed by focal adhesions proteins such as vinculin, talin, and integrins, as well as cadherins7–10. Generally, genetically encodable molecular tension sensors contain a deformable peptide linker with well-characterized extension in response to applied force flanked by two fluorescent proteins (FP). The extent of linker stretching due to mechanical force can measured by Förster resonance energy transfer (FRET) and subsequently be correlated to a quantifiable force. Measurement of tension across a protein typically utilizes Förster resonance energy transfer (FRET), though other readouts of signal output generated by molecular tension sensors include proximity imaging11, nanometal surface energy transfer12, catalytic amplification13 and contact quenching14. Until recently, the state of the art genetically-encoded FRET tension was TSMod, a distance dependent FRET-based sensor that can measure tensions in the 1–6 pN range7. The dynamic range of TSMod, however, is limited by the photophysical properties of the fluorescent protein pair, mTFP1 and venus(A206K)15. Recent studies reported improved FRET tension sensors by using more optimal fluorescent proteins, utilizing different linkers, and engineering FP termini to achieve closer distances that yielded higher FRET under no load16,17. A computational model has also been described to allow researchers to optimize force range or sensitivity based on experimental needs16. Nonetheless, these sensors are still constrained by the limitations of FRET, namely autofluorescence, direct acceptor excitation, photobleaching, and difficult in vivo imaging. We developed a genetically-encodable molecular tension sensor based on bioluminescence resonance energy transfer (BRET) that overcomes several limitations of FRET. BRET is a distance and orientation dependent phenomenon analogous to FRET but is initiated by a chemiluminescent reaction of a luciferase protein with its substrate instead of light excitation18. Upon luciferase excitation, non-radiative energy transfer can occur to excite a closely linked acceptor fluorescent protein that produces a distinct emission spectrum. Our BRET tension sensor, which we will refer to as BRET-TS, takes advantage of the uncharacteristically bright Nanoluciferase (NanoLuc), allowing in vitro and live cell detection of signal using standard plate readers and microscopes. The luminescent-fluorescent protein pair of NanoLuc19 and mNeonGreen20 has recently been shown to exhibit robust resonance energy transfer efficiency21. Here we report on using these proteins as a sensitive BRET donor-acceptor pair in a molecular tension sensor. Using BRET as a distance reporter in molecular tension sensors allows for more sensitive readouts due to the lack of autofluorescence and phototoxicity. BRET also offers the potential for use in vivo where light excitation is not able to penetrate into tissue. We demonstrate the utility of this tension sensor in vitro and in cell based assays, using vinculin as the model system to show an enhanced dynamic range compared to field standard FRET-based sensors.
RESULTS AND DISCUSSION
Sensor design and characterization
In the BRET-based molecular tension sensor (BRET-TS), the NanoLuc donor and mNeonGreen acceptor proteins flank a 40 amino acid flexible spider silk flagelliform (FL) domain (GPGGA)8 (Figure 1A). Flagelliform exhibits predictable changes in length under tension22. Both proteins are terminally truncated to enhance the base BRET efficiency by bringing them in closer proximity21. We first expressed recombinant BRET-TS and the commonly used FRET-TSMod to compare their spectral properties7.
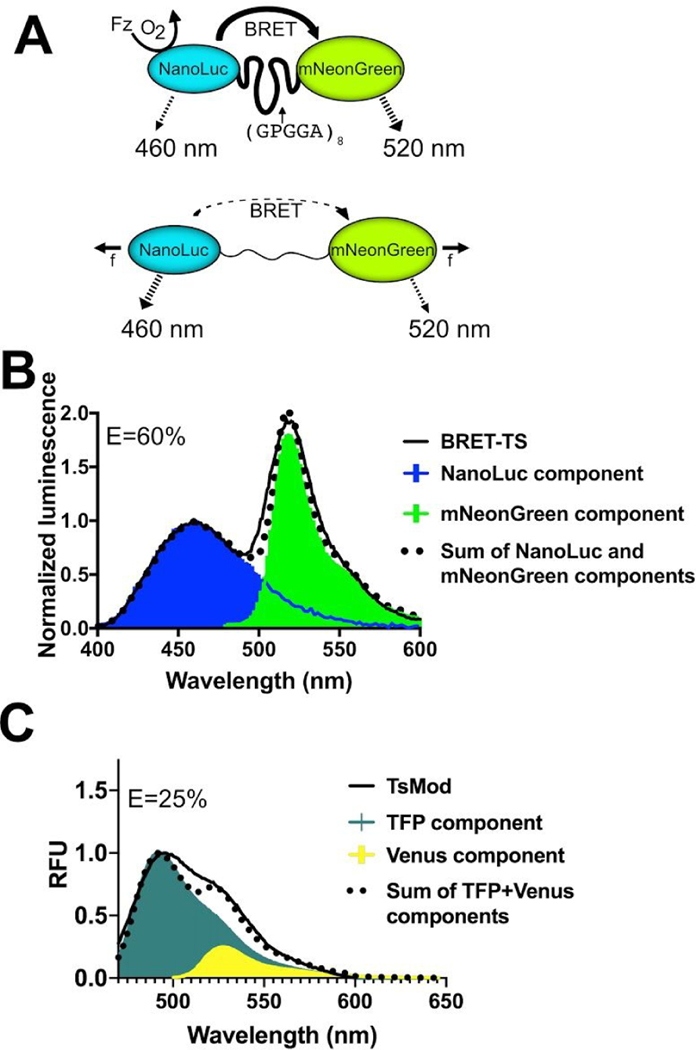
Figure 1.
Schematic of the genetically-encodable BRET molecular tension sensor (BRET-TS). (A) NanoLuc, the energy donor, is excited via addition of furimazine (Fz) in the presence of oxygen. In the absence of force (f), resonance energy transfer occurs to the fluorescent acceptor, mNeonGreen. With applied force, the donor-acceptor pair is separated, reducing resonance energy transfer. Unloaded spectral and resonance energy transfer properties of (B) BRET-TS in comparison to (C) FRET-based TSMod. Spectral components and resulting best-fit additive spectrum depicted. Axes in units of luminescence normalized at 460 nm and relative fluorescence units (RFU).
The apparent BRET efficiency E was calculated from spectral data using the ratiometric intensity method, as is common when detecting BRET. The following equation was used: E= ImNeonGreen@520nm/(INluc@460nm+ImNeonGreen@520nm) including a correction for the contribution of a small fraction of of NanoLuc emission at the mNeonGreen emission wavelength. Further details of calculation of apparent E are discussed in the Methods section. In the absence of tension, the unloaded BRET sensor boasts a robust ~60% apparent BRET efficiency upon addition of NanoLuc’s chemiluminescent substrate, furimazine (Figure 1B). Moreover, the emission maxima of NanoLuc (460 nm) and mNeonGreen (517 nm) are spectrally separated by 57 nm. In contrast, the unloaded TSMod FRET tension sensor expressed in the same context as the BRET-TS exhibits only 25% apparent energy transfer with extensive spectral overlap between mTFP and venus (Figure 1C)7. Recent iterations of FRET tension sensors using Clover and Ruby derived FPs display improved spectral separation but still only achieve unloaded energy transfer efficiencies of 45%16.
An apparent BRET efficiency of 60% approaches energy transfer efficiencies observed for unloaded spider silk peptides flanked by organic fluorophores Cy3 and Cy57,17,23 used to calibrate the sensors, despite the usually detrimental contribution of increased distance that FPs contribute. This is likely, in part, due to the smaller size of NanoLuc resulting in closer proximity to mNeonGreen. Based on crystal structures, NanoLuc (PDB: 5IBO) has a radius as small as 13 Å compared to traditional FPs like eGFP (PDB: 2Y0G) with a radius of ~24 Å. Moreover, when calculating BRET efficiencies using ratiometric intensities of acceptor to donor, the measured ratios depend not only on the energy transfer efficiency, but also on the bleedthrough of donor emission in the acceptor channel, differential detector sensitivities at the two wavelengths, and the ratio of acceptor to donor quantum yields. The mNeonGreen acceptor has a higher quantum yield than the NanoLuc donor, thus increasing the apparent BRET efficiency when measurements are made using ratiometric intensities. This is discussed in detail in the Methods section. Finally, both mNeonGreen and NanoLuc proteins in this sensor have their termini that flank the flagelliform peptide truncated, potentially impacting the assumption of random orientation in space. The enhanced apparent BRET efficiency at zero force translates to a larger dynamic range, as the sensor has the potential to detect a 60% change in energy transfer when force is applied, making it easier to detect higher forces and resolve the entire range of forces. The large spectral separation of the donor and acceptor is also desirable for ratiometric imaging, where spectral filters are used to separate emission from donor and acceptor.
Apparent energy transfer efficiencies E were calculated as described in Methods. Upon application of tension to the deformable peptide, we expect the spider silk linker to stretch, separating the protein pair and causing a decrease in the RET efficiency. Thus, we next aimed to ensure that BRET-TS exhibits the expected changes in RET with distance between the donor and acceptor. We initially characterized the distance dependence of BRET by inserting a series of rigid alpha helical linkers (HL) A(EAAAK)nA (Figure 2A) of known lengths between mNeonGreen and NanoLuc in the context of recombinant proteins expressed in E. Coli24. Expectedly, as the distance between the protein pair increases, a decrease in BRET is observed when measured in bulk using a standard plate reader (Figure 2B).
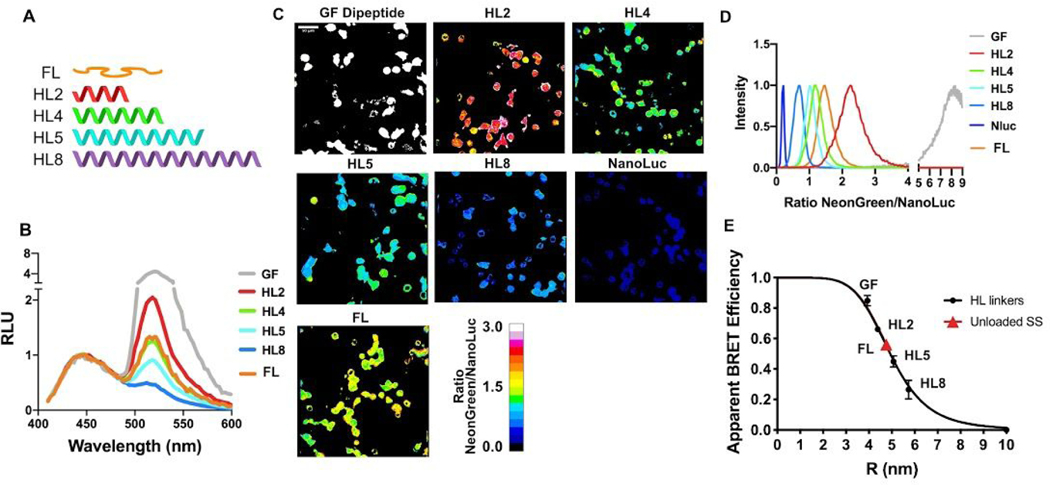
Figure 2.
Characterization of distance dependence of BRET-TS in vitro and in cells. (A) Rigid alpha helical linkers (HL) of varying lengths were inserted between the mNeonGreen-NanoLuc pair24 in addition to a minimal dipeptide GF linker. NanoLuc alone was also measured. (B) Spectral plate reader emissions of recombinantly expressed proteins, normalized to NanoLuc peak emission (460 nm). (C) HEK293T cells transfected with the various HL linker constructs inserted into a plasma membrane localized protein. Cells were imaged using NanoLuc and mNeonGreen specific spectral filters and ratiometric mNeonGreen:NanoLuc images are shown. (D) Histograms of a field of cells from C were calculated and normalized to 1 to derive the average BRET ratio for a given linker. (E) Apparent RET vs R curves comparing HL linker BRET efficiency derived from in vitro recombinant protein BRET-TS and cell-surface BRET-TS versus distance r. R was calculated from apparent BRET efficiencies corrected for the ratio of acceptor to donor quantum yields (see Methods) and using the measured R0 least squares fit to 1/R6 function.
We next encoded the series of alpha helical linkers sandwiched between mNeonGreen and NanoLuc into a cell surface expressed protein to characterize RET as a function of distance in cells. Constructs were transiently transfected into HEK293T or U2OS cells and BRET read out on a plate reader on via ratiometric imaging. Remarkably, the cellular BRET was easily detectable on a plate reader, underscoring the brightness and low background of NanoLuc (Figure S1). To detect BRET localization in live cells, furimazine was added to the media and the ratio of emission of mNeonGreen to NanoLuc was measured simultaneously or sequentially using an EM-CCD camera outfitted with spectral filters specific to NanoLuc and mNeonGreen emissions (Figure 2C). The pixel-by-pixel ratio of the two images was then calculated. Emission from NanoLuc alone as well as emission from the donor and acceptor connected by a short dipeptide glycine-phenylalanine (“GF”) linker were also measured for reference. Again, a drastic decrease in BRET is observed with increasing linker length (Figure 2D). Heterogeneity in the BRET signal, likely reflecting a diversity in mechanical microenvironments sensed by the cell-surface BRET-TS, is also apparent in the cell experiments that is masked in the in vitro bulk experiments. The cell surface construct (see methods for details) displaying BRET-TS may be differentially subjected to cellular forces, such as adhesive forces via its Protein G, EGF-like and CD8 domains25 and endocytic forces via the C-terminal domain of the Notch ligand DLL4. To ensure the BRET observed was due to intramolecular rather than intermolecular effects, we co-transfected cell surface receptors containing either NanoLuc or mNeonGreen alone. We did not observe any BRET signal, consistent with a lack of intermolecular BRET in this context (Figure S2A). Similar results were obtained when inserting a 3C proteolytic cleavage site in the recombinant protein version. Cleavage by HRV 3C protease abolished BRET resulting in only the expected NanoLuc emission (Figure S2B).
To determine if the ratios derived from in vitro and in cell linker experiments were similar, we plotted the BRET efficiency as a function of distance. We first calculated histograms of ratiometric images from Figure 2B to determine average NeonGreen to NanoLuc ratios. To convert BRET ratios to BRET efficiencies, we first corrected for the Nanoluc / NeonGreen emission overlap and calculated BRET efficiency using the ratio of intensities and the equation E=ratiomNG:NLuc/(1+ratiomNG:NLuc) (further details in Methods).
We next calculated the Förster radius (R0) of mNeonGreen-NanoLuc to be 51 Å. This was based on first experimentally calculating the spectral overlap (J = 2.84*1015 nm4M−1 cm−1) using the extinction coefficient of mNeonGreen and the normalized emission spectrum of NanoLuc. The Förster radius was then calculated using NanoLuc’s previously reported quantum yield26 and assuming a random orientation of the donor and acceptor. This is the highest reported Förster radius of any NanoLuc-FP pair27. Using this R0 and our experimental BRET efficiencies, we then calculated R values for the helical linkers using standard FRET equations and plotted them against BRET efficiency (Figure 2E). It should be noted that though apparent BRET efficiency is plotted on the y-axis, R was calculated using BRET efficiencies that have been corrected for the high acceptor to donor quantum yield ratio as described in methods. A similar curve is obtained using the R values previously calculated for these linkers24 (Figure S3). The BRET efficiencies measured in vitro and in cells were remarkably similar for a given linker, and we observed no significant difference between the image splitter and filter wheel methods of ratiometric imaging.
Measuring tension across vinculin
Finally, we wanted to validate BRET-TS in a known mechanosensing protein. We chose the focal adhesion protein vinculin that has previously been shown to experience tensions on the order of 1 to 6 pN using molecular tension sensors7. This system provides a good opportunity to benchmark the dynamic range of BRET-TS against state-of-the-art FRET tension sensors. We inserted the BRET-TS into a previously described site within the protein after residue 883 (VinTS). We also created a force-insensitive control construct lacking the carboxyl terminal F-actin binding tail (VinTL) (Figure 3A). As this tension sensor is 84 amino acids smaller than TSMod, we did not repeat previously reported functional characterization of vinculin with the inserted sensor7 aside from assessing colocalization with paxillin staining (Figure S4).
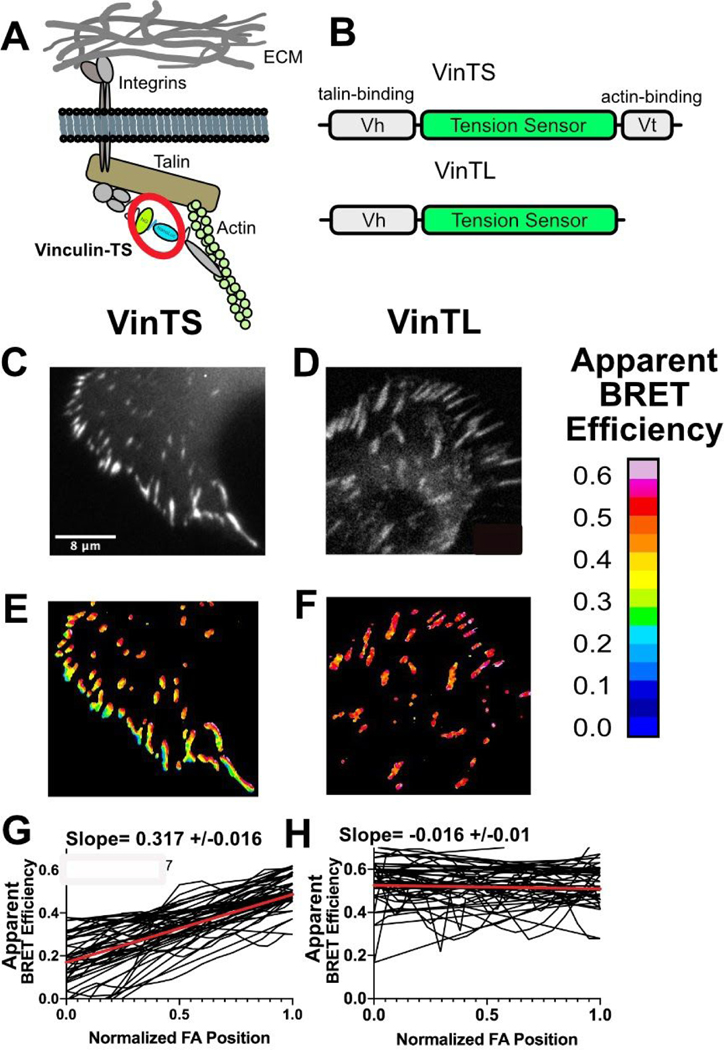
Figure 3.
Measuring tension across vinculin. (A) Schematic of vinculin with inserted BRET tension sensor (BRET-TS) in the context of a focal adhesion. (B) Vinculin constructs used in these studies: full length vinculin with inserted BRET-TS (VinTS) and actin binding deficient vinculin with BRET-TS (VinTL). (C–D) Unprocessed luminescent images of focal adhesions. (E–F) Processed, ratiometric images from (C–D). Scale bar displayed as apparent BRET efficiency (out of 1 maximum). (G–H) Manual line scans taken across focal adhesions, normalized for focal adhesion (FA) length (n=30). The red line represents the linear regression of all line scans combined. The slope of the line is given +/− the standard error of the linear regression calculation. Images and line scans are representative from images collected over numerous independent experiments.
Remarkably, luciferase signal from VinTS was clearly observable in a bulk plate reader assay in comparison to zero signal in untransfected cells (Figure S5). In contrast, direct excitation of mNeonGreen at 450 nm resulted in the same signal in both untransfected and transfected cells due to cellular autofluorescence. For imaging of VinTS in focal adhesions, constructs were transfected in U2OS cells and plated sparsely on fibronectin. The cells were subsequently imaged upon addition of furimazine. We observed a gradient in tension across peripheral focal adhesions28 in VinTS that was not observed in the force-insensitive mutant VinTL (Figure 3B–H). Tension gradients were not observed in the original FRET-TS TSMod due to insufficient dynamic range7, but were recently detected in improved FRET-TS’s16. BRET efficiency is lowest at the cell periphery, which corresponds to higher forces. BRET efficiency gradually increases (force decreases) moving towards the nucleus (Figure 3C). This gradient is abolished in the actin binding mutant lacking the carboxy-terminus of vinculin and in point mutants of the actin or talin binding domains (Figure 3H and S6)29. BRET-TS provides better resolution in highlighting the gradient across the focal adhesion compared to previous studies; we observe an average 31% change in BRET across peripheral focal adhesions, compared to 5% and 15% FRET gradients reported for TSMod and improved TSMods, respectively7,16. VinTS has already been used to dissect molecular mechanisms of collagen matrix formation in stem cells30 and measure the effect of osteocytes on migration potential of breast cancer cells31. The enhanced dynamic range of VinTS will allow further studies of focal adhesion tensions in cellular processes.
■ CONCLUSIONS
We developed a genetically-encodable BRET-based tension sensor that is smaller in size, offers better spectral separation, and a larger dynamic range for detecting pN forces over FRET based molecular tension sensors due to the desirable photophysical properties of the mNeonGreen-NanoLuc pair21. Moreover, BRET offers other key improvements over FRET-based sensors such as lack of phototoxicity, greatly simplified image processing, and increased signal-to-noise due to the use of bioluminescence as the energy donor. Though the enhanced dynamic range and potential for in vivo imaging of BRET-TS will be advantageous for most applications of molecular tension sensors, BRET-TS would not be suitable for measuring tensions related to cellular processes that happen in <10 seconds due to the longer exposure times required. Moreover, BRET-TS is not compatible with optical sectioning strategies utilizing laser scanning, such as confocal microscopy, and would necessitate the use of computational optical sectioning.
Further optimization of the force regime can be accomplished through substitution of the flexible spider silk flagelliform linker with other recently described molecular spring domains17,23. Furthermore, we believe this sensor will be aptly suited for live animal in vivo measurement of molecular scale tensions. Finally, this BRET pair should be widely applicable to use in other biosensors.
Supplementary Material
ACKNOWLEDGMENT
We would like to thank the Physical Sciences of Oncology Center at UMN for resources and support. Thanks to Fluorescence Innovations as well as Thomas Pengo of the University Imaging Centers for support and helpful discussions. Thanks to Brenton Hoffman for helpful discussions. We would also like to thank Hideki Aihara and Nick Levinson for use of their plate readers.
Funding Sources
This research was supported by an NIH NIGMS R35 GM119483 grant, an UMN ACS IRG-118198, and pilot funds from NIH U54 CA210190 grant. E.J.A. received salary support from a Biotechnology Training Grant NIH T32GM008347 and 3M Graduate Fellowship. W.R.G. is a Pew Biomedical Scholar.
ABBREVIATIONS
- BRET
Bioluminescence resonance energy transfer
- FRET
Förster resonance energy transfer
- FP
fluorescent protein
- TS
tension sensor
- RET
resonance energy transfer
- HL
helical linker
- FA
focal adhesion
- RLU
relative light units
- Nluc
NanoLuc
Footnotes
SUPPORTING INFORMATION AVAILABLE
The following files are available free of charge:
Supporting_information.pdf: The file contains methods, supporting figures and amino acid sequences.
REFERENCES
- (1).Janmey PA; Miller RT Mechanisms of Mechanical Signaling in Development and Disease. J. Cell Sci 2011, 124 (1), 9–18. 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Bonnans C; Chou J; Werb Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol 2014, 15 (12), 786–801. 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Insua-Rodríguez J; Oskarsson T. The Extracellular Matrix in Breast Cancer. A dv. Drug Deliv. Rev 2016, 97, 41–55. 10.1016/j.addr.2015.12.017. [DOI] [PubMed] [Google Scholar]
- (4).Wang N. Review of Cellular Mechanotransduction. J. Phys. D Appl. Phys 2017, 50 233002. 10.1088/1361-6463/aa6e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Humphrey JD; Dufresne ER; Schwartz MA Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol 2014, 15 (12), 802–812. 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Freikamp A; Cost AL; Grashoff C. The Piconewton Force Awakens: Quantifying Mechanics in Cells. Trends Cell Biol. 2016, 26 (11), 838–847. 10.1016/j.tcb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- (7).Grashoff C; Hoffman BD; Brenner MD; Zhou R; Parsons M; Yang MT; McLean MA; Sligar SG; Chen CS; Ha T; et al. Measuring Mechanical Tension across Vinculin Reveals Regulation of Focal Adhesion Dynamics. Nature 2010, 466 (7303), 263–266. 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kumar A; Ouyang M; Van den Dries K; McGhee EJ; Tanaka K; Anderson MD; Groisman A; Goult BT; Anderson KI; Schwartz MA Talin Tension Sensor Reveals Novel Features of Focal Adhesion Force Transmission and Mechanosensitivity. J. Cell Biol 2016, 213 (3), 371–383. 10.1083/jcb.201510012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sun Z; Guo SS; Fässler R. Integrin-Mediated Mechanotransduction. J. Cell Biol 2016, 215 (4), 445–456. 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Borghi N; Sorokina M; Shcherbakova OG; Weis WI; Pruitt BL; Nelson WJ; Dunn AR E-Cadherin Is under Constitutive Actomyosin-Generated Tension That Is Increased at Cell–cell Contacts upon Externally Applied Stretch. Proc. Natl. Acad. Sci. U.S.A 2012, 109 (31), 12568–12573. 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Iwai S; Uyeda TQP Visualizing Myosin–actin Interaction with a Genetically-Encoded Fluorescent Strain Sensor. P roc. Natl. Acad. Sci. U.S.A 2008, 105 (44), 16882–16887. 10.1073/pnas.0805513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Liu Y; Yehl K; Narui Y; Salaita K. Tension Sensing Nanoparticles for Mechano-Imaging at the Living/nonliving Interface. J. Am. Chem. Soc 2013, 135 (14), 5320–5323. 10.1021/ja401494e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ma VP-Y; Liu Y; Yehl K; Galior K; Zhang Y; Salaita K. Mechanically Induced Catalytic Amplification Reaction for Readout of Receptor-Mediated Cellular Forces. Angewandte Chemie. 2016, pp 5578–5582. 10.1002/ange.201600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Blakely BL; Dumelin CE; Trappmann B; McGregor LM; Choi CK; Anthony PC; Duesterberg VK; Baker BM; Block SM; Liu DR; et al. A DNA-Based Molecular Probe for Optically Reporting Cellular Traction Forces. Nat. Methods 2014, 11 (12), 1229–1232. 10.1038/nmeth.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Day RN; Booker CF; Periasamy A. Characterization of an Improved Donor Fluorescent Protein for Förster Resonance Energy Transfer Microscopy. J. Biomed. Opt 2008, 13 (3), 031203. 10.1117/1.2939094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).LaCroix AS; Lynch AD; Berginski ME; Hoffman BD Tunable Molecular Tension Sensors Reveal Extension-Based Control of Vinculin Loading. eLife 2018, 7, e33927. 10.7554/eLife.33927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Austen K; Ringer P; Mehlich A; Chrostek-Grashoff A; Kluger C; Klingner C; Sabass B; Zent R; Rief M; Grashoff C. Extracellular Rigidity Sensing by Talin Isoform-Specific Mechanical Linkages. Nat. Cell Biol 2015, 17 (12), 1597–1606. 10.1038/ncb3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Xu Y; Piston DW; Johnson CH A Bioluminescence Resonance Energy Transfer (BRET) System: Application to Interacting Circadian Clock Proteins. Proc. Natl. Acad. Sci U.S.A 1999, 96 (1), 151–156. 10.1073/pnas.96.1.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hall MP; Unch J; Binkowski BF; Valley MP; Butler BL; Wood MG; Otto P; Zimmerman K; Vidugiris G; Machleidt T; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol 2012, 7 (11), 1848–1857. 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Shaner NC; Lambert GG; Chammas A; Ni Y; Cranfill PJ; Baird MA; Sell BR; Allen JR; Day RN; Israelsson M; et al. A Bright Monomeric Green Fluorescent Protein Derived from Branchiostoma Lanceolatum. Nat. Methods 2013, 10, 407 10.1038/nmeth.2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Suzuki K; Kimura T; Shinoda H; Bai G; Daniels MJ; Arai Y; Nakano M; Nagai T Five Colour Variants of Bright Luminescent Protein for Real-Time Multicolour Bioimaging. Nat. Commun 2016, 7, 13718 10.1038/ncomms13718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Becker N; Oroudjev E; Mutz S; Cleveland JP; Hansma PK; Hayashi CY; Makarov DE; Hansma HG Molecular Nanosprings in Spider Capture-Silk Threads. Nat. Mater 2003, 2 (4), 278–283. 10.1038/nmat858. [DOI] [PubMed] [Google Scholar]
- (23).Brenner MD; Zhou R; Conway DE; Lanzano L; Gratton E; Schwartz MA; Ha T. Spider Silk Peptide Is a Compact, Linear Nanospring Ideal for Intracellular Tension Sensing. Nano Lett. 2016, 16 (3), 2096–2102. 10.1021/acs.nanolett.6b00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Arai R; Ueda H; Kitayama A; Kamiya N; Nagamune T. Design of the Linkers Which Effectively Separate Domains of a Bifunctional Fusion Protein. Protein Eng. 2001, 14 (8), 529–532. 10.1093/protein/14.8.529 [DOI] [PubMed] [Google Scholar]
- (25).Lovendahl KN; Hayward AN; Gordon WR Sequence-Directed Covalent Protein-DNA Linkages in a Single Step Using HUH-Tags. J. Am. Chem. Soc 2017, 139 (20), 7030–7035. 10.1021/jacs.7b02572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Shimomura O; Masugi T; Johnson FH; Haneda Y. Properties and Reaction Mechanism of the Bioluminescence System of the Deep-Sea Shrimp Oplophorus Gracilorostris. Biochemistry 1978, 17 (6), 994–998. 10.1021/bi00599a008. [DOI] [PubMed] [Google Scholar]
- (27).Looyenga B; VanOpstall C; Lee Z; Bell J; Lodge E; Wrobel K; Arnoys E; Louters L. Determination of GLUT1 Oligomerization Parameters Using Bioluminescent Förster Resonance Energy Transfer. Sci. Rep 2016, 6, 29130 10.1038/srep29130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sarangi BR; Gupta M; Doss BL; Tissot N; Lam F; Mège R-M; Borghi N; Ladoux B. Coordination between Intra-and Extracellular Forces Regulates Focal Adhesion Dynamics. Nano Lett. 2017, 17 (1), 399–406. 10.1021/acs.nanolett.6b04364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Rothenberg KE; Scott DW; Christoforou N; Hoffman BD Vinculin Force-Sensitive Dynamics at Focal Adhesions Enable Effective Directed Cell Migration. Biophys. J 2018, 114 (7), 1680–1694. 10.1016/j.bpj.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Gilchrist CL; Leddy HA; Kaye L; Case ND; Rothenberg KE; Little D; Liedtke W; Hoffman BD; Guilak F. TRPV4-Mediated Calcium Signaling in Mesenchymal Stem Cells Regulates Aligned Collagen Matrix Formation and Vinculin Tension. Proc. Natl. Acad. Sci. U.S.A 2019, 116 (6), 1992–1997. 10.1073/pnas.1811095116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li F; Chen A; Reeser A; Wang Y; Fan Y; Liu S; Zhao X; Prakash R; Kota D; Li B-Y; et al. Vinculin Force Sensor Detects Tumor-Osteocyte Interactions. Sci. Rep 2019, 9 (1), 5615 10.1038/s41598-019-42132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Studier FW Protein Production by Auto-Induction in High Density Shaking Cultures. Protein Expr. Purif 2005, 41 (1), 207–234. 10.1016/j.pep.2005.01.016 [DOI] [PubMed] [Google Scholar]
- (33).Chastagnier Y; Moutin E; Hemonnot A-L; Perroy J. Image Processing for Bioluminescence Resonance Energy Transfer Measurement—BRET-Analyzer. Front. Comput. Neurosci 2018, 11 (118). 10.3389/fncom.2017.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Quast RB; Fatemi F; Kranendonk M; Margeat E; Truan G. Accurate Determination of Human CPR Conformational Equilibrium by smFRET Using Dual Orthogonal Noncanonical Amino Acid Labeling. ChemBioChem. 2019, 20, 659 10.1002/cbic.201800607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.