Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Comorbidity, Drug-drug interaction, Side-effect
Abstract
Drug-drug interactions (DDI) potentially occurring between medications used in the course of COVID-19 infection and medications prescribed for the management of underlying comorbidities may cause adverse drug reactions (ADRs) contributing to worsening of the clinical outcome in affected patients. First, we conducted a meta-analysis to determine comorbidities observed in the course of COVID-19 disease associated with an increased risk of worsened clinical outcome from 24 published studies. In addition, the potential risk of DDI between medications used in the course of COVID-19 treatment in these studies and those for the management of observed comorbidities was evaluated for possible worsening of the clinical outcome. Our meta-analysis revealed an implication cardiometabolic syndrome (e.g. cardiovascular disease, cerebrovascular disease, hypertension, and diabetes), chronic kidney disease and chronic obstructive pulmonary disease as main co-morbidities associated with worsen the clinical outcomes including mortality (risk difference RD 0.12, 95 %-CI 0.05−0.19, p = 0.001), admission to ICU (RD 0.10, 95 %-CI 0.04−0.16, p = 0.001) and severe infection (RD 0.05, 95 %-CI 0.01−0.09, p = 0.01) in COVID-19 patients. Potential DDI on pharmacokinetic level were identified between the antiviral agents atazanavir and lopinavir/ritonavir and some drugs, used in the treatment of cardiovascular diseases such as antiarrhythmics and anti-coagulants possibly affecting the clinical outcome including cardiac injury or arrest because of QTc-time prolongation or bleeding. Concluding, DDI occurring in the course of anti-Covid-19 treatment and co-morbidities could lead to ADRs, increasing the risk of hospitalization, prolonged time to recovery or death on extreme cases. COVID-19 patients with cardiometabolic diseases, chronic kidney disease and chronic obstructive pulmonary disease should be subjected to particular carefully clinical monitoring of adverse events with a possibility of dose adjustment when necessary.
1. Introduction
The recent outbreak of the novel coronavirus officially known as Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) has progressed into global pandemic. Up to September 6, 2020 the World Health Organization (WHO) recorded 26,763,217 confirmed cases and 876,616 deaths in 216 countries worldwide [1]. An estimated 20–51 % of affected patients are reported to have at least one comorbidity [2,3]. These affected patients with underlying comorbidities may have a greater risk of poor clinical outcome including severity, mortality, and admission to ICU [[4], [5], [6]]. Again, it is expected that given the percentage of individuals with comorbidities affected by the COVID-19, the use of polypharmacy for treatment of existing chronic disease conditions might be a routine.
Since the inception of SARS-CoV-2 outbreak in the Chinese city of Wuhan in late 2019, several antiviral drugs and other medications currently utilized in clinics with known safety profile are repurposed in COVID-19 patients to reduce worsening of the symptoms [7,8]. On May 1, 2020, the US Food and Drug Administration (FDA) issued an emergency use authorization for the investigational antiviral drug remdesivir for the treatment of hospitalized adults and children with severe COVID-19 based on clinical trial data. Nonetheless, some of these drugs are known to cause severe drug-drug interactions (DDI) such as hydroxychloroquine and azathioprine leading to increased risk of QTc-time prolongations [9]. With respect to co-morbidities in COVID-19 patients there is an additional potential risk of DDI between antiviral agents and multiple medications prescribed to treat their chronic disease conditions. It was shown that in northern Italy COVID-19 patients experienced significant elevated plasma concentrations of direct oral anti-coagulants while on medications used in the course of COVID-19 [10]. Unfortunately, with the exception of hydroxychloroquine and QTc-time prolongation due to co-administration of other drugs, the issue of potential harmful DDI in COVID-19 comorbid patients seems to be of minor attention with a limited number of published studies currently available [[11], [12], [13], [14], [15]]. Also, of a public health concern is the use of self-medication being potentially harmful or without evidence of clinical benefit taking place particularly in low- and middle-income countries with restricted access to quality healthcare and where drug dispensing is less controlled in the communities [16,17]. We hypothesized that in addition to comorbidities, DDI may further worsen the clinical outcome of COVID-19 in these patients.
Herein, we first conducted a meta-analysis on COVID-19 clinical studies which characterized the epidemiological or clinical features of affected patients with comorbidities independent of pharmacological interventions. Secondly, the potential risk of DDI between drugs used in the course of COVID-19 and other medications prescribed for treatment of comorbidities were identified leading to potentially ADRs increasing the risk of poorer clinical outcome (e.g. hospitalization, prolonged time to recovery and death on extreme cases).
2. Methods
2.1. Search strategy and study criteria
Electronic databases of PubMed, Medline, Scopus and google scholar were searched for articles published before June 17, 2020 in English-language reporting on COVID-19. A combination of search terminologies (“COVID-19”, “coronavirus”, “nCOV”, SARS-CoV-2”) AND (“clinical characteristics”) AND (“epidemiological features”) AND (“chronic diseases”) AND (“comorbidities”) were used for the search. Additional studies were obtained by examining the references of selected articles. Selection criteria for the analysis focused exclusively on clinical studies characterizing the clinical or epidemiological features of COVID-19 patients. Only studies with confirmed SARS-CoV-2-RNA detection in respiratory specimen including nasopharyngeal swabs, bronchoalveolar lavage fluid, sputum, or bronchial aspiration as well as in plasma were included in the meta-analysis. Clinical signs of the infection such as fever, cough, myalgia, malaise, rhinorrhea, arthralgia, chest pain and dyspnea were also taken into consideration. Other clinical complications such as acute kidney and cardiac injuries were considered. We excluded studies conducted in children, pre-clinical models, case reports, letters, editorial commentaries, reviews, and meta-analysis.
2.2. Statistical analysis
The risk difference method was used to estimate weights of individual study outcome using the Mantel-Haenszel method with random-effect model in the R statistical software (version 3.4.2). The statistical heterogeneity between study outcomes were visualized using the forest plot and the inter-study heterogeneity estimated by calculating the Ԏ2, I2 and H2 statistics, and by computing Cochran’s Q test statistics [18,19]. An I2 values lower than 25 % was considered as low heterogeneity, values of 26–50 % indicated moderate heterogeneity and values greater than 50 % to indicate a high heterogeneity. A Cochran’s Q test statistics with p-value of < 0.05 was an indication of statistical significance heterogeneity. The trim and fill method was used to determine hypothetical missing studies as evidence of publication bias when necessary (Supplementary Fig. 1).
2.3. Potential drug-drug interactions
The data on drugs used in the course of COVID-19 and the primary indication were collected from www.ashp.org/COVID-19 as well as metabolizing enzymes involved in their biotransformation from www.drugbank.ca. The potential of drugs used in the course of COVID-19 infection reported in the included studies to interact with other drugs used for the management of comorbidities which could precipitate ADRs likely to further worsen clinical outcome of COVID-19 based on our meta-analysis was assessed using the www.covid19-druginteractions.org database. Here, potential DDIs are classified into four groups: (i) no clinically significant interaction expected; (ii) potential interaction likely to be of weak intensity with monitoring or dosage adjustment unlikely to be required; (iii) potential clinically significant interaction that may require close monitoring, alteration of drug dosage or timing of administration; and (iv) drugs should not be co-administered. We subsequently focused our analysis only on the latter. The clinical relevance of such DDI were risk ranked into five categories based on the quality of evidence as: (0) unlikely - no evidence of preclinical or clinically significant interaction, (1) very low - in vitro or animal studies, single case reports, parallel or crossover single dose pharmacokinetic (PK) study without area under plasma concentrations (AUCs), PK study in infected or healthy subjects, (2) low - multiple case reports, crossover or parallel steady state PK without AUCs, parallel or crossover single dose PK study with AUCs, metabolism study with probe substrates, observational PK in infected patients, (3) moderate - cross-over, parallel steady state PK study with AUCs and (4) high - data based on randomized, controlled interaction trial with clinical or validated surrogate endpoints.
The grading on quality of evidence of DDI was conducted for each medication prescribed for the treatment or management of comorbidities against individual COVID-19 therapies. Subsequently, the z-score was calculated and used to construct heatmaps in www. software.broadinstitute.org/morpheus.
3. Results
3.1. Study characteristics
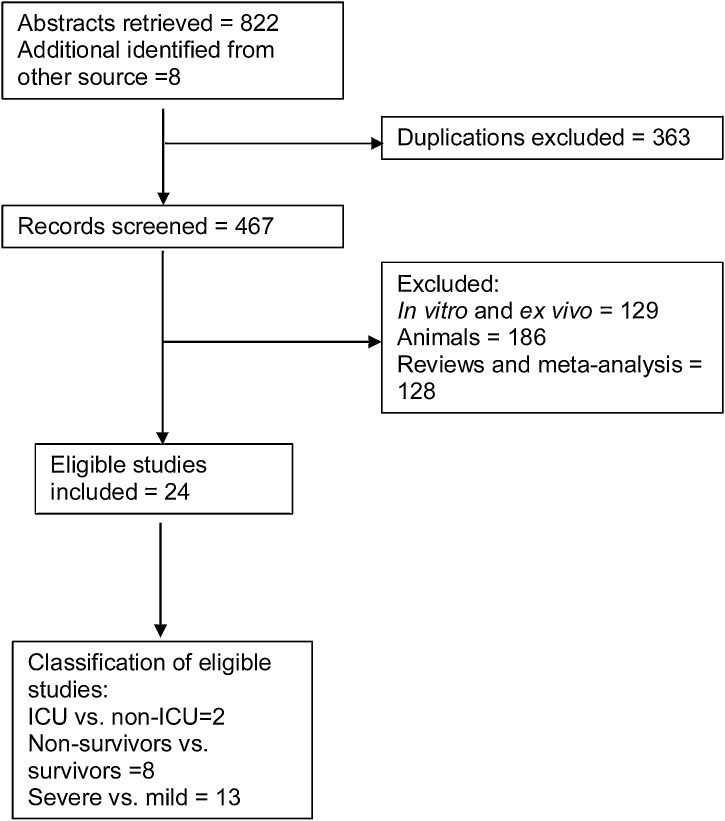
A literature search was conducted to extract eligible studies for the meta-analysis. Of 467 records screened for eligibility, 24 prospective and retrospective case studies with a total of 5,586 COVID-19 affected patients were included in the meta-analysis (Fig. 1). Data on the underlying comorbidities was drawn from the reported clinical characterization of the affected patients. Comorbidities reported include cardiovascular diseases, cerebrovascular disease, chronic kidney disease, chronic liver disease, chronic obstructive pulmonary disease (COPD), hypertension, diabetes, malignancy, human immunodeficiency virus (HIV) and others. The mean age of the affected patients ranged from 41 years to 63 years (Table 1 ).
Fig. 1.
Flow diagram indicating publications on clinical COVID-19 studies excluded and included in the meta-analysis.
Table 1.
Clinical characteristics of COVID-19 patients included in 24 eligible studies.
| Author (year) | Origin | Design | Age (years) | Number of Patients |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | CVD | CRV | CKD | CLD | Diabetes | Hypertension | Malignancy | COPD | ||||
| Cao et al., 2019 [47] | China | NA | 54 | 102 | 5 (5%) | 6 (6%) | 4 (4%) | 2 (2%) | 11 (11 %) | 28 (28 %) | 4 (4%) | 10 (10 %) |
| Chen et al., 2020 [48] | China | RD | 62 | 274 | 23 (8%) | NA | NA | NA | 47 (17 %) | 93 (34 %) | 7 (3%) | 18 (7%) |
| Deng et al., 2020 [49] | China | RD | NA | 225 | NA | NA | NA | NA | NA | NA | NA | NA |
| Feng et al., 2020 [50] | China | RD | 53 | 476 | 38 (8%) | 17 (4%) | NA | NA | 49 (10 %) | 113 (24 %) | 12 (3%) | 22 (5%) |
| Guan et al., 2020 [51] | China | PD | 47 | 1099 | 27 (3%) | 15 (1%) | 8 (1%) | NA | 81 (7%) | 165 (15 %) | 10 (1%) | 12 (1%) |
| Huang et al., 2020 [2] | China | PD | 49 | 41 | 6 (15 %) | NA | NA | 1 (2%) | 8 (20 %) | 6 (15 %) | 1 (2%) | 1 (2%) |
| Huang et al., 2020 [52] | China | RD | 44 | 202 | NA | NA | NA | NA | 19 (9%) | 29 (14 %) | NA | NA |
| Itelman et al., 2020 [53] | Israel | RD | 52 | 162 | NA | NA | 2 (1%) | NA | 30 (19 %) | 49 (30 %) | NA | 2 (1%) |
| Javanian et al., 2020 [54] | Iran | RD | 60 | 100 | 20 (20 %) | NA | 12 (12 %) | NA | 37 (37 %) | 32 (32 %) | 4 (4%) | 12 (12 %) |
| Liu et al., 2020 [55] | China | RD | 49 | 40 | NA | NA | NA | NA | 6 (15 %) | 6 (15 %) | NA | NA |
| Shi et al., 2020 [56] | China | RD | 63 | 671 | 60 (9%) | 22 (3%) | 28 (4%) | NA | 97 (15 %) | 199 (30 %) | 23 (3%) | 23 (3%) |
| Sun et al., 2020 [57] | China | RD | 44 | 55 | NA | NA | NA | NA | 5 (9%) | 8 (15 %) | NA | NA |
| Wan et al., 2020 [58] | China | RD | 47 | 135 | 7 (5%) | NA | NA | 2 (2%) | 12 (9%) | 13 (10 %) | 4 (3%) | NA |
| Wang et al., 2020 [6] | China | RD | 56 | 138 | 20 (15 %) | 7 (5%) | 4 (3%) | 4 (3%) | 14 (10 %) | 43 (31 %) | 7 (10 %) | 4 (3%) |
| Wang et al 2020 [59] | China | RD | 51 | 107 | 13 (12 %) | 6 (6%) | 3 (3%) | 6 (6%) | 11 (10 %) | 26 (24 %) | NA | 3 (3%) |
| Wu et al., 2020 [60] | China | RD | 43 | 280 | 57 (20 %) | NA | 3 (1%) | 7 (3%) | NA | NA | 5 (2%) | NA |
| Xie et al., 2020 [61] | China | RD | 60 | 79 | 7 (9%) | NA | NA | NA | 8 (10 %) | 14 (18 %) | NA | NA |
| Xu et al., 2020 [62] | China | RD | 41 | 62 | NA | 1 (2%) | 1 (2%) | 7 (11 %) | 1 (2%) | 5 (8%) | NA | 1 (2%) |
| Xu et al., 2020 [63] | China | RD | 46 | 703 | 35 (5%) | NA | 10 (1%) | 29 (4%) | 64 (9%) | 118 (17 %) | 9 (1%) | 13 (2%) |
| Yang et al., 2020 [4] | China | RD | 59.7 | 52 | 5 (10 %) | 7 (14 %) | NA | NA | 9 (17 %) | NA | 2 (4%) | 4 (8%) |
| Zhang et al., 2020 [64] | China | RD | 57 | 140 | 7 (5%) | NA | NA | NA | 17 (12 %) | 42 (30 %) | NA | 2 (1%) |
| Zheng et al., 2020 [65] | China | RD | 45 | 161 | 4 (3%) | 4 (3%) | NA | 4 (3%) | 7 (4%) | 22 (14 %) | NA | 6 (4%) |
| Zhao et al., 2020 [66] | China | RD | 46 | 91 | NA | NA | 1 (1%) | NA | 3 (3%) | NA | 3 (3%) | 1 (1%) |
| Zhou et al., 2020 [67] | China | RD | 56 | 191 | 15 (8%) | NA | 2 (1%) | NA | 36 (19 %) | 58 (30 %) | 2 (1%) | 6 (3%) |
*Median or average age (years. Abbreviations: cardiovascular disease (CVD), cerebrovascular disease (CRV), chronic kidney disease (CKD) and chronic liver disease. (CLD), retrospective design (RD), prospective design (PD), not specified (NS), not available (NA).
3.2. Meta-analysis
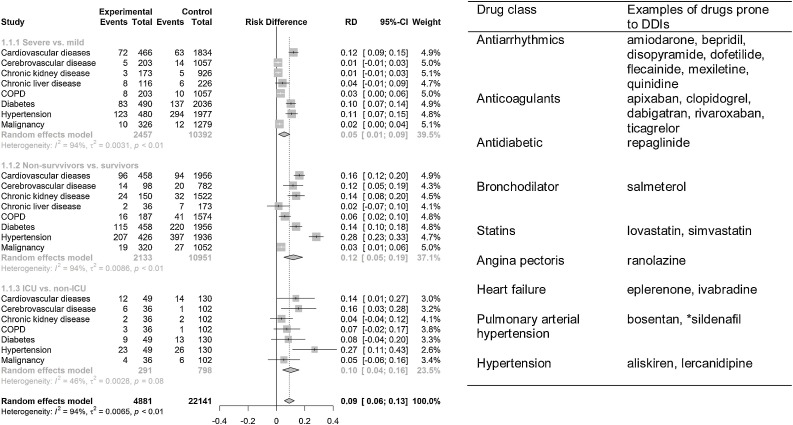
Based on the 24 identified eligible studies, a meta-analysis was conducted to determine comorbidities which may be associated with an increased risk of clinical outcome in COVID-19 affected patients. For the meta-analysis, we separated the comorbidities based on non-survivors vs. survivors, ICU vs. non-ICU and severity vs. mild cases depending on the clinical presentations of signs and symptoms of the COVID-19 patients as reported by individual studies. In general, we observed poorer clinical outcome for COVID-19 patients with co-morbidities in ascending order of severe vs. mild (risk difference (RD) 0.05, 95 % CI 0.01 – 0.09, p = 0.01), ICU vs. non-ICU (RD 0.10, 95 % CI 0.04−0.16, p = 0.001), and non-survivors vs. survivors (RD 0.12, 95 % CI 0.05 – 0.19, p = 0.001) (Fig. 2 ). The analysis on non-survivors vs. survivors group showed hypertension, cardiovascular disease, diabetes, cerebrovascular disease, chronic kidney disease and malignancies were associated with significant increase in risk of death among COVID-19 patients. Other diseases including COPD and chronic liver disease had no impact on the risk of death among infected patients, for details see Fig. 2. For cases admitted to ICU, affected patients with cerebrovascular disease showed a high risk (RD 0.16, 95 % CI 0.03 – 0.28, p = 0.01) but the data was insufficient to strengthen the outcome (Fig. 2). Similarly, the analysis on severe vs. mild COVID-19 infection indicated that hypertension, diabetes, and COPD were associated with increase severity of infection in patients as depicted in Fig. 2. Cardiovascular disease was a borderline risk factor in severe COVID-19 patients. The meta-analyses on individual studies included in respective groups are shown in supplementary Figs. 2–4.
Fig. 2.
Meta-analysis of comorbidities in COVID-19 patients and typical related drugs. Cardiometabolic syndrome (cardiovascular disease, hypertension, and diabetes) was associated with worse clinical outcome of COVID-19 in affected patients. Drugs used for management or treatment of comorbidities: antihypertensives, antiarrhythmics, lipid lowering drugs (statins) listed could increase poor clinical outcome in comorbid patients by potential interaction with drugs used in the course of COVID-19. *indication for both pulmonary hypertension and erectile dysfunction.
In subgroup analyses, low statistical heterogeneity was found in those (non-survivors vs. survivors) with chronic kidney disease (I2 26.0, Q 5.39) and diabetes (I2 21.0, Q 10.5), and high heterogeneity in patients with COPD (I2 52.0, Q 8.37) and cardiovascular disease (I2 70.0, Q 26.4). Patients (those in ICU vs. non-ICU) with diabetes (I2 84.8 %, Q 6.56) and hypertension (I2 83.1, Q 5.92) showed high heterogeneity. In addition, high heterogeneity was indicated in patients (those with severe vs mild) with diabetes (I2 56.2, Q 22.82), hypertension (I2 66.6, Q 27.0), and cardiovascular disease (I2 90.4, Q 62.74) as shown in Table 2 .
Table 2.
Results of meta-analysis and subgroup analysis on comorbidities in COVID-19 patients.
| Condition | Point estimate [95 % CI] | P value | Heterogeneity I2 (%) Q Ʈ2 |
||
|---|---|---|---|---|---|
| Non-survivors vs survivors | |||||
| Cardiovascular disease | 0.18 [0.1; 0.26] | <0.0001 | 70.0 | 26.41 | 0.010 |
| Cerebrovascular disease | 0.11 [0.04; 0.18] | 0.001 | – | 0.13 | – |
| Chronic kidney disease | 0.11 [0.04; 0.17] | 0.001 | 26.0 | 53.9 | – |
| Chronic liver disease | 0.01 [-0.07; 0.10] | 0.72 | – | 0.23 | – |
| COPD | 0.05 [-0.01; 0.11] | 0.10 | 52.0 | 8.37 | – |
| Diabetes | 0.14 [0.08; 0.19] | <00000.1 | 21.0 | 10.15 | – |
| Hypertension | 0.29 [0.23; 0.34] | <0.00001 | – | 6.78 | 0.010 |
| Malignancy | 0.04 [0.01; 0.06] | 0.008 | – | 1.91 | – |
| ICU vs non-ICU | |||||
| Cardiovascular disease | 0.14 [0.01; 0.27] | 0.004 | – | 0.01 | – |
| Chronic kidney disease | 0.04 [-0.02; 0.17] | 0.38 | – | – | – |
| COPD | 0.07 [-0.02; 0.17] | 0.12 | – | – | – |
| Diabetes | 0.01 [-0.33; 0.34] | 0.98 | 84.8 | 6.56 | 0.050 |
| Hypertension | 0.20 [-0.16; 0.56] | 0.28 | 83.1 | 5.92 | 0.060 |
| Malignancy | 0.05 [-0.06; 0.16] | 0.36 | – | – | – |
| Severe vs mild | |||||
| Cardiovascular disease | 0.10 [0.00; 0.20] | 0.05 | 90.4 | 62.74 | 0.015 |
| Cerebrovascular disease | 0.01 [-0.01; 0.03] | 0.32 | – | – | – |
| Chronic kidney disease | 0.01 [-0.01; 0.03] | 0.24 | – | – | – |
| Chronic liver disease | 0.03 [-0.02; 0.08] | 0.19 | – | 0.04 | – |
| COPD | 0.03 [0.00; 0.06] | 0.003 | – | 0.03 | – |
| Diabetes | 0.08 [0.02; 0.14] | 0.002 | 56.2 | 22.82 | 0.004 |
| Hypertension | 0.10 [0.08; 0.20] | 0.007 | 66.6 | 26.97 | 0.010 |
| Malignancy | 0.01 [0.00; 0.03] | 0.13 | – | 2.61 | – |
*COPD = chronic obstructive pulmonary disease.
3.3. Potential drug-drug interactions
From the meta-analysis, comorbidities associated with increased risk of worsen clinical outcome in COVID-19 patients were cardiovascular disease, cerebrovascular disease, hypertension, diabetes, chronic kidney disease and chronic obstructive pulmonary disease. Further, several drugs have been used in different countries in the course of COVID-19 infection as reported in various studies included in the meta-analysis. Hence, we further used the www.covid19-druginteractions.org database to estimate the potential interaction risk of antiarrhythmics, antihypertensives, anticoagulants, antidiabetics, lipid lowering medications (statins), and bronchodilators with drugs used in the course of COVID-19 patients. A list of 41 drugs used in the course of COVID-19, their primary indication as well as main metabolizing enzymes are documented in Table 3 . The use of hydroxychloroquine and lopinavir/ritonavir in COVID-19 was suspended or stopped in the WHO SOLIDARITY trial. According to the International Steering Committee interim trial report, hydroxychloroquine and lopinavir/ritonavir produced little or no decline in the mortality of hospitalized COVID-19 patients when compared to standard of care (www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19). However, these drugs are still used for the COVID-19 infection at some hospitals in other countries. Hence, both drugs were included in our DDI analysis.
Table 3.
Drugs used in the course of COVID-19 disease, classification, primary indication and main metabolic pathways.
| Drugs | Classification | Primary indication | Substrate of (enzyme/transporter)b | Inhibitor of | Inducer of |
|---|---|---|---|---|---|
| ACE Inhibitors, Angiotensin II Receptor Blockers (ARBs) | Renin angiotensin aldosterone system (RAAS) inhibitor | High blood pressure and heart failure | – | – | – |
| CYP2C9 | – | – | |||
| Alteplase | Plasmin activator | Acute ST elevation myocardial infarction (STEMI), pulmonary embolism | – | – | – |
| Anakinra | Disease modifying anti-rheumatic agent | RA | – | – | – |
| Ascorbic acid | Vitamin C | Vitamin C deficiency | – | – | – |
| Atazanavir | HIV protease inhibitors | HIV infection | CYP3A4 | CYP3A4 | – |
| Azithromycin | Macrolides | Multiple bacterial infections | – | – | – |
| Baloxavir | Antiviral | Influenza | – | – | – |
| Baricitinib | Disease-modifying anti-rheumatic agent | Moderate to severe RA | CYP3A4 | – | – |
| Bevacizumab | IgG1 antibody | Various cancer types | – | – | – |
| Chloroquine phosphate | Antimalarial (4-aminoquinoline derivative) | Malaria | CYP2C8, CYP3A4 | CYP2D6 | – |
| Colchicine | Antigout agents | Gout | CYP3A4, P-gp | – | – |
| Darunavir/cobicistat | HIV protease inhibitors | HIV infection | CYP3A | – | – |
| Emapalumab | Anti-interferon gamma | hemophagocytic lymphohistiocytosis | – | ||
| Famotidine | Histamine H2 antagonists | Peptic ulcer disease, gastroesophageal reflux disease, Zollinger-Ellison syndrome | – | ||
| Favipiravir | Antiviral | Influenza | Aldehyde oxidase | – | – |
| Fingolimod | Immunosuppressant | Multiple sclerosis | Sphingosine kinase, CYP4F2 | – | – |
| HMG-CoA reductase inhibitors (statins) | Antilipemic agent | Reduce risk of heart attack or stroke | CYP3A4, CYP3A5 | – | – |
| Hydroxychloroquine sulfate | Antimalarial (4-aminoquinoline derivative) | Malaria, auto-immune diseases (lupus, rheumatoid arthritis) | CYP3A4 | CYP2D6 | – |
| Inhaled prostacyclins (e.g. epoprostenol, iloprost) | Vasodilating agents | Pulmonary arterial hypertension | – | – | – |
| Interferon beta 1a | Interferon | Multiple sclerosis | – | – | – |
| Ivermectin | Anthelmintic | Multiple parasitic infections | P-gp | – | – |
| Lopinavir | HIV protease inhibitor | HIV infection | CYP3A | CYP3A4 | – |
| Methylprednisolone | Corticosteroid | Multiple conditions | 11beta-hydroxysteroid dehydrogenases and 20-ketosteroid reductases | – | – |
| N-acetylcysteine | Antioxidant | Acetaminophen overdose | – | – | – |
| Niclosamide | Anthelmintic | Tapeworm infestations | CYP1A2, UGT1A1 | – | – |
| Nitazoxanide | Antiprotozoal | GIT infections | – | – | – |
| Nitric oxide (inhaled) | Vasodilating Agent | Neonatal respiratory failure | – | – | – |
| NSAIDS (e.g. ibuprofen, indomethacin) | Nonsteroidal anti-inflammatory agent | Pain, fever, inflammation | CYP2C8/9, CYP2C19, UGT2B7 | – | – |
| Oseltamivir | Neuraminidase inhibitor | Influenza | Esterases | – | – |
| Peg-interferon alpha 2b | Interferon | Hepatitis C and melanoma | – | – | – |
| Remdesivir | Antiviral | *COVID-19 | CYP2C8, CYP2D6, CYP3A4 | – | – |
| Ribavirin | Hepatitis C | Adenosine kinase | |||
| Ritonavir | HIV protease inhibitors | HIV infection | CYP3A/CYP2D6 | CYP3A4, P-gp | – |
| Ruxolitinib | Antineoplastic Agents | Bone marrow disorders | CYP3A4 | P-gp | – |
| Sarilumab | Disease modifying anti -rheumatic agent | Moderate to severe RA in adults | – | – | – |
| Sildenafil | PDE5 inhibitor | Erectile dysfunction, pulmonary arterial hypertension | CYP3A4/CYP2C9 | – | – |
| Siltuximab | Monoclonal antibody | Multicentric Castleman's disease | – | – | – |
| Sirolimus | Immunosuppressive agent (mTOR inhibitor | Prevent rejection of kidney transplant | CYP3A4 | – | – |
| Tocilizumab | Disease-modifying antirheumatic agent | Moderate to severe rheumatoid arthritis (RA) in adults, systemic juvenile idiopathic arthritis-SJIA, other rheumatological conditions | Proteolytic enzymes | – | – |
| Umifenovir | Antiviral | Influenza | CYP3A4, UGT1A9, UGT2B7 | – | – |
FDA and EMA emergency use authorization.
Metabolizing enzymes information collected from Drugbank and product information, drug list data obtained from https://www.ashp.org/COVID-19.
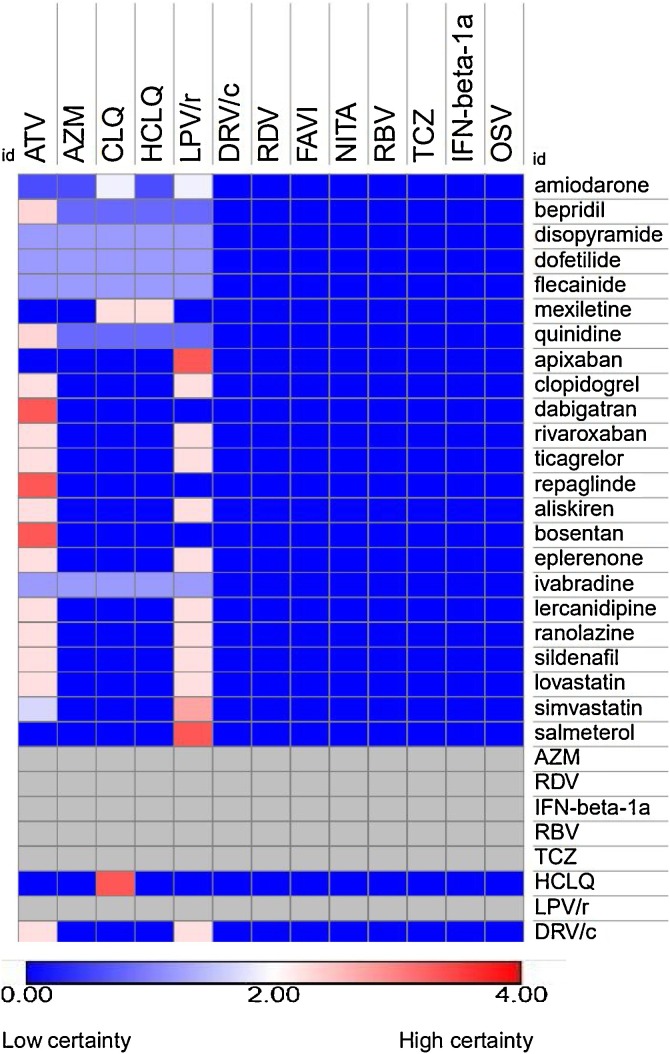
According to the analysis, co-administration of some drugs used for the treatment or management of comorbidities together with atazanavir and lopinavir/ritonavir (used as therapies for COVID-19) could increase the risk of adverse outcome of COVID-19 patients by evidence of potential pharmacokinetic interactions. E.g. an increase in plasma exposure of antiarrhythmics (e.g. amiodarone, bepridil, disopyramide, dofetilide, flecainide and quinidine), drugs prescribed for pulmonary hypertension (e.g. bosentan and sildenafil), angina pectoris (e.g. ranolazine), heart failure (e.g. eplerenone, ivabradine), erectile dysfunction (e.g. sildenafil), few anti-hypertensives (e.g. aliskiren and lercanidipine), antithrombotics and anticoagulants (e.g. ticagrelor and rivaroxaban), and statins (e.g. lovastatin and simvastatin) was detected due to a potential inhibition mainly of CYP3A4 by atazanavir or lopinavir/ritonavir (Fig. 3). Additionally, atazanavir and lopinavir/ritonavir may increase plasma concentrations of the anti-coagulant dabigatran by inhibiting the efflux drug transporter P-glycoprotein (P-gp). The HIV-protease inhibitor atazanavir was also shown before to inhibit CYP3A4, CYP2C8 and hepatic transporter OATP1B1 thereby increasing systemic exposure of antidiabetic drug repaglinide. The protease inhibitors lopinavir/ritonavir may also increase plasma exposure of the bronchodilator salmeterol via CYP3A4 inhibition. Azithromycin, chloroquine, or hydroxychloroquine used in the frame of COVID-19 treatment are prone to cause QTc-time prolongation in the presence of antiarrhythmics as a single agent or combined due to pharmacodynamic interactions. The summary of drugs used in the course of COVID-19 identified to cause clinically relevant interactions with other medications for the related co-morbidities are presented in Table 4 .
Fig. 3.
Heatmap of potential DDI between drugs used in the course of COVID-19 and co-medications. The co-medications are putatively used for treatment of identified comorbidities (hypertension, cerebrovascular, cardiovascular, diabetes and COPD) based on results of the meta-analysis. Anti-viral drugs LPV/r and AZM interact with drugs prescribed for cardiometabolic syndrome. Potential interactions were predicting using www.covid19-druginteractions.org database. Abbreviations: atazanavir (ATV), azithromycin (AZM) darunavir/cobicistat (DRV/c), lopinavir/ritonavir (LPV/r), remdesivir/GS-5734 (RDV), favipiravir (FAVI), chloroquine (CLQ), hydroxychloroquine (HCLQ), nitazoxanide (NITA), ribavirin (RBV), tocilizumab (TCZ), interferon β-1a (IFN-β-1a) and oseltamivir (OSV).
Table 4.
Potential DDI between drugs used in the course of COVID-19 and medications for comorbidities.
| Co-administered Drugs (CAD) | *CAD bioavailability (%) | *CAD Protein Binding (%) | Drug used the course of COVID-19 | Mechanism of interaction | Example of interaction effect on AUC of CAD | Consequences of interaction | b Recommendations |
|---|---|---|---|---|---|---|---|
| Apixaban | 50 | 92 - 94 | Lopinavir/ritonavir | CYP3A4 | Ketoconazole increases AUC of apixaban by 2-fold | Increased plasma concentration and bleeding | Avoid coadministration. Consider alternative anticoagulants |
| Amiodarone | 35 - 65 | 96 | Lopinavir/ritonavir | CYP3A4 inhibition | Indinavir increased amiodarone plasma concentration by 44 % via CYP3A4 inhibition | Increased amiodarone effects e.g. QTc-time prolongation, bradycardia, hypotension | Use with caution, monitor ECG, and adjust amiodarone |
| Bepridil | 60 | 99 | Atazanavir, lopinavir/ritonavir | – | – | Increased bepridil level effects. E.g. (QTc-time prolongation, hypotension | Do not co-administer |
| Bosentan | 50 | 98 | Atazanavir | – | Expected decreased atazanavir levels | Potential loss of antiviral activity | Do not co-administer bosentan with un-boosted atazanavir |
| Dabigatran | 3 - 7 | 35 | Atazanavir | P-gp inhibition | Dabigatran AUC increased by 110−127% via inhibition of intestinal P-gp by cobicistat | Increased risk of bleeding because of elevated dabigatran level | No dose adjustment if CrCL > 50 mL/min. avoid co-usage if CrCL < 50 mL/min |
| Eplerenone | 69 | 50 | Atazanavir, lopinavir/ritonavir | CYP3A4 inhibition | Ketoconazole as CYP3A4 inhibitor increases eplerenone AUC by 44 % | Increased plasma concentration, risk of hyperkalemia | Avoid co-administration |
| Lercanidipine | 10 | >98 | Atazanavir, lopinavir/ritonavir | CYP3A4 inhibition | – | Increased plasma concentration | Monitor and adjust lercanidipine levels |
| Mexiletine | 90 | 50 - 60 | Chloroquine, hydroxychloroquine | CYP2D6 inhibition | – | Possible increased mexiletine effect e.g. Cardiac arrythmias | Do not co-administer |
| Quinidine | 76 - 88 | 80 - 88 | Atazanavir | CYP3A4 inhibition | Enhanced quinidine effects e.g. cardiac arrhythmia | Use with caution. Monitor for toxicity | |
| Ranolazine | 73 | 62 | Atazanavir, lopinavir/ritonavir | CYP3A4 inhibition | Ketoconazole increased ranolazine AUC by 3.2-fold | QTc-time prolongation, cardiac arrythmias | Do not co-administer |
| Repaglinide | 56 | >98 | Atazanavir | CYP3A4 inhibition | Clarithromycin increases repaglinide AUC by 40 % | Increase risk of hypoglycemia | Monitor repaglinide clinical effect and lower the dose if necessary |
| Salmeterol | – | 96 | Lopinavir/ritonavir | CYP3A4 inhibition | – | Potential increased salmeterol effects. E.g. QT prolongation, palpitations, sinus tachycardia | Do not co-administer |
| sildenafil | 40 | 96 | Lopinavir/ritonavir | CY3A4 inhibition | Clarithromycin and ciprofloxacin increased sildenafil AUC by 128 % and 110 % | Increased sildenafil effects. E.g. hypotension, priapism, visual changes | Start sildenafil at 25 mg QOD-QD; adjust dose, not recommended to exceed 25 mg in a 48 h period |
| Simvastatin | 60 | 95 | Lopinavir/ritonavir | CYP3A4 inhibition | Simvastatin acid exposure increased by 30-fold when co-administered with ritonavir/saquinavir | Increased plasma concentration effects (e.g. myopathy, rhabdomyolysis) | Do not co-administer. Alternative agents e.g. atorvastatin (low dose), pravastatin |
| Lovastatin | 5 | >95 |
Bioavailability and protein binding information collected from Drugbank and product information.
Recommendations obtained from http://hivinsite.ucsf.edu/interactions.
We further estimated the potential interaction of combination therapies (e.g. azithromycin/nitazoxanide, hydroxychloroquine/azithromycin, and INF-β-1a/lopinavir-ritonavir/ribavirin) for COVID-19 because some of the included studies reported coadministration of these medications. In general, lack of evidence of clinically significant DDI was found. Potential interaction between other COVID-19 drugs (e.g. remdesvir, darunavir/cobistat, favipiravir, nitazoxanide, ribavirin, tocilizumab, sarilumab, IFN-β-1a, oseltamivir and anakinra) and co-medications prescribed for the treatment of existing comorbidities identified based on the meta-analysis were found to be of a low certainty.
4. Discussion
Comorbidities associated with poor clinical outcome of COVID-19 in affected patients are widely reported in other studies [[20], [21], [22]]. The results of our meta-analysis confirmed hypertension, cardiovascular disease, and diabetes being strongly associated with increased mortality and severe courses of COVID-19. Patients with cerebrovascular disease were more likely to be admitted to ICU or even die. Interestingly, in the set of studies included into the meta-analysis, chronic kidney disease and malignancies were associated with increasing the risk of mortality whilst COPD increases the severity of COVID-19 in affected patients. In general, patients with these underlying comorbidities have greater risk of upper respiratory tract infections and pneumonia because of dysfunctional innate and adaptive immune system [20,22].
Current treatment of COVID-19 primarily depends on supportive care, antiviral and immunomodulatory drugs. Given the distribution of population living with the comorbidities (hypertension, cardio-cerebrovascular, diabetes, chronic kidney disease), predominantly middle aged and elderly, polypharmacy and DDI might be apparent. Unfortunately, the potential risk of DDI is largely unknown since most studies on COVID-19 do not provide details on interaction between drugs used in the course of COVID-19 and co-medications used for the management of other comorbidities in these patients. The studies included in the meta-analysis indicated several medications used in the course of COVID-19 in infected patients with other underlying comorbidities. Hence, we evaluated the potential interaction of drugs for the treatment of these comorbidities with drugs for COVID-19 reported in studies included in the meta-analysis. Based on our findings, of a greater safety concern was prolonged cardiac repolarization and QT interval by pharmacokinetic interaction of atazanavir and lopinavir/ritonavir with some drugs, used in the treatment of cardiovascular diseases such as ivabradine in heart failure, ranolazine in symptomatic treatment of angina pectoris, the antiarrhythmics amiodarone disopyramide and quinidine or the formerly used calcium channel blocker bepridil (a drug with putative anti-viral properties) via inhibition of CYP3A4 which may further increase the risk of torsade de pointes (TdP) [[23], [24], [25]]. Consequences of such interaction may increase risk of hospitalization, prolonged time to recovery and finally sudden cardiac death in extreme cases. Other risk factors of QTc-time prolongation and TdP include hypokalemia and chronic heart failure. Furthermore, atazanavir and lopinavir/ritonavir could interact with antithrombotics and anticoagulants (e.g. ticagrelor, dabigatran and rivaroxaban) through CYP3A4 and P-glycoprotein to induce bleeding complication [10]. Interestingly, a recent retrospective study found the use of statins in hospitalized COVID-19 patients to be associated with a lower risk of all-cause mortality and a favorable recovery profile compared to the non-statin group [26]. However, with regards to DDI, statins (e.g. lovastatin and simvastatin) may induce myopathy as consequence of an elevated plasma concentration of these statins because of CYP3A4 inhibition by atazanavir and lopinavir/ritonavir. For example, the AUC of statins lovastatin and simvastatin increased in the presence of ritonavir by up to 20-fold [[27], [28], [29]]. Hence, the use of less DDI-proned statins should be preferred. In Asthma, plasma concentration of salmeterol could increase due to inhibition of CYP3A4 by lopinavir/ritonavir. Such combination may result in salmeterol related side-effects including QTc-time prolongation, palpitations, and tachycardia [28,30].
Adverse events detected in these patients while co-treatment with drugs used in the course of COVID-19 e.g. azithromycin, chloroquine, and hydroxychloroquine and anti-hypertensives are not based on pharmacokinetic interactions but on known risks of TdP by prolonged cardiac polarization and QT interval of such combinations [[31], [32], [33]]. Nonetheless, hydroxychloroquine and chloroquine are also known to be inhibitors of cytochrome P450 2D6 (CYP2D6) hence contributing to an increased risk of TdP of the older antiarrhythmics flecainide and mexiletine [[32], [33], [34], [35]]. Here, adjusting the recommended dose of hydroxychloroquine from 800 mg on day 1, followed by 400 mg daily for 4–7 days to a lower dose may be necessary to avoid potential adverse events (https://www.fda.gov/media/136537/download).
We additionally considered the potential interaction of combination therapies for COVID-19 azithromycin/nitazoxanide, hydroxychloroquine/azithromycin, tocilizumab/remdesivir, and triple combination (IFN-β-1a, lopinavir/ritonavir and ribavirin) used to tackle the pandemic. Studies have shown synergistic effects of these combinations therapies on inhibition of SARS-CoV-2 replication [[36], [37], [38], [39]]. Generally, DDI of such combinations are uncertain due to lack of evidence. The azithromycin/hydroxychloroquine combination related TdP may occur as side effect of single or both drugs [[31], [32], [33],37]. The antimalaria agent hydroxychloroquine is an inhibitor of P-glycoprotein [40]. However, pharmacokinetic interaction of azithromycin with hydroxychloroquine is unexpected because the former is not a sensitive substrate of P-glycoprotein [40,41]. Besides, hydroxychloroquine has long terminal elimination half-life (40–60 days) which may cause the risk of cardiac polarization and QT prolongation to persist even after discontinuation [34,42].
Prediction of DDI however could be hampered, since COVID-19 patients may experience phenoconversion whereby some genotypic extensive metabolizers transiently exhibit a decline in drug metabolizing enzyme activities comparable to that of poor metabolizers because of cytokine storm [43,44]. The problem of phenoconversion due to hyperactive immune system may increase the cardiac related side effects of drugs used in the course of COVID-19 (e.g. hydroxychloroquine) as consequence of prolonged plasma exposure [31,33,42]. Additionally, genetic polymorphism in drug metabolizing enzymes and transporters might worsen the side effects of drugs used for COVID-19 or in combination with other medications in individuals with defective genes.
On the other side, drugs used in the main regimens of hypertension, heart failure or diabetes did not show evidence of DDIs. In particular inhibitors of the renin angiotensin aldosterone system (RAAS) seem to be safe and concerns that the treatment with ACE-inhibitors could increase the risk of SARS-CoV-2 infections through elevation of the ACE-2 expression were not confirmed so far [45,46].
In conclusion, comorbidities including cardio-cerebrovascular diseases, hypertension, diabetes, and chronic kidney disease were associated with increased severity and mortality of COVID-19 in affected patients. DDI may be evident in these patients due to the use of polypharmacy as found in studies included in this meta-analysis. We have shown potential DDI particularly between antiretroviral drugs (atazanavir and lopinavir/ritonavir), and other drugs for treating comorbidity leading to TdP which might contribute to poorer clinical outcome (e.g. increased risk of hospitalization, prolonged time to recovery and death on extreme cases) in COVID-19 patients. This study cannot confirm whether the consequences of the DDI described change the expected course of COVID-19 since there are no clinical data available. To avoid adverse DDI, dose adjustment of drugs used in the course of COVID-19 prone to DDI or using an alternative drug for the management of related co-morbidity may be warranted to prevent risk of worsening clinical outcome. The findings of our study add to the knowledge on the potential risk of DDI in comorbid COVID-19 patients which is still an evolving area. It is worth noting that, this article is not intended to prevent the use of any medication but to outline the potential risk of specific DDIs which may further worsen the clinical outcome of COVID-19 patients with these comorbidities. Taken together, the choice of administration of medication in COVID-19 patients with comorbidities remains sole prerogative of the prescriber. However, we recommend that attention should be paid to symptoms that could indicate drug side effects in particular cardiac arrhythmia via DDI in these special population.
Funding
This work was supported by the Alexander von Humboldt Foundation.
Declaration of Competing Interest
The authors report no declarations of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.105250.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organisation . 2020. Coronavirus Disease (COVID-19) Situation Reports.www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P., Xiao W., Wang Y.-N., Zhong M.-H., Li C.-H., Li G.-C., Liu H.-G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. (Engl). 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA - J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Society of Health-Systems Pharmacists; 2020. Assessment of Evidence for COVID-19-related Treatments. https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evide nce-Table (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshad U., Pertinez H., Box H., Tatham L., Rajoli R.K.R., Curley P., Neary M., Sharp J., Liptrott N.J., Valentijn A., David C., Rannard S.P., O’Neill P.M., Aljayyoussi G., Pennington S.H., Ward S.A., Hill A., Back D.J., Khoo S.H., Bray P.G., Biagini G.A., Owen A. Prioritization of anti‐SARS‐Cov‐2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1909. cpt.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bun S., Taghji P., Courjon J., Squara F., Scarlatti D., Theodore G., Baudouy D., Sartre B., Labbaoui M., Dellamonica J., Doyen D., Marquette C., Levraut J., Esnault V., Bun S., Ferrari E. QT interval prolongation under hydroxychloroquine/ azithromycin association for inpatients with SARS‐CoV‐2 lower respiratory tract infection. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1968. cpt.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testa S., Prandoni P., Paoletti O., Morandini R., Tala M., Dellanoce C., Giorgi‐Pierfranceschi M., Betti M., Battista Danzi G., Pan A., Palareti G. Direct oral anticoagulant plasma levels’ striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. J. Thromb. Haemost. 2020;18:1320–1323. doi: 10.1111/jth.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elens L., Langman L.J., Hesselink D.A., Bergan S., Moes D.J.A.R., Molinaro M., Venkataramanan R., Lemaitre F. Pharmacologic treatment of transplant recipients infected with SARS-CoV-2. Ther. Drug Monit. 2020;42:360–368. doi: 10.1097/FTD.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartiromo M., Borchi B., Botta A., Bagalà A., Lugli G., Tilli M., Cavallo A., Xhaferi B., Cutruzzulà R., Vaglio A., Bresci S., Larti A., Bartoloni A., Cirami C. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19) Transpl. Infect. Dis. 2020 doi: 10.1111/tid.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Back D., Marzolini C., Hodge C., Marra F., Boyle A., Gibbons S., Burger D., Khoo S. COVID‐19 treatment in patients with comorbidities: Awareness of drug‐drug interactions. Br. J. Clin. Pharmacol. 2020 doi: 10.1111/bcp.14358. bcp.14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross S.B., Wilson M.G., Papillon‐Ferland L., Elsayed S., Wu P.E., Battu K., Porter S., Rashidi B., Tamblyn R., Pilote L., Downar J., Bonnici A., Huang A., Lee T.C., McDonald E.G. <scp>COVID‐SAFER</scp> : Deprescribing guidance for hydroxychloroquine drug interactions in older adults. J. Am. Geriatr. Soc. 2020 doi: 10.1111/jgs.16623. jgs.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montastruc J.-L., Toutain P.-L. A new drug–drug interaction between hydroxychloroquine and metformin? A signal detection study. Drug Saf. 2020 doi: 10.1007/s40264-020-00955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpern J.D., Gertner E. Off‐label therapies for COVID‐19—Are we all in this together? Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1862. cpt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daily Sabah; 2020. Hydroxychloroquine for COVID-19? Self-medicating with Malaria Drugs Could Be Lethal, Doctors Warn.https://www.dailysabah.com/life/health/hydroxychloroquine-for-covid-19-selfmedicating-with-malaria-drugs-could-belethal-doctors-warn (March 29, 2020) [Google Scholar]
- 18.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Awortwe C., Bruckmueller H., Cascorbi I. Interaction of herbal products with prescribed medications: a systematic review and meta-analysis. Pharmacol. Res. 2019;141:397–408. doi: 10.1016/j.phrs.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y., Liu X., Chen R., Tang C., Wang T., Ou C., Li L., Chen P., Sang L., Wang W., Li J., Li C., Ou L., Cheng B., Xiong S., Ni Z., Xiang J., Hu Y., Liu L., Shan H., Lei C., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Cheng L., Ye F., Li S., Zheng J., Zhang N., Zhong N., He J. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiffrin E.L., Flack J.M., Ito S., Muntner P., Webb R.C. Hypertension and COVID-19. Am. J. Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma R.C.W., Holt R.I.G. COVID‐19 and diabetes. Diabet. Med. 2020;37:723–725. doi: 10.1111/dme.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbett A.H., Lim M.L., Kashuba A.D. Kaletra (Lopinavir/Ritonavir) Ann. Pharmacother. 2002;36:1193–1203. doi: 10.1345/aph.1A363. [DOI] [PubMed] [Google Scholar]
- 24.Wolbrette D.L. Drugs that cause torsades de pointes and increase the risk of sudden cardiac death. Curr. Cardiol. Rep. 2004;6:379–384. doi: 10.1007/s11886-004-0041-8. [DOI] [PubMed] [Google Scholar]
- 25.Naksuk N., Lazar S., Peeraphatdit T.(Bee) Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur. Hear. J. Acute Cardiovasc. Care. 2020;9:215–221. doi: 10.1177/2048872620922784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X.-J., Qin J.-J., Cheng X., Shen L., Zhao Y.-C., Yuan Y., Lei F., Chen M.-M., Yang H., Bai L., Song X., Lin L., Xia M., Zhou F., Zhou J., She Z.-G., Zhu L., Ma X., Xu Q., Ye P., Chen G., Liu L., Mao W., Yan Y., Xiao B., Lu Z., Peng G., Liu M., Yang J., Yang L., Zhang C., Lu H., Xia X., Wang D., Liao X., Wei X., Zhang B.-H., Zhang X., Yang J., Zhao G.-N., Zhang P., Liu P.P., Loomba R., Ji Y.-X., Xia J., Wang Y., Cai J., Guo J., Li H. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fichtenbaum C.J., Gerber J.G., Rosenkranz S.L., Segal Y., Aberg J.A., Blaschke T., Alston B., Fang F., Kosel B., Aweeka F. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 28.Inaba T., Fischer N., Riddick D., Stewart D., Hidaka T. HIV protease inhibitors, saquinavir, indinavir and ritonavir: inhibition of CYP3A4-mediated metabolism of testosterone and benzoxazinorifamycin, KRM-1648, in human liver microsomes. Toxicol. Lett. 1997;93:215–219. doi: 10.1016/S0378-4274(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 29.Harper C.R., Jacobson T.A. Avoiding statin myopathy: understanding key drug interactions. Clin. Lipidol. 2011;6:665–674. doi: 10.2217/clp.11.57. [DOI] [Google Scholar]
- 30.Abramson M.J., Walters J., Walters E.H. Adverse effects of β-Agonists. Am. J. Respir. Med. 2003;2:287–297. doi: 10.1007/BF03256657. [DOI] [PubMed] [Google Scholar]
- 31.Jankelson L., Karam G., Becker M.L., Chinitz L.A., Tsai M.-C. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Hear. Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javelot H., El‐Hage W., Meyer G., Becker G., Michel B., Hingray C. COVID‐19 and (hydroxy)chloroquine–azithromycin combination: Should we take the risk for our patients? Br. J. Clin. Pharmacol. 2020;86:1176–1177. doi: 10.1111/bcp.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monzani A., Genoni G., Scopinaro A., Pistis G., Kozel D., Secco G.G. QTc evaluation in COVID‐19 patients treated with chloroquine/hydroxychloroquine. Eur. J. Clin. Invest. 2020;50 doi: 10.1111/eci.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 35.Somer M., Kallio J., Pesonen U., Pyykkö K., Huupponen R., Scheinin M. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br. J. Clin. Pharmacol. 2001;49:549–554. doi: 10.1046/j.1365-2125.2000.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtz N., Rolain J.-M., Colson P., La Scola B., Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., Seng P., Hocquart M., Eldin C., Finance J., Vieira V.E., Tissot-Dupont H.T., Honoré S., Stein A., Million M., Colson P., La Scola B., Veit V., Jacquier A., Deharo J.-C., Drancourt M., Fournier P.E., Rolain J.-M., Brouqui P., Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med. Infect. Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M., Yan W.-W., Chan W.-M., Chan J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherrmann J. Intracellular ABCB1 as a possible mechanism to explain the synergistic effect of hydroxychloroquine-azithromycin combination in COVID-19 therapy. AAPS J. 2020;22:86. doi: 10.1208/s12248-020-00465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin X., Skolnik S., Chen X., Wang J. Attenuation of intestinal absorption by major efflux transporters: quantitative tools and strategies using a Caco-2 model. Drug Metab. Dispos. 2011;39:265–274. doi: 10.1124/dmd.110.034629. [DOI] [PubMed] [Google Scholar]
- 42.Pussard E., Verdier F. Antimalarial 4-aminoquinolines: mode of action and pharmacokinetics. Fundam. Clin. Pharmacol. 1994;8:1–17. doi: 10.1111/j.1472-8206.1994.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki Y., Muraya N., Fujioka T., Sato F., Tanaka R., Matsumoto K., Sato Y., Ohno K., Mimata H., Kishino S., Itoh H. Factors involved in phenoconversion of CYP3A using 4β-hydroxycholesterol in stable kidney transplant recipients. Pharmacol. Rep. 2019;71:276–281. doi: 10.1016/j.pharep.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fosbøl E.L., Butt J.H., Østergaard L., Andersson C., Selmer C., Kragholm K., Schou M., Phelps M., Gislason G.H., Gerds T.A., Torp-Pedersen C., Køber L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324:168. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao J., Tu W., Cheng W., Yu L., Liu Y., Hu X., Liu Q. Institute of Radiation Medicine, China Academy of Medical Sc; 2019. Clinical Features and Short-term Outcomes of 102 Patients With Corona Virus Disease 2019 in Wuhan, China 1. Department of Cardiology, Zhongnan Hospital, Wuhan University, Wuhan, China 2. [Google Scholar]
- 48.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng Y., Liu W., Liu K., Fang Y.Y., Shang J., Zhou L., Wang K., Leng F., Wei S., Chen L., Liu H.G. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin. Med. J. (Engl). 2020;2019 doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., Xiong W., Yang D., Chen R., Lu F., Lu Y., Liu X., Chen Y., Li X., Li Y., Summah H.D., Lin H., Yan J., Zhou M., Lu H., Qu J. COVID-19 with different severities: a multicenter study of clinical features. Am. J. Respir. Crit. Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J.L., Liang Z., Peng Y., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang R., Zhu L., Xue L., Liu L., Yan X., Wang J., Zhang B., Xu T., Ji F., Zhao Y., Cheng J., Wang Y., Shao H., Hong S., Cao Q., Li C., Zhao X.A., Zou L., Sang D., Zhao H., Guan X., Chen X., Shan C., Xia J., Chen Y., Yan X., Wei J., Zhu C., Wu C. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itelman E., Wasserstrum Y., Segev A., Avaky C., Negru L., Cohen D., Turpashvili N., Anani S., Zilber E., Lasman N., Athamna A., Segal O., Halevy T., Sabiner Y., Donin Y., Abraham L., Berdugo E., Zarka A., Greidinger D., Agbaria M., Kitany N., Katorza E., Shenhav-Saltzman G., Segal G. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr. Med. Assoc. J. 2020;22:271–274. [PubMed] [Google Scholar]
- 54.Javanian M., Bayani M., Shokri M., Sadeghi-Haddad-Zavareh M., Babazadeh A., Yeganeh B., Mohseni S., Mehraein R., Sepidarkish M., Bijani A., Rostami A., Shahbazi M., Tabari A.M., Shabani A., Masrour-Roudsari J., Hasanpour A.H., Gholinejad H.E., Ghorbani H., Ebrahimpour S. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom. J. Intern. Med. 2020;0 doi: 10.2478/rjim-2020-0013. [DOI] [PubMed] [Google Scholar]
- 55.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F., Cao S., Liu X., Xiang Y., Zhao Q., Huang H., Yang B., Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun L., Shen L., Fan J., Gu F., Hu M., An Y., Zhou Q., Fan H., Bi J. Clinical Features of Patients with Coronavirus Disease 2019 (COVID-19) from a Designated Hospital in Beijing, China. J. Med. Virol. 2020:1–12. doi: 10.1002/jmv.25966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y., Huang X., Lv J., Luo Y., Shen L., Yang H., Huang G., Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020:1–10. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S., Jian M., Xu H., Prowle J., Hu B., Li Y., Peng Z. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit. Care. 2020;24:1–9. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J., Wang D., Liu C., Meng Y., Cui L., Yu J., Cao H., Li L. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J. Intern. Med. 2020;2:1–11. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 61.Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40:1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., Li S.B., Wang H.Y., Zhang S., Gao H.N., Sheng J.F., Cai H.L., Qiu Y.Q., Li L.J. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:1–7. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu P.P., Tian R.H., Luo S., Zu Z.Y., Fan B., Wang X.M., Xu K., Wang J.T., Zhu J., Shi J.C., Chen F., Wan B., Yan Z.H., Wang R.P., Chen W., Fan W.H., Zhang C., Lu M.J., Sun Z.Y., Zhou C.S., Zhang L.N., Xia F., Qi L., Zhang W., Zhong J., Liu X.X., Zhang Q.R., Lu G.M., Zhang L.J. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10:6372–6383. doi: 10.7150/thno.46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.jin Zhang J., Dong X., yuan Cao Y., dong Yuan Y., bin Yang Y., qin Yan Y., Akdis C.A., dong Gao Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur. J. Allergy Clin. Immunol. 2020:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 65.Zheng F., Tang W., Li H., Huang Y.X., Xie Y.L., Zhou Z.G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 66.Zhao X.Y., Xu X.X., Sen Yin H., Hu Q.M., Xiong T., Tang Y.Y., Yang A.Y., Yu B.P., Huang Z.P. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect. Dis. 2020;20:1–8. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.