Abstract
STUDY QUESTION
How does exposure to a testosterone rich environment affect the function and gene expression of human fallopian tube epithelium (hFTE)?
SUMMARY ANSWER
Elevated testosterone level alters several gene transcripts that regulate cilia expression and negatively impacts the rate of cilia beating.
WHAT IS KNOWN ALREADY
The presence of estrogen in the follicular phase of the menstrual cycle increases the human fallopian tube ciliary beating frequency. The luteal phase, triggered by ovulation and increasing progesterone, is marked by a decrease in ciliary beating. Women with polycystic ovarian syndrome (PCOS) may have twice the serum level of testosterone than ovulatory women. To date, the effect of elevated androgens on the function of the human fallopian tube is not well-understood. We chose to examine the impact of elevated testosterone on hFTE.
STUDY DESIGN, SIZE, DURATION
A prospective basic science study of human fallopian tube specimens from reproductive-aged women undergoing benign gynecologic surgery was performed. Fallopian tube removal at a large US academic center was collected and provided to us to continue with epithelium isolation and culturing. A total of 12 patients were analyzed in the study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Fallopian tube epithelium was isolated and exposed to two different conditions: normal with low testosterone concentration of 0.8 nM and PCOS-like, with high testosterone concentration of 2 nM. The study was conducted in both static and dynamic conditions in microfluidic devices for a total of 14 days, after which the tissue was collected for processing including RNA extraction, quantitative PCR and immunohistochemistry. After the first 7 days of each experiment, a sample of tissue from each condition was imaged to quantify cilia beating frequency.
MAIN RESULTS AND THE ROLE OF CHANCE
hFTE exposed to the 2 nM testosterone displayed slower cilia beating, inhibited estrogen signaling and decreased expression of the ciliary marker FOXJ1 when compared to stimulation with 0.8 nM testosterone.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
The in vivo response to elevated testosterone may differ from in vitro studies. RNA amount was limited from tissue cultured in the microfluidic devices as compared to static culture.
WIDER IMPLICATIONS OF THE FINDINGS
Understanding elevated testosterone in tubal function may explain an additional contribution to subfertility in women with PCOS and other hyper-androgen disorders, aside from oligo-ovulation. Furthermore, this adds to the body of literature of fallopian tube function using a microfluidic device.
STUDY FUNDING/COMPETING INTEREST(S)
NIH grants: UH3 ES029073 and R01 CA240301. There are no competing interests.
Keywords: fallopian tube, hyperandrogenism, estrogen, polycystic ovarian syndrome, microfluidic, cilia, FOXJ1
Introduction
The fallopian tubes are the site of fertilization but are also necessary for transport of the sperm, egg, and developing embryo, as well as tubal secretions which influence embryo development (Buhi et al., 2000; Hunter et al., 2005; Lyons et al., 2006a,b). The mechanisms involved in tubal transport include muscular contractions, flow of tubal secretions and the beating of cilia, which are hair-like projections extending into the tubal lumen (Jansen, 1984). It has been suggested that ciliary motion may play a critical role in fallopian tube transport (Lyons et al., 2006a,b). With widespread availability of IVF, the physiology of the human fallopian tube has become increasingly overlooked. However, for mammalian in vivo fertilization (IVF), the fallopian tube is essential for reproduction (Lyons et al., 2006a,b). When ciliary motion, and thus tubal function, is disrupted through trauma to the tubal epithelium by sexually transmitted infections, pelvic inflammatory disease or environmental exposures such as cigarette use, there is a significant negative impact on fertility (McGee et al., 1981; Cooper et al., 1986; Cooper et al., 1990; McGee et al., 1999; Bouyer et al., 2003). In addition, cigarette smoke inhibits aromatase activity, suggesting that it may play a role in reproduction through increased testosterone levels (Biegon et al., 2012).
The tubal epithelium responds to hormonal changes during the menstrual cycle (Novak, 1928; Critoph and Dennis, 1977a). Ovarian hormones regulate tubal epithelial structure and the expression of cilia (Lyons et al., 2006a,b). Estrogen stimulates epithelial cell hypertrophy, secretion and ciliogenesis, while high levels of progesterone, as seen in the luteal phase, are associated with epithelial atrophy and deciliation (Verhage et al., 1979). Animal studies found an increase in cilia beating frequency (CBF) following copulation, presumed to be due to high estrogen levels and the need for gamete transport (Borell et al., 1957). This finding was corroborated in human fallopian tube specimens with an increase in CBF after ovulation, when estrogen predominates, and decreased CBF later in the progesterone dominant luteal phase (Critoph and Dennis, 1977b).
Ciliary beating frequency in response to estrogen and progesterone was studied in an ex vivo model using human fallopian tube epithelium (hFTE) specimens and a stepwise exogenous hormone preparation (Zhu et al., 2016). A static culture model was able to maintain viability of hFTE for the duration of the 14 days experiment. After 7 days of hormone exposure, the CBF was significantly increased with estrogen exposure in a dose–response pattern, with a 25% increase in CBF in the tissue exposed to higher estrogen and in the absence of progesterone. However, when the hFTE was exposed to estrogen combined with progesterone, the CBF decreased by 30%. One effect not considered in this study was the influence of androgens, including testosterone, on CBF and overall tubal function.
In ovarian steroidogenesis, estrogen is made in the granulosa cells by the aromatization of precursor androgens, which originate from ovarian theca cells (Stocco, 2008). Ovarian androgens are continuously produced throughout the menstrual cycle (Lebbe and Woodruff, 2013). Testosterone peaks a couple of days after estrogen (Salonia et al., 2008) suggesting that in physiological conditions it does not interfere with estrogen action. Ovulation is a sentinel event changing the hormonal environment of the human reproductive tract. However, in anovulatory or oligo-ovulatory women, the lack of cyclical progesterone production, and thus prolonged estrogen and testosterone exposure, can have various clinical consequences including subfertility and endometrial hyperplasia, which can result in endometrial cancer (Kim et al., 2013). Clinically significant disruptions in ovulation most often occur in women with polycystic ovarian syndrome (PCOS) and during the perimenopausal transition.
PCOS is the most common endocrinopathy of reproductive-aged women with an incidence of up to one in eight women of reproductive age. Despite being a common diagnosis worldwide, there is no universally accepted definition of the condition. In fact, several criteria are used to determine the diagnosis based on the presence of hyperandrogenism, oligoamenorrhea or amenorrhea, and polycystic morphology of the ovaries on ultrasound (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). Among these, hyperandrogenism is the most consistently agreed upon criterion between expert panels. Testosterone is the most frequently tested androgen in the clinical diagnosis of PCOS. Although there is no cutoff for the definition of hyperandrogenemia, women with PCOS have been noted to have two times the level of testosterone than ovulatory women (Winters et al., 2000). The paracrine and endocrine actions of ovarian testosterone on the human fallopian tube are of interest as there is evidence that ovarian hormones are very highly concentrated in the ovarian follicular fluid, which is found in the fallopian tubes after ovulation (Emori and Drapkin, 2014). Furthermore, the event of ovulation is a risk factor for high grade serous ovarian cancer that originates in the fallopian tube (Fathalla, 2013). There is emerging evidence of elevated circulating testosterone increasing the risk of epithelial ovarian cancers (Ose et al., 2017). Exploring the sequelae of elevated testosterone exposure on fallopian tube health and function may be important to understanding the lifetime health risks for women living with PCOS and/or hyperandrogenism.
This study sought to investigate the impact of androgens on hFTE using ex vivo models that were previously validated in a microfluidic device (Zhu et al., 2016; Xiao et al., 2017). Our objective was to evaluate the impact of elevated testosterone on hFTE function and gene expression in static and dynamic conditions used for primary human explant culture. Given the importance of tubal cilia to reproduction, we choose to examine cilia beating and cilia related genes as a marker of overall tubal health.
Materials and methods
Chemical and reagents
Seventeen beta-estradiol (E2) was purchased from innovative research of America (NE-121). Testosterone (T1500), rhFSH (F4021) and fetuin (F3385) were purchased from Sigma (St. Louis, USA). F12 was purchased from Gibco (1967615). Alpha minimum essential medium (MEM) was purchased from Corning (10-022-CV). ITS (5 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml selenium) was purchased from Sigma (11074547001).
Human fallopian tissue
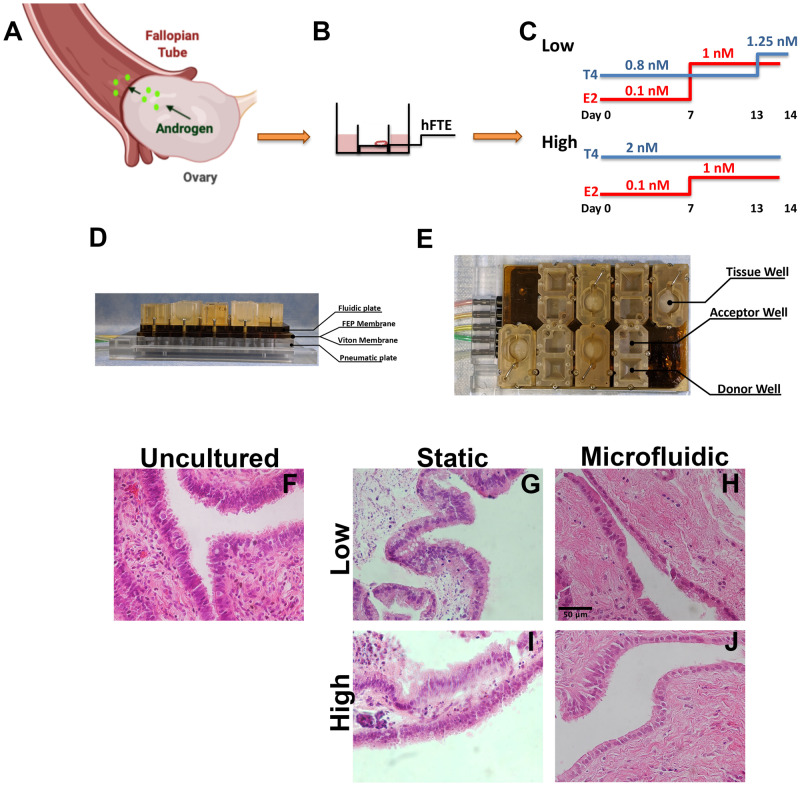
Human fallopian tube specimens were obtained from hysterectomies and salpingectomies done for benign indications in pre-menopausal women, aged 35–50, at Northwestern University Prentice Women’s Hospital (Chicago, IL, USA) from 1 October 2018 to 30 November 2019. Written informed consent was obtained from each subject prior to surgery. Each portion of fallopian tube received was cut lengthwise and the inner epithelium and underlying stroma were removed with blunt dissection using forceps. The tissue was dissected in MEM and Nutrient Mixture F-12 medium (50:50) with 10% fetal bovine serum within a 24-h period from arrival. The tissue was then transferred to MEM with 0.3% bovine serum albumin, 0.5 mg/ml fetuin, 1% penicillin/streptomycin and ITS medium. The epithelium was then isolated from the underlying stroma, again with blunt dissection using forceps, and fashioned into 2 × 2 mm sections for culture. Tissues from the same patient were divided into normal and PCOS-like conditions and cultured on 0.4 µm pore Millicell inserts (Millipore, MA, USA). After 24 h, the static condition experiments continued in static culture in 12-well plates while microfluidic experiments were transferred to the microfluidic devices for dynamic culture. Both static and microfluidic conditions were maintained in incubators at 37°C and 5% CO2 for 14 days in one of the two experimental conditions. The physiologic media condition, referred to as ‘normal’ contained estrogen (E2) 0.1 nM and testosterone 0.8 nM on Days 0–6 then E2 1 nM and testosterone 0.8 nM Days 7–13 and finally E2 1 nM and testosterone 1.25 nM for 1 day prior to the end of the experiment on Day 14. The hyperandrogenic media conditions referred to as ‘PCOS’ contained E2 0.1 nM and testosterone 2 nM on Days 0–6 then E2 1 nM and testosterone 2 nM Days 7–14 until the experiment ended on Day 14. In this model, recombinant follicle-stimulating hormone (rFSH) was present in the media from Days 0 to 14, to replicate the follicular phase of the menstrual cycle. After 14 days of culture, the tissue was either frozen for RNA extraction or fixed in 4% paraformaldehyde for immunohistochemistry.
Ethical committee approval
Written informed consent was obtained from each subject and experiments, procedures and methods were performed in accordance with the IRB-approved guidelines and regulations (IRB# STU00205203).
RNA sequencing
RNA was isolated from cells using Qiagen RNAeasy Mini kit (#74104) as per manufacturer’s protocol. RNA libraries (three technical replicates) were created. RNA quality determination, mRNA enrichment, library construction, sequencing and transcriptome statistical analysis were performed at the Genomics Core Facility at Northwestern University as previously described (Colina et al., 2019).
Microfluidic systems
Prior to all microfluidic experiments, all systems were sterilized and subsequently assembled under sterile conditions. The single microfluidic platform (Solo-MFP) system operation was previously described (Xiao et al., 2017) (Fig. 1). All fallopian tube epithelium isolated as above was cultured on 0.4 µm pore Millicell inserts and the microfluidic experiments were placed into the Solo-MFP tissue culture modules containing 200 µl of the corresponding culture media, either normal or PCOS. An additional 2 ml of media was placed in each donor module, for circulation throughout the Solo-MFP system. Acceptor modules were sampled daily, and the collected media stored at −20°C prior to analysis. The flow rate was between 35 and 50 µl/h. The donor modules were refilled daily.
Figure 1.
Experimental design to model androgen exposure from polycystic ovarian syndrome (PCOS). (A) Schematic of fallopian tube interaction with ovarian-derived androgen. (B) Transwell insert with human fallopian tube epithelium (hFTE) tissues exposed at the air/liquid interface. (C) Hormonal treatments for Low (0.8 nM) vs. PCOS-like High (2 nM) androgen conditions. Estrogen (E2) was the same in both conditions, whereas testosterone was two times higher than control. (D and E) Microfluidic device using pneumatic pumps for tissue perfusion, fluorinated ethylene-propylene (FEP) and Viton membranes. (F) Hematoxylin and eosin staining for uncultured hFTE showing a dense and structured stroma vs. (G and I) hFTE cultured for 14 days in static conditions. (H and J) In the microfluidic system, the hFTE tissue maintained stroma more similar to the uncultured. Scale bar is 50 µm and all pictures have same magnification.
Immunohistochemistry
After 14 days, cultured tissue was fixed for 24 h in 10% paraformaldehyde. Tissues were then dehydrated in increasing concentrations of 50–70% ethanol. Dehydrated tissues were then embedded in paraffin using an automated tissue processor. Later 5 µm serial sections were created for hematoxylin and eosin (H&E) and immunohistochemistry staining as previously described (Russo et al., 2018). Antibodies used are described in Supplementary Table SI. In brief, tissues were incubated with primary antibodies overnight at 4°C and secondary for 1 h at room temperature.
Imaging and quantification of CBF
After 7 days of culture, the hFTE was imaged using a Nikon CFI SR HP Plan Apo Lamba S 100xSil Objective 1.35NA on a Nikon Ti2 microscope stand and captured with a Photometrics Prime 95B 25 mm camera with 5 ms frame rate. Five movies in different areas of the sample were taken. The movies were analyzed within ImageJ FIJI software by creating kymograph images to determine the CBF in Hertz (Hz) (Zhu et al., 2016).
Immunoassay analysis
Vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) concentrations in media collected from both the static and microfluidic experiments were measured using commercial ELISA kits (RayBiotech, Peachtree Corners, GA, USA) according to the manufacturer’s protocols. A 100 µl of conditioned media from hFTE from three subjects at Day 14 of treatment, were loaded on ELISA plates in duplicate.
Quantitative PCR
Tissues were homogenized in lysis buffer plus beta-mercaptoethanol (RLT buffer) using zirconia beads (Invitrogen, Waltham, MA, USA) and the Mini-BeadBeater cell/tissue disrupter (BioSpec, Bartlesville, OK, USA). Tissues were centrifuged and supernatant processed for RNA extraction as described by RNeasy kit manufacturer’s instructions (Qiagen, Germantown, MD, USA). iScript™ cDNA synthesis kit (BioRad, Hercules, CA, USA) and SYBR green (Roche, Madison, WI, USA) were used according to manufacturer’s instructions. All quantitative PCR (qPCR) runs were performed on the BioRad machine. Primers used are listed in Supplementary Table SII.
Statistical analysis
All data are represented as mean ± SE. Statistical analysis was carried out using Prism software (GraphPad, La Jolla, CA, USA). All conditions were tested in three replicates in at least triplicate experiments. Statistical significance was determined by Student’s t-test, or one-way ANOVA with Dunnett’s post hoc test or two-way ANOVA. *P ≤ 0.05 was considered significant.
Results
A PCOS mimetic protocol for human fallopian tube in a microfluidic system
An in vitro model for studying the role of fallopian tube epithelium in PCOS was created to simulate a key feature of the disease, the presence of increased androgen, with a concentration of testosterone approximately two times higher (2 nM) than what is detected in control (0.8 nM). The hFTE was isolated from tissue collected from women having surgery for benign gynecological conditions and cultured in the normal (Low) or PCOS-like (High) conditions for 14 days (Fig. 1C). The same culture medium and concentration of hormones were used for tissues in static versus microfluidic conditions. In both cases, the tissues were added to transwells (Fig. 1A and B) inserted into 12-well plates (static) or into microfluidics wells (Fig. 1D and E). In the microfluidic system, the tissues maintained its structure with a more compact stroma that resembled uncultured tissue as shown through H&E staining (Fig. 1F–J).
Hormonal responses for fallopian tube epithelium in static or dynamic cultures
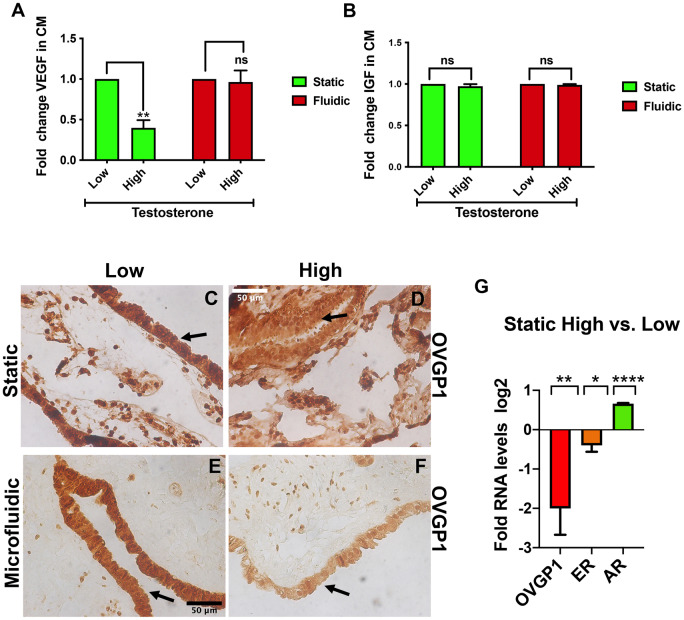
Previously, hFTE was cultured in the microfluidic device in the presence of hormones to model the normal reproductive cycle. VEGF has been shown to be induced in the presence of the estrogen-dominated phases (Zhu et al., 2016) and to play a role in embryo implantation and is expressed and secreted by hFTE (Zarezade et al., 2015). In order to compare the role of elevated testosterone levels, the amount of VEGF-A secretion was quantified. Reduced VEGF-A secretion was found in PCOS-like (High) vs. normal (Low) in the static condition, but not in microfluidic conditions potentially due to the effect of increased flow (Fig. 2A). In the microfluidic system, secretions are distributed by the constant flow of the pumps and this results in the generation of gradients that might result in increased dilution, limiting detection. IGF has been shown to be secreted by fallopian tube and to play a role in embryo pre-implantation (Winger et al., 1997) and development (Kirby et al., 1996). IGF-1 secretion was not changed (Fig. 2B). The reduced VEGF-A secretion suggests an anti-estrogenic effect of testosterone. In order to confirm the impact on estrogen receptor signaling, a well-known estrogen-dependent target, OVGP1 (estrogen target oviduct-specific glycoprotein 1), was measured and the protein expression was downregulated in the presence of testosterone in static and fluidic conditions (Fig. 2C–F). OVGP1 downregulation was also confirmed by qPCR in the static condition where estrogen receptor and the estrogen-dependent target, OVGP1, were both downregulated in the presence of testosterone (Fig. 2G).
Figure 2.
Androgen-induced response on human fallopian tube static vs. dynamic conditions. (A) ELISA for vascular endothelial growth factor-A (VEGF-A) in conditioned media from hFTE treated with low testosterone vs. high, show a significant decrease in VEGF-A after 14 days. (B) Insulin-like growth factor-1 (IGF-1) secretion was unchanged in the presence of high testosterone. (C and D) Decreased oviduct-specific glicoprotein-1 (OVGP1) protein, in static culture, after exposure to high testosterone is compared to low testosterone using immunohistochemistry (IHC). Scale bar is 50 µm and all pictures have same magnification. (E and F) Decreased OVGP1 protein in the presence of high testosterone as compared to low using IHC in the microfluidic. (G) Reduction of OVGP1 expression confirmed by quantitative PCR (qPCR) in static tissues treated for 14 days. Experiments include four different patients and graph are represented as mean ± SEM. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001
Androgen and estrogen receptor localization were analyzed using immunohistochemistry in normal (Low) and PCOS-like (High) conditions. In both Low static and Low fluidic culture conditions, the androgen receptor was localized more in the cytoplasm, while in the PCOS-like High conditions, there was more receptor localized in the nucleus (Figs 3A and B and 3E and F). In contrast, estrogen receptor was localized less in the nucleus in High condition treated hFTE as compared to Low (Figs 3C and D and 3G–F). The receptor localization further suggests that the increased testosterone concentration was activating androgen receptor and thereby its nuclear accumulation, while inhibiting estrogen receptor activity and causing it to reside in the cytosol.
Figure 3.
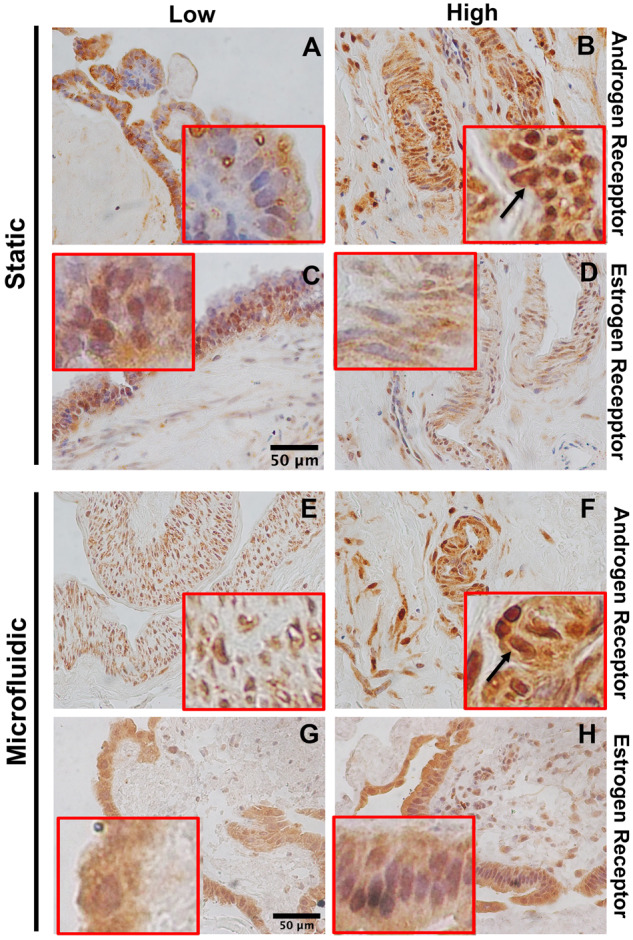
Estrogen and androgen receptor expression in responses to hFTE static vs. dynamic conditions in response to high androgen. IHC for androgen receptor (A and B) and estrogen receptor (C and D) in static conditions. IHC for androgen receptor (E and F) and estrogen receptor (G and H) in microfluidic conditions (E–H). Scale bar 50 µm. Magnification is the same for all pictures. Red squares show a 4× magnification of the image to visualize receptor localization.
Androgen stimulation alters cilia genes profile
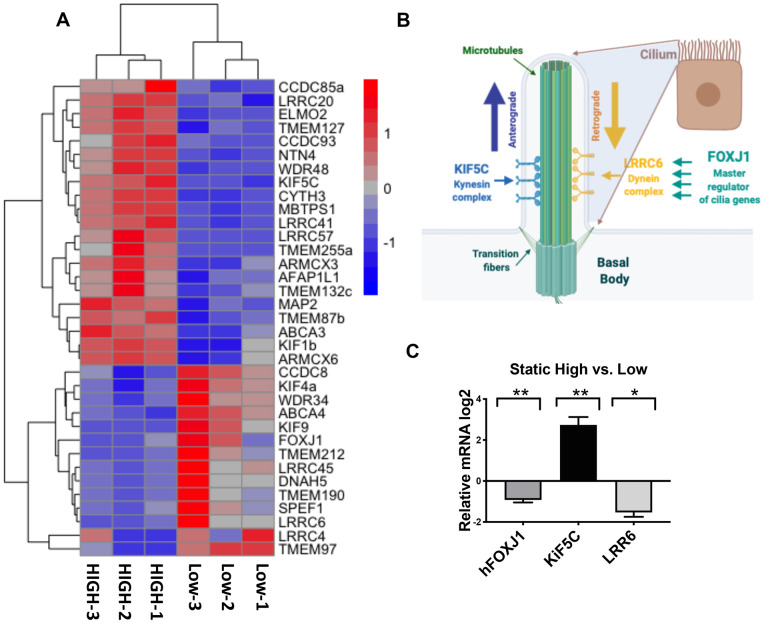
Fallopian tube epithelium expresses androgen receptors (Horne et al., 2009); however, very few analyses have been reported on the signaling pathways of androgens in the fallopian tube, particularly using primary human tissue. To gain a better insight into the androgen-regulated pathways, RNA-sequencing (RNAseq) was performed from RNA extracted from tissues treated with High vs. Low testosterone concentrations. Unbiased two-way hierarchical clustering analysis revealed transcripts were most similar between individual patients (Supplementary Fig. S1). The RNAseq identified 556 transcripts differentially and significantly altered upon exposure to high testosterone concentrations. GSEA (gene set enrichment analysis) and Cytoscape clustering revealed treatment with high testosterone-regulated pathways associated with ciliary function and assembly (Supplementary Figs S2 and S3) with FOXJ1 and LRRC6 appearing in the GSEA leading edge analysis. Based on the data analysis, we generated a heat map of cilia transcripts regulated upon high testosterone stimulation in hFTE that were involved in cilia function and cilia machinery, including FOXJ1, LRRC6 and KIF5C (Fig. 4A). Other members of the LRRC dynein family were also regulated. Dyneins are involved in the retrograde intraciliary transport and are balanced with kinesins that regulate anterograde transport (Fig. 4B). A member of the kinesins KIF5C showed up in our top 20 upregulated genes (Fig. 4A). FOXJ1, LRRC6 and KIF5C were therefore further validated in static conditions using qPCR (Fig. 4C).
Figure 4.
Exposure to PCOS-like (high) androgen concentrations alters expression of transcripts that encode for cilia in hFTE. (A) Heatmap of cilia mRNA expression in in PCOS-like (High) vs. control (Low). These data were generated using hFTE from three subjects treated with control or PCOS-like androgen levels in static culture for 14 days. Only significant genes with false discovery rate (FDR) adjusted P-value < 0.05 were included. (B) Schematic showing the role of FOXJ1 as master regulator of cilia genes and of LRRC6 and KIF5C regulation of intra-cilia transport that is critical for proper cilia function. (C) qPCR validation of FOXJ1, KIF5C, LRRC6 mRNA expression from three independent patient samples. *P ≤ 0.05; **P ≤ 0.01.
Androgen stimulation alters the frequency of cilia beating
In order to further validate the RNAseq, we analyzed the expression and localization of cilia proteins in hFTE tissues by immunohistochemistry. In particular acetylated tubulin was chosen to stain cilia to confirm the cilia structure. FOXJ1, which is a master regulator of cilia function, was also selected as this transcription factor regulates expression of important proteins that constitute cilia. In both static (Fig. 5A and B) and fluidic cultures (Fig. 5E–H), cilia structures were present based on acetylated tubulin staining in Low and High conditions. However, FOXJ1 expression and localization in the nucleus, which indicates its activity, was reduced with increased testosterone in High conditions (Fig. 5C and D) and (Fig. 5G and H). This suggests that cilia function may be altered by elevated testosterone. After 7 days of culture, we imaged the hFTE using a Nikon Ti2 microscope and found that elevated testosterone exposure reduces CBF (Fig. 6A and B).
Figure 5.
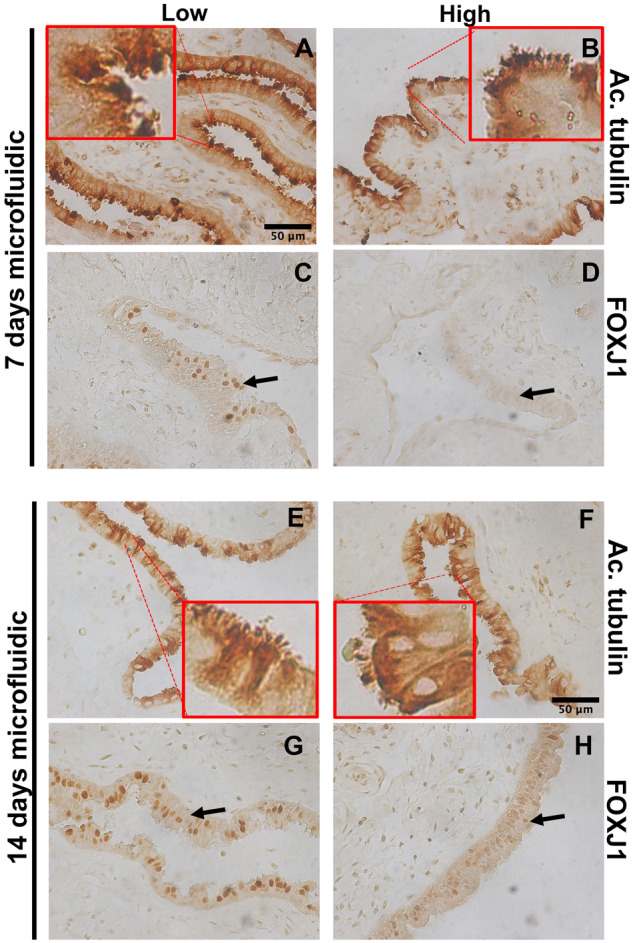
High androgen alters protein expression of FOXJ1, a regulator of cilia expression, in fluidic cultures. (A and B) IHC of acetylated tubulin (Ac. Tubulin), which marks cilia, in low vs. high testosterone-treated hFTE after 7 days treatment and 14 days. (C and D) IHC of FOXJ1 shows reduced expression and nuclear localization in high testosterone-treated hFTE after 7 days treatment. (E and F) IHC of acetylated tubulin (Ac. Tubulin), in low vs. high testosterone-treated hFTE after 14 days of treatment. (G and H) IHC of FOXJ1 shows reduced expression and nuclear localization in high testosterone-treated hFTE after 14 days treatment. Scale bar is 50 µm and magnification is the same for all pictures. Red squares show a 4× magnification of the image to visualize receptor localization.
Figure 6.
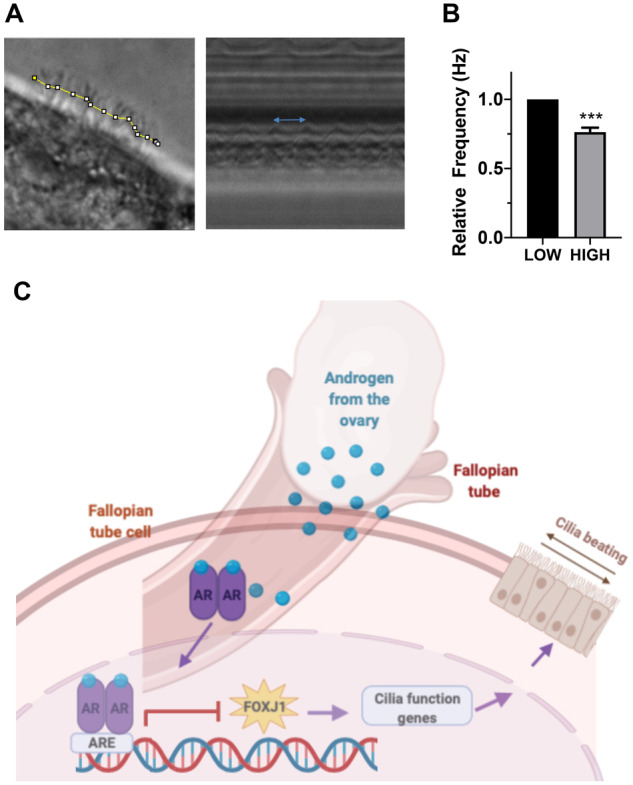
High androgen reduces cilia beating frequency. (A and B) Tubal cilia were imaged using a spinning disc Nikon Ti2 microscope which showed that high androgen exposure significantly reduced cilia beating. In the left panel of ‘A’, the yellow line indicates a section of moving cilia selected for quantification. The blue arrow in the right hand panel shows the distance between two waveforms. Experiments shown in ‘B’ include four unique subjects. The graphs are represented as mean ± SEM. (C) Schematic representing the androgen signaling pathway with ovarian over-production of androgen in PCOS patients and the stimulation of receptors on the hFTE. Activation of the receptor caused translocation to the nucleus to inhibit transcription of cilia genes, FOXJ1 (as well as LRRC6 and KIF5C) that are critical for proper cilia assembly and movement.***P < 0.001.
Discussion
This study investigated the impact of androgens on ex vivo models of primary hFTE. We chose to focus on testosterone as this is most clinically relevant and is documented as the predominantly elevated androgen in women with PCOS (Stocco, 2008). Excessive testosterone stimulation of hFTE altered the genomic profile, specifically of genes related to ciliary assembly and function. In addition, higher testosterone concentration altered CBF (Cilia Beating Frequency) and hormonal signaling. These results suggest that the hyperandrogenic environment, found in PCOS patients, may impact human fallopian tube function in ways that can modulate reproduction, as compared to a physiologic ovulatory environment.
Herein, we addressed the role of androgen on hFTE in static and dynamic culture and showed that human fallopian tube maintains a healthier and compact structure, when cultivated in microfluidic conditions (Fig. 1), consistent with other studies showing that dynamic condition preserves fallopian and ovarian health (Zhu et al., 2016; Xiao et al., 2017). Our model is based on isolation of the epithelium monolayer that allows a reduced thickness of the tissue that is critical for high-resolution microscopy of CBF in addition to tissue exposure to nutrients and hormone stimulation.
In vivo study of fallopian tube epithelial function is contraindicated in humans as the potential for trauma to the fallopian tubes can predispose to scarring, infection, ectopic pregnancy and infertility (Lyons et al., 2006a,b). Therefore, building upon previous work using ex vivo cultures of hFTE, we designed experiments using exogenous hormones and investigated their role on hFTE explants. We also chose to use exogenous hormones as opposed to co-culturing with murine ovaries as a way to test our hypothesis and eliminate the impact of other ovarian derived hormones or peptides which could affect tubal function. Limitations of this study include limited sample size and consistency in terms of age, diagnosis and potential contraception or treatment of benign gynecologic conditions, as well as the intrinsic variability between human specimens. Future work could examine the relevance of our findings using either a mouse or rat model of PCOS, both of which have been developed using exposure to either dehydroepiandrosterone or testosterone by repeated injection or ingestion that gives rise to ovaries with some biological characteristics of human disease (Paixao et al., 2017).
Androgen exposure in the fallopian tube epithelium reduced estrogen receptor as well as the estrogen target OVGP1, which is produced by fallopian secretory cells. OVGP1 facilitates sperm capacitation and motility for fertilization (Erickson-Lawrence et al., 1989; Verhage et al., 1997) suggesting a potential role for testosterone on sperm function through reduction of OVGP1. VEGF-A, which increases during periovulatory stages (Lam et al., 2003a,b), was reduced with high concentration androgen treatment, consistent with less nuclear estrogen receptor and therefore less estrogen receptor action.
RNA-sequencing analysis of hFTE after high testosterone exposure revealed repression of FOXJ1, a master regulator of ciliogenesis, that modifies the expression of other cilia-related genes (Yu et al., 2008). FOXJ1 affects the assembly of the cilium by anterograde transport (Johnson and Rosenbaum, 1992) and cilia turnover via retrograde transport (Rosenbaum and Witman, 2002; Scholey, 2003; Ishikawa and Marshall, 2011). Dulohery et al. (2019) characterized the alterations to the fallopian tube after surgical removal in female-to-male transsexuals who had undergone testosterone therapy for 1–3 years prior to surgery, as compared to normally cycling controls and found luminal accumulations of thick mucus-like secretions and cellular debris, which caused ciliary clumping and potential luminal blockage. The abundance of viscous secretions in the lumen may be a mechanism by which hyperandrogenism impairs fertility and decreases ciliary beating frequency by ciliary entrapment. However, this was not tested, and mucus accumulation may be actually a consequence of cilia impairment. This study corroborates our own as both studies show the immediate and long-term impact of testosterone on fallopian tube epithelium to be disruptive to normal function.
Tubal transport may play the dominant role in transport of gametes and contributes to early embryo development (Lyons et al., 2006a,b). Cilia beating is an integral component of the reproductive function of fallopian tubes, but tubal contraction and secretions are also required (Jansen, 1984). Nevertheless, experiments that blocked tubal contractility still allowed for transport of ovum suggesting that the cilia alone can facilitate this task (Halbert et al., 1976; Halbert et al., 1989). Genetic conditions, such as primary ciliary dyskinesia, impair ciliary motion and affected women with varying degrees of infertility based on the specific genes involved and thus the degree of ciliary motility (Halbert et al., 1997; Raidt et al., 2015). In women with primary ciliary dyskinesia, who present with subfertility (Afzelius et al., 1978; Pedersen, 1983; Lurie et al., 1989), rare cases of successful pregnancy may be explained by the presence of additional tubal factors in the absence of normal ciliary function, which allow for gamete and embryo transport (McComb et al., 1986; Halbert et al., 1997). In a study of 36 women with primary ciliary dyskinesia, only 61.1% were found to be infertile. However, in the infertile patients, mutations in LRRC50 and LRRC6 that encode for cilia machinery have been found (Vanaken et al., 2017) suggesting that the gene profile of ciliary components is a critical contribution to infertility in addition to cilia beating. Furthermore, common sexually transmitted reproductive pathogens cause deciliation and acute and chronic inflammation in the fallopian tube (McGee et al., 1981; Cooper et al., 1986). CBF is significantly lower in the surviving cilia of fallopian tubes showing evidence of edema, dilation or distal obstruction as a result of salpingitis (Leng et al., 1998). This deciliation appears to be irreversible which significantly increases infertility and ectopic pregnancy risk (Cooper et al., 1990).
There is a paucity of published data on the risk of ectopic pregnancy in women with PCOS. One retrospective cohort study found a higher ectopic pregnancy risk in women with PCOS who underwent controlled ovarian hyperstimulation (COH) with subsequent embryo transfer compared to women without PCOS (Wang et al., 2013). Higher estrogen levels in women without PCOS also conferred a higher ectopic pregnancy risk than women with lower estrogen levels, although women with PCOS in both the high and low estrogen groups had a higher risk of ectopic than women without PCOS. This risk was ameliorated when frozen embryo transfers were performed, where serum hormone levels are more similar to natural conception cycles than COH. Testosterone levels were not included in their analyses. Women with PCOS tend to have higher estrogen levels during COH and are at higher risk of ovarian hyperstimulation syndrome (Luke et al., 2010). The derangement of serum ovarian hormones in COH may also include elevated testosterone, which increases with estrogen levels prior to ovulation. Interestingly, in our study, higher androgen levels resulted in reduced estrogen receptor nuclear localization and OVGP1 expression suggesting that despite higher steroid levels of estrogen, the fallopian tube epithelium of PCOS women may respond to hormones differently due to higher androgen exposure. Given the effect of testosterone on tubal cilia in our ex vivo models, we are left to question the clinical reproductive significance and whether women with hyperandrogenism have an increased risk of ectopic pregnancy or other fallopian tube dysfunction. Unfortunately, no specimens included in this study were obtained from women with a clinical diagnosis of PCOS at the time of surgery. This could be viewed as a limitation but due to the heterogeneity of PCOS phenotypes and differences in diagnostic criteria, using PCOS tubal specimens may have added to the patient variability. Further women of reproductive age with PCOS are not frequent among surgical cases for bilateral salpingectomy for sterilization or hysterectomy for benign indications. Potential PCOS specimens may be captured by including specimens from surgery for ectopic pregnancy, hydrosalpinges or endometrial cancer, but these fallopian tubes may also confer a high degree of tubal damage. We sought to reduce selection bias by limiting our specimens to healthy tubal specimens. If the epithelium appeared damaged or scant in amount on initial dissection, that tissue was not used for experiment and was banked for potential future research. In addition, another interesting question that we are currently addressing is the role of anti-androgenic drugs (bicalutamide) and environmental endocrine disrupting factors (phthalates) on human fallopian tube health and cilia function. Furthermore, the uncovered role of testosterone on cilia assembly and function may suggest an interesting implication on the effect on androgen on lung function. Sex disparity has been demonstrated in lung diseases, but the role of testosterone has been shown to be controversial. In a mouse model, testosterone exacerbated pulmonary fibrosis (Voltz et al., 2008).
In summary, this study demonstrated that elevated testosterone exposure impaired tubal ciliary function and gene expression. This direct effect on ciliary function, hormone profile and gene expression were observed at a relatively low dose of testosterone and short duration of time. The in vitro models sustained tissue integrity and viability for 14 days of culture on both static and microfluidics systems, again adding to the literature the validity of using microfluidic systems to understand nuances of human reproduction and factors that contribute to impaired fertility. This study adds to our understanding of the mechanisms of subfertility and reproductive health risks in women living with hyperandrogenic disorders, including PCOS.
Supplementary Material
Acknowledgements
We thank the University of Illinois at Chicago and Northwestern University tissue bank for providing valuable samples for our study. We thank the Northwestern University Center for Advanced Microscopy (supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center) for their assistance with the cilia imaging.
Authors’ roles
T.J.-B. contributed on experimental execution, paper figures and writing. J.C. contributed on paper figures. B.C.I. and J.C. provided the microfluidic devices. M.U., J.J.K. and W.K.T. are part of the Northwestern Reproductive Science Center and collaborated on the experimental design, subject recruitment and provision of human fallopian tube specimens. J.E.B. supervised the project. A.R. generated the project idea and contributed on experimental design, paper figures and writing.
Funding
We thank the National Institutes of Health (NIH) for funding UH3 (ES029073) and R01 (CA240301).
Conflict of interest
The authors declare no conflict of interest.
References
- Afzelius BA, Camner P, Mossberg B.. On the function of cilia in the female reproductive tract. Fertil Steril 1978;29:72–74. [DOI] [PubMed] [Google Scholar]
- Biegon A, Alia-Klein N, Fowler JS.. Potential contribution of aromatase inhibition to the effects of nicotine and related compounds on the brain. Front Pharmacol 2012;3:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borell U, Nilsson O, Westman A.. Ciliary activity in the rabbit fallopian tube during oestrus and after copulation. Acta Obstet Gynecol Scand 1957;36:22–28. [DOI] [PubMed] [Google Scholar]
- Bouyer J, Coste J, Shojaei T, Pouly JL, Fernandez H, Gerbaud L, Job-Spira N.. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol 2003;157:185–194. [DOI] [PubMed] [Google Scholar]
- Buhi WC, Alvarez IM, Kouba AJ.. Secreted proteins of the oviduct. Cells Tissues Organs 2000;166:165–179. [DOI] [PubMed] [Google Scholar]
- Colina J, Varughese P, Karthikeyan S, Salvi A, Modi D, Burdette JE.. Reduced PAX2 expression in murine fallopian tube cells enhances estrogen receptor signaling. Carcinogenesis 2019;Bgz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MD, McGraw PA, Melly MA.. Localization of gonococcal lipopolysaccharide and its relationship to toxic damage in human fallopian tube mucosa. Infect Immun 1986;51:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MD, Rapp J, Jeffery-Wiseman C, Barnes RC, Stephens DS.. Chlamydia trachomatis infection of human fallopian tube organ cultures. J Gen Microbiol 1990;136:1109–1115. [DOI] [PubMed] [Google Scholar]
- Critoph FN, Dennis KJ.. The cellular composition of the human oviduct epithelium. Br J Obstet Gynaecol 1977. a;84:219–221. [DOI] [PubMed] [Google Scholar]
- Critoph FN, Dennis KJ.. Ciliary activity in the human oviduct. Br J Obstet Gynaecol 1977. b;84:216–218. [DOI] [PubMed] [Google Scholar]
- Dulohery K, Trottmann M, Bour S, Liedl B, Alba-Alejandre I, Reese S, Hughes B, Stief CG, Kolle S.. How do elevated levels of testosterone affect the function of the human fallopian tube and fertility?—New insights. Mol Reprod Dev 2020;87:30–44. [DOI] [PubMed] [Google Scholar]
- Emori MM, Drapkin R.. The hormonal composition of follicular fluid and its implications for ovarian cancer pathogenesis. Reprod Biol Endocrinol 2014;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Lawrence MF, Turner TT, Ross P, Thomas TS, Oliphant G.. Sulfated oviductal glycoproteins in the rabbit: quantitation by competitive enzyme-linked immunosorbent assay. Biol Reprod 1989;40:1299–1310. [DOI] [PubMed] [Google Scholar]
- Fathalla MF. Incessant ovulation and ovarian cancer—a hypothesis re-visited. Facts Views Vis Obgyn 2013;5:292–297. [PMC free article] [PubMed] [Google Scholar]
- Halbert SA, Becker DR, Szal SE.. Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol Reprod 1989;40:1131–1136. [DOI] [PubMed] [Google Scholar]
- Halbert SA, Patton DL, Zarutskie PW, Soules MR.. Function and structure of cilia in the fallopian tube of an infertile woman with Kartagener’s syndrome. Hum Reprod 1997;12:55–58. [DOI] [PubMed] [Google Scholar]
- Halbert SA, Tam PY, Blandau RJ.. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science 1976;191:1052–1053. [DOI] [PubMed] [Google Scholar]
- Horne AW, King AE, Shaw E, McDonald SE, Williams AR, Saunders PT, Critchley HO.. Attenuated sex steroid receptor expression in fallopian tube of women with ectopic pregnancy. J Clin Endocrinol Metab 2009;94:5146–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RH, Einer-Jensen N, Greve T.. Somatic cell amplification of early pregnancy factors in the fallopian tube. Ital J Anat Embryol 2005;110:195–203. [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF.. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 2011;12:222–234. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Endocrine response in the fallopian tube. Endocr Rev 1984;5:525–551. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Rosenbaum JL.. Polarity of flagellar assembly in Chlamydomonas. J Cell Biol 1992;119:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE.. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013;34:130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby CJ, Thatcher WW, Collier RJ, Simmen FA, Lucy MC.. Effects of growth hormone and pregnancy on expression of growth hormone receptor, insulin-like growth factor-I, and insulin-like growth factor binding protein-2 and -3 genes in bovine uterus, ovary, and oviduct. Biol Reprod 1996;55:996–1002. [DOI] [PubMed] [Google Scholar]
- Lam PM, Briton-Jones C, Cheung CK, Lok IH, Yuen PM, Cheung LP, Haines C.. Vascular endothelial growth factor in the human oviduct: localization and regulation of messenger RNA expression in vivo. Biol Reprod 2003. a;68:1870–1876. [DOI] [PubMed] [Google Scholar]
- Lam PM, Briton-Jones C, Cheung CK, Po LS, Cheung LP, Haines C.. Increased mRNA expression of vascular endothelial growth factor and its receptor (flt-1) in the hydrosalpinx. Hum Reprod 2003. b;18:2264–2269. [DOI] [PubMed] [Google Scholar]
- Lebbe M, Woodruff TK.. Involvement of androgens in ovarian health and disease. Mol Hum Reprod 2013;19:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Z, Moore DE, Mueller BA, Critchlow CW, Patton DL, Halbert SA, Wang SP.. Characterization of ciliary activity in distal Fallopian tube biopsies of women with obstructive tubal infertility. Hum Reprod 1998;13:3121–3127. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Morbeck DE, Hudson SB, Coddington CC 3rd, Stern JE.. Factors associated with ovarian hyperstimulation syndrome (OHSS) and its effect on assisted reproductive technology (ART) treatment and outcome. Fertil Steril 2010;94:1399–1404. [DOI] [PubMed] [Google Scholar]
- Lurie M, Tur-Kaspa I, Weill S, Katz I, Rabinovici J, Goldenberg S.. Ciliary ultrastructure of respiratory and fallopian tube epithelium in a sterile woman with Kartagener’s syndrome. A quantitative estimation. Chest 1989;95:578–581. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O.. The effect of ovarian follicular fluid and peritoneal fluid on Fallopian tube ciliary beat frequency. Hum Reprod 2006. a;21:52–56. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O.. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update 2006. b;12:363–372. [DOI] [PubMed] [Google Scholar]
- McComb P, Langley L, Villalon M, Verdugo P.. The oviductal cilia and Kartagener’s syndrome. Fertil Steril 1986;46:412–416. [PubMed] [Google Scholar]
- McGee ZA, Jensen RL, Clemens CM, Taylor-Robinson D, Johnson AP, Gregg CR.. Gonococcal infection of human fallopian tube mucosa in organ culture: relationship of mucosal tissue TNF-alpha concentration to sloughing of ciliated cells. Sex Transm Dis 1999;26:160–165. [DOI] [PubMed] [Google Scholar]
- McGee ZA, Johnson AP, Taylor-Robinson D.. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis 1981;143:413–422. [DOI] [PubMed] [Google Scholar]
- Novak J. Die menstruation und ihre störungen. Wien und Berlin: J. Springer, 1928. [Google Scholar]
- Ose J, Poole EM, Schock H, Lehtinen M, Arslan AA, Zeleniuch-Jacquotte A, Visvanathan K, Helzlsouer K, Buring JE, Lee IM. et al. Androgens are differentially associated with ovarian cancer subtypes in the ovarian cancer cohort consortium. Cancer Res 2017;77:3951–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixao L, Ramos RB, Lavarda A, Morsh DM, Spritzer PM.. Animal models of hyperandrogenism and ovarian morphology changes as features of polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol 2017;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen H. Absence of dynein arms in endometrial cilia: cause of infertility? Acta Obstet Gynecol Scand 1983;62:625–627. [DOI] [PubMed] [Google Scholar]
- Raidt J, Werner C, Menchen T, Dougherty GW, Olbrich H, Loges NT, Schmitz R, Pennekamp P, Omran H.. Ciliary function and motor protein composition of human fallopian tubes. Hum Reprod 2015;30:2871–2880. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB.. Intraflagellar transport. Nat Rev Mol Cell Biol 2002;3:813–825. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- Russo A, Czarnecki AA, Dean M, Modi DA, Lantvit DD, Hardy L, Baligod S, Davis DA, Wei JJ, Burdette JE.. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene 2018;37:1976–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Pontillo M, Nappi RE, Zanni G, Fabbri F, Scavini M, Daverio R, Gallina A, Rigatti P, Bosi E. et al. Menstrual cycle-related changes in circulating androgens in healthy women with self-reported normal sexual function. J Sex Med 2008;5:854–863. [DOI] [PubMed] [Google Scholar]
- Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol 2003;19:423–443. [DOI] [PubMed] [Google Scholar]
- Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids 2008;73:473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaken GJ, Bassinet L, Boon M, Mani R, Honore I, Papon JF, Cuppens H, Jaspers M, Lorent N, Coste A. et al. Infertility in an adult cohort with primary ciliary dyskinesia: phenotype-gene association. Eur Respir J 2017;50:1700314. [DOI] [PubMed] [Google Scholar]
- Verhage HG, Bareither ML, Jaffe RC, Akbar M.. Cyclic changes in ciliation, secretion and cell height of the oviductal epithelium in women. Am J Anat 1979;156:505–521. [DOI] [PubMed] [Google Scholar]
- Verhage HG, Fazleabas AT, Mavrogianis PA, O’Day-Bowman MB, Donnelly KM, Arias EB, Jaffe RC.. The baboon oviduct: characteristics of an oestradiol-dependent oviduct-specific glycoprotein. Hum Reprod Update 1997;3:541–552. [DOI] [PubMed] [Google Scholar]
- Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC.. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2008;39:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wei Y, Diao F, Cui Y, Mao Y, Wang W, Liu J.. The association between polycystic ovary syndrome and ectopic pregnancy after in vitro fertilization and embryo transfer. Am J Obstet Gynecol 2013;209:139.e1–9. [DOI] [PubMed] [Google Scholar]
- Winger QA, de los Rios P, Han VK, Armstrong DT, Hill DJ, Watson AJ.. Bovine oviductal and embryonic insulin-like growth factor binding proteins: possible regulators of "embryotrophic" insulin-like growth factor circuits. Biol Reprod 1997;56:1415–1423. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Talbott E, Guzick DS, Zborowski J, McHugh KP.. Serum testosterone levels decrease in middle age in women with the polycystic ovary syndrome. Fertil Steril 2000;73:724–729. [DOI] [PubMed] [Google Scholar]
- Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 2017;8:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Ng CP, Habacher H, Roy S.. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet 2008;40:1445–1453. [DOI] [PubMed] [Google Scholar]
- Zarezade N, Saboori Darabi S, Ramezanali F, Amirchaghmaghi E, Khalili G, Moini A, Aflatoonian R.. mRNA expression of VEGF and its receptors in fallopian tubes of women with ectopic pregnancies. Int J Fertil Steril 2015;9:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Xu Y, Rashedi AS, Pavone ME, Kim JJ, Woodruff TK, Burdette JE.. Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle. Mol Hum Reprod 2016;22:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.