Abstract
Enteric redmouth disease caused by the bacterial pathogen Yersinia ruckeri is the main reason for antimicrobial prescription, and a cause of substantial economic losses and decreased animal welfare in aquaculture. Given the importance of the intestinal microbiota in digestion and disease, our aim was to investigate whether synbiotic feed supplementation strategies could improve feed performance and disease resistance. Four experimental synbiotic feeds formulated with pre- and probiotics were tested against a commercially available probiotic control feed. Each experimental feed was evaluated for feed performance, effects on gross as well as intestinal morphometrics, and finally their effect on resistance against a waterborne experimental infection with Yersinia ruckeri serotype O1, biotype 2. While co-supplementing Pediococcus acidilactici with citrus flavonoids or bacterial paraprobiotics significantly improved utilization of feed lipid content relative to the control group, a decrease in lipid utilization was observed for feeds that combined P. acidilactici with yeast paraprobiotics. No significant improvements on disease resistance were observed. Still, synbiotic formulations including P. acidilactici led to reduced risks relative to that of the control group, while an increased relative risk was observed for a Bacillus-based formulation. In conclusion, two of the synbiotic supplements significantly improved lipid utilization and contributed to minor increases in disease resistance.
Subject terms: Microbiology, Applied microbiology, Pathogens
Introduction
As worldwide production of fish in aquaculture continues to grow, intense production schedules increase the risk of disease outbreaks. Enteric redmouth disease, caused by the bacterial pathogen Yersinia ruckeri1,2, currently accounts for more than 90% of the prescribed antimicrobials used in Danish aquaculture. In 2017, the total amount used was 1697 kg active substance3, for a total production of 48.300 tons4. Exceptionally high water temperatures presumeably led to increased outbreak severity and induced an increase of antimicrobial prescription of more than 100% from 2017 to 20183. Prophylactic measures including vaccines5–8 and improved vaccination strategies9,10 have been developed and implemented. In spite of these efforts outbreaks continue to occur.
In an effort to improve feed performance and disease resistance, and thereby reduce the need for antimicrobial treatments and improve both animal welfare and farm revenue, feed supplements promoting disease resistance may add positively to existing prophylaxis and treatments strategies. In line with this concept, the aim of the present study was to test if feed performance and resistance towards infection with Y. ruckeri could be improved through synbiotic feed supplements.
Pre- pro- and synbiotic feed additives all have the same overall aim: the establishment of an intestinal microbiota that is beneficial to the health of the host. This is considered to occur through three modes of action. While prebiotics have recently been redefined as “substrates that are selectively utilized by host microorganisms conferring a health benefit”11, probiotics are defined as “live microorganisms, which when consumed in adequate amounts, confer a health effect on the host”12,13. Synbiotic feed supplementation is the co-supplementation of pre- and probiotics that benefits host health or welfare by selectively stimulating the growth of implanted, as well as other beneficial bacteria14. A separate and more recent derivative from the probiotics, paraprobiotics are defined as inactivated microbial cells or fractions thereof15,16. While microbial by nature, these do not match the probiotic definition. Throughout this study, paraprobiotics are considered to belong to the prebiotic category of feed supplements. They will, however, be referenced to and discussed as paraprobiotics.
Prebiotic supplementation of fish feed has proven beneficial for various compounds and for several species of fish. This includes postitive effects on different feed performance parameters following supplementation with a variety of compounds, including yeast cell wall components. Examples include yeast cell wall supplementation of Japanese seabass (Lateolabrax japonicus)17, fructose- and mannose-oligosaccharide (MOS) supplementation in Atlantic salmon (Salmo salar)18, as well as both MOS supplementation19–21 and incorporation of various organic acids in rainbow trout feeds either alone22–25, or in combination with β-glucans26. In addition to this, improved disease resistance towards bacterial infection has been reported for yeast cell wall supplements in Japanese seabass17, as well as citrus flavonoid, β-glucan, co-supplementation of β-glucan and organic acids in rainbow trout26–28.
Several probiotics have also proved beneficial in fish feeds. Proven effects include inhibitory activity against Aeromonas hydropila, A. salmonicida, Vibrio anguillarum and Y. ruckeri as demonstrated in vitro for Lactococcus lactis29, as well as reduced mortality in rainbow trout fed Lactobacillus plantarum supplements following Lactococcus garvieae infection. Studies performed using rainbow trout supplemented with Pediococcus acidilactici suggest that this probiotic is capable of colonizing the rainbow trout gastrointestinal (GI) tract30,31 and that it induces an increase in culturable, autochthonous bacterial counts from the GI tract, as well32. Paraprobiotics, potentially offering advantages found with probiotics, without the same regulatory restrictions on their use, have seen increasing intention in recent years16. Previous studies on grouper (Epinephelus coioides) and rainbow trout have demonstrated positive effects on feed performance following feed supplementation with inactivated Bacillus pumilus and Enerococcus faecalis, respectively33,34. In addition, in vitro studies have indicated an ability of yeast paraprobiotics to adsorb pathogenic bacteria, potentially inducing a beneficial effect on disease resistance35.
Previous investigations on combined synbiotic feed supplements in fish have been promising. When co-supplemented as a synbiotic mixture, P. acidilactici and galacto-oligosaccharides resulted in significantly increased final weights, weight gains, daily growth rates and feed conversion ratios in rainbow trout, while no significant differences were observed for neither the pre- nor the probiotic component when these were administered alone32. Synbiotic supplementation of P. acidilactici and MOS also had a positive, albeit not statistically significant, effect on the relative risk during infection with Y. ruckeri compared to control fed fish36.
To investigate the potential synergies of combining proven pre- and probiotics further, four experimental, proprietary synbiotic feed formulations were evaluated in the present study. Three were formulated with P. acidilactici as the probiotic component, as well as either citrus flavonoids, bacterial paraprobiotics or yeast paraprobiotics. The fourth feed was formulated with a novel Bacillus spp. probiotic candidate and yeast paraprobiotics. In order to assess the effects of the synbiotic strategy, the control group was fed a commercial feed supplemented with probiotic P. acidilactici. The experimental setup consisted of a 63-day experimental feeding period, of which the initial 34 days were feeding only, and the final 29 days included an experimental, waterborne infection with Y. ruckeri serotype O1, biotype 237. Throughout the experimental period, feed performance and morphometrics were monitored.
Materials and methods
Fish
Rainbow trout eggs (AquaSearch FRESH strain, all-female, AquaSearch OVA, Billund, Denmark) were hatched and reared at the Bornholm Salmon Hatchery (Nexø, Denmark). The hatchery has a disease-free record and upon arrival, the eggs were disinfected using Desamar K30. Prior to experimental feeding, the fish were transported to the BioMar A/S research facilities (Hirtshals, Denmark), where the fish were sorted into 30 fiberglass tanks (150 L) in a fully recirculated system with biological filtering and physical filtering mechanisms, including a UV-filter. Subsequently, an 8-day acclimatization period was allowed. Water quality parameters were recorded daily throughout the experimental period (Table 1).
Table 1.
Physical and chemical water quality parameters.
| NH4+ (mg/L) | NO2 (mg/L) | NO3 (mg/L) | pH | Temp. (°C) | |
|---|---|---|---|---|---|
| Mean | 0.55 | 0.18 | 18.09 | 7.69 | 14.09 |
| Standard deviation ( ±) | 0.69 | 0.44 | 11.78 | 0.44 | 1.29 |
Experimental feeding and setup
Following the acclimatization period, experimental feeding was initiated. Five different feed groups were established. Each type of feed is described in Table 2. Five 150 L tanks holding 64–68 individuals (mean weight with standard deviation = 2.06 ± 0.07 g) were included per feed group, of which four were subjected to experimental infection, and one was kept as a non-infected control. During the experimental phase, all feed group identities were blinded.
Table 2.
Experimental feed content for both pellet sizes.
| Control | PECF | PEBP | PEYP | BAYP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Probiotic | P. acidilactici | P. acidilactici | P. acidilactici | P. acidilactici | Bacillus candidate | |||||
| Prebiotic | - | Citrus flavonoids 0.15% | Bacterial paraprobiotic 0.003% | 0.15% yeast paraprobiotic | 0.15% yeast paraprobiotic | |||||
| Pellet size (mm) | 1.1 | 1.5 | 1.1 | 1.5 | 1.1 | 1.5 | 1.1 | 1.5 | 1.1 | 1.5 |
| Protein (%) | 49.6 | 53.8 | 51.5 | 53.5 | 49.9 | 54.7 | 49.7 | 54.6 | 48.8 | 54.7 |
| Lipid/fat (%) | 17.3 | 20.2 | 15 | 20.4 | 16.2 | 20.13 | 18 | 19.8 | 19.1 | 20.2 |
The total feeding period was 63 days, of which the experimental infection ran for the final 29 days. Daily feeding was 3.7% of total biomass up until the experimental infection, and 1.7% during the infection period. This decrease in feed percentage was made to accommodate a decrease in appetite during the infection, and to avoid the strain of excessive waste feed on the tank and filter setup. To accommodate increasing fish size, the fish were fed 1.1 mm pellets for the first 7 days of the feeding period, while 1.5 mm pellets were fed for the remainder of the experiment. Feed lipid and protein content was measured using near-infrared spectroscopy.
Feed performance
Through days 0–34 of the feeding period leading up to the experimental infection, feed performance parameters were tracked for all five tanks in each feed group. Based on initial and endpoint bulk weight, counts of fish per tank, consumed feed and feed composition, the following parameters were calculated38,39:
- Relative growth rate (RGR):
- Economic feed conversion ratio (eFCR):
- Specific growth rate (SGR):
- Lipid efficiency ratio (LER):
- Protein efficiency ratio (PER):
Sampling
All sampling was performed from tanks designated as non-infected controls to avoid interference with the experimental infection. Weight and fork length was recorded for ten convenience netted fish from each feed group at three different time points: day 0, day 34, and day 63. These time points correspond to immediately prior to feeding, immediately prior to start of the experimental infection in the infection tanks and the end of the experiment, respectively. In addition to weight and length measurements, ten individuals were convenience netted per group for sampling of intestinal tissue at day 0 and day 63. Immediately prior to sampling, each individual was euthanized in an overdose of benzocaine (Aquacen, Cenavisa, Spain). The gastrointestinal tract was subsequently exposed by a ventral midline incision. Adhering to the gross morphological description of the Atlantic salmon gastrointestinal tract40, tissue samples for histological examination were obtained from two distinct sections: A) spanning the distal section of the second mid-intestinal and posterior segments, and B) from the distal section of the first segment of the mid-intestine (see Supplementary Fig. S1). Following sampling, the tissues were immediately placed in 10% neutral buffered formalin for 24 h, and then changed to 70% ethanol for further storage.
Morphometrics
At each of the three time points mentioned in the previous section, weight and length were utilized to calculate Fulton’s condition factor (K)41,42 for each individual:
-
6.Fulton’s condition factor (K):
For each sampling time point, a relative condition factor was calculated. All individual condition factors were divided by the control group mean value at each time point.
Intestinal samples taken from the GI tract were processed for histological sectioning at the pathology lab of the Division of Diagnostics & Scientific Advice, Danish Technical University, Denmark. Following paraffin embedding, sections were cut and the tissues were stained using hematoxylin and eosin (H&E). Micrographs of one full H&E stained section of the posterior segment of the GI tract from each of the ten fish from the BAYP and PECF feed groups were captured using a Zeiss Axioplan2 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) fitted with a Zeiss Axiocam 702 mono (Carl Zeiss Microscopy GmbH, Jena, Germany) camera controlled by Zen 2 (Blue edition, Carl Zeiss Microscopy GmbH, Göttingen, Germany) software. Intestinal fold height was recorded from base to tip for all non-complex folds to avoid measuring posterior annulo-spiral valve heights, as described by Escaffre, et al.43. These measurements were performed using the segmented line tool in ImageJ (version 2.0.0-rc-68/1.52 h), following calibration of measurement tool using a calibration slide (0.01 mm, OMAX Microscope).
Experimental Y. ruckeri infection
Bath infection was performed as previously described37. Briefly, cryopreserved Y. ruckeri serotype O1, biotype (strain 07111224) were streaked onto 5% blood agar plates and incubated at room temperature for 48 h. Subsequently Luria–Bertani media were inoculated with single colonies, and incubated for 36 h at room temperature with mechanical shaking or magnetic stirring. Prior to infection, all cultures were pelleted by centrifugation (3488 G, 15 min), the growth medium supernatant was discarded, and the bacteria were resuspended in tank water to 1:10 of their initial volume.
Fish in all infection tanks were anaesthetized and moved to designated infection containers holding 9 l of tank water. The infection was started by addition of 1 l of the 10X concentrated bacterial suspension to the respective infection containers to a final dose of 9.15 × 108 colony forming units (CFU)/ml, as determined by plating of serially diluted infection suspension onto 5% blood agar plates and counting the colonies, accounting for dilutions. Following the 3-h infection period, all fish were netted and returned to their respective holding tanks and closely monitored several times per day in accordance with the experimental animal license for 29 days. Upon reaching the humane endpoints specified by the experimental animal license, fish were netted and euthanized in an overdose of benzocaine. The dorsal surface of the fish was sterilized in ethanol, and an incision through to the anterior kidney was made using a sterile scalpel and a smear from the anterior kidney onto 5% blood agar was made using a sterile inoculation loop. Resulting colonies were tested using a Bionor mono-aqua Y. ruckeri agglutination kit (Bionor AS, Skien, Norway) to verify that the pathogen could be re-isolated, satisfying Koch’s postulate. In this case, the individual was entered as an “event” in subsequent analyses, otherwise the individual would be censored from the time it left the experimental setup. Experimental feeding continued throughout the experimental infection period.
Statistical analyses
All statistical analyses were performed using R (version 3.5.2 – “Eggshell Igloo”)44 run via RStudio (version 1.1.463). The following packages were used: ggplot245, ggradar46, ggpubr47, survminer48, tibble49, RColorBrewer50, survival51, scales52, readxl53, fmsb54 and dplyr55.
A markdown.html document is available as supplementary material for full description of all analyses.
All statistical analyses were performed to either confirm or reject the null-hypothesis, that the data sets in question are equal, using an alpha-level of 0.05 as threshold.
All feed performance parameters including fish weight, length and Fulton’s condition factor data were checked for underlying Gaussian distribution of data through a Shapiro-Wilks test, as well as by visual inspection of QQ-plots (see supplementary markdown.html). Once this distribution was confirmed, data were analyzed by one-way ANOVA. If the result of the ANOVA was P < 0.05, multiple comparisons were performed using a Tukey post hoc test (95% family-wise conficence level). The performance data were summarized in radar chart and table form.
Intestinal fold heights were checked for underlying Gaussian distribution of data, as detailed for the performance parameters. As this distribution could not be proven, an unpaired Mann–Whitney U (non-parametric) test was used to compare feed groups at each time point.
Survival data were plotted using the Kaplan–Meier method. The survival curves for the quadruple replicate in each feed group were analyzed using the log-rank method, and as no statistically significant differences were demonstrated, the data for each feed code replicate was pooled. Given the ten possible comparisons, a Bonferroni-correction for multiple comparisons result in an alpha-level 0.05/10 = 0.005 for pairwise comparisons of survival curves.
Finally, the resulting survival curves for each feed code were compared using the log-rank method. Hazard-ratio analysis was then performed using the Cox proportional hazards method.
Ethics Statement
The protocols involving use of experimental animals in this study were approved by the Danish Animal Experiments Council under license number 2015-15-0201-00645. The granted license is in accordance with the Danish law on animal experiments, as well as EU directive 2010/63.
Results
Feed performance data
All mean feed performance results following the initial 34 days of experimental feeding are shown in Table 3.
Table 3.
Feed performance data for each experimental feed group. All values are group mean ± standard deviation. Statistically significant differences between feed groups following ANOVA are indicated by different lowercase letters. See “Materials and methods” section for details on statistical analyses.
| Control | PECF | PEBP | PEYP | BAYP | |
|---|---|---|---|---|---|
| RGR | 151.8 ± 4.3 | 149.6 ± 4.5 | 152.6 ± 4.0 | 149.9 ± 4.9 | 151.2 ± 5.3 |
| eFCR | 0.62 ± 0.02 | 0.62 ± 0.02 | 0.62 ± 0.02 | 0.63 ± 0.02 | 0.62 ± 0.02 |
| SGR | 2.73 ± 0.03 | 2.71 ± 0.03 | 2.74 ± 0.02 | 2.71 ± 0.02 | 2.72 ± 0.04 |
| LER | 9.33 ± 0.2d | 10.80 ± 0.4a | 10.0 ± 0.2c | 8.87 ± 0.3b,d | 8.43 ± 0.3b |
| PER | 3.25 ± 0.1 | 3.14 ± 0.1 | 3.26 ± 0.1 | 3.21 ± 0.1 | 3.30 ± 0.1 |
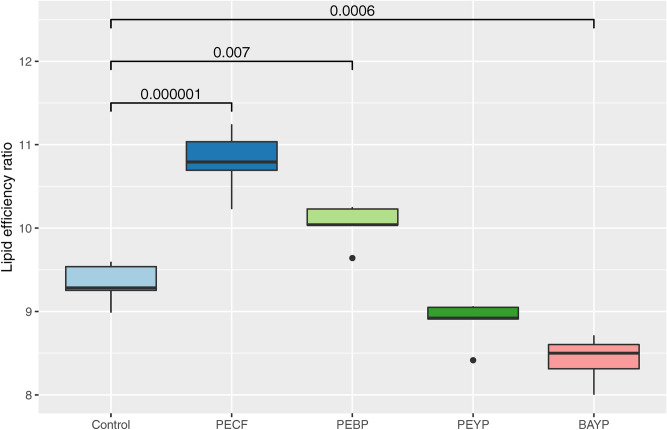
Across all calculated parameters, statistically significant differences were observed between group LERs. The PECF (P = 0.000001) and PEBP (P = 0.007) feed groups both displayed significantly elevated LERs relative to the control group (16% and 7%, respectively), while the PEYP was not significantly different from the control group. The BAYP group had a significantly lowered LER relative to the control (P = 0.0006). The LER values for each group are shown in Fig. 1.
Figure 1.
Lipid efficiency ratios for each group. The figure shows the median values with 25% and 75% quartiles (box) for all group replicates. Whiskers indicate largest and smallest observation equal to or less than 1.5 × interquartile range above and below the box, respectively. Outliers beyond these are shown individually. The P values shown are all based on comparisons with the control group, and are obtained by one-way ANOVA followed by Tukeys post hoc test for multiple comparisons (95% family-wise confidence level).
Morphometrics
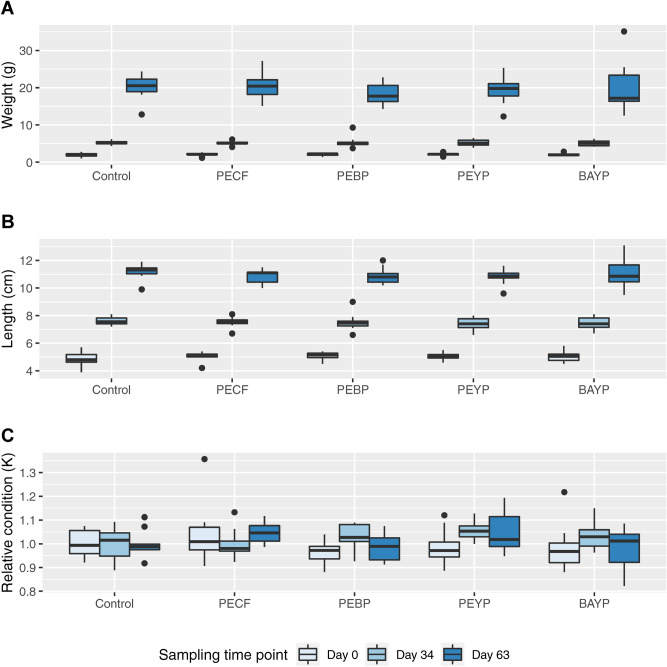
Recorded gross measurements of weight and length, as well as their resulting condition factors are shown as boxplots in Fig. 2. Growth measured by either parameter was similar in all groups, or showed only very subtle differences, with no statistically significant differences identified between groups at any time point.
Figure 2.
Gross morphometrics boxplot. Development of weight (A), length (B) and relative condition factor (C) throughout the experimental period. For details on sampling, measurements and calculation of K, see Materials & Methods. Each boxplot shows median value with 25% and 75% quartiles (box). Whiskers indicate largest and smallest observation equal to or less than 1.5 × interquartile range above and below the box, respectively. Outliers beyond these are shown individually.
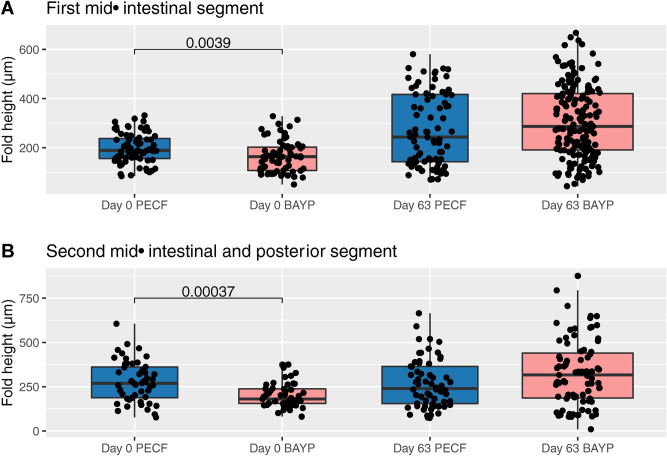
Given the results from the lipid utilization, influences of feed supplements on intestinal morphology were assessed. The PECF and BAYP supplemented feed groups, representing the highest and lowest recorded LER, respectively, were selected for microscopical examination. The results are shown in Fig. 3. Comparing samples taken prior to experimental feeding and samples taken at the conclusion of the experimental feeding period, intestinal fold height was significantly higher in the PECF supplemented feed group, prior to any experimental feeding, for both the first and second mid-intestinal segments (P = 0.0039 and P = 0.00037, respectively). No differences were seen following the experimental feeding period.
Figure 3.
Intestinal morphometrics boxplot. Measured intestinal heights from (A) the first mid-intestinal segment, and (B) the second mid-intestinal and posterior segments immediately prior to the start of the feeding period, and at the end of the entire experimental period. For details on sampling and measurements, see Materials and Methods. Each boxplot shows median value with 25% and 75% quartiles (box). Whiskers indicate largest and smallest observation equal to or less than 1.5 × interquartile range above and below the box, respectively. Outliers beyond these are shown individually. Given that the numbers of individual measurements vary between groups, as well as time points, the width of each boxplot is increased with increasing numbers of measurements as an arbitrary measure to reflect these differences. Groups were compared for each segment at each sampling time point by Mann–Whitney U test. Statistically significant differences are indicated by P values.
Experimental infection
A single individual was lost during the initial feeding phase, prior to the experimental infection. The survival curves obtained by pooling replicates following initial analyses reflect all mortalities observed during the experimental infection and are shown in Fig. 4. Endpoint mortalities for each group were: Control = 19.3%, PECF = 22%, PEBP = 20.2%, PEYP = 22.5% and BAYP = 19.1% (See supplementary Markdown.html). No significant differences were observed between any of the survival curves (P = 0.22), and no further pairwise comparisons were therefore warranted. A Cox proportional hazards analysis found no significant changes in relative risk for any of the synbiotic groups when compared to the probiotic control group (P > 0.1). However, all synbiotic formulations that include P. acidilactici showed a reduced risk level, whereas the BAYP group showed a slightly increased relative risk. While these ratios were not statistically significant, the pattern is clear from the results shown in Fig. 5.
Figure 4.
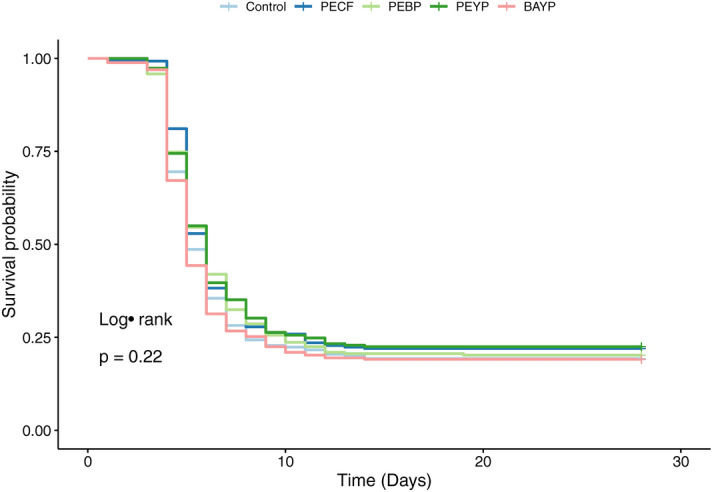
Survival curves from experimental infection. Kaplan–Meier plot of survival data showing computed survival probabilities for each feed group over time. All censored events are shown as ticks.
Figure 5.
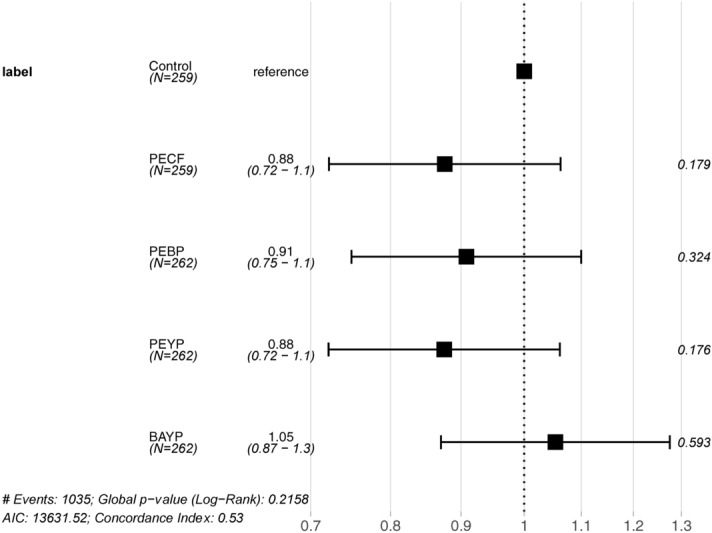
Forest plot of proportional hazards. Results from the Cox proportional hazards analysis. The control group has been set as reference. Each line shows the ratio of the hazard estimate of the given feed group relative to that of the control group ± 95% confidence interval, as well as the P value for that comparison.
Discussion
With the aim of improving disease resistance and feed performance in rainbow trout, four experimental, synbiotic feed formulations were tested and compared to results obtained using a commercial probiotic feed. While individual combinations of pre- and probiotics featured individual strengths, general trends were also identified.
The main difference between feed groups in the present study, was observed with regards to feed performance. The primary outcome of the present study was that LER can be increased significantly (7–16%) through a combination of P. acidilactici as the probiotic component, and either citrus flavonoids or bacterial paraprobiotics as prebiotic components, as compared to inclusion of dietary P. acidilactici alone. This is a substantial and statistically significant improvement of feed utilization. Furthermore, given the general shift from marine- to plant-based sourcing of feed components39 and the potential effects of such a shift on fish muscle composition56, an increased efficiency in conversion of lipid to biomass must be considered highly beneficial in terms of efficient utilization of feed components and product quality. In a previous study, Baba et al. found a statistically significant increase in serum protein concentration, as well as significant reductions in serum triglyceride and cholesterol concentrations in Mozambique tilapia (Oreochromis mossambicus) fed crude citrus essential oil extracts57. Weight gain, SGR and FCR were, however, unaffected by the supplement. While the outer peel of citrus fruits have been shown to generally contain the highest concentrations of flavonoids, citrus peel essential oils remain a crude mix of many components57,58. In a recent study, prebiotic supplementation with citrus flavonoids alone had no apparent effects on weight gain, FCR, SGR, LER or PER in rainbow trout26. A plausible explanation for the improved LER observed in this study, could be the additional inclusion of probiotics, providing a synergistic improvement of lipid utilization.
A study on E. faecalis paraprobiotics has previously demonstrated improved final weight, weight gain and specific growth rate in rainbow trout34. While these were not found to be significantly elevated in the present study, the improved LER observed here likely reflects a similar beneficial effect of a bacterial paraprobiotic supplement on feed performance. It must be noted, that as with the abovementioned citrus oil extracts, paraprobiotic preparations should be considered crude in nature, and detailed information on their exact composition can be sparse, potentially resulting in variation between products and suppliers, as recently noted for yeast derived products59.
When comparing the probiotic control and the synbiotic alternatives, the results from the present study strongly indicate synergistic effects from the PECF and PEBP formulations. This is evident when comparing the lipid utilization of these two groups to that of the control and the PEYP groups. While supplementation with MOS, a major yeast cell wall component, has proven beneficial in previous performance studies in Atlantic salmon and rainbow trout18–20,60, the PEYP group in the present study was shown to have an LER similar to that of the control group. Potential antagonistic effects of the BAYP composition, however, cannot be dissected here due to the lack of a Bacillus-only group, but the significantly decreased LER observed in this group further underlines the effects of the composition of synbiotic feed supplements.
Whether the observed differences in LER affects total body composition in terms of fat percentage or -composition, was not investigated in the present study, but should be investigated in future studies.
Previous studies have reported intestinal morphological changes following probiotic30,61, as well as MOS feed supplementation20,62. While all experimental feeds in the present study are formulated with a probiotic supplement, differences in the prebiotic component of the synbiotic formulation could potentially affect the intestinal morphology. To examine this, a histological comparison of the intestinal fold heights for PECF and BAYP groups were performed, as these represented the highest and lowest LER recorded in the present study, respectively. A positive correlation has previously been demonstrated between fold height and epithelial surface in rainbow trout43. Differences in fold height could therefore indicate differences in total surface area within the intestinal sections examined. Interestingly, the only statistically significant differences found between the two groups were prior to experimental feeding, at day 0. All fish used in this setup were obtained from the same source and were fed the same feed at day 0. Furthermore, as shown in Fig. 2 there were no observed differences in length, weight or relative condition factor at day 0. The observed significant difference in intestinal fold height was unexpected, but could potentially be a result of the scattered nature of the observations, although P-values (< 0.01) indicate a low probability of the null hypothesis to be true. At day 63, following the experimental feeding period, no statistically significant differences were observed. While no micromorphological measurements were made in the present study, a plausible explanation for the observed increases in LER relative to the probiotic control feed group is that the synbiotic formulations of the PEBP and PECF promote a more beneficial metabolic profile for the intestinal microbiota32.
With regards to disease resistance, no differences were observed between groups for the survival curves from the experimental infection study. From the Cox proportional hazards analysis, however, a trend was observed, as all P. acidilactici-based synbiotic formulations conferred a decrease in relative risk, when compared to the control group. While not statistically significant, this trend was clear and consistent. As the probiotic control group feed was also formulated with P. acidilactici, this trend is attributed to the additional prebiotic components in these synbiotic formulations. No difference was found between the PEBP and PEYP groups, and as such whether the paraprobiotics were yeast or bacterial in nature did not seem to play a role. No mechanistic investigations were performed regarding the specifics of any inhibitory function of the included paraprobiotics on Y. ruckeri. However, as the binding between yeast paraprobiotics and various bacterial pathogens has been suggested to be strain-specific35, further studies are warranted to address whether or not improvements could be made with various bacterial paraprobiotics.
While no differences, significant or other, in hazard ratios were found for the PECF, PEBP and PEYP, a change of probiotic component did not yield the same results, as a noticeable difference was seen in the hazard ratio analysis for the PEYP and BAYP feed groups. The exact nature of the Bacillus probiotic used in this study is proprietary information. In a previous study, however, Ramos et al. tested a commercial probiotic supplement based on Bacillus subtilis and B. cereus toyoi in rainbow trout and found the combination to be “harmful”61.
Citrus flavonoids have been suggested to conduct an antimicrobial activity against Gram-negative bacteria63, as well as suppression of bacterial mechanisms involved in infection64. Furthermore, a previous study has shown that citrus flavonoid supplemented feed conferred a significant reduction in relative risk in trout following a similar waterborne infection with Y. ruckeri26. Rodriguez-Estrada et al. have previously demonstrated a seemingly dose-dependent reduction in mortality in rainbow trout fed MOS, inactivated E. faecalis or a combination of both following intraperitoneal injection of A. salmonicida34. While the statistical significance of these previous results did not appear to carry over entirely into the synbiotic formulations investigated in the present study, the observed apparent decrease in risk of the PECF, PEBP and PEYP feed groups compared to the probiotic control group, could argueably be due to a synergistic protective effect of the combined feed components.
In conclusion, a substantial, statistically significantly increase in feed lipid utilization was demonstrated for synbiotic co-supplementation of P. acidilactici and either citrus flavonoids (P = 0.000001) or bacterial paraprobiotics (P = 0.007) relative to the probiotic control feed. Improving the LER by 7–16%, these feed formulations could make a noticeable difference throughout the production value chain. In addition to this significant feed performance improvement, a clear but non-significant tendency towards reduced risk during infection was observed for synbiotic feed supplements combining P. acidilactici and either citrus flavonoids, bacterial paraprobiotics or yeast paraprobiotics. This demonstrates that considerable effects on feed performance, as well as minor, non-significant effects on disease resistance can be obtained applying the PECF or PEBP synbiotic feed supplements in rainbow trout feed. Whether additional synergistic effects can be achieved by adding multiple prebiotic components, such as co-supplementing both citrus flavonoids and bacterial paraprobiotics or even further complementing with additional prebiotics needs to be addressed in further studies.
Supplementary information
Acknowledgements
The presented study was funded by GUDP (``Præ-Pro-Fisk'', grant no. 34009-17-1218) and BioMar A/S. The authors would like to thank Anni Nielsen, Miguel Martin and the staff at the BioMar Research Facility, Hirtshals, Denmark for their expert assistance during this study. Elisabeth Wairimu Petersen and Betina Gjedsted Andersen at the Veterinary Pathology Lab, University of Copenhagen are acknowledged for the preparation of the histological sections.
Author contributions
Conceived the study: T.F., E.A., J.T., A.M.B.. Designed the work: K.R.V., M.O., T.F., A.M.B. Acquisition and analysis of data: K.R.V., T.F. Interpretation of data: K.R.V., T.F., A.M.B. Drafting the manuscript: K.R.V. Substantial revision of manuscript: K.R.V., T.F., A.M.B. Approval of submitted manuscript: K.R.V., M.O., T.F., E.A., J.T., A.M.B.
Data availability
The datasets generated during and/or analysed during the current study are summarized in the present publication. Raw data can be made available from the corresponding author on reasonable request.
Competing interests
Torunn Forberg, Elisabeth Aasum and John Tinsley are all employed at different branches of Biomar Group, who produce, market and sell fish feed supplements with some of the ingredients tested in the current investigation. Furthermore, Biomar provided parts of the funding for this study. Kasper Rømer Villumsen, Maki Ohtani and Anders Miki Bojesen all declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/28/2021
A Correction to this paper has been published: 10.1038/s41598-021-88891-4
Contributor Information
Kasper Rømer Villumsen, Email: krv@sund.ku.dk.
Anders Miki Bojesen, Email: miki@sund.ku.dk.
Supplementary information
is available for this paper at 10.1038/s41598-020-73812-8.
References
- 1.Ross AJ, Rucker RR, Ewing WH. Description of a bacterium associated with redmouth disease of rainbow trout (Salmo gairdneri) Can. J. Microbiol. 1966;12:763–770. doi: 10.1139/m66-103. [DOI] [PubMed] [Google Scholar]
- 2.Rucker RR. Redmouth disease of rainbow trout (Salmo gairdneri) Bull. Off. Int. Epizoot. 1966;65:825–830. [PubMed] [Google Scholar]
- 3.Institut, S. S. & National Food Institute, T. U. o. D. DANMAP 2018. (2019).
- 4.Statistics_Denmark. Akvakultur efter fiske- og skaldyrsarter, enhed, anlægstype og tid, https://web.archive.org/save/https://www.statistikbanken.dk/10207 (2019).
- 5.Villumsen KR, Neumann L, Ohtani M, Strøm HK, Raida MK. Oral and anal vaccination confers full protection against enteric redmouth disease (erm) in rainbow trout. PLoS ONE. 2014;9:e93845. doi: 10.1371/journal.pone.0093845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtani M, Villumsen KR, Koppang EO, Raida MK. Global 3D Imaging of Yersinia ruckeri Bacterin Uptake in Rainbow Trout Fry. PLoS ONE. 2015;10:e0117263. doi: 10.1371/journal.pone.0117263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olesen NJ. Detection of the antibody response in rainbow trout following immersion vaccination with Yersinia ruckeri bacterins by ELISA and passive immunization. J. Appl. Ichthyol. 1991;7:36–43. doi: 10.1111/j.1439-0426.1991.tb00592.x. [DOI] [Google Scholar]
- 8.Tobback E, Decostere A, Hermans K, Haesebrouck F, Chiers K. Yersinia ruckeri infections in salmonid fish. J Fish Dis. 2007;30:257–268. doi: 10.1111/j.1365-2761.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 9.Jaafar RM, et al. Secondary immune response of rainbow trout following repeated immersion vaccination. J. Fish. Dis. 2018;41:117–123. doi: 10.1111/jfd.12682. [DOI] [PubMed] [Google Scholar]
- 10.Chettri JK, et al. Comparative evaluation of administration methods for a vaccine protecting rainbow trout against Yersinia ruckeri O1 biotype 2 infections. Vet. Immunol. Immunopathol. 2013;154:42–47. doi: 10.1016/j.vetimm.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Gibson GR, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 12.Hill C, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 13.FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria (2001).
- 14.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota—introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 15.Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin R, Langella P. Emerging health concepts in the probiotics field: streamlining the definitions. Front. Microbiol. 2019;10:1047. doi: 10.3389/fmicb.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu HH, et al. Efficacy and tolerance of yeast cell wall as an immunostimulant in the diet of Japanese seabass (Lateolabrax japonicus) Aquaculture. 2014;432:217–224. doi: 10.1016/j.aquaculture.2014.04.043. [DOI] [Google Scholar]
- 18.Grisdale-Helland B, Helland SJ, Gatlin DM. The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar) Aquaculture. 2008;283:163–167. doi: 10.1016/j.aquaculture.2008.07.012. [DOI] [Google Scholar]
- 19.Staykov Y, Spring P, Denev S, Sweetman J. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss) Aquacult Int. 2007;15:153–161. doi: 10.1007/s10499-007-9096-z. [DOI] [Google Scholar]
- 20.Dimitroglou A, et al. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum) J. Anim. Sci. 2009;87:3226–3234. doi: 10.2527/jas.2008-1428. [DOI] [PubMed] [Google Scholar]
- 21.Torrecillas S, et al. Effects of dietary concentrated mannan oligosaccharides supplementation on growth, gut mucosal immune system and liver lipid metabolism of European sea bass (Dicentrarchus labrax) juveniles. Fish. Shellfish Immunol. 2015;42:508–516. doi: 10.1016/j.fsi.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Hernández AJ, Satoh S, Kiron V. Supplementation of citric acid and amino acid chelated trace elements in low-fish meal diet for rainbow trout affect growth and phosphorus utilization. J. World Aquacult. Soc. 2012;43:688–696. doi: 10.1111/j.1749-7345.2012.00589.x. [DOI] [Google Scholar]
- 23.Gao Y, Storebakken T, Shearer KD, Penn M, Øverland M. Supplementation of fishmeal and plant protein-based diets for rainbow trout with a mixture of sodium formate and butyrate. Aquaculture. 2011;311:233–240. doi: 10.1016/j.aquaculture.2010.11.048. [DOI] [Google Scholar]
- 24.Pandey A, Satoh S. Effects of organic acids on growth and phosphorus utilization in rainbow trout Oncorhynchus mykiss. Fish. Sci. 2008;74:867–874. doi: 10.1111/j.1444-2906.2008.01601.x. [DOI] [PubMed] [Google Scholar]
- 25.Acar U, et al. Effects of different levels of pomegranate seed oil on some blood parameters and disease resistance against Yersinia ruckeri in rainbow trout. Front. Physiol. 2018;9:596. doi: 10.3389/fphys.2018.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romer Villumsen K, et al. Citrus flavonoids, beta-Glucan and organic acid feed additives decrease relative risk during Yersinia ruckeri O1 biotype 2 infection of rainbow trout (Oncorhynchus mykiss) Peer J. 2020;8:e8706. doi: 10.7717/peerj.8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz S, Ergun S. Trans-cinnamic acid application for rainbow trout (Oncorhynchus mykiss): I. Effects on haematological, serum biochemical, non-specific immune and head kidney gene expression responses. Fish. Shellfish Immunol. 2018;78:140–157. doi: 10.1016/j.fsi.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 28.Yılmaz S, Ergun S, Çelik EŞ, Yigit M. Effects of dietary humic acid on growth performance, haemato-immunological and physiological responses and resistance of rainbow trout, Oncorhynchus mykiss to Yersinia ruckeri. Aquac. Res. 2018;49:3338–3349. doi: 10.1111/are.13798. [DOI] [Google Scholar]
- 29.Balcázar JL, et al. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture. 2008;278:188–191. doi: 10.1016/j.aquaculture.2008.03.014. [DOI] [Google Scholar]
- 30.Merrifield DL, Harper GM, Dimitroglou A, Ringo E, Davies SJ. Possible influence of probiotic adhesion to intestinal mucosa on the activity and morphology of rainbow trout (Oncorhynchus mykiss) enterocytes. Aquac. Res. 2010;41:1268–1272. doi: 10.1111/j.1365-2109.2009.02397.x. [DOI] [Google Scholar]
- 31.Merrifield DL, et al. Assessment of the effects of vegetative and lyophilized Pediococcus acidilactici on growth, feed utilization, intestinal colonization and health parameters of rainbow trout (Oncorhynchus mykiss Walbaum) Aquac. Nutr. 2011;17:73–79. doi: 10.1111/j.1365-2095.2009.00712.x. [DOI] [Google Scholar]
- 32.Hoseinifar SH, Mirvaghefi A, Amoozegar MA, Merrifield DL, Ringo E. In vitro selection of a synbiotic and in vivo evaluation on intestinal microbiota, performance and physiological response of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Nutr. 2017;23:111–118. doi: 10.1111/anu.12373. [DOI] [Google Scholar]
- 33.Yan YY, Xia HQ, Yang HL, Hoseinifar SH, Sun YZ. Effects of dietary live or heat-inactivated autochthonous Bacillus pumilus SE5 on growth performance, immune responses and immune gene expression in grouper Epinephelus coioides. Aquac. Nutr. 2016;22:698–707. doi: 10.1111/anu.12297. [DOI] [Google Scholar]
- 34.Rodriguez-Estrada U, Satoh S, Haga Y, Fushimi H, Sweetman J. Effects of Inactivated Enterococcus faecalis and mannan oligosaccharide and their combination on growth, immunity, and disease protection in rainbow trout. N. Am. J. Aquac. 2013;75:416–428. doi: 10.1080/15222055.2013.799620. [DOI] [Google Scholar]
- 35.Posadas GA, et al. Yeast pro- and paraprobiotics have the capability to bind pathogenic bacteria associated with animal disease. Transl. Anim. Sci. 2017;1:60–68. doi: 10.2527/tas2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani M, et al. Assessing effects of dietary supplements on resistance against Yersinia ruckeri infection in rainbow trout (Oncorhynchus mykiss) using different infection models. Aquaculture. 2020 doi: 10.1016/j.aquaculture.2019.734744. [DOI] [Google Scholar]
- 37.Ohtani M, et al. Effects of fish size and route of infection on virulence of a Danish Yersinia ruckeri O1 biotype 2 strain in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2019;503:519–526. doi: 10.1016/j.aquaculture.2019.01.041. [DOI] [Google Scholar]
- 38.Lugert V, Thaller G, Tetens J, Schulz C, Krieter J. A review on fish growth calculation: multiple functions in fish production and their specific application. Rev. Aquac. 2016;8:30–42. doi: 10.1111/raq.12071. [DOI] [Google Scholar]
- 39.Ytrestøyl T, Aas TS, Åsgård T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture. 2015;448:365–374. doi: 10.1016/j.aquaculture.2015.06.023. [DOI] [Google Scholar]
- 40.Løkka G, Austbø L, Falk K, Bjerkås I, Koppang EO. Intestinal morphology of the wild atlantic salmon (Salmo salar) J. Morphol. 2013;274:859–876. doi: 10.1002/jmor.20142. [DOI] [PubMed] [Google Scholar]
- 41.Ricker WE. Computation and interpretation of biological statistics of fish populations. Michigan, USA: Department of the Environment, Fisheries and Marine Service; 1975. [Google Scholar]
- 42.Nash RDM, Valencia AH, Geffen AJ. The origin of Fulton's condition factor—setting the record straight. Fisheries. 2006;31:236–238. [Google Scholar]
- 43.Escaffre A-M, Kaushik S, Mambrini M. Morphometric evaluation of changes in the digestive tract of rainbow trout (Oncorhynchus mykiss) due to fish meal replacement with soy protein concentrate. Aquaculture. 2007;273:127–138. doi: 10.1016/j.aquaculture.2007.09.028. [DOI] [Google Scholar]
- 44.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. (R Foundation for Statistical Computing, 2018).
- 45.Wickham H. ggplot2: elegant graphics for data analysis. Ggplot2: Elegant Graphics Data Anal. 2009;10:1–212. doi: 10.1007/978-0-387-98141-3. [DOI] [Google Scholar]
- 46.ggradar: Create radar charts using ggplot2 (2019).
- 47.ggpubr: 'ggplot2' Based Publication Ready Plots (2019).
- 48.survminer: Drawing Survival Curves using 'ggplot2' (2019).
- 49.tibble: Simple Data Frames (2019).
- 50.RColorBrewer: ColorBrewer Palettes (2014).
- 51.A Package for Survival Analysis in S version 2.38 (2015).
- 52.scales: Scale Functions for Visualization (2018).
- 53.readxl: Read Excel Files (2019).
- 54.fmsb: Functions for Medical Statistics Book with some Demographic Data. R package version 0.7.0. (2019).
- 55.dplyr: A Grammar of Data Manipulation (2019).
- 56.Beheshti Foroutani M, et al. Minimizing marine ingredients in diets of farmed Atlantic salmon (Salmo salar): effects on growth performance and muscle lipid and fatty acid composition. PLoS ONE. 2018;13:e0198538. doi: 10.1371/journal.pone.0198538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baba E, Acar Ü, Öntaş C, Kesbiç OS, Yılmaz S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture. 2016;465:13–18. doi: 10.1016/j.aquaculture.2016.08.023. [DOI] [Google Scholar]
- 58.Wang SC, et al. Characterization and metabolic diversity of flavonoids in citrus species. Sci. Rep.-UK. 2020 doi: 10.1038/s41598-017-10970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shurson GC. Yeast and yeast derivatives in feed additives and ingredients: sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 2018;235:60–76. doi: 10.1016/j.anifeedsci.2017.11.010. [DOI] [Google Scholar]
- 60.Refstie S, Baeverfjord G, Seim RR, Elvebø O. Effects of dietary yeast cell wall beta-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture. 2010;305:109–116. doi: 10.1016/j.aquaculture.2010.04.005. [DOI] [Google Scholar]
- 61.Ramos MA, et al. Commercial Bacillus probiotic supplementation of rainbow trout (Oncorhynchys mykiss) and brown trout (Salmo trutta): growth, immune responses and intestinal morphology. Aquac. Res. 2017;48:2538–2549. doi: 10.1111/are.13090. [DOI] [Google Scholar]
- 62.Torrecillas S, Montero D, Izquierdo M. Improved health and growth of fish fed mannan oligosaccharides: potential mode of action. Fish. Shellfish Immunol. 2014;36:525–544. doi: 10.1016/j.fsi.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 63.Mandalari G, et al. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007;103:2056–2064. doi: 10.1111/j.1365-2672.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- 64.Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010;109:515–527. doi: 10.1111/j.1365-2672.2010.04677.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are summarized in the present publication. Raw data can be made available from the corresponding author on reasonable request.