Abstract
Coffee is one of the most important commodities worldwide. The industrial processing of coffee cherries generates a considerable volume of by-products such as wastewater, coffee pulp, mucilage, and husk. These by-products have sugars and nutrients that can be converted into value-added products via microbial action. In this study, for the first time, we evaluated the potential of coffee pulp and coffee wastewater as substrate for alcoholic fermentation produce a distilled beverage. The must composed by dry or wet coffee pulp and coffee wastewater added of commercial sucrose or sugarcane molasses was fermented by S. cerevisiae. After a screening step, a larger fermentation was carried out with the wet pulp added of sucrose due to its higher alcoholic fermentation efficiency. The distilled beverage contained 38% (v/v) ethanol and 0.2 g/L of acetic acid. The contaminants furfural, hydroxymethylfurfural and ethyl carbamate were below detection level. Among the 48 volatile compounds detected, the majority (21) were ethyl esters usually associated with floral and sweet aromas. Ethyl decanoate (996.88 µg/L) and ethyl dodecanoate (1088.09 µg/L) were the most abundant esters. Coffee spirit presented taste acceptance of 80% and sugarcane spirit, 70%. The tasters indicated an aroma acceptance of 86% for the coffee spirit and 78% for the sugarcane spirit. The results of this work demonstrate the potential for using coffee by-products to produce a good quality distilled beverage. Considering our results, especially sensorial analysis, we can infer that the produced coffee beverage represents a new alternative for adding value to the coffee production chain.
Keywords: Coffee pulp, Fermentation, Wastewater
Introduction
Coffee is an important global commodity and represents a significant fraction of the economy in many countries. According to the International Coffee Organization (2019), the global coffee output of 2017/2018 was 168 million bags (60 kg). However, this output considers only the coffee bean, which corresponds to about 20% of the total volume of the cherry. The remaining 80% of the cherry is skin, pulp, and mucilage, which are removed during the coffee processing (Guardia Puebla et al. 2013). Coffee cherries are processed either by dry, wet, or semi-dry method, depending on climate characteristics of the production regions. The dry method consists of direct drying of whole cherries. The wet method mechanically removes pulp and husk of the cherries; mucilage is then removed by spontaneous fermentation in water tanks, and beans are washed to remove any mucilage left and dried. Alternatively, the semi-dry is an intermediary method that also uses depulpers to remove the husk and part of the mucilage (Poltronieri and Rossi 2016).
The wet and semi-dry methods include stages where water is used to wash away the undesirable parts of the cherry. The semi-washed coffee generates about 1 m3 of wastewater per ton of fresh fruit, without including finish fermentation and washing, while the fully washed method results in more than 20 m3 of wastewater per ton of cherry (Chanakya and Alwis 2004). This wastewater used to carry away the coffee husk and pulp is rich in suspended organic material. The main constituents of coffee pulp are carbohydrates, fibers, and protein, that represents, respectively, 50, 18 and 10% of its dry weight. Besides these components, coffee pulp also contains tannins, pectin, polyphenols and minerals (Pandey et al. 2000).
If not treated properly these coffee by-products are easily susceptible to spontaneous fermentations, pH decrease, and can cause the eutrophication of receiving body waters. The development of added value products from coffee by-products has been studied by many authors such as the production of enzymes (Cerda et al. 2017) and phenolic compounds (Burniol-Figols et al. 2016). Coffee by-products have also been reported as substrates with potential for alcoholic fermentation, for example, the spent coffee grounds (Machado et al. 2018; Sampaio et al. 2013) was used to produce distilled beverages with good sensory acceptance and desirable volatile compounds profile. In the work of Bonilla-Hermosa et al. (2014), our group verified that the sugar and nutrient of the coffee wastewater, coffee husk and pulp could be fermented by different yeasts, leading to a satisfactory ethanol yield and production of aromatic volatile compounds. To the best of our knowledge, the present work is the first report on the use of pulp and wastewater from coffee processing as a substrate for fermentation and production of a distilled beverage. Therefore, the aim of this study was to evaluate the use of coffee pulp and coffee wastewater mixture as substrate for alcoholic fermentation and production of a distilled beverage.
Materials and methods
Raw material
Coffee wastewater and coffee pulp from the wet processing of coffee beans variety Catuaí 99 vermelho were supplied by a coffee-producing unit located in the municipality of Machado, Southern of the Minas Gerais state (Brazil). The Catuaí variety was chosen because is one of the main variety of Coffeea arabica grown in the Minas Gerais state (Botelho et al. 2010). The sugar content (sucrose, glucose and fructose) was determined by HPLC as described further here. The obtained materials were packed in sterile plastic bags and frozen at − 20 °C. In the case of treatments using dried coffee pulp, the drying process was performed at 65 °C until constant weight followed by manual grinding and storing in hermetic glass flasks (Bonilla-Hermosa et al. 2014).
Yeast strain and inoculum preparation
The yeast used in this study was Saccharomyces cerevisiae LNF CA11 (LNF—Latino América®, Bento Gonçalves—Brazil) in its active dry form, which is widely used in Brazil for cachaça production. To reactivate the yeast, 0.1 g was added in 1 mL of YPD (Yeast Extract-Peptone-Dextrose) broth (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose) and kept at 28 °C for 60 min. After reactivation, the yeast adaptation to the medium was performed using YPD containing coffee wastewater and increasing concentrations of glucose (YPDCoffee). First, the yeast was transferred to a flask containing 1 mL of YPDCoffee 2 °Brix, where it remained for 24 h at 28 °C. After 24 h of incubation, the material was transferred to a flask containing 9 mL of YPDCoffee at 8 °Brix and incubated at 28 °C/24 h. Afterward, the content was added to an Erlenmeyer with 90 mL of YPDCoffee at 12 °Brix and incubated at 28 °C/24 h. Lastly, the Erlenmeyer content was centrifuged at 4 °C/6805 RCF for 10 min to obtain the biomass which was washed twice with 0.1% sterile peptone water with subsequent centrifugation (Andrade et al. 2017).
Fermentation for must selection
Different musts composed by coffee wastewater, dry or wet coffee pulp, commercial sugar (sucrose) or sugarcane molasses were inoculated with S. cerevisiae LNF CA11 to evaluate the fermentation efficiency. Considering the initial sugar content of the raw material (coffee pulp: 4.7% glucose, 6.4% fructose, 0.5% sucrose; coffee pulp: 0.49% glucose, 0.66% fructose and 0.04% sucrose), commercial sucrose or sugarcane molasses were added until the must reached 160 g/L of total sugars. Specifically, the studied musts were: must 1 (M1), 100 mL of coffee wastewater, 10 g of dried coffee pulp with sucrose addition; must 2 (M2), 100 mL of coffee wastewater, 10 g of dried pulp with sugarcane molasses; must 3 (M3), 100 mL of coffee wastewater, 10 g of wet pulp with sucrose; must 4 (M4), 100 mL of coffee wastewater, 10 g of wet pulp with sugarcane molasses. All experiments were performed in duplicate.
The musts were sterilized in an autoclave for 15 min at 121 °C, then inoculated with the yeast biomass obtained as described above and incubated at 28 °C. Samples were collected right after the inoculation and at the end of the fermentation for the analysis of sugar and ethanol by high-performance liquid chromatography (HPLC). Ethanol yield (YP/S), ethanol conversion efficiency (Ef), sugar conversion (Conv) and ethanol volumetric productivity (Qp) were calculated as described below to evaluate the fermentation kinetics (Duarte et al. 2010).
where Pf is the ethanol concentration, Si and Sf are the initial and final concentration of sugars, and tf the fermentation time.
Distilled beverage production
The larger volume fermentation for the production of the distilled beverage was carried out with the must that presented the highest sugar consumption and ethanol production, as well as the best fermentation kinetics parameters. Inocula for this fermentation was prepared as described in item 2.3 and the fermentation was carried out according to Amorim et al. (2016). Samples were collected at the beginning and the end of the fermentation for analyses in liquid chromatography (sugars, acetic acid, and ethanol). All experiments were performed in duplicate.
Distillation
The fermented must was distilled as described by Amorim et al. (2016) and Campos et al. (2010). The 3 fractions of the distillate were collected separately, being the first fraction “head” corresponding to 10% of the distillate; the second fraction “heart” corresponding to the beverage itself was collected and stored in glass bottles; the third fraction “tail” corresponding to 10% of the distillate was discarded. The distilled beverage was submitted to the analyses of the volatile compounds by gas chromatography (HS SPME GC–MS); sugar, ethanol, acetic acid, furfural, hydroxymethylfurfural, and ethyl carbamate by HPLC and finally, sensory analysis.
Sugars, acids and alcohols analyses
Samples of the musts were analyzed to determine sugars (glucose, fructose, and sucrose) and ethanol while the distillate was analyzed for its composition of ethanol and acetic acid. Prior to analyses, the musts samples were centrifuged twice at 6805 RCF, 4 °C/10 min, and filtered in 0.22 µm filters (Duarte et al. 2010). For the analyses, it was used a Shimadzu chromatographer (Shimadzu Corp. Japan) equipped with a UV–Vis (SPD-10Ai) detector and a refractive index detector (RID-10A). Separations occurred on a Supelcogel 8H (7.8 mm × 30 cm) column using sulfuric acid 0.005 M as mobile phase in a flow of 0.5 mL/min with the oven maintained at 30 °C. The identification of the compounds was performed by comparing the retention time of standards with the retention time of peaks in the samples injected under the same conditions. Quantification was done by external calibration (Andrade et al. 2017; Duarte et al. 2010).
Furfural, hydroxymethylfurfural and ethyl carbamate analyses
The distilled beverage was analyzed to verify the presence of furfural, hydroxymethylfurfural and ethyl carbamate. For the determination of ethyl carbamate, it was used the methodology proposed by Santiago et al. (2014), with the previous derivatization of the sample. Analyses were carried out using a Shimadzu high-performance liquid chromatographer equipped with two high-pressure pumps model LC 6AD and an RF-10AXL fluorescence detector. Separations were performed using an Agilent—Zorbax Eclipse AAA (4.6 × 150 mm, 5 μm) column connected to an Agilent—Zorbax Eclipse AAA (4.6 × 12.5 mm, 5 μm) pre-column. Excitation and emission wavelengths employed were 233 and 660 nm, respectively. The mobile phase was composed of 20 mmol/L sodium acetate solution (Solvent A) and acetonitrile (Solvent B). The flow used throughout the analysis was 0.75 mL/min with elution in a gradient from 0 to 5 min (40–60% B); 5 to 10 min (60–70% B); 10 to 18 min (70–80% B); 18 to 19.5 min (80–90% B); 19.5 to 25 min (90–40% B); 25 to 30 min (40% B). Quantification of CE was done using external calibration curves.
Furthermore, furfural and hydroxymethylfurfural were analyzed according to the methodology described by Souza et al. (2009) with some minor modifications. Samples and standards were filtered on a 0.45 µm polyethylene membrane and directly injected into the Shimadzu chromatographic system, equipped with two high-pressure pumps model SPD-M20A, a diode array detector and a Zorbax Eclipse XDB-C18 (4.6 × 250 mm, 5 μm) column connected to an Agilent—Zorbax Eclipse XDBC18 4-Pack (4.6 × 12.5 mm, 5 μm) pre-column. The solvents used as mobile phase were: 2% acetic acid solution in water (Solvent A) and methanol:water:acetic acid (70:28:2% v/v/v) (Solvent B). Elution was in a 0.8 mL/min flow and a gradient from 0 to 25 min (0–40% B); 25 to 40 min (40–55% B); 40 to 43 min (55–60% B); 43 to 50 min (60–100% B); 50 to 55 min (100–0% B); 55 to 60 min (0% B). The wavelength used was 280 nm. The compounds quantification was done by external calibration with analytical curves obtained from a stock solution diluted to concentrations ranging from 0.1 to 25 mg/L. The identity of the analytes was confirmed by the retention time and peak profile of the samples compared to the standards. All injections were done in triplicate.
Volatile compounds analysis by HS SPME GC–MS
Volatile organic compounds (VOCs) were analyzed from 1 mL of sample diluted in 4 mL of distilled water containing 0.25 g NaCl. The 15 mL vials containing the samples were kept at 60 °C and the VOCs extraction from the headspace was performed with a DVB/CAR/PDMS 50–30 µm (Supelco) fiber in a manual holder for 25 min. After extraction, the fiber was maintained in the injector for 5 min for the desorption of the VOCs. The analyses were carried out in a gas chromatograph GC–MS QP2010SE (Shimadzu) coupled to a mass spectrometer equipped with a Carbowax (30 m × 0.20 mm id × 0.25 μm) column. The injector temperature was maintained at 230 °C and injections were in splitless mode. Helium was used as the carrier gas with a flow rate of 1.2 mL/min. Oven temperature was set at 50 °C/5 min, followed by a heating ramp of 5 °C/min until 200 °C, keeping the final temperature for 10 min. The mass spectra were acquired using scan mode (45 a 1000 m/z) from 5 min of analysis (solvent cut time) (Amorim et al. 2016). Compounds were identified using the NIST library version 2011 and the identity confirmed by linear retention index calculated using an alkanes homologous series (C8-C40). Concentrations were expressed as equivalents of 4-nonanol, used as the internal standard at a final concentration of 125 μg/L (Duarte et al. 2010).
Sensory analysis
The sensory analysis was performed by 50 untrained testers, 29 women and 21 men with ages ranging from 18 to 52 years. Besides the beverage produced with coffee by-product, it was included a sample of sugarcane spirit produced in the study of Amorim et al. (2016). This beverage was used as a beverage with recognized quality due to its high acceptance scores in the sensory analysis. Each taster received two random samples containing 5 mL of each beverage and analyzed them according to their appearance, aroma, taste and global impression, according to a hedonic scale ranging from 9 to 1: (9) like extremely, (8) like very much, (7) like moderately, (6) like slightly, (5) neither like nor dislike, (4) dislike slightly, (3) dislike moderately, (2) dislike very much and (1) dislike extremely. The acceptance percentage, percentage of tasters who did not reject the product, was obtained considering scores higher than 5.
Statistical analysis
HPLC data were analyzed with ANOVA and Scott-Knott test using the Sisvar 5.6 software (Lavras, Brazil). Sensory analysis data were submitted to principal component analysis using XLSTAT® software (Addinsoft).
Results and discussion
Must selection
Before the fermentation in a larger volume to produce the distilled beverage, the screening step was carried out to verify which of the musts would allow a better alcoholic fermentation efficiency. For this evaluation, the musts were characterized by HPLC before and after their fermentation. Overall, the total sugar concentration in the musts was higher for those supplemented with commercial sucrose and, among the sugars, sucrose was the most abundant in all musts (Table 1). Similar to that observed for sucrose, the monosaccharides glucose and fructose were also detected in higher concentrations in musts added of commercial sucrose, but without significant difference (p < 0.05). Considering the type of coffee pulp used (dry or wet), musts prepared with dry pulp (M1 and M2) presented higher initial sugar concentration (p < 0.05) than musts M3 and M4 prepared with wet pulp (Table 1). This higher sugar concentration in the musts with dry pulp occurred due to the greater amount (in weight) of coffee pulp added, since in the case of the wet pulp the moisture content is approximately 82%, as previously reported by Bonilla-Hermosa et al. (2014). After 48 h of fermentation, the residual sugars of the fermented musts followed the same profile found at the beginning of the fermentation with 7.94 g/L and 4.77 g/L for musts M1 (dried pulp + sucrose) and M3 (wet pulp + sucrose) (Table 1). The ethanol production after 48 h of fermentation was in general proportional to the sugar concentration in each must. Those musts (M1 and M3) supplemented with commercial sucrose resulted in significantly higher ethanol concentrations (p < 0.05) (Table 1). The fermentation of musts M1 and M3 resulted in 69.07 g/L and 67.19 g/L of ethanol, respectively; while in M2 (dried pulp + sugarcane molasses) and M4 (wet pulp + sugarcane molasses), the ethanol concentrations were respectively, 33.24 g/L and 30.41 g/L (Table 1).
Table 1.
Concentrations of sugars and ethanol by HPLC in fermented coffee pulp and kinetics parameters for S. cerevisiae CA11
| Compounds | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| Non-fermented musts (0 h) | ||||
| Sucrose | 103.82 ± 5.54a | 47.06 ± 0.78d | 92.82 ± 1.25b | 58.83 ± 0.43c |
| Glucose | 26.03 ± 0.22a | 13.30 ± 0.57a | 18.00 ± 0.89a | 6.66 ± 0.06a |
| Fructose | 37.98 ± 0.88a | 20.43 ± 1.51a | 25.65 ± 0.79a | 8.04 ± 0.60b |
| Total sugars1 | 173.33 ± 5.17a | 83.28 ± 2.90c | 141.38 ± 0.36b | 76.64 ± 0.98c |
| Fermented musts (48 h) | ||||
| Sucrose | 0.65 ± 0.03a | 0.18 ± 0.00c | 0.39 ± 0.07b | 0.23 ± 0.05c |
| Glucose | 0.18 ± 0.02b | 0.11 ± 0.00b | 0.26 ± 0.02a | 0.03 ± 0.00c |
| Fructose | 7.07 ± 1.19a | 3.01 ± 0.05b | 4.09 ± 0.31b | 2.25 ± 0.04b |
| Total sugars1 | 7.94 ± 1.17a | 3.32 ± 0.05b | 4.77 ± 0.36a | 2.53 ± 0.10b |
| Ethanol | 69.07 ± 2.42a | 33.24 ± 2.47b | 67.19 ± 0.26a | 30.41 ± 0.27b |
| Kinetics parameters | ||||
| Yp/s (g/g) | 0.42 ± 0.03b | 0.42 ± 0.02b | 0.49 ± 0.00a | 0.41 ± 0.01b |
| Efic (%) | 82.0 ± 6.01b | 81.45 ± 3.14b | 96.43 ± 0.38a | 80.47 ± 1.69b |
| Conv (%) | 95.41 ± 0.81a | 96.02 ± 0.08a | 96.63 ± 0.24a | 96.70 ± 0.08a |
| Qp (g/L/h) | 1.44 ± 0.05a | 0.69 ± 0.05b | 1.40 ± 0.01a | 0.63 ± 0.01b |
Data expressed as mean value ± standard deviation of duplicates. Values followed by the same latter in the superscript do not statistically differ among the treatments by the Scott–Knott test (p > 0.05)
1Considering sucrose mathematically converted to fructose and glucose
From the obtained data it was possible to notice that the supplementation with commercial sucrose would be preferential when compared to the addition of sugarcane molasses, once the ethanol content was higher for supplementation with sucrose. However, to obtain a more detailed view on sugars consumption, ethanol production and consequently, a more accurate decision about the better must for fermentation, the parameters sugar conversion (Conv), ethanol yield (YP/S), ethanol conversion efficiency (Ef) and ethanol volumetric productivity (Qp) were analyzed. The “Conv” parameter was similar and did not showed statistical difference (p < 0.05) for all musts with values around 96%, reinforcing that (based on the total sugar consumption) is possible to cultivate S. cerevisiae in musts containing coffee pulp and wastewater. This value is similar to that reported in studies using S. cerevisiae in musts such as sugarcane (Amorim et al. 2016), grapes (Vernocchi et al. 2015) and honey (Pereira et al. 2013), which are fermentative process focused on the yield of ethanol. As Conv only indicates the total sugar used by yeast, the ethanol conversion efficiency (Ef) and ethanol yield (Yp/s) were calculated to verify how much of the sugars were specifically converted into ethanol. While the fermentations of musts M1, M2 and M4 resulted in Ef values around 80–82% without significant difference (p > 0.05), the fermentation Ef of must M3 (wet pulp + sucrose) was approximately 96%, corresponding to a Yp/s of 0.49 g/g (Table 1). These values found for the fermentation of must M3 are indicative of a high efficiency alcoholic fermentation and comparable to fermentation of sugarcane juice reported by Amorim et al. (2016) using the same Saccharomyces strain. Being the efficient alcoholic fermentation the focus of the study, the must M3 (wet pulp + sucrose) was selected for the production of a wastewater and coffee pulp distillate.
Ethanol and acetic acid
The distilled beverage obtained from the fermentation of must M3 presented an ethanol content of 38% v/v. The Brazilian legislation defines that to be named “aguardente” or spirit (of any substrate) the distilled beverage must present an alcoholic content ranging from 38 to 54% v/v at 20 °C (Brasil 2009); therefore, the produced distilled beverage is in agreement with the standards required by the Brazilian legislation.
Among the acids produced during fermentation, acetic acid has been, quantitatively, the main component of the acidic fraction, and is expressed as volatile acidity. The acetic acid content detected in the distillate was 0.2 g/L (52.36 mg/100 mL anhydrous alcohol – a.a.). In general, acetic acid affects the acidity of the beverage and contributes to an undesirable aroma due to its “vinegar” aroma descriptor (Czerny et al. 2008). Compared to the sugarcane spirit produced by Amorim et al. (2016) using the same strain S. cerevisiae, which presented the acetic acid concentration of 0.018 g/L (4.74 mg/100 mL a.a.), the beverage produced with wastewater and coffee pulp resulted in a higher volatile acidity. However, the beverage is still within the Brazilian legislation requirement where the acetic acid concentration should not exceed 150 mg/100 mL a.a. (Brasil 2005).
Furfural, hydroxymethylfurfural and ethyl carbamate quantification
Considering the substrates used in this work, the produced distillate was analyzed to check the presence of organic contaminants, furfural, hydroxymethylfurfural, and ethyl carbamate. All analyzed contaminants were below the detection limit which was respectively for furfural, hydroxymethylfurfural and ethyl carbamate, 0.017 mg/100 mL a.a., 0.011 mg/100 mL a.a. and 1.86 µg/L.
Some aldehydes are considered organic contaminants in alcoholic beverages, which is the case of furfural and hydroxymethylfurfural, and their presence is undesirable. They are formed by the chemical decomposition of pentoses and hexoses, or by pyrogenation of organic matter deposited on the bottom of distillers. The contaminations can be avoided by keeping the must to be distilled clean and free of organic matter in suspension. Before the distillation, the must was left undisturbed to allow the sedimentation of solid material, a procedure that probably helped in the non-detection of these contaminants in the wastewater and coffee pulp beverage. Another procedure that assists in the elimination of these beverage contaminants is the separation of the fractions with the discard of the “head” which correspond to 10% of the distillate volume.
Another contaminant analyzed in the beverage was ethyl carbamate, an organic contaminant that has been widely studied in various beverages. This carcinogenic compound is naturally found in low concentrations in different alcoholic beverages and some fermented foods (d’Avila et al. 2016; Santiago et al. 2014). Due to its high toxicity and common presence in alcoholic beverages, its detection and quantification has become relevant. According to the Brazilian legislation (Brasil 2005), the maximum limit for ethyl carbamate content is 210 μg/L. This compound is produced in low levels (ng/L or ng/kg or mg/L) from several precursors, such as hydrocyanic acid, urea, citrulline, and N-carbamyl amino acids (including carbamyl phosphate by reaction with ethanol) (Beland et al. 2005). However, the pathways of formation and precursors of ethyl carbamate in foods and beverages have not been completely elucidated yet, as they depend on the type of food and their processing.
Evaluation of volatile compounds by HS–SPME–GC–MS
The HS-SPME-GC- MS analysis resulted in the identifications of 48 compounds (Table 2). Different chemical classes were detected, such as higher alcohols, terpenes, volatile acids, aldehydes, ketones, and esters, being esters the most abundant group.
Table 2.
Concentration of volatile compounds (μg/L) in distilled beverage produced from coffee pulp by HS SPME GC–MS
| Number | Compound | LRI calc | LRI lit | Concentration (µg/L) | Descriptors |
|---|---|---|---|---|---|
| Alcohols (9) | |||||
| 1 | 2-Methyl-1-propanol | 1095 | 1048f | 44.63 ± 0.21 | Maltya, unpleasant |
| 2 | 1-Butanol | 1146 | 1145f | 2.45 ± 0.02 | Solventa |
| 3 | 2-Methyl-1-butanol | 1212 | 1212f | 1269.16 ± 5.23 | Bananaa |
| 3-Methyl-1-butanol | |||||
| 4 | 2-Methyl-1-decanol | 1500 | N.I | 13.28 ± 0.56 | – |
| 5 | 1-Octanol | 1562 | 1567f | 8.15 ± 0.12 | Coco, walnut oilb |
| 6 | 1-Decanol | 1767 | 1809f | 8.75 ± 0.31 | Sweet, fattyg |
| 7 | 2-Phenylethanol | 1925 | 1931f | 226.79 ± 4.74 | Sweet, rosesa |
| 8 | 1-Dodecanol | 1973 | 1940f | 24.16 ± 1.41 | Floral, waxyg |
| 9 | 1-Eicosanol | 2179 | N.I | 6.34 ± 0.27 | – |
| Monoterpene alcohols (5) | |||||
| 10 | Citronellol | 1172 | N.I | 22.75 ± 0.11 | Citrusc |
| 11 | Linalool | 1516 | 1550f | 36.52 ± 1.02 | Bergamota |
| 12 | Nerolidol* | 2407 | N.I | 16.94 ± 0.38 | – |
| 13 | d-Nerolidol* | 1673 | 1634f | 5.81 ± 0.09 | – |
| 14 | α-Terpineol | 1706 | 1696g | 11.91 ± 0.20 | Pinusb |
| Volatile acids (2) | |||||
| 15 | 1-Decanoic acid | 2329 | 2278g | 454.98 ± 5.83 | Waxy, rancid, tallowb |
| 16 | Octanoic acid | 2098 | 2047f | 200.85 ± 0.92 | Rancidb |
| Esters (26) | |||||
| 17 | Linalool acetate | 1735 | N.I | 1.57 ± 0.10 | Citrusa |
| 18 | Phenylethyl acetate | 1830 | 1820f | 296.17 ± 7.54 | Rosesb |
| 19 | Farnesyl acetate | 2282 | N.I | 13.43 ± 1.24 | |
| 20 | Isoamyl acetate | 1126 | 1105f | 62.04 ± 0.54 | Banana, appled |
| 21 | Citronellol acetate | 1670 | N.I | 10.69 ± 1.20 | Citronellac |
| 22 | Ethyl butanoate | 1036 | 1031h | 25.28 ± 0.42 | Fruity, sweet, applea |
| 23 | Ethyl 3-metylbutanoate | 1138 | 1035h | 5.21 ± .012 | Fruity, berriesa |
| 24 | Ethyl decanoate | 1543 | 1620f | 39.67 ± .84 | Fruityb |
| 25 | Butyl octanoate | 1559 | N.I | 6.25 ± 0.05 | – |
| 26 | Methyl decanaote | 1602 | 1603f | 20.98 ± 0.57 | – |
| 27 | Isoamyl octanoate | 1666 | 1658f | 141.08 ± 0.90 | Oilyg |
| 28 | Ethyl 9-decenoate | 1699 | 1694f | 850.98 ± 5.31 | Roseg |
| 29 | Propyl decanoate | 1731 | N.I | 2.34 ± 0.05 | – |
| 30 | Ethyl undecanoate | 1748 | 1725f | 13.18 ± 0.70 | – |
| 31 | Isobutyl decanoate | 1762 | 1773f | 11.84 ± 0.21 | – |
| 32 | Methyl salicylate | 1792 | 1820g | 48.57 ± 0.98 | – |
| 33 | Ethyl dodecanoate | 1852 | 1848f | 1088.09 ± 6.83 | Floral, fruityd |
| 34 | Isoamyl decanoate | 1780 | 1779f | 109.58 ± 0.51 | – |
| 35 | Ethyl hydrocinnamato | 1900 | N.I | 26.24 ± 0.90 | – |
| 36 | Ethyl 9-hexadeceoate* | 1905 | N.I | 22.45 ± 0.18 | – |
| 37 | Ethylicosanoate | 2056 | N.I | 11.71 ± 0.14 | – |
| 38 | Ethyl hexanoate | 1239 | 1241f | 193.89 ± 2.30 | Green appleb |
| 39 | Ethyl heptanoate | 1339 | 1338f | 8.55 ± 0.06 | Fruity, pineappled |
| 40 | Methyl octanaote | 1395 | 1385f | 5.18 ± 0.21 | – |
| 41 | Ethyl octanoate | 1443 | 1445f | 996.88 ± 1.42 | Fruityb |
| 42 | Isoamyl hexanoate | 1465 | 1445f | 8.28 ± 0.08 | Sweet, fruityg |
| Aldehydes (2) | |||||
| 43 | Decanal | 1507 | 1500g | 9.97 ± 0.14 | Sweet waxy, orangeg |
| 44 | Dodecanal | 1720 | 1729g | 4.65 ± 0.04 | Floral, waxyg |
| Ketones (2) | |||||
| 45 | 1-Menthone* | 1472 | N.I | 15.2 ± 0.06 | – |
| 46 | β-Damascenone | 1835 | 1805h | 55.81 ± 0.48 | Honey, sweetg |
| Others (2) | |||||
| 47 | d-Limonene | 1198 | 1222f | 17.83 ± 0.33 | Citrus, herbale |
| 48 | 2,3-Dihydrofarnesol* | 2285 | N.I | 13.43 ± 0.65 | – |
Data expressed as mean value ± standard deviation of duplicates
LRIcalc, linear retention index based on a series of n-hydrocarbons reported according to their elution order on a Carbowax column
LRIlit, linear retention index from literature
aCzerny et al. (2008), bMeilgaard (1975), cRibéreau-Gayon et al. (2006), dHu et al. (2018), ePalassarou et al. (2017), fMartines et al. (2018), gGurbuz et al. (2006), and hPino and Queris (2011)
The higher abundance of esters is generally associated with superior quality beverages. Among the ethyl esters were detected 21 compounds, being ethyl dodecanoate (1088.09 µg/L), ethyl octanoate (996.88 µg/L) and ethyl 9-decenoate (850.98 µg/L) the most abundant. Esters are associated with pleasant descriptions, such as roses, fruity and floral (Hu et. al. 2018). These compounds are common in many alcoholic beverages, including wine, other spirits and fruit distillates (Amorim et al. 2016; Palassarou et al. 2017; Vernocchi et al. 2015). Ethyl octanoate was reported by Sampaio et al. (2013) in similar concentration to the distillate produced in this study, 842 µg/L (Table 2). On the other hand, this concentration was higher than the 239.4 µg/L and 698.0 µg/L found in two different spent coffee ground spirits by Machado et al. (2018). The profile of these three main ethyl esters reinforces the potential of studied coffee by-products for use in alcoholic fermentation and generation of desirable volatile aromatic compounds.
Besides ethyl esters, five acetates were also found in the beverage, with phenylethyl acetate being the most abundant (296.17 µg/L) (Table 2). This concentration was more than twice as high as that found by Sampaio et al. (2013) in the spent coffee grounds distillate. Phenylethyl acetate is an impactful compound in the beverages with aroma descriptor associated with roses. Isoamyl acetate, responsible for conferring banana aroma in to the distillate (Hu et al. 2018) was found in the concentration of 62 µg/L. Other authors also reported the presence of this compound in other distilled beverages such as, whey and cachaça (Amorim et al. 2016; Dragone et al. 2009). It is also worth mentioning that the high concentration of ethyl esters found in the beverage produced with coffee pulp is similar to those reported in studies such as Sampaio et al. (2013) and Santiago et al. (2014), in which high-quality sugarcane spirits also presented esters as the main group of volatile compounds.
Among other compounds that positively influence the aroma of distilled beverages, there are higher alcohols. Higher alcohols can be synthesized by yeasts through an anabolic glucose pathway or a catabolic pathway of corresponding amino acids (valine, leucine, iso-leucine and phenylalanine). Consequently, higher alcohols are released to the medium as secondary products from yeast metabolism and are responsible for secondary aroma in beverages. The higher alcohols that are formed by the metabolism of yeasts form amino acids – naturally, occur in higher concentrations in distilled beverages (Sampaio et al. 2013). In the beverage produced in this study, the higher alcohols with highest concentrations were 2-methyl-1-butanol and 3-methyl-1-butanol (isoamyl alcohols), with a concentration of 1269.16 µg/L. These compounds constitute most of the higher alcohols in distilled beverages and define the sensory character of the beverage (Czerny et al. 2008). In a distilled beverage produced from spent coffee grounds by Sampaio et al. (2013) these compounds were found high concentrations, as well as in the sugarcane spirit produced by Amorim et al. (2016) with the same yeast used in this study. In both studies, the final beverages were submitted to sensory analysis and presented a good acceptance by the trained and untrained tasters. As the major alcohols in the beverage, 2-methyl-1-butanol and 3-methyl-1-butanol can be considered positive contributors to the sensorial quality of the beverage as discussed below. The positive impact of these is associated with their aroma descriptors such as “banana” described by Czerny et al. (2008). Another higher alcohol also detected in a high concentration was the 2-phenylethanol, with 226.76 µg/L (Table 2). This compound presence in low concentrations may contribute to the floral and sweet aroma of the distillate (Amorim et al. 2016; Hu et al. 2018). The aroma character of this compound changes with its oxidation and additional oxidation produces esters with honey aroma. Besides alcohols with a positive impact in the sensorial characteristics of distilled beverages, an interesting fact is that 1-butanol was found in the lowest concentration (2.45 µg/L) among the measured alcohols. In beverages, this compound is associated with “solvent” odor (Czerny et al. 2008) and may exert a negative effect on the aroma of the final product. The concentration found in distilled coffee pulp beverage was lower than the 4.89 µg/L reported by Amorim et al. (2016) in the sugarcane spirit fermented by the same strain used here.
The monoterpene alcohols found in the beverage were linalool, citronellol, nerolidol, α-terpineol and d-nerolidol (Table 2). The monoterpene alcohols are described with “citrus”, “bergamot”, “pinus” and “citronella” aromas by Czerny et al. (2008), and strongly impact the final aroma of beverages due to their low perception threshold. Among them, the linalool was detected in the highest concentration, 36.52 µg/L (Table 2). Considering that terpenes are either derived from the substrate or released by enzymatic reactions during the fermentation as described by Penã-Alvarez et al. (2004), the linalool content found in the beverage may be from the used substrate. The concentration found in our beverage is higher than the value found by Sampaio et al. (2013) in coffee spent ground distillate. Citronellol was detected in a concentration of 22.75 µg/L, almost two times more than the concentration reported by Machado et al. (2018) in spent coffee ground distilled beverage. Unlike citronellol, nerolidol was found in the distillate of spent coffee ground by Machado et al. (2018) in an amount approximately 8 times higher than the one we measured in our distillate (16.94 µg/L). α-Terpineol, which common aromatic descriptor is “pinus”, was found in a concentration of 11.91 µg/L (Table 2).
Regarding the volatile acids, interestingly, only 2 compounds were found, 1-decanoic acid (456.98 µg/L) and octanoic acid (200.85 µg/L) (Table 2). Their concentrations were lower than those reported in other coffee by-products by Sampaio et al. (2013) and Machado et al. (2018). These compounds are frequently associated with negative impacts on the sensorial quality of beverages. The aroma of octanoic acid is described as “rancid” while decanoic acid descriptors are “waxy, rancid and tallow”. These acids are related to rancid and fat aromas such as in the whey distillate produced by Dragone et al. (2009).
Aldehydes (related to the hangover) and ketones were also detected in the beverage. According to Perestrelo et al. (2006), aldehydes are formed from unsaturated fatty acids while ketones are formed by the condensation of active fatty acids. Aldehydes may also be produced from their corresponding alcohols during fermentation (Perestrelo et al. 2006), so they were identified in low concentrations.
Sensory analysis
The sensory analysis was performed in comparison to a sugarcane spirit previously produced by Amorim et al. (2016). This sugarcane spirit showed a desirable aromatic compounds profile and good acceptance among the tasters in its previous sensory analysis. From the comparative sensory analysis, it was verified that the coffee pulp beverage presented a considerable higher acceptance (scores higher than 5 in the hedonic scale) percentage for aroma, taste and global impression. While the sugarcane spirit presented 70% of acceptance in relation to “taste”, the coffee pulp spirit showed 80%. In the “aroma” evaluation, the tasters indicated an acceptance of 86% for the coffee pulp spirit and 78% for the sugarcane spirit. Similarly, to the “aroma”, the “global impression” of the coffee pulp spirit (84%) was 8% higher than the sugarcane spirit (76%). The difference between both beverages for the “appearance” attribute was 4% (74% for the coffee pulp spirit and 78% for the sugarcane spirit). These differences, found mainly for the “aroma” and “taste” attributes, are directly related to the composition of volatiles previously described, which when compared to that reported by Amorim et al. (2016) shows differences in the diversity as well as the concentration of the common compounds in the two beverages.
It was detected a large number of esters and terpenes in the distilled beverage of coffee pulp, which are compounds associated with floral and fruity aromas. Even more, many of these compounds present a low perception threshold that strongly impacts the sensory quality of the beverage. Terpene-like notes have already been related to the aroma of green coffee beans. As shown in the volatile compound profile of the coffee pulp spirit, it was identified a considerable abundance of terpenes in the beverage. When questioned as to which aroma could be used to describe the coffee pulp spirit, a large percentage of the tasters pointed out the presence of an aroma that recalled them to brewed coffee. Also, considering the general acceptance for coffee, probably this aroma of coffee in the coffee pulp spirit positively impacted in its higher acceptance.
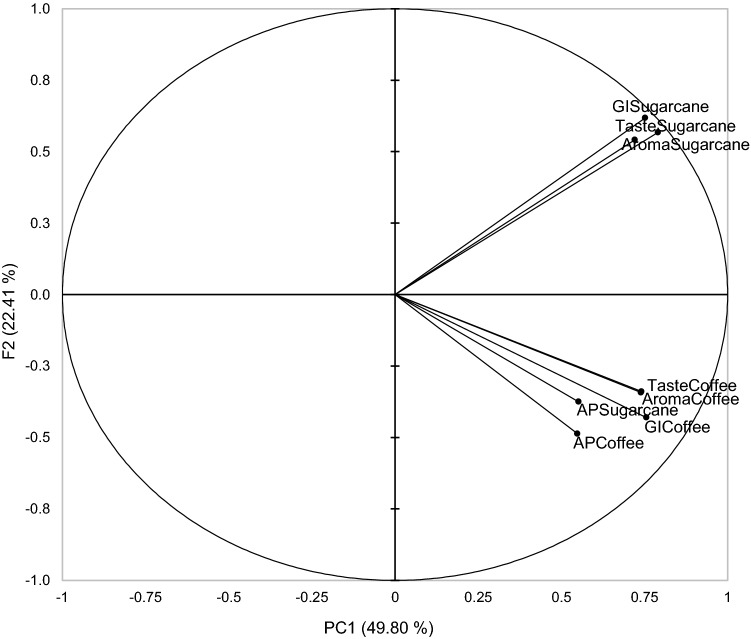
The Principal Component Analysis (PCA) of the sensory evaluation data (Fig. 1) demonstrated that the first two components PC1 and PC2 accounted for 72.21% of the variance. The “aroma”, “taste” and “global impression” attributes of the coffee pulp spirit were in the right lower quadrant (positive side of PC1 and negative of PC2), while these same attributes for the sugarcane spirit were grouped in the right superior quadrant (positive side of PC1 and PC2). For the “appearance” attribute, both beverages were grouped together, which is due to the fact that they are distilled beverages with identical clear visual; thus, they did not generate a different perception by the tasters.
Fig. 1.
Principal component analysis (PCA) of sensory attributes of coffee pulp and wastewater spirit and sugarcane spirit. AP appearance, GI global impact
The world coffee production in 2017/2018 was 168 million 60 kg bags (IOC 2019). Brazil was responsible for 76% of this production. However, this value considers only the final green coffee beans, being most part of the cherry weight removed during the coffee processing. Indeed, for every 2 tons of processed coffee, about 1 ton of pulp is generated (Murthy and Naidu 2012). Also, the wet processing releases up to 20 m3 of wastewater per ton of cherry in the fully washed method (Chanakya and Alwis 2004). Considering the volume of coffee produced in Brazil, the amount of by-products generated, the amount of fermentable sugar available in these by-products and the quality of the distilled beverage produced in this work, the use of by-products represents a great economic potential for generation of profit in the coffee production chain.
Conclusion
Considering the results found in this work, especially in the analysis of volatile and sensory compounds, we can conclude that the evaluated coffee by-products can be used to produce a good quality distilled beverage. Also, we can infer that the production of a distilled beverage represents an interesting alternative for adding value to the coffee production chain since currently, the by-products used here do not represent a source of profit for coffee farmers. In a scenario of search for sustainability and value aggregation to the coffee production chain, the use of coffee pulp and wastewater for alcoholic fermentation represents itself as an interesting alternative to be exploited, for example, in the coffee producing units, generating a differentiated product that can be attractive to the industry because it is a distilled beverage with coffee aroma.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors also would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq), Fundação de Amparo à Pesquisa de MG (FAPEMIG) and National Institute of Coffee Science and Technology for their support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amorim JC, Schwan RF, Duarte WF. Sugar cane spirit (cachaça): effects of mixed inoculum of yeasts on the sensory and chemical characteristics. Food Res Int. 2016;85:76–83. doi: 10.1016/j.foodres.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Andrade RP, Melo CN, Genisheva Z, Schwan RF, Duarte WF. Yeasts from Canastra cheese production process: isolation and evaluation of their potential for cheese whey fermentation. Food Res Int. 2017;91:72–79. doi: 10.1016/j.foodres.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Beland FA, Benson RW, Mellick PW, Kovatch RM, Roberts DW, Fang JL, et al. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem Toxicol. 2005;43:1–19. doi: 10.1016/j.fct.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Bonilla-Hermosa VA, Duarte WF, Schwan RF. Utilization of coffee by-products obtained from semi-washed process for production of value-added compounds. Bioresour Technol. 2014;166:142–150. doi: 10.1016/j.biortech.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Botelho CA, Rezende JC, Carvalho GR, Carvalho AM, Andrade VT, Barbosa CR (2010) Adaptabilidade e estabilidade fenotípica de cultivares de café arábica em Minas Gerais. Pesquisa Agropecuária Brasileira, Brasília vol 45, no 12, pp 1404–1411
- BRASIL (2005) Ministério da Agricultura Pecuária e Abastecimento. Instrução Normativa n◦. 13 de 29 de junho de
- BRASIL (2009) Ministério da Agricultura Pecuária e Abastecimento. Decreto n° 6.871 de 4 de junho de
- Burniol-Figols A, Cenian K, Skiadas IV, Gavala HN. Integration of chlorogenic acid recovery and bioethanol production from spent coffee grounds. Biochem Eng J. 2016;116:54–64. doi: 10.1016/j.bej.2016.04.025. [DOI] [Google Scholar]
- Campos CR, Silva CF, Dias DR, Basso LC, Amorim HV, Schwan RF. Features of Saccharomyces cerevisiae as a culture starter for the production of the distilled sugar cane beverage, cachaça in Brazil. J Appl Microbiol. 2010;108(6):1871–1879. doi: 10.1111/j.1365-2672.2009.04587.x. [DOI] [PubMed] [Google Scholar]
- Cerda A, Gea T, Vargas-García MC, Sánchez A. Towards a competitive solid state fermentation: Cellulases production from coffee husk by sequential batch operation and role of microbial diversity. Sci Total Environ. 2017;589:56–65. doi: 10.1016/j.scitotenv.2017.02.184. [DOI] [PubMed] [Google Scholar]
- Chanakya HN, Alwis AAP. Environmental issues and management in primary coffee processing. Process Saf Environ. 2004;82(4):291–300. doi: 10.1205/095758204323162319. [DOI] [Google Scholar]
- Czerny M, Christlbauer M, Christlbauer M, Fischer A, Granvogl M, Hammer M, et al. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur Food Res Technol. 2008;228(2):265–273. doi: 10.1007/s00217-008-0931-x. [DOI] [Google Scholar]
- de Souza PP, de Oliveira LC, Catharino RR, Eberlin MN, Augusti DV, Siebald HG, Augusti R. Brazilian cachaça: “Single shot” typification of fresh alembic and industrial samples via electrospray ionization mass spectrometry fingerprinting. Food Chem. 2009;115(3):1064–1068. doi: 10.1016/j.foodchem.2008.12.026. [DOI] [Google Scholar]
- d'Avila GB, Cardoso MDG, Santiago WD, Rodrigues LMA, da Silva BL, Cardoso RR, et al. Quantification of ethyl carbamate in cachaça produced in different agro-industrial production systems. J Inst Brew. 2016;122(2):299–303. doi: 10.1002/jib.322. [DOI] [Google Scholar]
- Dragone G, Mussatto SI, Oliveira JM, Teixeira JA. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009;112(4):929–935. doi: 10.1016/j.foodchem.2008.07.005. [DOI] [Google Scholar]
- Duarte WF, Dias DR, Oliveira JM, Vilanova M, Teixeira JA, Silva JBA, Schwan RF. Raspberry (Rubus idaeus L.) wine: yeast selection, sensory evaluation and instrumental analysis of volatile and other compounds. Food Res Int. 2010;43(9):2303–2314. doi: 10.1016/j.foodres.2010.08.003. [DOI] [Google Scholar]
- Guardia Puebla Y, Rodríguez Pérez S, Jiménez Hernández J, Sánchez-Girón Renedo V. Performance of a UASB reactor treating coffee wet wastewater. Rev Ciênc Téc Agropecu. 2013;22(3):35–41. [Google Scholar]
- Gurbuz O, Rouseff JM, Rouseff RL. Comparision of aroma volatiles in commercial Merlot and Carbenet Sauvignon wines using gas chromatography—olfactometry and gas chromatography-mass spectrometry. J Agric Food Chem. 2006;54:3990–3996. doi: 10.1021/jf053278p. [DOI] [PubMed] [Google Scholar]
- Hu K, Jin GJ, Mei WC, Li T, Tao YS. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018;239:495–501. doi: 10.1016/j.foodchem.2017.06.151. [DOI] [PubMed] [Google Scholar]
- ICO (2019). International coffee organization. https://www.ico.org. Accessed 15 Feb 2019
- Machado E, Mussatto S, Teixeira J, Vilanova M, Oliveira J. Increasing the sustainability of the coffee agro-industry: spent coffee grounds as a source of new beverages. Beverages. 2018;4(4):105. doi: 10.3390/beverages4040105. [DOI] [Google Scholar]
- Martines N, Garcia R, Davide M, Freintas AMC, da Silva MG, Cabrita MJ. An anciant winemaking tehcnology: Exploring the volatile composition of amphora wines. LWT Food Sci Technol. 2018;96:288–295. doi: 10.1016/j.lwt.2018.05.048. [DOI] [Google Scholar]
- Meilgaard MC. Flavor chemistry of beer. II. Flavor and threshold of 239 aroma volatiles. Tech Q Master Brew Assoc Am. 1975;12:151–168. [Google Scholar]
- Murthy PS, Naidu MM. Suistainable management of coffee industry by-products and value addition—a review. Resour Conserv Recycl. 2012;66:45–58. doi: 10.1016/j.resconrec.2012.06.005. [DOI] [Google Scholar]
- Palassarou M, Melliou E, Liouni M, Michaelakis A, Balayiannis G, Magiatis P. Volatile profile of Greek dried white figs (Ficus carica L.) and investigation of the role of β-damascenone in aroma formation in fig liquors. J Sci Food Agric. 2017;97:5254–5270. doi: 10.1002/jsfa.8410. [DOI] [PubMed] [Google Scholar]
- Pandey A, Soccol CR, Nigam P, Brand D, Mohan R, Roussos S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem Eng J. 2000;6(2):153–162. doi: 10.1016/S1369-703X(00)00084-X. [DOI] [PubMed] [Google Scholar]
- Peña-Alvarez A, Dĺaz L, Medina A, Labastida C, Capella S, Vera LE. Characterization of three Agave species by gas chromatography and solid-phase microextraction–gas chromatography–mass spectrometry. J Chromatogr A. 2004;1027(1–2):131–136. doi: 10.1016/j.chroma.2003.10.082. [DOI] [PubMed] [Google Scholar]
- Pereira AP, Mendes-Ferreira A, Oliveira JM, Estevinho LM, Mendes-Faia A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimisation of mead production. Food Microbiol. 2013;33(1):114–123. doi: 10.1016/j.fm.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Perestrelo R, Fernandes A, Albuquerque FF, Marques JC, Câmara JS. Analytical characterization of the aroma of Tinta Negra Mole red wine: identification of the main odorants compounds. Anal Chim Acta. 2006;563(1–2):154–164. doi: 10.1016/j.aca.2005.10.023. [DOI] [Google Scholar]
- Pino JA, Queris O. Characterization of odor-active compounds in guava wine. J Agric Food Chem. 2011;59(9):4885–4890. doi: 10.1021/jf2011112. [DOI] [PubMed] [Google Scholar]
- Poltronieri P, Rossi F. Challenges in specialty coffee processing and quality assurance. Challenges. 2016;7(2):19. doi: 10.3390/challe7020019. [DOI] [Google Scholar]
- Ribéreau-Gayon P, Glories Y, Maujean A, Dubourdieu D (2006) Alcohols and other volatile compounds. In: Handbook of enology, vol2, 2nd ed. Wiley, Bordeaux, pp 51–61
- Sampaio A, Dragone G, Vilanova M, Oliveira JM, Teixeira JA, Mussatto SI. Production, chemical characterization, and sensory profile of a novel spirit elaborated from spent coffee ground. LWT Food Sci Technol. 2013;54(2):557–563. doi: 10.1016/j.lwt.2013.05.042. [DOI] [Google Scholar]
- Santiago WD, Das Graças Cardoso M, Duarte FC, Saczk AA, Nelson DL. Ethyl carbamate in the production and aging of cachaça in oak (Quercus sp.) and amburana (Amburana cearensis) barrels. J Inst Brew. 2014;120(4):507–511. [Google Scholar]
- Vernocchi P, Patrignani F, Ndagijimana M, Lopez CC, Suzzi G, Gardini F, Lanciotti R. Trebbiano wine produced by using Saccharomyces cerevisiae strains endowed with β-glucosidase activity. Ann Microbiol. 2015;65(3):1565–1571. doi: 10.1007/s13213-014-0995-8. [DOI] [Google Scholar]