Abstract
The effects of the inoculation method of Saccharomyces bayanus BV818 and non-Saccharomyces yeast Metschnikowia agaves P3-3 and the fermentation temperature on the volatile profiles of red pitaya wine were investigated in the present study. Although the growth of P3-3 was inhibited by BV818 in the mixed inoculations, simultaneous and sequential inoculations promoted the production of seven volatiles, including higher alcohols (propan-1-ol, 3-methyl-1-butanol and phenethyl alcohol), esters (ethyl decanoate and diethyl succinate), acid (2-ethylhexanoic acid), and ketone (acetoin). Sequential inoculation produced the largest total content of volatile compounds and exhibited the best in the global aroma. The red pitaya wine produced in different inoculations can be separated by its main volatile components. Furthermore, the highest total content was yielded at 25 °C for alcohols and at 21 °C for esters and acids. Within an experimental range of 17 °C to 29 °C, the contents of benzaldehyde and acetoin decreased with the increase in temperature, whereas the change in 4-ethyl-2-methoxyphenol content was the opposite. The similarly high total contents of volatiles and global aroma score were yielded via sequential inoculation at 21 °C and 25 °C. Therefore, the desired red pitaya wine can be effectively produced by modulating the inoculation method and fermentation temperature.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04484-5) contains supplementary material, which is available to authorized users.
Keywords: Non-saccharomyces, Inoculation method, Fermentation temperature, Pitaya wine, Volatile compound
Introduction
Pitaya (Hylocereus sp.), also known as dragon fruit, is a member of the Cactaceae family. Pitaya is native to Latin America and West Indies and is cultivated in tropical and subtropical regions of the world. This fruit is planted over 34,000 hectares with an output of 100,000 tons annually in China, and the sales revenue exceeded 7 billion yuan. However, storage of this fruit is difficult, so it is often processed and consumed as dried fruit or wine (Gong et al. 2017b).
Pitaya can be divided into red (Hylocereus lemairei, also called Hylocereus polyrhizus), white (Hylocereus undatus), and yellow (Hylocereus megalanthus) depending on the color of peel and pulp. Red pitaya has attracted great attention because of its bright red–purple color and strong antioxidant activity produced by its high anthocyanin content. Such antioxidant activity protects the body against oxidative damage (Sandate-Flores et al. 2017). These characteristics made red pitaya an ideal wine-making material.
Saccharomyces yeasts are widely used in the winemaking industry, but the single inoculation of this strain may result in the simplification of wine flavor (Padilla et al. 2016). Increasingly more studies have revealed that mixed inoculations of Saccharomyces and non-Saccharomyces yeast strains improve the levels of volatile components in wines, thereby yielding products with complex, intense, and characteristic flavor (Englezos et al. 2018; Wang et al. 2017). The mixed culture fermentation can modify the chemical compositions and the overall character of fruit wine because an interaction occurs between Saccharomyces and non-Saccharomyces strains. Moreover, the inoculation method of Saccharomyces and non-Saccharomyces strains can induce various changes in the chemical components of the wine (Lee et al. 2012; Sun et al. 2016). Therefore, theoretically an improved wine can be acquired via appropriate inoculation method in mixed culture fermentation. Nevertheless, the aroma of red pitaya wine, the use of mixed inoculation for producing red pitaya wine, and the effects of inoculation method on the volatile profiles of red pitaya wine are rarely reported.
Fermentation parameters, such as temperature, are important factors that impact the formation of volatiles during wine making. Temperature can alter the rate of fermentation by influencing the metabolism of carbon and nitrogen sources, thereby affecting the chemical and organoleptic qualities of the final wine product (Malherbe et al. 2004). Low temperature increases positive aromas and maintains a high level of varietal aromas. In contrast, at an elevated temperature, the growth of yeast and utilization of sugar can be accelerated. Findings from Peng et al. (2015) on apple wine revealed that changing the fermentation temperature was able to remarkably influence the production of the key volatile components, and 20 °C was found to be the most suitable fermentation temperature in terms of volatile and sensory profiles. Sun et al. (2016) on cherry wine suggested that compared to the wine fermented at 25 °C, fermentations at 20 °C and 30 °C decreased its sensory quality. Nevertheless, the effects of fermentation temperature on the volatile and sensory profiles of red pitaya wine are rarely studied, thus must be explored to improve the processing technique and the quality of final product.
The current study aims to enrich the knowledge on the volatile profile of red pitaya wine, and evaluate the effects of the inoculation method of Saccharomyces bayanus strain BV818 and non-Saccharomyces yeast Metschnikowia agaves P3-3 and the fermentation temperature on the formation of volatiles in red pitaya wine.
Materials and methods
Chemicals
The chemical standards (Sigma-Aldrich, Saint Louis, MO, USA) used in this work were analytical reagent grade.
Yeast strains
Commercial S. bayanus strain BV818 was purchased from Angel (Yichang, China). Non-Saccharomyces yeast M. agaves P3-3 was deposited in the Laboratory of Food Biotechnology at Hainan University. The strain M. agaves P3-3 was isolated from the papaya and owned a high β-glucosidase activity.
Red pitaya wine fermentation
Red pitaya (Jindu no.1) was harvested from fruit orchards in Dongfang city, Hainan Province, China, at optimal maturity. The soluble solid content of the red pitaya juice was adjusted from 13 to 22°Brix using sucrose. The pH of the red pitaya juice was adjusted from 4.6 to 3.8 using citric acid. The total acidity (expressed as tartaric acid) of the red pitaya juice was 5.13 g/L. 100 mg/L sodium metabisulphite was added to prevent bacterial contamination.
In this study, the samples were prepared as follows: (I) individually inoculating S. bayanus BV818 in the pure-culture fermentation (PF), (II) individually inoculating M. agaves P3-3 in PF, (III) simultaneously inoculating S. bayanus BV818 and M. agaves P3-3 in the co-culture fermentation (CF), (IV) the S. bayanus strain BV818 was inoculated 48 h after the strain M. agaves P3-3 in sequential fermentation (SF). The strain S. bayanus BV818 was inoculated at 106 cells/mL, and the strain M. agaves P3-3 was inoculated at 107 cells/mL.
The yeast strains were statically pre-cultured in yeast extract peptone dextrose (YEPD) medium (20 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract) at 25 °C for 48 h. The cells were harvested through centrifugation (1500 × g, 4 °C, 10 min), washed once with 50 mL of sterile water, and then resuspended in sterile water.
The fermentations were conducted in 250 mL conical flasks that contained 190 mL of adjusted red pitaya juice. The PFs and CF were statically conducted at 25 °C. The SFs were conducted at 17 °C, 21 °C, 25 °C, and 29 °C, respectively. The fermentations were conducted three times.
Analysis of physicochemical properties
Total soluble solid, pH, and total acidity of the red pitaya juices and total soluble solid, reducing sugar, titratable acidity, volatile acidity, and alcoholic content of the red pitaya wines were determined according to Zhu et al. (2014). Total soluble solid was assessed using a hand refractometer (WYT, HaoChuang, Chengdu, China). pH of red pitaya juices was determined by using a pH meter (SevenEasy S20, METTLER TOLEDO, Zurich, Switzer-land). Residual sugar and titratable acidity were determined by titration, and the results were expressed as glucose and tartaric acid, respectively. Volatile acidity was obtained by distillation combined with titration, and the results were expressed as acetic acid. Alcoholic content was obtained by distillation combined with a portable densimeter (DA-130 N, KEM, Tokyo, Japan). Each measurement was conducted in triplicates.
Volatile compound analysis
Headspace-solid phase microextraction (HS-SPME) combined with gas chromatography-mass spectrometry (GC–MS) was used to the analysis of volatile compounds according to the previous study (Lin et al. 2018) with the following modifications. 8 mL of sample and 3 g of NaCl were placed in a 15 mL vial sealed with silicone septa (Sigma Chemical Co., Saint Louis, MO, USA). A HP-INNOWAX capillary column (60 m × 0.25 mm inner diameter, 0.25 μm film thickness, Agilent Technologies, Santa Clara, CA) was used. The GC oven temperature program was set as follows: initial temperature of 40 °C (held for 5 min), then increased to 120 °C at 4 °C/min, and finally increased to 230 °C at 5 °C/min (held for 5 min). The internal standard (IS, 10 μL of 250 mg/L 2-octanol) was used to quantify the volatiles.
Sensory analysis
Sensory evaluation was performed according to the previous study (Lin et al. 2018) with the following modification. The fermented red pitaya wines were evaluated by a well-trained panel of 15 members (6 men and 9 women, ranging from 20 to 50 years old). 20 mL of wine sample was presented to the panelists at room temperature of 22 ± 1 °C. Seven important sensory terms including floral, fruity, sweet, sweaty, fatty, spicy, and global aroma were selected by the panelists during discussion to describe and differentiate the attributes of the samples, and a scale from 0 to 5 was used to score the intensity of each attribute, where 0 indicated that the attribute was not perceived, and the intensity of the perceived attribute gradually enhanced from the values 1 to 5.
Statistical analysis
One-way ANOVA and Tukey’s Honestly Significant Difference (HSD) tests were used to determine significant differences. Differences at p < 0.05 were considered statistically significant. Principal component analysis (PCA) was conducted using PLS_TOOLBOX version 5.02 under Matlab version 7.0.
Results and discussion
Effect of inoculation method on the biomass evolution of yeasts
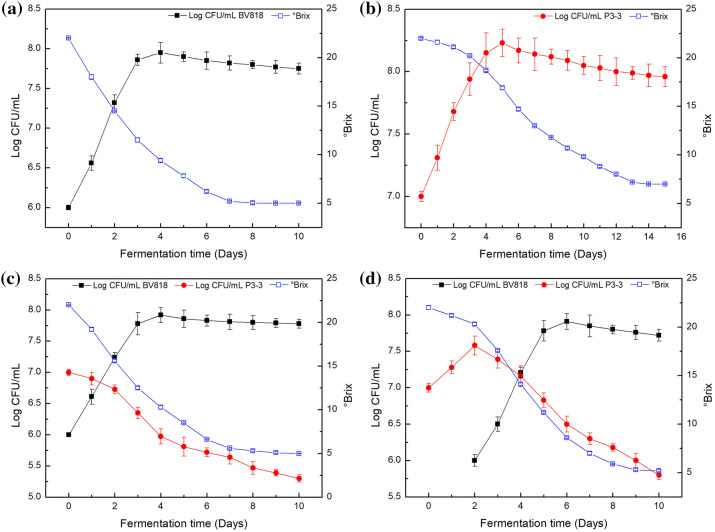
The growth kinetics of yeast strains in pure and mixed culture fermentations are shown in Fig. 1. In PFs, BV818 achieved the maximum viable population (7.95 Log CFU/mL) in four days, whereas P3-3 took five days to yield a maximum population of 8.23 Log CFU/mL (Fig. 1a and b). In the mixed inoculations, BV818 quickly adapted to the red pitaya juices and displayed similar growth patterns to that in PF (Fig. 1c, d). However, compared to that in PF, the maximum populations of BV818 decreased to 7.92 Log CFU/mL and 7.91 Log CFU/mL in CF and SF, respectively. This result may be ascribed to the nitrogen competition of the two strains. With regard to the growth of P3-3, its proliferation followed the same pattern as in PF in the initial two days in SF, but it was restricted and decreased to 5.80 Log CFU/mL when BV818 was inoculated. An even greater dropping trend of P3-3 growth was observed in CF, in which its population continuingly declined to 5.30 Log CFU/mL. The growth of P3-3 was significantly affected by the proliferation of BV818. This condition could be caused by the formation of some killer toxins to P3-3 (Albergaria et al. 2010; Ciani et al. 2010). These findings were consistent with those in grape and cherry wine fermentations, in which Metschnikowia species strains were used (Medina et al. 2012; Sadoudi et al. 2012; Sun et al. 2014).
Fig. 1.
Growth kinetics and °Brix changes during the red pitaya wine making. The red pitaya wines were produced by a BV818 pure-culture fermentation, b P3-3 pure-culture fermentation, c co-culture fermentation, and d sequential fermentation. The YEPD agar plate was used for the viable count of the yeast cells in the pure-culture fermentations (PFs). The WL nutrient agar medium (HB0300, Hopebio, Qingdao, China) was applied to the identification of wine yeast colonies. The lysine medium (HB8814, Hopebio, Qingdao, China) was used to differentiate non-Saccharomyces yeast cells cultured in the mixed culture fermentation. The fermentations were conducted until the residual sugar was lower than 4 g/L
Effect of inoculation method on the physicochemical properties of red pitaya wine
In BV818 PF, the alcoholic fermentation was completed in seven days, whereas it took a longer fermentation time (thirteen days) with a slower utilization rate of sugar in P3-3 PF (Fig. 1a, b). Simultaneously, the °Brix values reached stability at approximately 5% and 7% in BV818 and P3-3 PFs, respectively. Compared to P3-3 PF, the mixed inoculations with BV818 shortened the fermentation time to seven and eight days in CF and SF, respectively (Fig. 1c, d).
For the general characteristics of the red pitaya wines yielded by different inoculations (Table 1 columns 2–5), the single inoculation of BV818 bringing about the highest contents of titratable acidity and ethanol, whereas the single inoculation of P3-3 led to the opposite results. The red pitaya wines produced by CF and SF displayed medium levels of the chemical parameters. Non-Saccharomyces yeasts were commonly considered negative starters due to their high production of undesirable metabolites, such as acetic acid (Comitini et al. 2011; Sun et al. 2016). The non-Saccharomyces yeast strain P3-3 used in this study yielded a low concentration of volatile acidity, suggesting a promising oenological feature of the strain. These results were consistent with those in Comitini et al. (2011) and Sun et al. (2014).
Table 1.
General physicochemical properties of the red pitaya wines resulting from different inoculations (columns 2–5) and fermentation temperatures (columns 5–8)
| BV818 | P3-3 | CF | SF (25 °C) | SF (17 °C) | SF (21 °C) | SF (29 °C) | |
|---|---|---|---|---|---|---|---|
| Reducing sugar (g/L) | 1.72 ± 0.06D | 2.74 ± 0.08A | 2.03 ± 0.04C | 2.17 ± 0.06C | 2.68 ± 0.11A | 2.43 ± 0.10B | 2.19 ± 0.04C |
| Titratable acidity (g/L) | 7.08 ± 0.12A | 5.75 ± 0.08C | 6.43 ± 0.11B | 6.53 ± 0.12B | 6.58 ± 0.05B | 6.51 ± 0.04B | 6.37 ± 0.08B |
| Volatile acidity (g/L) | 0.68 ± 0.05A | 0.56 ± 0.03A | 0.60 ± 0.02A | 0.61 ± 0.06A | 0.63 ± 0.04A | 0.62 ± 0.04A | 0.65 ± 0.06A |
| Ethanol (% vol) | 10.8 ± 0.03A | 8.9 ± 0.08D | 10.5 ± 0.04B | 10.4 ± 0.05BC | 10.3 ± 0.02C | 10.4 ± 0.04BC | 10.4 ± 0.06BC |
Values shown represent the averages of triplicate samples (data are mean ± SD)
Values with different superscript capital letters in the same row are significantly different (p < 0.05)
BV818: wine samples inoculated with S. bayanus BV818 alone at 25 °C
P3-3: wine samples inoculated with M. agaves P3-3 alone at 25 °C
CF: wine samples inoculated with BV818 and P3-3 simultaneously at 25 °C
SF (25 °C), SF (17 °C), SF (21 °C) and SF (29 °C): wine samples inoculated with P3-3 prior to BV818 48 h at 17 °C, 21 °C, 25 °C and 29 °C, respectively
Effect of inoculation method on the volatile profiles of red pitaya wine
The application of the SPME/GCMS method allowed for the identification and quantification of 35 volatile compounds (Table 2). This finding differed from that reported by Gong et al. (2017a, b), who identified 55 and 16 volatiles in red pitaya wines. This difference could be attributed to the different wine samples and analysis methods.
Table 2.
Mean concentration (μg/L) of volatile compounds detected in the red pitaya wines resulting from different inoculations (columns 7–10) and fermentation temperatures (columns 10–13)
| Code | Compounds | RI# | RI (literature)## | Identified### | Odour description* | BV818 | P3-3 | CF | SF (25 °C) | SF (17 °C) | SF (21 °C) | SF (29 °C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||||||
| A1 | Propan-1-ol | 1031 | 1024a | MS, RI, S | Alcohol, pungent | 148 ± 5C | 98 ± 4D | 192 ± 7A | 179 ± 3AB | 156 ± 10C | 162 ± 11BC | 147 ± 6C |
| A2 | 2-Methyl-1-propanol | 1093 | 1099a | MS, RI, S | Wine, solvent, bitter | 666 ± 7D | 295 ± 7F | 571 ± 6E | 792 ± 8B | 802 ± 7B | 725 ± 11C | 857 ± 9A |
| A3 | Butan-1-ol | 1148 | 1153b | MS, RI, S | Medicine, fruit | 20 ± 1D | 43 ± 2A | 39 ± 2AB | 30 ± 1C | 19 ± 2D | 23 ± 2D | 38 ± 1B |
| A4 | 3-Methyl-1-butanol | 1236 | 1220b | MS, RI, S | Whiskey, malt, burnt | 4498 ± 31F | 2043 ± 11G | 5698 ± 51E | 6381 ± 18B | 5865 ± 20D | 6533 ± 49A | 6014 ± 52C |
| A5 | Hexan-1-ol | 1350 | 1354a | MS, RI, S | Resin, flower, green | 40 ± 2F | 92 ± 4A | 54 ± 3DE | 58 ± 1CD | 47 ± 2EF | 65 ± 3C | 76 ± 2B |
| A6 | 6-Methyl-2-heptanol | 1380 | - | MS, S | 15 ± 1C | - | 10 ± 1D | 22 ± 1AB | 15 ± 2C | 25 ± 1A | 20 ± 1B | |
| A7 | 2-Nonanol | 1510 | - | MS, S | Cucumber | 34 ± 1B | 18 ± 1C | 33 ± 3B | 52 ± 1A | 33 ± 2B | 53 ± 2A | 37 ± 2B |
| A8 | 2,3-Butanediol | 1540 | 1553c | MS, RI, S | Butter, cream | 55 ± 2CD | 63 ± 2AB | 61 ± 2BC | 50 ± 2D | 69 ± 2A | 58 ± 3BC | 37 ± 2E |
| A9 | Octan-1-ol | 1560 | 1556a | MS, RI, S | Chemical, metal, burnt | 43 ± 1C | 51 ± 2B | 42 ± 2C | 44 ± 3C | 62 ± 3A | 40 ± 1C | 53 ± 1B |
| A10 | 3,7-Dimethyl-(R)-6-octen-1-ol | 1694 | 1717d | MS, RI, S | Sweet, Rose | 70 ± 3CD | 24 ± 1E | 67 ± 3D | 78 ± 1AB | 84 ± 4A | 74 ± 2BC | 68 ± 2CD |
| A11 | Phenethyl alcohol | 1924 | 1923a | MS, RI, S | Honey, spice, rose, lilac | 3303 ± 40E | 2022 ± 26F | 4470 ± 38D | 5893 ± 27A | 5612 ± 22B | 5205 ± 31C | 5288 ± 41C |
| Esters | ||||||||||||
| E1 | Ethyl acetate | 868 | - | MS, S | Pineapple | 6736 ± 45D | 3174 ± 22E | 6723 ± 19D | 7283 ± 21C | 7480 ± 22B | 8643 ± 31A | 7551 ± 18B |
| E2 | Isobutyl acetate | 1014 | 1020c | MS, RI, S | Fruit, apple, banana | 31 ± 2E | 76 ± 3A | 66 ± 3B | 45 ± 1D | 61 ± 4BC | 57 ± 2C | 39 ± 1D |
| E3 | Isoamyl acetate | 1128 | 1131b | MS, RI, S | Banana | 53 ± 2CD | 48 ± 2DE | 56 ± 1C | 68 ± 2B | 43 ± 1E | 87 ± 4A | 31 ± 1F |
| E4 | Ethyl hexanoate | 1231 | 1239a | MS, RI, S | Apple peel, fruit | 20 ± 1E | 55 ± 1B | 64 ± 1A | 44 ± 2C | 63 ± 1A | 58 ± 2B | 28 ± 2D |
| E5 | Ethyl lactate | 1364 | 1364e | MS, RI, S | Fruit | 1210 ± 10C | 358 ± 6E | 1233 ± 7C | 1278 ± 11B | 1345 ± 8A | 1320 ± 14A | 1178 ± 8D |
| E6 | Ethyl octanoate | 1441 | 1437a | MS, RI, S | Fruit, fat | 69 ± 2E | 162 ± 7A | 156 ± 10A | 123 ± 4C | 158 ± 5A | 141 ± 2B | 92 ± 2D |
| E7 | Isoamyl lactate | 1606 | - | MS, S | 69 ± 2AB | - | 56 ± 3C | 60 ± 3C | 62 ± 4BC | 75 ± 3A | 58 ± 1C | |
| E8 | Ethyl decanoate | 1633 | 1634a | MS, RI, S | Grape | 120 ± 4E | 46 ± 3F | 191 ± 7C | 206 ± 3B | 240 ± 3A | 232 ± 2A | 171 ± 4D |
| E9 | Diethyl succinate | 1677 | 1688b | MS, RI, S | Wine, fruit | 35 ± 2D | 23 ± 1E | 42 ± 1C | 55 ± 2B | 71 ± 2A | 52 ± 1B | 67 ± 1A |
| E10 | Ethyl phenylacetate | 1774 | 1771f | MS, RI, S | Fruit, sweet | 429 ± 9C | 12 ± 0E | 411 ± 7D | 478 ± 6B | 483 ± 7B | 511 ± 5A | 446 ± 5C |
| E11 | Phenethyl acetate | 1790 | 1808f | MS, RI, S | Rose, honey, tobacco | 201 ± 3G | 714 ± 9A | 415 ± 6B | 340 ± 4D | 287 ± 4E | 367 ± 5C | 245 ± 2F |
| E12 | Ethyl myristate | 2042 | 2020 g | MS, RI, S | Ether | 149 ± 4BC | - | 138 ± 2C | 143 ± 2C | 178 ± 6A | 159 ± 4B | 124 ± 5D |
| E13 | Ethyl hexadecanoate | 2250 | 2257 h | MS, RI, S | Wax | 68 ± 4B | 11 ± 1C | 74 ± 4B | 88 ± 3A | 93 ± 5A | 96 ± 2A | 70 ± 1B |
| Acids | ||||||||||||
| AC1 | Acetic acid | 1450 | 1446 h | MS, RI, S | Sour | 4595 ± 38C | 1197 ± 16G | 4702 ± 42B | 4397 ± 21D | 3306 ± 31F | 5022 ± 19A | 3656 ± 22E |
| AC2 | Isobutyric acid | 1585 | 1597e | MS, RI, S | Rancid, butter, cheese | 27 ± 2E | 69 ± 3A | 55 ± 1B | 48 ± 2C | 50 ± 2BC | 52 ± 3BC | 36 ± 1D |
| AC3 | Hexanoic acid | 1853 | 1851a | MS, RI, S | Sweat | 301 ± 7BC | 216 ± 11E | 286 ± 16BCD | 268 ± 3D | 311 ± 10AB | 285 ± 4CD | 332 ± 7A |
| AC4 | 2-Ethylhexanoic acid | 1956 | - | MS, S | 887 ± 15D | 730 ± 12E | 911 ± 17D | 975 ± 8C | 1034 ± 12B | 906 ± 4D | 1086 ± 11A | |
| AC5 | Heptanoic acid | 1975 | 1970b | MS, RI, S | - | 21 ± 1A | 18 ± 2A | 14 ± 2B | 10 ± 1C | 19 ± 1A | 12 ± 1BC | |
| AC6 | Octanoic acid | 2058 | 2065a | MS, RI, S | Sweat, cheese | 878 ± 16D | 625 ± 21F | 778 ± 14E | 956 ± 6C | 945 ± 8C | 1044 ± 13B | 1177 ± 12A |
| AC7 | Nonanoic acid | 2179 | 2192i | MS, RI, S | Green, fat | - | 17 ± 2BC | 20 ± 2B | 27 ± 3A | 15 ± 2BC | 31 ± 2A | 13 ± 1C |
| AC8 | Decanoic acid | 2288 | 2300i | MS, RI, S | Rancid, fat | 475 ± 11C | 145 ± 9E | 410 ± 5D | 520 ± 7B | 408 ± 8D | 580 ± 8A | 426 ± 5D |
| Aldehydes and ketones | ||||||||||||
| AK1 | Benzaldehyde | 1530 | 1529 h | MS, RI, S | Almond, burnt sugar | - | 14 ± 1B | 21 ± 2A | 14 ± 1B | 21 ± 1A | 19 ± 2A | 10 ± 1C |
| AK2 | Acetoin | 1274 | 1287j | MS, RI, S | Butter, cream | 58 ± 3C | 33 ± 2E | 69 ± 2AB | 65 ± 2B | 72 ± 3A | 68 ± 1AB | 45 ± 2D |
| Phenols | ||||||||||||
| P1 | 4-Ethyl-2-methoxyphenol | 2025 | - | MS, S | Spice, clove | 55 ± 2E | 210 ± 5A | 106 ± 2B | 87 ± 3C | 72 ± 2D | 75 ± 2D | 110 ± 3B |
The red pitaya wines kept at 18 °C for 40 days were sampled for the analysis of volatile compounds. Values shown represent the averages of triplicate samples (data are mean ± SD). Values with different superscript capital letters in the same row are significantly different (p < 0.05)
-: not detected/founded
#The retention indices of compounds were determined on the INNOWAX column, relative to C6-30 n-alkane standards
##RIs reported in literatures. (a) Xiao et al. 2014a; (b) Niu et al. 2011; (c) Xiao et al. 2011; (d) Peña et al. 2005; (e) Weldegergis et al. 2007; (f) Isogai et al. 2005; (g) Erdemoglu et al. 2003; (h) Xiao et al. 2014b; (i) Tabanca et al. 2001; (j)Viegas and Bassoli 2007
###Identified volatile compounds by mass spectra (MS), retention indice (RI) and standard compounds (S)
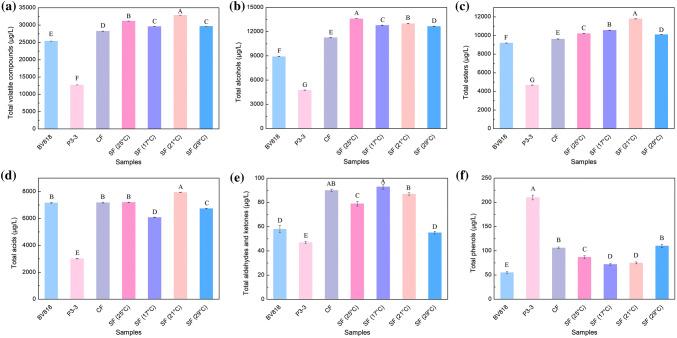
The effects of inoculation method on the concentration and type of volatile compounds in the red pitaya wines are shown in Table 2 (columns 7–10) and Fig. 2. The wines yielded by PFs displayed relatively low total contents of the volatile compounds, and P3-3 PF generated the lowest value. The CF and SF evidently increased the total concentrations of the volatiles, and the largest content was achieved by SF. These findings suggested that certain synergistic interactions might occur in the two yeast strains. Regarding the relative content of the volatiles, esters were the main components in the BV818 PF wine sample, but the main constituents in P3-3 PF, CF, and SF were alcohols. Compared to that in BV818 PF, the proportion of acids decreased in the other three methods. The wine produced by P3-3 PF displayed the highest relative contents of aldehydes, ketones, and phenols. In summary, modifying the inoculation method affected the global volatile profiles of the red pitaya wine and might consequently influenced the wine style and quality like those for papaya wine (Lee et al. 2010) and dry white wine (Puertas et al. 2018).
Fig. 2.
Concentrations of total volatiles, alcohols, esters, acids, aldehydes and ketones, and phenols produced by different inoculations. BV818: wine samples inoculated with S. bayanus BV818 alone at 25 °C. P3-3: wine samples inoculated with M. agaves P3-3 alone at 25 °C. CF: wine samples inoculated with BV818 and P3-3 simultaneously at 25 °C. SF (25 °C), SF (17 °C), SF (21 °C) and SF (29 °C): wine samples inoculated with P3-3 prior to BV818 48 h at 17 °C, 21 °C, 25 °C and 29 °C, respectively. Data are the averages of three independent experiments and error bars represent ± SD. Values with different capital letters in the columns are significantly different (p < 0.05)
In the present study, pure and mixed inoculations produced the alcohols at concentrations below 300 mg/L, which can actively influence the wine flavor (Duarte et al. 2010). Compared to those produced by PFs, the CF considerably increased the concentrations of propan-1-ol, 3-methyl-1-butanol, and phenethyl alcohol. More diverse types of alcohols yielded by SF exhibited higher contents than those produced by PFs. Propan-1-ol, 3-methyl-1-butanol, and phenethyl alcohol were the main higher alcohols in many kinds of wines, such as Chinese Baijiu (Li et al. 2017), red wine (Ma et al. 2017), and beer (Ocvirk et al. 2018). 3-Methyl-1-butanol is formed through deamination and decarboxylation reactions from leucine (Molina et al. 2009). 3-Methyl-1-butanol negatively contributes to wine quality when the content is higher than its odor threshold. On the contrary, a high level of phenethyl alcohol, which is synthesized through the transamination of l-phenylalanine, contributes a pleasant odor of the wine (Swiegers et al. 2005). These findings were in agreement with that reported in Sun et al. (2014), which found that mixed fermentation with Metschnikowia species could enhance the production of the three main higher alcohol compounds in cherry wine.
Among the identified esters, acetate esters were the most representative aroma family in fruit wines (Dzialo et al. 2017). Acetate esters are considered more effective to the perceived aroma than the ethyl esters derived from medium-chain fatty acids (Dzialo et al. 2017). The concentrations of more than half of the esters yielded by CF were higher than those generated by BV818 PF. Similar behavior but with more types of esters were increased by SF. Compared to BV818 PF, the SF remarkably enhanced the contents of most detected esters except isoamyl lactate and ethyl myristate. The increase in ester production could be attributed to the enhanced contents of precursor alcohols or organic acids. These results were consistent with those in Sadoudi et al. (2012), which found that the sequential inoculation with M. pulcherrima produced higher levels of most esters than the inoculation of S. cerevisiae alone.
Acids actively contributed to the flavor of wine at levels below their odor thresholds. Nevertheless, unpleasant sweaty, sour, and thin odors could be aggravated at a high content of acids (Hernanz et al. 2009). A total of two medium-chain fatty acids (heptanoic acid and nonanoic acid), which were absent in the wine fermented by BV818 alone, appeared in the condition of the mixed inoculations with P3-3. The CF over PFs elevated the concentrations of acetic acid and 2-ethylhexanoic acid, but the concentrations of another two medium-chain fatty acids, octanoic acid and decanoic acid, were increased by SF besides 2-ethylhexanoic acid.
A small amount of benzaldehyde was observed in P3-3 PF, CF, and SF. Compared to that of BV818 PF, the concentration of acetoin increased by 19% and 12% in CF and SF, respectively. Only one phenol was identified in this study. The concentration of 4-ethyl-2-methoxyphenol in CF and SF was 93% and 58% higher than that in BV818 PF, respectively. Strains of wine yeast, wild yeast (Saccharomyces cerevisiae), and non-Saccharomyces yeast exhibited distinguished phenolic off-flavors productivity in the winemaking environment (Shinohara et al. 2000). The enhancement of 4-ethyl-2-methoxyphenol content in mixed fermentation could be due to the strain P3-3 that natively possesses a relatively strong ability of accumulating this compound (Table 2).
PCA was performed on the 35 volatile compounds shown in Table 2. The loadings of the volatiles and the distribution of the wine samples are shown in Fig. 3. Mixed inoculations brought about differences in the composition of volatile compounds from pure inoculations. The SF was situated in the first quadrant and was related more to four groups of compounds, including six alcohols (A2 2-methyl-1-propanol, A4 3-methyl-1-butanol, A6 6-methyl-2-heptanol, A7 2-nonanol, A10 3,7-dimethyl-(R)-6-octen-1-ol, and A11 phenethyl alcohol), six esters (E1 ethyl acetate, E5 ethyl lactate, E8 ethyl decanoate, E9 diethyl succinate, E10 ethyl phenylacetate, and E13 ethyl hexadecanoate), two acids (AC4 2-ethylhexanoic acid and AC8 decanoic acid), and one ketones (AK2 acetoin). The characteristic aroma of CF, mainly related to A1 propan-1-ol, A8 2,3-butanediol, E4 ethyl hexanoate, and AK1 benzaldehyde, was relatively close to that of SF. The wine samples produced by P3-3 and BV818 PFs were situated in the second and fourth quadrants, respectively, and far from those volatile compounds. These results demonstrated that mixed inoculation of P3-3 and BV818 enhanced the concentrations of volatile compounds and improved the aroma characteristic of red pitaya wine.
Fig. 3.
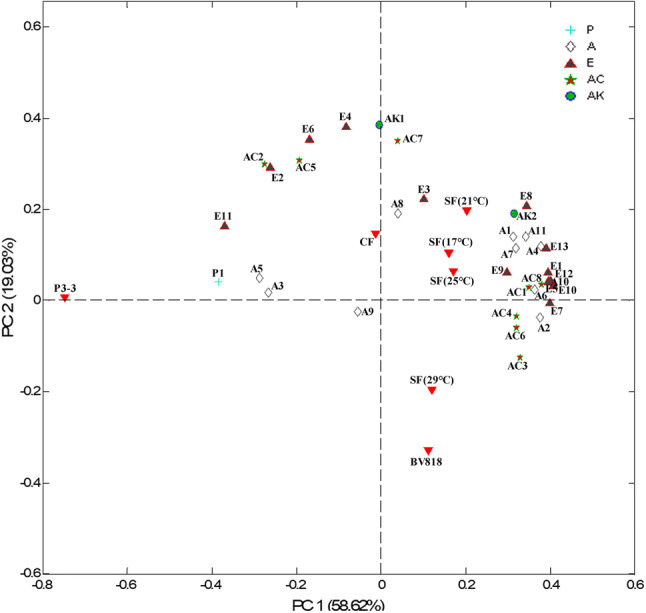
Loadings of the volatile compounds and the distribution of the wine samples in the first two PCs. BV818: wine samples inoculated with S. bayanus BV818 alone at 25 °C. P3-3: wine samples inoculated with M. agaves P3-3 alone at 25 °C. CF: wine samples inoculated with BV818 and P3-3 simultaneously at 25 °C. SF: wine samples inoculated with P3-3 prior to BV818 48 h at the corresponding fermentation temperatures (17 °C, 21 °C, 25 °C and 29 °C). P: Phenols, A: Alcohols, E: Esters, AC: Acids, AK: Aldehydes and ketones
The odor activity values (OAVs) were used to further investigate the contribution of each compound to the flavor of the red pitaya wines (Table 3). The OAVs of ten types of volatile compounds were above 1 in this test. 3,7-Dimethyl-(R)-6-octen-1-ol was the only alcohol that exhibited high concentrations and surpassed its odor threshold. Therefore, an intensely sweet and rose odor could be captured in the wines yielded by BV818 PF, CF, and SF. Isoamyl acetate, ethyl hexanoate, and ethyl octanoate presented OAVs > 1 in the four red pitaya wine samples, thus contributing fruity and fatty odors to wines. An OAV ≥ 1 of ethyl acetate was only found in SF, thereby contributing an intense pineapple odor to the wine yielded by sequential inoculation. The OAVs of ethyl decanoate and phenethyl acetate produced by CF and SF were ≥ 1, thus bringing out intense grape, rose, honey and tobacco odors to the wines produced by mixed inoculations. Although the concentrations of octanoic acid (a sweat and cheese odor), decanoic acid (a rancid and fat odor), and 4-ethyl-2-methoxyphenol (a spice and clove odor) differed in four wines, all of their OAVs were ≥ 1. However, high concentrations of acids and phenols could negatively affect the wine flavor.
Table 3.
OAVs (≥ 1) for the main volatile compounds in the red pitaya wines resulting from different inoculations (columns 5–8) and fermentation temperatures (columns 8–11)
| Code | Compounds | Odour description* | Odour threshold (μg/L)a | BV818 | P3-3 | CF | SF (25 °C) | SF (17 °C) | SF (21 °C) | SF (29 °C) |
|---|---|---|---|---|---|---|---|---|---|---|
| A10 | 3,7-Dimethyl-(R)-6-octen-1-ol | Sweet, Rose | 40 | 1.7 | 0.6 | 1.7 | 2.0 | 2.1 | 1.9 | 1.7 |
| E1 | Ethyl acetate | Pineapple | 7500 | 0.9 | 0.4 | 0.9 | 1.0 | 1.0 | 1.2 | 1.0 |
| E3 | Isoamyl acetate | Banana | 30 | 1.8 | 1.6 | 1.9 | 2.3 | 1.4 | 2.9 | 1.0 |
| E4 | Ethyl hexanoate | Apple peel, fruit | 14 | 1.4 | 3.9 | 4.6 | 3.1 | 4.5 | 4.1 | 2.0 |
| E6 | Ethyl octanoate | Fruit, fat | 20 | 3.5 | 8.1 | 7.8 | 6.2 | 7.9 | 7.1 | 4.6 |
| E8 | Ethyl decanoate | Grape | 200 | 0.6 | 0.2 | 1.0 | 1.0 | 1.2 | 1.2 | 0.9 |
| E11 | Phenethyl acetate | Rose, honey, tobacco | 250 | 0.8 | 2.9 | 1.7 | 1.4 | 1.1 | 1.5 | 1.0 |
| AC6 | Octanoic acid | Sweat, cheese | 500 | 1.8 | 1.3 | 1.6 | 1.9 | 1.9 | 2.1 | 2.4 |
| AC8 | Decanoic acid | Rancid, fat | 150 | 3.2 | 1.0 | 2.7 | 3.5 | 2.7 | 3.9 | 2.8 |
| P1 | 4-Ethyl-2-methoxyphenol | Spice, clove | 11 | 5.0 | 19.1 | 9.6 | 7.9 | 6.5 | 6.8 | 10.0 |
*https://www.flavornet.org/flavornet.html
aOAVs were calculated by the ratio of the concentration of a compound to its odor threshold value
Effect of inoculation method on the sensory properties of red pitaya wine
As shown in Figure S1A, the wines produced by BV818 and P3-3 PFs showed differences in the senses except for the “floral” and “fruity” features. These results indicated that the wines fermented with different genera of the yeasts could result in different sensory properties, depending on the function of yeast strains during fermentation. Compared to PFs, CF and SF reinforced the floral, fruity, and sweet aromas of the red pitaya wine. This finding could be resulted from the large quantities of 3,7-dimethyl-(R)-6-octen-1-ol, ethyl acetate, isoamyl acetate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, and phenethyl acetate produced in CF and SF. The wine produced by SF showed the highest score in “sweaty” rating due to its large content of octanoic acid. The mixed inoculations with P3-3 over BV818 PF increased the “fatty” and “spicy” ratings without influencing the global aroma of the wines yielded by CF and SF. These results suggested that the mixed inoculation with non-Saccharomyces strains could enhance the sensory complexity and aromatic intensity of the red pitaya wine. This result was consistent with PCA, suggesting that the flavor of the red pitaya wines produced by CF and SF are relatively similar in this test.
On the basis of the above results, compared to BV818 and P3-3 PFs, the moderate levels of physicochemical properties were found in the red pitaya wines yielded by CF and SF. Although the growth of P3-3 was inhibited by BV818 in mixed inoculations, synergistic interactions might occur between the two strains. Thus, high concentrations of volatile components were accumulated by CF and SF. Although SF and CF presented a very similar profile, SF scored the highest in three positive perceptions (floral, fruity, and sweet) and global aroma (Figure S1A). Furthermore, SF produced the highest total content of volatiles. Therefore, the strong flavor and excellent fusion of notes in the red pitaya wine produced by SF were able to yield the best sensory profile (pure, elegant, and harmonious fruit and wine aroma, coupled with full-bodied, mellow, and refreshing taste). Moreover, as far as technology is concerned, a slightly longer initial fermentation stage plays an important role in the wine quality, such as the presentation of color and the formation of aroma (Sun et al. 2016). Therefore, the sequential inoculation was used in the following trials, which focused on the effects of fermentation temperature on the volatile and sensory profiles of the red pitaya wine.
Effect of fermentation temperature on the physicochemical properties of red pitaya wine
Table 1 (columns 5–8) shows the general physicochemical properties of the red pitaya wines via SF at different temperatures. No evident changes in titratable acidity, volatile acidity, and ethanol were found at the four temperatures. This result differed from that on apple wine, which was produced by single inoculation of S. cerevisiae (Peng et al. 2015). This discrepancy highlights that the importance of raw material and non-Saccharomyces yeast might play a role in alcoholic fermentation along with Saccharomyces strains at various temperatures.
Effect of fermentation temperature on the volatile profiles of red pitaya wine
The volatile profiles of the red pitaya wines via SF at different temperatures are presented in Table 2 (columns 10–13). The highest total amount of volatiles was found at 21 °C, and the lowest amount was produced at 17 °C. A relatively cold condition generally renders a greater preservation of volatiles (Beltran et al. 2008). However, low temperature can also weaken the growth and the metabolism of yeasts and decrease the production of volatile compounds, such as higher alcohol concentration (Beltran et al. 2008). On the contrary, high temperature accelerates the yeast metabolism, yet physical loss of volatiles in wines can be aggravated (Yilmaztekin et al. 2013). The proportion of the five groups of volatiles varied at different temperatures. The wine fermented at 25 °C exhibited the highest proportion of alcohols, whereas the largest proportions of esters and acids were produced at 21 °C. The concentrations of benzaldehyde and acetoin decreased with increasing temperature. A similar trend was observed for the changes in the main volatiles (OAVs ≥ 1) ethyl hexanoate, ethyl octanoate, and ethyl decanoate. Conversely, the concentration of the main contributor 4-ethyl-2-methoxyphenol kept on rising from 17 to 29 °C. The concentrations of the main volatiles isoamyl acetate, phenethyl acetate, and decanoic acid dramatically increased when the temperature rose from 17 to 21 °C and gradually decreased thereafter between 21 and 29 °C.
According to the PCA result, the SFs at 17 °C, 21 °C, and 25 °C produced similarities in the characteristic aroma of the red pitaya wine. However, the wine sample fermented at 29 °C, which was mainly related to A2 (2-methyl-1-propanol), AC3 (hexanoic acid), AC4 (2-ethylhexanoic acid), and AC6 (octanoic acid), was located at the different quadrants. These results revealed that changing the fermentation temperature could modulate the aroma character and flavor profile of the red pitaya wine.
Effect of fermentation temperature on the sensory properties of red pitaya wine
As shown in Figure S1B, the wine fermented at 21 °C was characterized by intense notes of floral, fruity, sweet, and fatty, moderate sweatiness, and relatively slight spiciness. The highest rating of fatty might negatively influence the global aroma of this wine. Compared to those produced at 21 °C, wine samples yielded at 25 °C were more intense in the spicy sense but rated less in attributes including “fruity”, “sweet”, “sweaty”, and “fatty”. The high score of global aroma (pure, elegant, and harmonious fruit and wine aroma) produced at 25 °C (a slightly higher than that produced at 21 °C, p > 0.05) was benefited from the optimal combination of various perceptions. The moderate intensity of the notes was produced at 17 °C. The wine made at 29 °C scored the highest level of sweaty and spicy senses and was rated the lowest of the other features. The low levels of active odors and high levels of undesirable odors resulted in the lowest score of global aroma at 29 °C. These findings were consistent with those of volatile profiles, suggesting that the flavor properties of wines produced at 21 °C and 25 °C were relatively similar in this work. Therefore, the fermentation temperatures ranging from 21 to 25 °C appear suitable for making the desirable red pitaya wine.
Conclusions
Results showed a positive effect on the production of the red pitaya wine by mixed inoculations of S. bayanus BV818 and M. agaves P3-3. Mixed inoculations influenced the yeast-yeast interactions and consequently the final wine quality, especially the enhancement of the aroma intensity, complexity, and global sensory quality. Fermentation temperature modulated the volatile production of the red pitaya wine. An appropriate temperature was advantageous to the aroma profile and sensory quality of the red pitaya wine. To our knowledge, this study is the first to use the M. agaves strain in mixed inoculation fermentation of fruit wine. Although different ratios of yeasts are needed to fully investigate the effects of mixed inoculation on the wine flavor, this study can provide knowledge on the aroma properties of the red pitaya wine and establish foundations for its production with the desired flavor profile.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by the Natural Science Foundation of Hainan province (grant number 317056), the Scientific Research Foundation of Hainan University (grant number KYQD1660) and the Education Department of Hainan Province (grant number Hnky2019-7).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xue Lin, Email: linxiaoxuelx@163.com.
Xiaoping Hu, Email: xiaopinghuxph@163.com.
Qingke Wang, Email: 81508624@qq.com.
Congfa Li, Email: licongfalcf@163.com.
References
- Albergaria H, Francisco D, Gori K, Arneborg N, Gírio F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl Microbiol Biotechnol. 2010;86:965–972. doi: 10.1007/s00253-009-2409-6. [DOI] [PubMed] [Google Scholar]
- Beltran G, Novo M, Guillamón JM, Mas A, Rozès N. Effect of fermentation temperature and culture media on the yeast lipid composition and wine volatile compounds. Int J Food Microbiol. 2008;121:169–177. doi: 10.1016/j.ijfoodmicro.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Ciani M, Comitini F, Mannazzu I, Domizio P. Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010;10:123–133. doi: 10.1111/j.1567-1364.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Duarte WF, Dias DR, Oliveira JM, Vilanova M, Teixeira JA, Almeida e Silva JB, Schwan RF. Raspberry (Rubus idaeus L.) wine: yeast selection, sensory evaluation and instrumental analysis of volatile and other compounds. Food Res Int. 2010;43:2303–2314. doi: 10.1016/j.foodres.2010.08.003. [DOI] [Google Scholar]
- Dzialo MC, Park R, Steensels J, Lievens B, Verstrepen KJ. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol Rev. 2017;41:S95–S128. doi: 10.1093/femsre/fux031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englezos V, Rantsiou K, Cravero F, Torchio F, Pollon M, Fracassetti D, Ortiz-Julien A, Gerbi V, Rolle L, Cocolin L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018;257:350–360. doi: 10.1016/j.foodchem.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Erdemoglu N, Sener B, Demirci B, Baser KHC. The glycosidically bound volatile compounds of Taxus baccata. Chem Nat Compd. 2003;39:195–198. doi: 10.1023/A:1024870031960. [DOI] [Google Scholar]
- Gong X, Ma LN, Li LJ, Yuan Y, Peng SD, Lin M. Analysis of aroma compounds of pitaya fruit wine. IOP Conf Ser Earth Environ Sci. 2017 doi: 10.1088/1755-1315/100/1/012125. [DOI] [Google Scholar]
- Gong X, Yang Y, Ma L, Peng S, Lin M. Fermentation and characterization of pitaya wine. IOP Conf Ser Earth Environ Sci. 2017 doi: 10.1088/1755-1315/100/1/012029. [DOI] [Google Scholar]
- Hernanz D, Gallo V, Recamales ÁF, Meléndez-Martínez AJ, González-Miret ML, Heredia FJ. Effect of storage on the phenolic content, volatile composition and colour of white wines from the varieties Zalema and Colombard. Food Chem. 2009;113:530–537. doi: 10.1016/j.foodchem.2008.07.096. [DOI] [Google Scholar]
- Isogai A, Utsunomiya H, Kanda R, Iwata H. Changes in the aroma compounds of sake during aging. J Agr Food Chem. 2005;53:4118–4123. doi: 10.1021/jf047933p. [DOI] [PubMed] [Google Scholar]
- Lee PR, Ong YL, Yu B, Curran P, Liu SQ. Profile of volatile compounds during papaya juice fermentation by a mixed culture of Saccharomyces cerevisiae and Williopsis saturnus. Food Microbiol. 2010;27:853–861. doi: 10.1016/j.fm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Lee PR, Chong ISM, Yu B, Curran P, Liu SQ. Effects of sequentially inoculated Williopsis saturnus and Saccharomyces cerevisiae on volatile profiles of papaya wine. Food Res Int. 2012;45:177–183. doi: 10.1016/j.foodres.2011.10.011. [DOI] [Google Scholar]
- Li W, Wang JH, Zhang CY, Ma HX, Xiao DG. Regulation of Saccharomyces cerevisiae genetic engineering on the production of acetate esters and higher alcohols during Chinese Baijiu fermentation. J Ind Microbiol Biotechnol. 2017;44:949–960. doi: 10.1007/s10295-017-1907-2. [DOI] [PubMed] [Google Scholar]
- Lin X, Wang Q, Hu X, Wu W, Zhang Y, Liu S, Li C. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds in pineapple wine. J Food Sci Technol. 2018;55:4119–4130. doi: 10.1007/s13197-018-3338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Huang S, Du L, Tang P, Xiao D. Reduced production of higher alcohols by Saccharomyces cerevisiae in red wine fermentation by simultaneously overexpressing BAT1 and deleting BAT2. J Agric Food Chem. 2017;65:6936–6942. doi: 10.1021/acs.jafc.7b01974. [DOI] [PubMed] [Google Scholar]
- Malherbe S, Fromion V, Hilgert N, Sablayrolles JM. Modeling the effects of assimilable nitrogen and temperature on fermentation kinetics in enological conditions. Biotechnol Bioeng. 2004;86:261–272. doi: 10.1002/bit.20075. [DOI] [PubMed] [Google Scholar]
- Medina K, Boido E, Dellacassa E, Carrau F. Growth of non-Saccharomyces yeasts affects nutrient availability for Saccharomyces cerevisiae during wine fermentation. Int J Food Microbiol. 2012;157:245–250. doi: 10.1016/j.ijfoodmicro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Molina AM, Guadalupe V, Varela C, Swiegers JH, Pretorius IS, Agosin E. Differential synthesis of fermentative aroma compounds of two related commercial wine yeast strains. Food Chem. 2009;117:189–195. doi: 10.1016/j.foodchem.2009.03.116. [DOI] [Google Scholar]
- Niu Y, Zhang X, Xiao Z, Song S, Eric K, Jia C, Yu H, Zhu J. Characterization of odor-active compounds of various cherry wines by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2287–2293. doi: 10.1016/j.jchromb.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Ocvirk M, Mlinarič NK, Košir IJ. Comparison of sensory and chemical evaluation of lager beer aroma by gas chromatography and gas chromatography/mass spectrometry. J Sci Food Agric. 2018;98:3627–3635. doi: 10.1002/jsfa.8840. [DOI] [PubMed] [Google Scholar]
- Padilla B, Gil JV, Manzanares P. Past and future of non-Saccharomyces yeasts: from spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front Microbiol. 2016;7:411. doi: 10.3389/fmicb.2016.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña RM, Barciela J, Herrero C, García-Martín S. Optimization of solid-phase microextraction methods for GC-MS determination of terpenes in wine. J Sci Food Agr. 2005;85:1227–1234. doi: 10.1002/jsfa.2121. [DOI] [Google Scholar]
- Peng B, Li F, Cui L, Guo Y. Effects of fermentation temperature on key aroma compounds and sensory properties of apple wine. J Food Sci. 2015;80:S2937–2943. doi: 10.1111/1750-3841.13111. [DOI] [PubMed] [Google Scholar]
- Puertas B, Jimenez-Hierro MJ, Cantos-Villar E, Marrufo-Curtido A, Carbú M, Cuevas FJ, Moreno-Rojas JM, González-Rodríguez VE, Cantoral JM, Ruiz-Moreno MJ. The influence of yeast on chemical composition and sensory properties of dry white wines. Food Chem. 2018;253:227–235. doi: 10.1016/j.foodchem.2018.01.039. [DOI] [PubMed] [Google Scholar]
- Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyer D, Gallardo-Chacón JJ, Ballester J, Vichi S, Guérin-Schneider R, Caixach J, Alexandre H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012;32:243–253. doi: 10.1016/j.fm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Sandate-Flores L, Rostro-Alanis MJ, Mancera-Andrade EI, Esquivel-Hernandez DA, Brambila-Paz C, Parra-Saldívar R, Welti-Chanes J, Escobedo-Avellaneda Z, Rodríguez-Rodríguez J. Using high hydrostatic pressures to retain the antioxidant compounds and to reduce the enzymatic activity of a pitaya-pineapple (Stenocereus sp.-Fragaria ananassa) beverage. J Food Sci Technol. 2017;54:611–619. doi: 10.1007/s13197-016-2482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Kubodera S, Yanagida F. Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation. J Biosci Bioeng. 2000;90:90–97. doi: 10.1016/S1389-1723(00)80040-7. [DOI] [PubMed] [Google Scholar]
- Sun SY, Gong HS, Jiang XM, Zhao YP. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2014;44:15–23. doi: 10.1016/j.fm.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Sun SY, Gong HS, Zhao YP, Liu WL, Jin CW. Sequential culture with Torulaspora delbrueckii and Saccharomyces cerevisiae and management of fermentation temperature to improve cherry wine quality. J Sci Food Agric. 2016;96:1880–1887. doi: 10.1002/jsfa.7293. [DOI] [PubMed] [Google Scholar]
- Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius IS. Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine R. 2005;11:139–173. doi: 10.1111/j.1755-0238.2005.tb00285.x. [DOI] [Google Scholar]
- Tabanca N, Kirimer N, Demirci B, Demirci F, Başer KH. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J Agric Food Chem. 2001;49:4300–4303. doi: 10.1021/jf0105034. [DOI] [PubMed] [Google Scholar]
- Viegas MC, Bassoli DG. Utilizacao do indice de retencao linear para caracterizacao de compostos volateis em cafe soluvel utilizando GC-MS e coluna HP-Innowax. Quim Nova. 2007;30:2031–2034. doi: 10.1590/S0100-40422007000800040. [DOI] [Google Scholar]
- Wang XC, Li AH, Dizy M, Ullah N, Sun WX, Tao YS. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017;228:550–559. doi: 10.1016/j.foodchem.2017.01.113. [DOI] [PubMed] [Google Scholar]
- Weldegergis BT, Tredoux AG, Crouch AM. Application of a headspace sorptive extraction method for the analysis of volatile components in South African wines. J Agric Food Chem. 2007;55:8696–8702. doi: 10.1021/jf071554p. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Dai S, Niu Y, Yu H, Zhu J, Tian H, Gu Y. Discrimination of Chinese vinegars based on headspace solid-phase microextraction-gas chromatography mass spectrometry of volatile compounds and multivariate analysis. J Food Sci. 2011;76:C1125–1135. doi: 10.1111/j.1750-3841.2011.02356.x. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Yu D, Niu Y, Chen F, Song S, Zhu J, Zhu G. Characterization of aroma compounds of Chinese famous liquors by gas chromatography-mass spectrometry and flash GC electronic-nose. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;945–946:92–100. doi: 10.1016/j.jchromb.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Liu S, Gu Y, Xu N, Shang Y, Zhu J. Discrimination of cherry wines based on their sensory properties and aromatic fingerprinting using HS-SPME-GC-MS and multivariate analysis. J Food Sci. 2014;79:C284–294. doi: 10.1111/1750-3841.12362. [DOI] [PubMed] [Google Scholar]
- Yilmaztekin M, Cabaroglu T, Erten H. Effects of fermentation temperature and aeration on production of natural isoamyl acetate by Williopsis saturnus var saturnus. Biomed Res Int. 2013;2013:870802. doi: 10.1155/2013/870802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JC, Niu YW, Feng T, Liu SJ, Cheng HX, Xu N, Yu HY, Xiao ZB. Evaluation of the formation of volatiles and sensory characteristics of persimmon (Diospyros kaki L.f.) fruit wines using different commercial yeast strains of Saccharomyces cerevisiae. Nat Prod Res. 2014;28:1887–1893. doi: 10.1080/14786419.2014.955492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.