Abstract
Matching the general trend of replacing synthetic additives with healthier natural products, the present research studies the effects of different concentrations of chokeberry extract which substitute carmoisine dye in jelly candies. Also, the colour and antioxidant properties of the aforementioned extract and their changes at various pH and in presence of different mineral salts from foods are analysed. The phenolic content of the extract was determined using HPLC and spectrophotometric methods. A high concentration of polyphenols was found in the chokeberry extract, of which around 97% were flavonoids. Catechin, epicatechin, ferulic acid and its methyl esther, protocatechuic, gallic and para-hydroxybenzoic acids were the major phenolics identified in the extract. The total antioxidant activity decreased in acidic media, while close-to-neutral and alkaline pH values did not exhibit any effect on this parameter. Furthermore, the green/red colour parameter, the chroma and the hue angle were enhanced in the most acidic media (pH 2.3 and 3.5). From the studied salts, CaCl2 and KNO3 had the most significant effects on colour. The chokeberry extract proved to be suitable as replacement of carmoisine dye in jelly candies, as the physicochemical and microbiological properties comply with the regulated requirements. More than that, the extract improved the antioxidant and sensory properties of jellies in all studied concentrations and the best total sensory score was obtained for 1.5% extract. After 5 and 50 days of storage, the microbiological properties were improved in candies prepared with aronia extracts compared to carmoisine, as the total viable count registered important diminutions.
Keywords: Aronia, Carmoisine, Antioxidant, CIELab colour parameters, Total viable count, Storage
Introduction
Many studies proved that certain synthetic food additives may cause immediate or long-term health problems, when consumed for long periods of time (Borzelleca and Hallagan 1988). The ingestion of artificial dyes such as tartrazine and erythrosine combined with a diet rich in trans-fats, nitrites, nitrates and reduced intake of fibres is related to malignancies, specifically in the oesophagus, breast, rectum, stomach, and ovaries (Polonio and Peres 2009). Carmoisine (also named azorubine, or E 122) may induce allergic reactions, intensifying of asthma symptoms, intolerance in people sensitive to salicylates, children hyperactivity and is suspected for carcinogenic properties connected to urinary bladder cancer (Ghendov-Mosanu et al. 2016). On the other hand, jelly candies are multi-coloured sweets with gelatinous structure, especially popular among children, the production of which involves the use of various colouring additives to obtain an attractive appearance (Tamer et al. 2013). The replacement of the synthetic food dyes with colourings of natural origin is therefore a current concern.
The use of natural ingredients as food additives is becoming more and more popular across the food industry due to their health properties. However, the choice of natural food colorants on the market is limited, this being caused by the insufficient exploitation of renewable natural resources and by-products of food processing. Due to the mentioned trend, the producers of colorants are continuously extending their product portfolio. Thus, the necessity of food dyes and antioxidants of natural origin for the food industry is increasing the demand of natural plant extracts. Many studies have shown that wood berries are a source of natural colourants and, at the same time, candidates for dietary interventions when it comes to mitigating chronic inflammation. Recent studies place the black chokeberry (Aronia melanocarpa, also named aronia) amongst the berries with the highest antioxidant activity and polyphenol content. They contain high levels of flavonoids, the majority of which are proanthocyanidins and anthocyanins, which might have potential health benefits (Savikin et al. 2014). The bioactive value of berries results from their composition in substances such as vitamins, pro-vitamins and related compounds, minerals, phytosterols and phenolic compounds (Savikin et al. 2014). Thus, the use of chokeberry extract in food production will not only act as a technological aid, but will also improve the product in terms of its effect on consumers’ health.
Previous studies have come to the conclusion that in order to fully understand the influence of processing on the bioactive compounds, researches on different technological treatments should be performed (Tolic et al. 2015). Therefore, in the present study a hydroalcoholic extract from chokeberry fruits is prepared and characterized, after which the influence of pH and different salts on its properties (such as antioxidant activity and colour parameters) is analysed in order to predict possible interactions in complex food matrices. Except this, the main objective was to test the mentioned extract as a food dye in jelly candy, in order to improve this product’s properties and storage period, and make it healthier. Analyses of the chokeberry’s extract influence on the physicochemical, microbiological, sensory characteristics and antioxidant activity, as well as the change of jellies during storage were also performed.
Materials and methods
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) and sinapic acid were purchased from Alfa Aesar (Germany); Folin-Ciocalteu reagent—from Merck (Germany); DPPH (2,2-diphenyl-1-picrylhydrazyl), (+)-catechin, caffeic acid, syringic acid, ferulic acid and ferulic acid methyl ester, gallic acid, protocatechuic acid, gentisic acid, para-hydroxybenzoic acid, salicylic acid, para-coumaric acid, quercetin, meta-hydroxybenzoic acid, vanillic acid, epicatechin, cis-resveratol—Sigma (Germany, Japan, China); procyanidin B1, procyanidin B2, polydatin, hyperoside—Extrasynthese (France); trans-resveratrol—TCI Europe (Belgium). All the spectrophotometric measurements were made using Analytic Jena Specord 200 Plus spectrophotometer.
Extraction
The ethanolic extract was prepared from mature Aronia melanocarpa berries harvested in Moldova. The chokeberries were dried at 60.0 ± 1.0 °C to 8.05 ± 0.13% moisture content, milled to powder with the median particle (d50) of 140 μm, then sieved. The obtained powder was subjected to extraction in 50% hydroethanolic solvent, with solid:liquid ratio 1:10, under stirring at 60 rpm for 30 min. After the filtration of extract, the polyphenol composition and the antioxidant activity were determined, and also, the trials involving the influence of salts and pH were performed. For the use in jelly production, the extract was concentrated in a rotary evaporator at 65.0 ± 1.0 °C until the mass fraction of dry substance reached 75.0 ± 0.1%. The extract was stored in glass bottles at 4.0 ± 1.0 °C, in the dark.
Characterization of chokeberry extract
Total polyphenols and total flavonoids
The content of total polyphenols was determined by the reaction with Folin-Ciocalteu reagent following the method described by Singleton and Rossi (1965). The results for total polyphenols were calculated from a calibration curve using gallic acid and expressed as equivalents of gallic acid per litre (mg GAE/L). The total content of flavonoids was determined using precipitation with formaldehyde at pH < 0.8 (Filimon et al. 2020), followed by the reaction with Folin-Ciocalteu reagent, according to the method described by Spranger et al. (2008).
Absorbance at 280 nm
The absorbance at 280 nm (Abs 280) is a parameter which reflects the total polyphenol content and was determined according to the method described by Ribereau-Gayon et al. (2006). The results were expressed as mg equivalents of gallic acid per litre (mg GAE/L) from a calibration curve.
The content of anthocyanins by difference of pH
The content of total and monomeric anthocyanins was determined by pH differential method described by Giusti and Wrolstad (2001). Each sample was brought at pH 1 (with KCl/HCl buffer) and 4.5 (with CH3COONa/HCl buffer), and at each pH, two reads of the absorbance were performed: at 520 nm and 700 nm (Giusti and Wrolstad 2001; Cristea et al. 2019). The results were calculated according to Cristea et al. (2019).
Total cinnamic acids
The content of total cinnamic acids was determined using the method described by Demir et al. (2014). The results were expressed as mg caffeic acid equivalents per litre (mg CAE/L) based on a calibration curve with standard of caffeic acid.
Total flavonols
The content of total flavonols was determined spectrophotometrically, at 360 nm after acidification with HCl. The results were expressed as mg quercetin equivalents per litre (mg QE/L) based on a calibration curve with standard of quercetin (Demir et al. 2014).
Antioxidant activity
The antioxidant activity of the extracts was measured by two assays:
ABTS· (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical) scavenging activity, following the method described by Re et al. (1999). The results were expressed as mmol trolox equivalents per litre (mmol TE/L) from a calibration curve.
DPPH· (2,2-diphenyl-1-picrylhydrazyl radical) scavenging activity, according to Brand-Williams et al. (1995). The results were expressed as % of inhibition.
Colour parameters (CIEL*a*b*)
The CIEL*a*b* colour parameters were assessed according to method OIV-MA-AS2-11 (2006). The transmittance was measured every nm between 380 and 780 nm, using a 1 mm optical glass cuvette and the reference was distilled water. The illuminant was D65 and the observer 10°. The luminosity (L*), the green/red parameter (a*), the blue/yellow parameter (b*), the chroma (C*), and the hue angle (H*) were determined (OIV-MA-AS2-11 2006).
HPLC of polyphenols
The polyphenol composition was analysed with Agilent 1100 Series HPLC with LiCrospher 100 RP (18; 5 μm) in LiChroCart 250-4 guard column thermostatted at 35 °C. The injection volume was 20 μL and the run time 90 min. The phases consisted of ultrapure water, methanol and trifluoroacetic acid as follows: A: H2O:MeOH:TFA (98:1:1) and B: H2O:MeOH (50:50) + TFA (1%), with a flow rate of 1.5 mL/min. The detection was carried out at 256 nm, 280 nm, 324 nm, and 365 nm. The gradient of elution was 100% (A): for 10 min; 82% (A): 18% (B) for the next 10 min; 70% (A): 30% (B) for 10 min; 65% (A): 35% (B) for 6 min; 40% (A): 60% (B) for 15 min; 20% (A): 80% (B) for 5 min; 100% (B) for 15 min, and 100% (A) for 10 min.
Studies of factors influencing the properties of chokeberry extract
Studies on the influence of salts
The influence of some salts, often used in different food processing technologies, was studied by adding NaCl, CaCl2, or KNO3 to the extract in the concentrations: 0.001 M, 0.01 M, and 0.1 M. The extracts were kept at t = 4 ± 1 °C for 12 h, and then the antioxidant activity and the CIEL*a*b* colour parameters were measured.
Studies on pH influence
The extracts were brought to some pH values found in different foods: 2.3; 3.5; 5.6; 7.3, and 8.0 using various buffers, and then stored at t = 4 °C for 12 h. Control samples were prepared by diluting the extracts with the same volumes of ethanol 50% as the ones of the buffers used for pH adjustment (Cristea et al. 2019). Afterwards, the antioxidant activity and the colour parameters were determined.
Jelly candies preparation
Sugar, sorbitol, molasses, and water (25% of the total amount of sucrose and sorbitol) were used to prepare the syrup having 78.0 ± 1.0% dry substance. A suspension was prepared using one part corn starch and three parts water and was added to the cooked syrup. Afterwards, swelled gelatine (ratio 1:2), heated citric acid and carmoisine (0.03%) or chokeberry extract were added to the mixture at 64.0 ± 1.0 °C. The obtained jelly-like mass fraction with about 70% dry matter was poured into moulds and cooled to room temperature. The candies were removed from the moulds, dried at 45.0 ± 1.0 °C for 24 h, cooled, packed, and stored in the refrigerator at 5.0 ± 1.0 °C. The samples with chokeberry extract were obtained by replacing the carmoisine synthetic dye with 1.0%, 1.5%, or 2.0% extract. The specific quantities of the manufacturing recipe were: 357.14 g sugar, 392.86 g molasses, 57.14 g gelatine, 42.86 g corn starch, 35.71 g sorbitol, 10.00 g citric acid, 104.29 g water, and either 0.3 g of carmoisine for the control sample, or 10 g, 15 g, 20 g of aronia extract for 1% aronia extract sample, 1.5% aronia extract sample, 2% aronia extract sample, respectively.
Jelly candies analysis
Physicochemical analysis
The physicochemical parameters of the jelly candy were determined following the procedures described in the Official Methods of Analysis of AOAC International (2016). The mass fraction of dry matter was quantified by refractometry. The mass fraction of reducing substances was determined by reaction with ferricyanide. The acidity was determined by titration with sodium hydroxide using phenolphthalein as indicator (Official Methods of Analysis of AOAC International 2016).
Microbiological analysis
The microbiological analysis was carried out in accordance with the rules on microbiological criteria for foodstuffs (G.D. of R.M. no. 221 2009). The total viable count (TVC) of mesophilic aerobic organotrophic bacteria was determined after incubation at 37 °C for 4–72 h.
Sensory analysis
The sensory analysis of the products was carried out following the procedure described in the standard SR ISO 6658:2017 of the International Organization for Standardization. The sensory attributes: appearance, taste, odour, colour and consistency were appreciated using the 5 points system by an expert panel formed of food technology specialists.
In vitro antioxidant activity of the candies
The antioxidant activity was measured in vitro, in conditions of gastric digestion, by the DPPH· radical scavenging assay. The gastric digestion was simulated with pepsin (150 mg/100 g of product) at pH = 2.0 ± 0.1 (1.5 M HCl) and 37.0 ± 0.1 °C, stirring 2 h at 600 rpm. After simulation of digestion, the samples were centrifuged at 6000 rpm for 10 min, filtered and then the DPPH· radical scavenging activity was measured at room temperature (20 ± 1 °C), according to Brand-Williams et al. (1995).
Statistical analysis
The mean values and the standard deviations were calculated from three independent experiments. One-way ANOVA and post hoc Tukey test were used to distinguish between means and evaluate the results. The considered significance level was p ≤ 0.05. All calculations were made using SPSS Statistics 23 and Microsoft Office Excel 2007. Mathematical modelling and informational analysis were done using MATLAB (Matlab and Statistics Toolbox Release 2015a; MathWorks, Natick, MA, USA). The informational analysis of the obtained experimental data allows the evaluation of the mutual influences of the measured parameters. It is based on two main concepts: entropy and information, and is expressed in bits, as unit of measure. The higher the values of the mutual information, the lower are the uncertainties and hence, the higher the predictions (Fellin 2005, Scafetta 2001).
Results and discussion
Characterization of the chokeberry extract
The results of the analysis of the phenolic composition and the antioxidant activity of the extract used for candy manufacturing are presented in Table 1.
Table 1.
Composition, antioxidant activity and individual polyphenols identified in the ethanolic chokeberry extract
| Parameter | Value |
|---|---|
| Total polyphenols (Folin-Ciocalteu) (mg GAE/L extract) | 4441 ± 243 |
| Total flavonoids (mg GAE/L extract) | 4293 ± 209 |
| Total polyphenols by Abs280 (mg GAE/L extract) | 3470 ± 21 |
| Cinnamic acids (mg CAE/L extract) | 580 ± 21 |
| Flavonols (mg QE/L extract) | 501 ± 15 |
| Total anthocyanins (mg/L extract) | 102 ± 2 |
| Monomeric anthocyanins (mg/L extract) | 61 ± 2 |
| ABTS• scavenging antioxidant activity (mmol TE/L extract) | 31.61 ± 1.02 |
| DPPH· scavenging antioxidant activity (% inhibition) | 72.95 ± 0.24 |
| Catechin (mg/100 mL) | 7.8 ± 0.0 |
| Epicatechin (mg/100 mL) | 3.95 ± 0.32 |
| Ferulic acid (mg/100 mL) | 3.71 ± 2.57 |
| Protocatechuic acid (mg/100 mL) | 1.50 ± 0.07 |
| Ferulic acid methyl ester (mg/100 mL) | 1.32 ± 0.54 |
| Gallic acid (mg/100 mL) | 0.36 ± 0.05 |
| p-hydroxybenzoic acid (mg/100 mL) | 0.23 ± 0.04 |
| Quercetin (mg/100 mL) | 0.21 ± 0.11 |
| Procyanidin B2 (mg/100 mL) | 0.17 ± 0.06 |
| m-hydroxybenzoic acid (mg/100 mL) | 0.11 ± 0.01 |
| Sinapic acid (mg/100 mL) | 0.10 ± 0.01 |
| Hyperoside (mg/100 mL) | 0.10 ± 0.01 |
| Procyanidin B1 (mg/100 mL) | 0.09 ± 0.05 |
| Vanillic acid (mg/100 mL) | 0.08 ± 0.01 |
| Chlorogenic acid (mg/100 mL) | 0.08 ± 0.06 |
| Syringic acid (mg/100 mL) | 0.04 ± 0.01 |
| p-coumaric acid (mg/100 mL) | 0.04 ± 0.01 |
| Gentisic acid (mg/100 mL) | 0.01 ± 0.00 |
| Cis-resveratrol (mg/100 mL) | 0.01 ± 0.00 |
| Trans-resveratrol (mg/100 mL) | 0.008 ± 0.003 |
| Salicylic acid | Traces |
| Caffeic acid (mg/100 mL) | – |
| Polydatine (mg/100 mL) | – |
A high content of polyphenols was found in the chokeberry extract, of which approximately 97% are flavonoids, but also, important quantities of cinnamic acids and flavonols. Other authors have also found high concentrations of polyphenols, e.g. Ovaskainen et al. (2008) found the highest content of polyphenols in chokeberry in a study in which 143 plant species were analysed (Ovaskainen et al. 2008).
The main phenolic compounds identified in the chokeberry extract were catechin (7.8 mg/100 mL) and epicatechin (3.95 mg/100 mL), but also ferulic acid and its methyl esther, protocatechuic and gallic acids, para- and meta-hydroxybenzoic acids, quercetin, procyanidin B2 and B1 etc. Also, high contents of total and monomeric anthocyanins were found (Table 1). The major anthocyanins in chokeberry are glycosides of cyanidin (Kulling and Rawel 2008). According to Savikin et al. (2014), the main components of chokeberry teas are cyanidin-galactoside and cyanidin-arabinoside (concentrations above 2.3 mg/100 mL), while cyanidin-glucoside and cyanidin-xyloside are present in amounts lower than 1.3 mg/100 mL (Savikin et al. 2014). Other authors have also analysed different parts of the chokeberry shrub and have found that cyanidin 3-galactoside was the major anthocyanin in all three Aronia melanocarpa cultivars of the following “Moskva”, “Hugin”, and “Nero”. They found minor differences in antioxidant content between the three aforementioned cultivars of Aronia melanocarpa and one cultivar of Aronia prunifolia, and were comprised between 21.2 and 35.7 DPPH IC50 (μg/mL) in the 80% ethanolic extract (Wangesteen et al. 2014). Same authors detected high contents of chlorogenic acid in the chokeberry decoction (5.24 mg/100 mL) and infusion (4.02 mg/100 mL) in a research covering three species of berries, namely: chokeberry, bilberry, and black currant. Also, it was shown that the antioxidant activity and the total polyphenol content depend on the cultivar, berry size, yield, and extraction solvent (Wangesteen et al. 2014).
Researchers from Croatia have evaluated the polyphenol content and the antioxidant activity of various chokeberry products, namely juices, powders, teas, capsules, and dry berries. It was found that all products contain high amount of phenolics (3002–6639 mg/L and 1494–5292 mg/100 g dry weight, depending on the product) and a lower amount of total anthocyanins (150–1228 mg/L and 141–2468 mg/100 g dry weight). The antioxidant activity of the examined juices and other chokeberry products was also found to be high (12.09–40.19 mmol TE/L or 58.49–191.31 mmol TE/100 g dry weight) (Tolic et al. 2015). The values found by the Croatian authors are comparable to the ones found in the present study.
The influence of different salts on the antioxidant activity and colour parameters of chokeberry extract
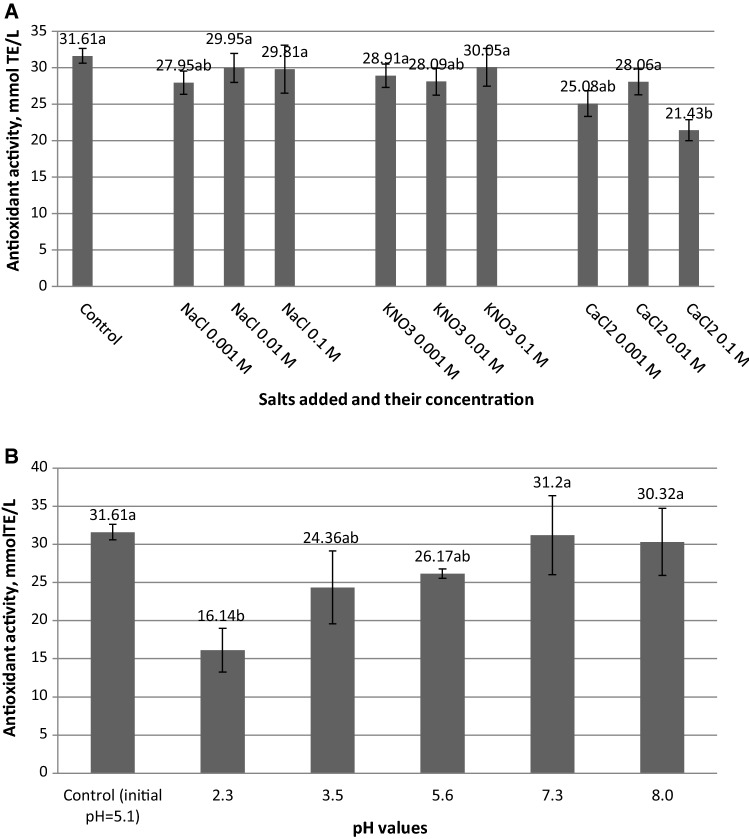
The pH and the ionic strength are key parameters in the gelation process, impacting greatly both the sol–gel transition and the concentration of the thickening agent. Both the antioxidant activity and the colour of the extract are influenced by pH and ionic strength variations. The cause of this phenomenon lies in the fact that anthocyanins exist in different mesomeric structures. Cation flavylium (AH+), for example, with its many resonance structures is dominant at low pH. As the pH increases, the carbinol pseudo base, which is in tautomeric equilibrium with its colorless chalcone, forms proportionally with the decrease in the AH+ concentration. At pH bigger than 5, anhydrous blue quinoidal base begins to form. Each of these structures has different antioxidant capacity. The ionic strength also influences the colour of the extract due to the direct involvement of the electrolyte ions in the tautomeric balance. The sol–gel transition is also influenced by pH value and ionic strength, because the condensation process can be electrocratically stabilized, maintaining the total gel volume. In case this stabilization does not occur, the connections in the three-dimensional structure may break, causing the spontaneous release of the liquid (syneresis) (Sturza and Deseatnicova 2012). Consequently, the following two sections of this study will focus on the analysis of the influence of ionic strength and pH on the antioxidant activity and colour of chokeberry extract. The changes of the antioxidant activity after the addition of NaCl, KNO3 and CaCl2 are shown in Fig. 1a.
Fig. 1.
Effects of salts (a) and pH (b) on the antioxidant activity of chokeberry extract (errors bars present the standard deviation of three determinations and different letters designate statistically different results)
No significant changes of the antioxidant activity were observed at the addition of sodium chloride or potassium nitrate. High concentration of CaCl2 changed the antioxidant activity by lowering its value from 31.61 mmol TE/L to 21.43 mmol TE/L, which was a statistically significant decrease. Previous studies proved that the addition of calcium chloride led to a reduction of the antioxidant activity also in the grape marc extract (Cristea et al. 2019) and this phenomenon was explained by the ability of certain flavonoids to entrap cations, which results in a change of their antioxidant properties (Jabbari and Gharib 2012).
Table 2 summarizes the change of colour parameters after the addition of NaCl, CaCl2 and KNO3 at different concentrations. Generally, the addition of these three salts had little effect on the colour of the extract. CaCl2 added at the concentration of 0.1 M had the most significant effect by lowering the blue/yellow parameter, increasing the green/red parameter, the hue angle and chroma. A similar increase in colour intensity was observed in the case of the grape marc extract (Cristea et al. 2019). Also in red cabbage hydroethanolic extract, the addition of calcium chloride increased the green/red parameter, the hue angle and chroma (Patras 2019). The colour increase can be explained either by the reactions of polymerization and complexation among anthocyanins, other phenolics and calcium ions (Ngo and Zhao 2009) or by the pH change caused by the addition of calcium chloride. Further studies on mentioned influences on colour could give more information on the possibilities to apply these results in the formulation of products containing both calcium and anthocyanins from different sources, as fruit yogurts or dairy products coloured with natural dyes.
Table 2.
Change of CIEL*a*b* colour parameters of chokeberry extract in the presence of different salts and different concentrations (standard deviations are based on three replicates)
| Salt and concentration | L* | a* | b* | C* | H* |
|---|---|---|---|---|---|
| Control | 42.36 ± 0.13a | 41.79 ± 0.74b | 24.90 ± 1.29d | 48.65 ± 2.08c | 1.47 ± 0.01a |
| NaCl, 0.001 M | 43.91 ± 0.52a,b | 41.00 ± 0.34b | 22.78 ± 0.21b | 46.90 ± 0.30b | 1.61 ± 0.02b |
| NaCl, 0.01 M | 44.05 ± 0.51b | 42.39 ± 1.54b | 22.93 ± 0.10c | 48.20 ± 1.32b,c | 1.66 ± 0.08b,c |
| NaCl, 0.1 M | 43.95 ± 0.54a,b | 41.17 ± 0.54b | 22.52 ± 0.34b | 46.93 ± 0.62b | 1.64 ± 0.01b |
| KNO3, 0.001 M | 45.85 ± 0.37c | 38.64 ± 0.34a | 21.01 ± 0.27a | 43.98 ± 0.45a | 1.65 ± 0.01b,c |
| KNO3, 0.01 M | 44.75 ± 1.31b,c | 38.40 ± 0.25a | 21.05 ± 0.19a | 43.80 ± 0.16a | 1.64 ± 0.03b |
| KNO3, 0.1 M | 46.16 ± 0.07d | 41.11 ± 0.21b | 25.15 ± 0.36d | 48.20 ± 0.36b,c | 1.43 ± 0.02a |
| CaCl2, 0.001 M | 44.41 ± 0.40b,c | 40.71 ± 0.20b | 22.73 ± 0.04b | 46.62 ± 0.19b | 1.60 ± 0.01b |
| CaCl2, 0.01 M | 44.29 ± 0.25b,c | 41.46 ± 0.48b | 22.71 ± 0.09b | 47.27 ± 0.41b,c | 1.64 ± 0.03b |
| CaCl2, 0.1 M | 42.35 ± 0.15a | 45.01 ± 0.23c | 23.48 ± 0.04c | 50.77 ± 0.21d | 1.74 ± 0.01c |
The results of the informational analysis shown in Fig. 2c confirmed that increasing concentration of calcium chloride had the strongest influence, i.e. 0.47 bits, on all tested parameters. In a similar way, the addition of potassium nitrate (Fig. 2b) impacted four of the six tested parameters, namely: antioxidant activity, green/red parameter (a*), blue/yellow parameter (b*), and chroma (C*). On the other hand, in the case of sodium chloride (Fig. 2a), the information analysis had the same value—0.47 bits—only in the case of blue/yellow parameter (b*). For the other five parameters, the influence of the salt concentration is small and constitutes 0.01 bits.
Fig. 2.
Informational analysis of the salt concentrations and pH influences on the colour parameters and antioxidant activity of the chokeberry extract a NaCl; b KNO3; c CaCl2 and d pH
The effect of pH on the antioxidant activity and colour parameters
The results presented in Fig. 1b show that the antioxidant activity decreases in the acidic medium, the higher the acidity, the lower is the antioxidant activity. Neutral and alkaline media did not change significantly the radical scavenging abilities of chokeberry extract at the studied pHs. Similar results were obtained for grape marc extract (Cristea et al. 2019) and were prior documented in the studies of Altukaya et al. (2016) on the antioxidant activity of lettuce extract and Saeedeh et al. (2007), who have evaluated the same parameter in drumstick and mint leaves, as well as carrot tuber extracts. This effect was explained by the deprotonation and stabilization of the polyphenols in alkaline solution and subsequent increased ability to donate electrons (Altukaya et al. 2016).
Concerning the colour parameters (Table 3), the highest decrease in luminosity was observed for the close-to-neutral pH = 7.3 and alkaline pH = 8.0, but also the acidic pH = 3.5 has decreased this parameter and darkened the extract. The green/red parameter, chroma and hue angle were significantly enhanced in the most acidic medium (pH = 2.3). These modifications can be explained by the stabilization of the flavylium cation in acidic medium. Furthermore, the tones have evolved towards more blue shades in the most acidic media (pH 2.3 and 3.5). On the other hand, at pH = 5.6, pH = 7.3 and pH = 8.0, the blue/yellow parameter increased, demonstrating a shift towards yellow. Moreover, the green/red parameter a* decreased at these values which suggests a shift from red shades towards green, and as a result an overall decrease in chromaticity and overall colour quality. The changes of colour parameters were statistically significant. These results indicate that the extract would be most suitable for use in acidic foods, specifically the ones with a pH lower than the one of the aronia extract, i.e. 5.1, if the colour is expected to become more intense. The revealed colour changes at low pH or in presence of salts can be useful in the production of different food with added chokeberry, in order to adapt the product’s chromatic properties to consumer preferences.
Table 3.
CIEL*a*b* parameters’ dependence on pH (results are expressed as means ± standard deviation, different letters designate significantly different results between pairs of test and control for each value of pH)
| CIEL*a*b* parameters | L* | a* | b* | C* | H* |
|---|---|---|---|---|---|
| Control for 2.3 | 75.7 ± 0.1a | 19.1 ± 0.1a | 8.1 ± 0.1a | 20.8 ± 0.1a | 2.23 ± 0.04a |
| pH = 2.3 | 74.6 ± 1.0a | 37.8 ± 3.6b | 7.0 ± 0.1b | 38.4 ± 3.6b | 5.34 ± 0.42b |
| Control for 3.5 | 65.5 ± 0.1a | 19.1 ± 0.1a | 11.3 ± 0.2a | 22.2 ± 0.2a | 1.49 ± 0.02a |
| pH = 3.5 | 57.2 ± 2.2b | 23.8 ± 0.5b | 12.5 ± 0.1b | 26.9 ± 0.4b | 1.74 ± 0.07b |
| Control for 5.6 | 54.4 ± 0.2a | 35.5 ± 0.1a | 16.4 ± 0.3a | 39.1 ± 0.2a | 2.01 ± 0.01a |
| pH = 5.6 | 55.4 ± 0.5b | 31.5 ± 0.4b | 17.7 ± 0.2b | 36.2 ± 0.4b | 1.59 ± 0.1b |
| Control for 7.3 | 54.4 ± 0.2a | 35.5 ± 0.1a | 16.4 ± 0.3a | 39.1 ± 0.2a | 2.01 ± 0.01a |
| pH = 7.3 | 45.5 ± 2.4b | 21.6 ± 0.4b | 20.8 ± 0.1b | 30.0 ± 0.4b | 0.70 ± 0.02b |
| Control for 8.0 | 67.4 ± 0.3a | 24.5 ± 0.5a | 11.5 ± 0.2a | 27.1 ± 0.5a | 1.96 ± 0.01a |
| pH = 8.0 | 62.0 ± 0.1b | 11.5 ± 0.1b | 15.0 ± 0.2b | 19.0 ± 0.1b | 0.28 ± 0.02b |
The informational analysis (Fig. 2d) confirmed that, overall, the pH of the aronia fruit extracts had the strongest influence on the luminosity (L*) and the blue/yellow component (b*), with values of mutual information of 0.19 bits in both cases, followed by chroma (C*) and green/red parameter (a*) (− 0.09 bits), and the hue (H*) and antioxidant activity (0.02 bits).
Jelly candy production trials
Table 4 presents several physicochemical and microbiological quality parameters, the sensorial profile and the antioxidant activity of the candies with addition of 1%, 1.5% and 2% aronia extract, compared to the control with carmoisine. The evolution during storage of the most unstable quality parameters (mass fraction of the dry matter, titratable acidity and total viable count) was also estimated by evaluation on the 5th day and on the 50th day from production date.
Table 4.
Physicochemical and microbiological quality indicators, sensorial profile and antioxidant activity of jelly candy with addition of aronia extract compared to the control with carmoisine (the results are presented as means ± standard deviation)
| Quality indicators | Jelly candy | |||||||
|---|---|---|---|---|---|---|---|---|
| Control (carmoisine) | 1.0% aronia extract | 1.5% aronia extract | 2.0% aronia extract | |||||
| After 5 days | After 50 days | After 5 days | After 50 days | After 5 days | After 50 days | After 5 day | After 50 days | |
| The mass fraction of the dry matter (%) | 71.1 ± 0.26a | 69.46 ± 0.19b | 72.12 ± 0.25c | 70.05 ± 0.60c | 73.45 ± 0.19d | 70.71 ± 0.35a | 73.81 ± 0.24e | 71.9 ± 0.19c |
| Titratable acidity, degrees | 14.1 ± 0.1a | 14.4 ± 0.2a | 15.7 ± 0.1b | 16.2 ± 0.2cde | 16.1 ± 0.1d | 16.5 ± 0.1ef | 16.5 ± 0.1f | 16.7 ± 0.1cfg |
| The mass fraction of reducing substances (%) | 13.55 ± 0.10a | n.d. | 14.87 ± 0.11b | n.d. | 14.93 ± 0.28b | n.d. | 15.11 ± 0.36b | n.d. |
| TVC (total viable count), % of the maximum admissible number* | 5.5 ± 0.2a | 26.3 ± 0.3b | 4.5 ± 0.2ac | 22.7 ± 0.3d | 3.4 ± 0.4ac | 21.2 ± 2.0d | 3.3 ± 0.3c | 18.0 ± 0.2e |
| DPPH· antioxidant activity (%) | − 27.62 ± 1.63a | 5.37 ± 0.45b | 8.93 ± 0.65c | 10.63 ± 0.57d | ||||
| Sensory profile total score | 21.9 ± 0.5a | 22.2 ± 0.7ac | 23.4 ± 0.5b | 22.9 ± 0.4bc | ||||
| Appearance | 4.5 ± 0.1a | 4.1 ± 0.2b | 4.5 ± 0.1a | 4.5 ± 0.1a | ||||
| Taste | 4.0 ± 0.1a | 4.6 ± 0.1b | 4.8 ± 0.1b | 4.8 ± 0.1b | ||||
| Odour | 3.8 ± 0.1a | 4.3 ± 0.2b | 4.4 ± 0.1b | 4.5 ± 0.1b | ||||
| Colour | 4.6 ± 0.2ac | 4.2 ± 0.2ab | 4.7 ± 0.2c | 4.1 ± 0.1b | ||||
| Consistency | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | ||||
n. d. not determined
*nutrient agar
The results proved that the mass fraction of the dry matter, the mass fraction of reducing substances and the titratable acidity are enhanced in aronia containing jellies compared to carmoisine containing control, and their values are getting higher when the concentration of the natural extract in jelly is increasing (Table 4). After 5 days of storage, the presence of 1% extract increased the mass fraction of solids with 1.4%, while the 1.5% extract, with 3.3% and the 2% extract with 3.8% compared to the control sample containing 0.03% carmoisine. After 50 days of storage, the mass fraction of all samples was smaller than after 5 days storage, but again it was increased in presence of aronia extract and the greater the extract concentration, the higher the mass fraction of dry matter. The explanation for the decrease of mass fraction of the dry matter during storage is the fact that the recipe of the jelly candy includes gelatin and sorbitol, which have the ability to bind water. For this reason, during the storage period of 50 days, there is a non-essential increase of the mass of the product, contributing to the decrease of the dry substance content.
The titratable acidity also increased with the concentration of the aronia and on the 5th day of storage varied between 15.7° of acidity (for 1% aronia) and 16.5° of acidity (for 2% aronia), while after 50 days—between 16.2° and 16.7° of acidity and is higher than the control’s. The increase of titratable acidity is due to the organic acids content of the chokeberry extract: chlorogenic acid, neoclorogenic acid, caffeic acid and derivatives thereof (Sikora et al. 2008). Simultaneously, the mass fraction of reducing substances slightly increased with the addition of extract and was within the range 14.87–15.11%, thus it did not exceed the maximum value stipulated by regulations; e.g. the Moldovan national regulation for this category of products limit the mass fraction of reducing substances at 60% (G.D. No. 204 2009). The analysis of the results showed that the physicochemical characteristics of the candies were within the permissible values stated in the national product regulations on the 5th day and the 50th day from the date of production (G.D. No. 204 2009).
The microbiological results showed that after 5 and 50 days of storage, all the products had the TVC corresponding to the European Union allowed value (Regulation of EC no. 1441/2007). The total number of germs is smaller in presence of natural extract, compared with the control and TVC decreases with increasing concentration of chokeberry extract (Table 4), which confirms its recognized antimicrobial activity (Puupponen-Pimia et al. 2008). After 50 days of storage, the TVC registered a general increase and the chokeberry extract 1%, 1.5% and 2% reduced it with 14%, 19.4% and 31.6%, respectively, compared to the control. Therefore, replacing carmoisine with aronia extract may allow a longer period of preservation for jelly candies.
The sensory analysis proved that taste and odour of all aronia jellies were improved compared to the control with carmoisine, with at least 0.6 and 0.5 points, respectively (Table 4). But, only the colour of the candy with 1.5% extract was slightly superior to the candy containing carmoisin. The consistency of all samples was the same, and the appearance of 1.5% and 2% aronia containing jellies was identical to the control. The best total sensory score was obtained for candy with 1.5% chokeberry fruit extract, followed by the 2% and then 1% extract, but all scores are higher than the control’s. The jelly containing 1.5% aronia extract can be considered as optimal from the sensory point of view.
It is known that the presence of bioactive compounds influences the stability and antioxidant capacity of the food (Savvin and Bolotov 2008). In present research, the antioxidant capacity of the jelly candies subjected to gastric digestion was investigated. Negative values of the antioxidant activity, i.e. − 27.62 ± 1.63%, were found in the control sample prepared with synthetic dye, indicating an oxidising action, while the samples containing aronia extract had positive, antioxidant activities, which increased with the augmentation of the extract’s concentration in jelly candies. The jellies with 1.5% extract revealed an antioxidant activity of 8.93 ± 0.65% (Table 4).
Bermudez-Soto et al. (2007) explored the effect of gastric and pancreatic digestions on the major polyphenols found in chokeberry juice. The authors found that the gastric digestion had “no substantial effect on any of the major phenolic compounds in chokeberry, namely anthocyanins, flavan-3-ols, flavonols and caffeic acid derivatives”. However it was found that the same compounds are modified during pancreatic digestion. The authors concluded that alkaline conditions are the main responsible factor for the changes. Thus, considering the impact of alkaline medium and previously published results, a study on the effect of pancreatic digestion on chokeberry containing candies is also recommended, as the specific matrix of jelly candies may influence the transformations and antioxidant properties of resulting compounds.
The results revealed by the present study confirmed that the chokeberry extract can have a double role in foods: dye and antioxidant. Harnessing these properties could lead to the development of high-value food ingredients prepared from locally-grown plants which can be found in important quantities in different areas and are not valorised at their real potential. For example, the black chokeberry (Aronia melanocarpa) plantations in the forest districts occupy an area of 157.8 ha in the Republic of Moldova (Calalb 2008).
Conclusion
Within the global tendency to replace the synthetic additives with natural compounds extracted from plants in order to obtain healthier foods, the present research studied the properties of an underused fruit extract and its changes in presence of different pHs and inorganic salts usually present in foods, as well as the effects of the substitution of carmoisine dye with different concentrations of the aforementioned extract in jelly candies.
The chokeberry extract proved to have a high radical scavenging capacity and to contain important concentrations of polyphenols, of which, around 97% were flavonoids.
The pH is a factor strongly influencing the extract’s properties only in the case of high acidic media. The total antioxidant activity decreases significantly at pH 2.3 and the green/red parameter, the chroma and the hue angle were enhanced. From the studied salts, CaCl2 and KNO3 had the most significant effects on colour. All colour changes of aronia extract depending on pH or salts can be useful in the production of different food, in order to adequately modify the chromatic properties.
The physicochemical and microbiological properties of aronia jelly candies comply with the regulated requirements for such products. The extract improved the antioxidant and sensory properties of jellies in all studied concentrations and the best total sensory score was obtained for 1.5% extract. After 5 and 50 days of storage, the microbiological properties were improved in the candies prepared with aronia extracts, comparing to carmoisine, as the total viable count registered important diminutions. Present study recommends the use of chokeberry extract as replacement of carmoisine dye in the production of jelly candies.
Acknowledgements
Aliona Ghendov-Mosanu and Elena Cristea were recipients of Eugen Ionescu scholarships offered by AUF and the Ministry of Foreign Affairs of Romania. The authors would also like to thank the project AUF BECO-2012-53-U-56135FT205. This work was benefited from support through the Projects: AUF - DRECO - S0446 SaIN (Agence Universitaire de la Francophonie, 2017-2019) and 16.80013.5107.22/Ro “The substitution of synthetic food additives with bioactive components extracted from natural renewable resources” (Academy of Science of Moldova, Moldovan Government and UEFISCDI, BM 35/2016 PN III-P3-220).
Compliance with ethical standards
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Altukaya A, Gokmen V, Skibsted LH. pH dependent antioxidant activity of lettuce (L. sativa) and synergism with added phenolic antioxidants. Food Chem. 2016;190:25–32. doi: 10.1016/j.foodchem.2015.05.069. [DOI] [PubMed] [Google Scholar]
- Bermudez-Soto M-J, Tomas-Barberan F-A, Garcia-Conesa M-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007;102:865–874. doi: 10.1016/j.foodchem.2006.06.025. [DOI] [Google Scholar]
- Borzelleca JF, Hallagan JB. Chronic toxicity/carcinogenicity studies on FD, C yellow no.5 (tartrazine) in rats. Food Chem Toxic. 1988;26(3):179–187. doi: 10.1016/0278-6915(88)90117-2. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Calalb T. Comparative phenolic potential of the callus masses and the Aronia melanocarpa fruits. Elliot Rev Farm. 2008;1(4):23–28. [Google Scholar]
- Cristea E, Sturza R, Jauregi P, Niculaua M, Ghendov-Moşanu A, Patras A. Influence of pH and ionic strength on the color parameters and antioxidant properties of an ethanolic red grape marc extract. J Food Biochem. 2019;43(4):e12788. doi: 10.1111/jfbc.12788. [DOI] [PubMed] [Google Scholar]
- Demir N, Yioldiz O, Alpaslan M, Hayaloglu AA. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci Technol. 2014;57(1):126–133. doi: 10.1016/j.lwt.2013.12.038. [DOI] [Google Scholar]
- Fellin W. Analyzing uncertainty in engineering. Berlin: Springer; 2005. [Google Scholar]
- Filimon RV, Filimon RM, Patraş A, Rotaru L. Grape quality and ornamental potential of interspecific cultivars for temperate climate vineyards. J Hortic Sci Biotechnol. 2020;95(1):65–75. doi: 10.1080/14620316.2019.1631127. [DOI] [Google Scholar]
- G. D. of R. M. no. 204 from March 11, 2009 regarding the approval of Technical Regulation on Confectionery. Official Monitor of the Republic of Moldova, 11.03.2009, no. 57-58 (in Romanian)
- G. D. of R. M. no. 221 from March 16, 2009 regarding the approval of rules on microbiological criteria for foodstuffs. Official Monitor of the Republic of Moldova, 24.03.2009, no. 59-61, art. 272 (in Romanian)
- Ghendov-Mosanu A, Sturza R, Chiriţa E, Patras A. Valorization of winemaking by-products in the production of jelly candies. Ital Food Mater Mach. 2016;04(2016):12–15. [Google Scholar]
- Giusti MM, Wrolstad RE (2001) Characterisation and measurement of anthocyanins by UV-visible spectroscopy. Current protocols in food analytical chemistry, F1.2.1-F1.2.13
- Jabbari M, Gharib F. Solvent dependence on antioxidant activity of some water-insoluble flavonoids and their cerium(IV) complexes. J Mol Liq. 2012;168:36–41. doi: 10.1016/j.molliq.2012.02.001. [DOI] [Google Scholar]
- Kulling SE, Rawel HM. Chokeberry (Aronia melanocarpa)—a review on the characteristic components and potential health effects. Planta Med. 2008;74:1625–1634. doi: 10.1055/s-0028-1088306. [DOI] [PubMed] [Google Scholar]
- Ngo T, Zhao Y. Stabilization of anthocyanins on thermally processed red d’Anjou pears through complexation and polymerization. LWT Food Sci Technol. 2009;42:1144–1152. doi: 10.1016/j.lwt.2009.02.013. [DOI] [Google Scholar]
- Official Methods of Analysis of AOAC International (2016), 20th edn
- OIV (2006) Method OIV-MA-AS2-11: R2006, Determination of chromatic characteristics according to CIELab. Compendium of international analysis of methods—OIV chromatic characteristics, 1–16
- Ovaskainen ML, Torronen R, Koponen JM, Sinkko H, Hellstrom J, Reinivuo HE. Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr. 2008;138(3):562–566. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- Patras A. Stability and colour evaluation of red cabbage waste hydroethanolic extract in presence of different food additives or ingredients. Food Chem. 2019;275:539–548. doi: 10.1016/j.foodchem.2018.09.100. [DOI] [PubMed] [Google Scholar]
- Polonio ML, Peres F. Consumption of food additives and its health effects: challenges for Brazilian public health. Cad Saude Publica. 2009;25(8):1653–1666. doi: 10.1590/S0102-311X2009000800002. [DOI] [PubMed] [Google Scholar]
- Puupponen-Pimia R, Nohynec L, Alacomy H, Oksman-Caldentey K. The action of berry phenolics against human intestinal pathogens. Bio Factors. 2008;23(4):243–251. doi: 10.1002/biof.5520230410. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Regulation of EC no. 1441/2007 amending Regulation of EC no. 2073/2005 on microbiological criteria for foodstuffs (2007) Official Journal of the European Union, L 322, December 7
- Ribereau-Gayon P, Glories Y, Maujean A, Dubourdieu D. Handbook of enology. Chichester: Wiley; 2006. [Google Scholar]
- Saeedeh AD, Vishlakshi Devi D, Urooj A. Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chem. 2007;100:1100–1105. doi: 10.1016/j.foodchem.2005.11.014. [DOI] [Google Scholar]
- Savikin K, Zdunic G, Jankovic T, Godevac D, Stanoikovic T, Plievliakusic D. Berry fruit teas: phenolic composition and cytotoxic activity. Food Res Int. 2014;62:677–683. doi: 10.1016/j.foodres.2014.04.017. [DOI] [Google Scholar]
- Savvin P, Bolotov V. Investigation of antioxidant properties of jelly marmalade. Chem Plant Raw Mater. 2008;4:177–179. [Google Scholar]
- Scafetta N (2001) An entropic approach to the analysis of time series. Dissertation University of North Texas
- Sikora E, Cieslik E, Topolska K. The sources of natural antioxidants. Acta Sci Pol Technol Aliment. 2008;7(1):5–17. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Spranger I, Sun B, Mateus AM, de Freitas V, Ricardo-da-Silva J. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008;108:519–532. doi: 10.1016/j.foodchem.2007.11.004. [DOI] [PubMed] [Google Scholar]
- SR ISO 6658:2017 (2017) Sensory analysis—methodology—general guidance
- Sturza R, Deseatnicova O (2012) Physico-chemical and colloidal processes in food systems. Monograph UTM, Chisinau (in Romanian)
- Tamer C, Incedayi B, Copur O, Karinca M. A research on the fortification applications for jelly confectionery. J Food Agric Environ. 2013;11(2):152–157. [Google Scholar]
- Tolic M-T, Jurcevic I-L, Krbavcic I-P, Markovic K, Vahcic N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol Biotechnol. 2015;53–2:171–179. doi: 10.17113/ftb.53.02.15.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangesteen H, Braunlich M, Nikolic V, Malterud KE, Slimestad R, Barsett H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: antioxidant and enzyme inhibitory effects. J Funct Foods. 2014;7:746–752. doi: 10.1016/j.jff.2014.02.006. [DOI] [Google Scholar]