Abstract
Purpose
To assess the efficacy and clinical outcomes of preimplantation genetic testing for monogenic diseases (PGT-M), following blastomere biopsy prior or following vitrification.
Methods
A cohort-historical study of all consecutive patients admitted to IVF in a large tertiary center for PGT-M and PCR cycle from September 2016 to March 2020. Patients were divided into 4 groups: Group A1 consisted of patients undergoing day-3 embryos biopsy followed by a fresh transfer of unaffected embryos. Group A2 consisted of Group A1 patients that their surplus unaffected embryos were vitrified, thawed, and transferred in a subsequent FET cycle. Group B1 consisted of patients that their day-3 embryos were vitrified intact (without biopsy) for a subsequent FET cycle. Later embryos were thawed and underwent blastomere biopsies, and the unaffected embryos were transferred, while the surplus unaffected embryos were re-vitrified for a subsequent FET cycle. Group B2 consisted of Group B1 patients that their surplus unaffected embryos were re-vitrified, thawed, and transferred in a subsequent FET cycle. The laboratory data and clinical results were collected and compared between groups.
Results
A total of 368 patients underwent 529 PGT-M cycles in our center: 347 with day-3 embryos biopsied before undergoing vitrification (Group A1) and 182 following vitrification and thawing (Group B1). There were no between group differences in embryo survival rate post-thawing, nor the ongoing implantation and pregnancy rates.
Conclusion
In PGT-M cycles, the timing of embryos vitrification, whether prior or following blastomere biopsy, has no detrimental effect on post-thawing embryo survival rate, nor their potential ongoing implantation and pregnancy rates.
Keywords: Preimplantation genetic testing, Blastomere biopsy, Vitrification, Pregnancy rate, PGT-M, Day-3 biopsy
Introduction
Couples at risk for transmitting a serious genetic disease may benefit from preimplantation genetic testing (PGT), enabling the birth of healthy unaffected offspring [1]. PGT is performed either on oocytes (polar bodies) or cleavage-stage (blastomeres) or blastocyst-stage (trophectoderm cells) embryos. According to the European Society of Human Reproduction and Embryology (ESHRE) PGD Consortium data collection XIV–XV [2] on PGT cycles for monogenic diseases (PGT-M), day-3 cleavage-stage embryo biopsy is the most frequently used (93% of cycles), while the use of blastocyst biopsy remained low (2%), and PCR was the most widely used first-line method of DNA amplification (93% of cycles).
Couples undergoing assisted reproductive technique (ART) treatment for PGT-M are generally fertile with good ovarian reserve [3]. Moreover, since the goal of ART in these couples is to enable the recruitment of multiple healthy fertilizable oocytes, they undergo a more vigorous ovarian stimulation, which often necessitates gonadotropin-releasing hormone (GnRH)-agonist trigger and freeze-all, aiming to avoid severe ovarian hyperstimulation syndrome (OHSS) [4]. While cycle segmentation was initially offered to couples undergoing final follicular maturation with GnRH agonist [4], or those undergoing PGT with repeated implantation failure [3], nowadays, it is widely applied worldwide in vitrified PGT for aneuploidy cycles (PGT-A) [5, 6], with excellent outcome.
The optimal timing of day-3 embryo biopsy and its effect on embryo survival and implantation potential following cryopreservation and thawing have not been established. In 1999, Joris et al. [7] studied good morphological quality derived from abnormal fertilization. They demonstrated significantly lower number of blastomeres and lower survival rates after slow freezing and thawing of embryos following drilling-only or embryo biopsy. Shinar et al. [8] retrospectively evaluated the effect of day-3 biopsy for PGT, before and after slow freezing, on embryo survival rates. Cryopreservation preceding biopsy was shown to result in better embryo survival compared with biopsy before cryopreservation with comparable pregnancy rates per transfer cycle.
Zhang et al. [9] studied the effect of vitrification on day-3 cleavage embryo derived from abnormally fertilized oocytes. They demonstrated that the survival rate after warming in the non-biopsied cleavage control group was significantly higher than in the biopsied cleavage group, probably because most of the biopsied embryos were destroyed due to blastomere escaping from the zona pellucida while warming. On the other hand, Kahraman et al. [10] compared the post-warming survival rates of biopsied and non-biopsied day-3 embryos vitrified on day 4. They demonstrated similar survival, implantation, and clinical pregnancy live birth rates between those vitrified without or following day-3 blastomere biopsy.
Prompted by the aforementioned observations, we sought to assess the efficacy and clinical outcomes of PGT-M following blastomere biopsy of fresh vs thawed day-3 cleavage-stage embryos.
Patients and methods
We reviewed the computerized files of all consecutive patients admitted to our IVF-PGT-M program, for the prevention of single gene disorders based on multiplex PCR programs designed for haplotyping using informative microsatellites markers [11], from September 2016 to March 2020. We included only patients undergoing their first three IVF- PGT-M attempt, who had at least one day-3 embryo available for genetic evaluation. The study was approved by the IRB of the Sheba Medical Center ethical committee (IRB approval no. SMC-7352-20).
All the usual indications for IVF/ICSI and accepted protocols for ovarian stimulations (OS) previously described [3, 12] were included. Laboratory procedures and molecular diagnosis were thoroughly presented elsewhere [11]. Embryos underwent vitrification, using a vitrification kit (SAGE Vitrification Kit, SAGE Media, USA), prior or following blastomere biopsy. Ongoing pregnancy was defined when the pregnancy had completed ≥ 8 weeks of gestation following fetal heartbeat.
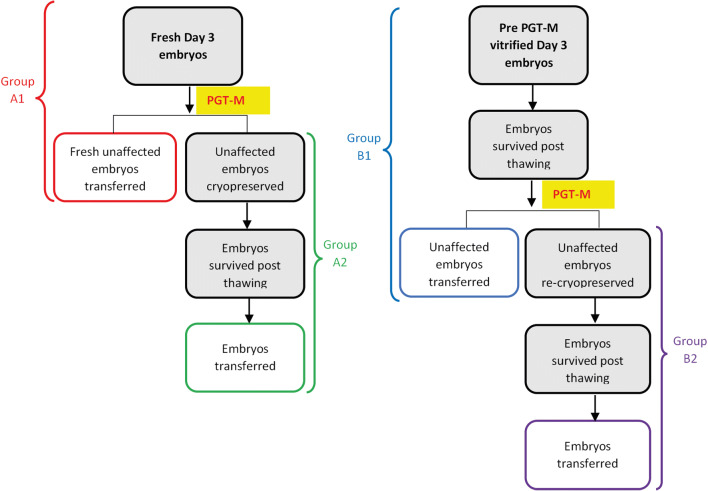
In our PGT-M program, we use blastomere biopsies on either fresh day-3 embryos followed by the transfer of unaffected embryos 1–2 days later, and the surplus unaffected embryos are vitrified; or alternatively, cleaved day-3 embryos are vitrified for a subsequent frozen-thawed embryo transfer (FET) cycle. In the latter, embryos are thawed and undergo blastomere biopsies, and the unaffected embryos are transferred 1–2 days later, and the surplus unaffected embryos are re-vitrified for a subsequent FET cycle. Accordingly, in the present study, patients were divided into four groups (Fig. 1): Group A1 consisted of patients undergoing day-3 embryos biopsy followed by a fresh transfer of unaffected embryos 1–2 days later. Group A2 consisted of Group A1 patients that their surplus unaffected embryos were vitrified, thawed, and transferred in a subsequent FET cycle. Group B1 consisted of patients that their cleaved day-3 embryos were vitrified intact (without biopsy) for a subsequent FET cycle. In the latter, embryos were thawed and underwent blastomere biopsies, and the unaffected embryos were transferred 1–2 days later, while the surplus unaffected embryos were re-vitrified for a subsequent FET cycle. Group B2 consisted of Group B1 patients that their surplus unaffected embryos were re-vitrified, thawed, and transferred in a subsequent FET cycle.
Fig. 1.
Workflow of study groups’ allocations
The selection of treatment allocation (biopsy timing and vitrification) was the decision of the treating physician/embryologist and largely dependent on the patients’ characteristics, the mode of final follicular maturation triggering (hCG vs GnRH-agonist trigger), the day of ovum pick-up (OPU), and the number of embryos available for blastomere biopsy.
Molecular diagnoses of each embryo within the 4 study groups are classified as previously described [11]:“ Complete diagnosis – unaffected or affected embryo according to the genetic disorder examined; Incomplete diagnosis - suspected allele dropout or recombination; PCR failure – no DNA is available for diagnosis; Abnormal – the embryo has abnormal assembly of alleles – i.e. any structure different from one maternal and one paternal alleles matching the known haplotype, e.g. trisomy, monosomy or uniparental disomy.”
The Chi-square/t-test tests were used were appropriate. Significance was accepted at P < 0.05.
Figures were prepared using Adobe Illustrator version CC 2020/24 (Adobe, USA).
Results
Three hundred and sixty-eight patients underwent 529 PGT-M cycles in our center: 347 with day-3 embryos biopsied before undergoing vitrification (Group A1) and 182 following vitrification and thawing (Group B1). The indications for PGT-M cycles were single gene disorders (n = 472), chromosomal abnormalities (n = 47), and sex selection (n = 10).
The laboratory/embryological data are presented in Table 1. While the mean number of oocyte retrieved (17.23 + 8.9 vs 9.9 + 4.8, p < 0.001, respectively) and 2PN (10.7 + 5.9 vs 7.2 + 3.9, p < 0.001, respectively) were significantly higher in Group B1, fertilization rate was significantly lower (65% + 20% vs 74% + 20%, p < 0.001, respectively), with no difference in the mean number of biopsied embryos (5.9 + 2.9 vs 5.6 + 3.3, p = 0.37, respectively), as compared with A1.
Table 1.
Patents’ laboratory/embryological data following the first embryo transfer
| Fresh day-3 blastomere Bx | FET day-3 blastomere Bx | P value | |
|---|---|---|---|
| Group A1 | Group B1 | ||
| No. of patients | 236 | 132 | |
| Total no. of cycles | 347 | 182 | |
| Mean age (years) | 32.6 ± 4.8 | 32.3 ± 4.5 | 0.425 |
| Indications for PGT-M | |||
| Single gene disorders | 301 | 171 | |
| Chromosomal abnormalities | 37 | 10 | |
| Sex selection | 9 | 1 | |
| Mean OPU | 9.9 ± 4.8 | 17.23 ± 8.9 | < 0.001 |
| Mean 2PN | 7.2 ± 3.9 | 10.7 ± 5.9 | < 0.001 |
| Mean FR | 0.74 ± 0.2 | 0.65 ± 0.2 | < 0.001 |
| Mean no of day-3 biopsied embryos | 5.6 ± 3.3 | 5.9 ± 2.9 | 0.368 |
| Total number of 2PN | 2509 | 1940 | |
| Total number of embryos cryopreserved | 1547 | ||
| Total number of embryos thawed | 1162 | ||
| Embryos survived post-thawing (%) | 1082/1162 (93.1) | ||
| Total number of embryos biopsied | 1964 | 1078 | |
| Total number of embryos with complete diagnosis | 1639 | 880 | |
| Embryos with complete diagnosis per embryo biopsied (%) | 1639/1964 (83.5) | 880/1078 (81.6) | 0.208 |
| No. of ETs | 253 | 152 | |
| No. of embryos transferred | 324 | 207 | |
| Ongoing PR (%) | 75/253 (29.6) | 40/152 (26.3) | 0.467 |
| Ongoing implantation rate (%) | 84/324 (25.9) | 46/207 (22.2) | 0.327 |
FET frozen-thawed embryo transfer
The percentage of embryos with complete diagnosis per embryo biopsied (%), the ongoing implantation, and the pregnancy rates were comparable between the two groups (Table 1).
When comparing the second ET in both groups (A2 and B2), there were no between group differences in the embryo survival rate post-thawing, nor the ongoing implantation and pregnancy rates (Table 2).
Table 2.
Patents’ laboratory/embryological data following the second embryo transfer
| Fresh day-3 blastomere Bx | FET day-3 blastomere Bx | P value | |
|---|---|---|---|
| Group A2 | Group B2 | ||
| Total number of unaffected embryos cryopreserved | 219 | 102 | |
| Embryos survived post-thawing (%) | 105/110 (95.4) | 43/44 (97.7) | 0.448 |
| No. of ETs | 92 | 38 | |
| No. of embryos transferred | 110 | 43 | |
| Ongoing PR (%) | 22/92 (23.9) | 10/38 (26.3) | 0.775 |
| Ongoing implantation rate (%) | 24/105 (22.8) | 10/43 (23.3) | 0.958 |
FET frozen-thawed embryo transfer
Moreover, no differences in ongoing implantation and pregnancy rates were observed when comparing the four study groups: A1, blastomere biopsy and a fresh transfer; A2, vitrification post-biopsy, thawing, and transfer; B1, thawing followed by blastomere biopsy and transfer; and B2, re-vitrification of embryo that underwent blastomere biopsy after thawing, re-thawing, and transfer.
Discussion
In the present study of patients undergoing IVF treatment cycle, utilizing PGT based on multiplex PCR programs, ongoing implantation, and pregnancy rates per transfer were comparable between those undergoing day-3 embryos biopsied before vitrification (Group A) or following vitrification and thawing (Group B).
The present study is in agreement with previously reported high post-vitrification survival rates of non-biopsied cleaved stage embryos [13, 14]. Additionally, the observed implantation (22.2–25.9%) and pregnancy (26.3–29.6%) rates in our study correspond to the figures reported by the ESHRE PGT-M consortium of 22% and 23% implantation and overall clinical pregnancy rates following day-3 biopsy, respectively [15].
As expected, Group B1 patient undergoing day-3 embryos vitrification (without biopsy) for a subsequent FET cycle yielded significantly higher numbers of oocytes and 2PN embryos, as compared with Group A1. These are the patients with more vigorous stimulation, prone to develop OHSS, as reflected by higher number of oocytes and 2PN embryos, who were more frequently allocated to freeze-all. This might also explain their lower fertilization rate. It is well established, for example, that patients suffering from polycystic ovary syndrome are known to achieve higher oocytes yield, with lower fertilization rate (reduced quality) and same number of top-quality embryos for transfer [16].
These observations justified the more conservative approach applying the freeze-all policy in patients who vigorously respond to OS and are at risk to develop severe OHSS.
While in the past, embryos cryopreservation was carried by the slow freezing method following warming and embryo transfer [17], nowadays, technology improvement and the use of embryos vitrification have dramatically improved post-thawing embryo survival, as compared with the slow freezing method [18]. In the present study, pre-biopsy thawing survival rate was 93.1% of vitrified intact embryos, 95.4% of embryos vitrified following blastomere biopsy, and 97.7% of embryos that underwent double vitrification, the first before and the second after blastomere biopsy. Moreover, the timing of embryos vitrification has no effect on the genetic evaluation, with the same percentage of embryos with complete diagnosis per embryo biopsied in the different groups (Table 1). Our observations suggest that the timing of embryos vitrification, whether prior or following blastomere biopsy, has no detrimental effect on post-thawing embryo survival rate, nor their potential ongoing implantation and pregnancy rates.
A thorough search of the literature revealed 4 studies that relate to the optimal timing of day-3 embryo biopsy and its effect on embryo survival and implantation potential, following cryopreservation and thawing. Joris et al. [7] studied human embryos of good morphological quality derived from abnormal fertilization, in an attempt to evaluate the influence of the embryo biopsy procedure on survival after cryopreservation. Three small groups of embryos were evaluated, control (n = 20), drilling-only (n = 16), and biopsy (n = 29). The blastomeres’ number and survival rate were significantly lower following slow freezing and thawing in the drilling-only and in the embryo biopsy groups, as compared with the control group. Moreover, since they used embryos derived from abnormal fertilization, no transfer was attempted, and no data on the effect of timing of biopsy on embryos’ implantation potential could be achieved. On the contrary, Shinar et al. [10] have retrospectively studied all of their PGT patients with good-quality embryos available for cryopreservation by the slow freezing method. Of the 65 patients included, 44 patients had 145 embryos that were biopsied before cryopreservation and 21 patients had 228 embryos that were biopsied after thawing. While mean survival of embryos and intact embryo survival were significantly greater in the latter group, no in-between group differences were observed in pregnancy rates per transfer cycle.
Zhang et al. [9] studied 50 abnormally fertilized embryos randomly allocated to the control and biopsy groups. They demonstrated that the survival rate after warming in the non-biopsied vitrified cleavage control group was significantly higher than in the biopsied vitrified cleavage group (23/25 (92%) vs 16/25 (64%), respectively). Moreover, the high osmotic potential of the medium caused the blastomeres to shrink dramatically, resulting in blastomeres escaping the zona pellucida during the cooling and warming procedures, in 6 out of 9 destroyed embryos. On the other hand, and in accordance with our study, Kahraman et al. [10] compared the post-warming survival rates of biopsied and non-biopsied day-3 embryos vitrified on day 4. The embryo survival rate after warming in the biopsied and non-biopsied groups was similar (53/59 (89.8%) versus 55/64 (85.9%), respectively), with comparable implantation, clinical pregnancy and live-birth rates.
Of notice, the aforementioned studies consisted of small sample sizes of up to 373 and 108 slow-freezed or vitrified embryos, respectively, while we studied > 3000 vitrified embryos. Moreover, while cryopreservation carried by the slow freezing method had a detrimental effect on post-thawing survival rate on biopsied embryos [7, 8], when summarizing all vitrified embryos in Zhang et al. [9] and Kahraman et al. [10] studies, 69/84 (84.1%) of the biopsied embryos and 78/89 (87.6%) of the non-biopsied embryos survived thawing. Figures that were not significantly different (p = 0.3), and were in agreement with our observation. Limitations of this study are the retrospective analysis. The study patients underwent up to 3 IVF-PGT-M attempts, which might introduce confounders of repeated measures. Moreover, women included in the study were treated by different OS protocols, so follicles/oocytes exposed to different gonadotrophins were included.
Conclusion
It might be therefore concluded that in PGT-M cycles, the timing of embryos vitrification, whether prior or following blastomere biopsy, has no detrimental effect on post-thawing embryo survival rate, nor their potential ongoing implantation and pregnancy rates. Further prospective studies of sibling embryos are warranted.
Acknowledgment
The authors would like to thank Ms. Moran Madari for her contributions to data collection and secretarial assistance.
Code availability
Not applicable.
Authors’ contribution
All authors read and approved the final manuscript.
Data availability
Raw data were generated at the Sheba Medical Center. Derived data supporting the findings of this study are available within the article and its supplementary materials.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Handyside AH, Pattinson JK, Penketh RJ, Delhanty JD, Winston RM, Tuddenham EG. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1989;1(8634):347–349. doi: 10.1016/S0140-6736(89)91723-6. [DOI] [PubMed] [Google Scholar]
- 2.De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C. ESHRE PGD consortium data collection XIV-XV: cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013. Hum Reprod. 2017;32(10):1974–1994. doi: 10.1093/humrep/dex265. [DOI] [PubMed] [Google Scholar]
- 3.Orvieto R, Brengauz M, Feldman B. A novel approach to normal responder patient with repeated implantation failures--a case report. Gynecol Endocrinol. 2015;31:435–437. doi: 10.3109/09513590.2015.1005595. [DOI] [PubMed] [Google Scholar]
- 4.Orvieto R. Ovarian hyperstimulation syndrome- an optimal solution for an unresolved enigma. J Ovarian Res. 2013;6(1):77. doi: 10.1186/1757-2215-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theobald R, SenGupta S, Harper J. The status of preimplantation genetic testing in the UK and USA. Hum Reprod. 2020;35(4):986–998. doi: 10.1093/humrep/deaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing S, Luo K, He H, et al. Obstetric and neonatal outcomes in blastocyst-stage biopsy with frozen embryo transfer and cleavage-stage biopsy with fresh embryo transfer after preimplantation genetic diagnosis/screening. Fertil Steril. 2016;106(1):105–112.e4. doi: 10.1016/j.fertnstert.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Joris H, Van den Abbeel E, Vos AD, Van Steirteghem A. Reduced survival after human embryo biopsy and subsequent cryopreservation. Hum Reprod. 1999;14(11):2833–2837. doi: 10.1093/humrep/14.11.2833. [DOI] [PubMed] [Google Scholar]
- 8.Shinar S, Kornecki N, Schwartz T, Mey-Raz N, Amir H, Almog B, Shavit T, Hasson J. Timing embryo biopsy for PGD – before or after cryopreservation? Gynecol Endocrinol. 2016;32:756–758. doi: 10.1080/09513590.2016.1177010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Trokoudes KM, Pavlides C. Vitrification of biopsied embryos at cleavage, morula and blastocyst stage. Reprod BioMed Online. 2009;19(4):526–531. doi: 10.1016/j.rbmo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Kahraman S, Candan ZN. Outcomes of vitrified-warmed day-4 embryos after day-3 cleavage-stage biopsy. Reprod BioMed Online. 2010;21(5):636–641. doi: 10.1016/j.rbmo.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Feldman B, Aizer A, Brengauz M, Dotan K, Levron J, Schiff E, Orvieto R. Pre-implantation genetic diagnosis-should we use ICSI for all? J Assist Reprod Genet. 2017;34(9):1179–1183. doi: 10.1007/s10815-017-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orvieto R, Feldman N, Lantsberg D, Manela D, Zilberberg E, Haas J. Natural cycle frozen-thawed embryo transfer-can we improve cycle outcome? J Assist Reprod Genet. 2016;33(5):611–615. doi: 10.1007/s10815-016-0685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alpha Scientists In Reproductive Medicine The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod BioMed Online. 2012;25:146–167. doi: 10.1016/j.rbmo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 14.ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online. 2017;35(5):494–510. doi: 10.1016/j.rbmo.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Harper JC, Coonen E, De Rycke M, et al. ESHRE PGD consortium data collection X: cycles from January to December 2007 with pregnancy follow-up to October 2008. Hum Reprod. 2010;25(11):2685–2707. doi: 10.1093/humrep/deq228. [DOI] [PubMed] [Google Scholar]
- 16.Kim MK, Park JK, Jeon Y, Choe SA, Lee HJ, Kim J, Chang EM, Kim JW, Lyu SW, Kim JY, Kwak IP, Lee WS, Yoon TK. Correlation between morphologic grading and Euploidy rates of blastocysts, and clinical outcomes in in vitro fertilization preimplantation genetic screening. J Korean Med Sci. 2019;34(4):e27. doi: 10.3346/jkms.2019.34.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305(5936):707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 18.Lin TK, Su JT, Lee FK, Lin YR, Lo HC. Cryotop vitrification as compared to conventional slow freezing for human embryos at the cleavage stage: survival and outcomes. Taiwan J Obstet Gynecol. 2010;49(3):272–278. doi: 10.1016/S1028-4559(10)60060-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at the Sheba Medical Center. Derived data supporting the findings of this study are available within the article and its supplementary materials.