Abstract
The application of natural antibrowning agents for fresh-cut products has been recently considered. This study manifested the efficiency of coconut liquid endosperm, coconut water (CW), from mature tall (cooking) coconut (C-CW) and yellow dwarf coconut (Y-CW) on browning inhibition of ‘Gala’ apple wedges during storage at 4 ± 1 °C for 9 days. The apple wedges were immersed in water (control), C-CW or Y-CW for 1 min. The visual appearance, superficial colour attributes, browning pigment concentration, total phenols concentration, polyphenoloxidase (PPO) activity and reducing antioxidant capacity of apple wedges were monitored. Moreover, antioxidant activity of both C-CW and Y-CW was also observed. Antioxidant activity of Y-CW was higher than that of C-CW. Both of the CW immersions maintained visual appearance, whiteness and lightness values as well as delayed the increased yellowness and brownness values of hypanthium (flesh) and mesocarp (core) of apple wedges. The browning pigment concentration and PPO activity were obviously lowered by both CW immersions. Total phenols concentration and antioxidant activity of C-CW and Y-CW immersed apple wedges were higher than those of control samples. In conclusion, both of the mature coconut liquid endosperms are feasible natural agent inhibiting browning incidence of fresh-cut fruits during storage.
Keywords: Polyphenol oxidase, Coconut water, Browning incidence, Fresh-cut apples
Introduction
The demand of fresh-cut products has been recently increased regarding to the increment of health concern and modern life style. Fresh-like quality and desirable appearance are the main factors influencing consumer’s acceptability and marketability of fresh-cut products. It is commonly recognised that fresh-cut product quality attributes change rapidly after processing due to tissue damages. The minimal process stimulates metabolic rate, wound-ethylene production and undesirable biochemical reactions limiting marketable quality and shelf-life of fresh-cut products. Browning incidence is one of main problems leading undesirable visual appearance. It occurs rapidly within few minutes after cut and exposed to aerobic atmosphere (McEvily et al. 1992). The browning incidence of fresh-cut products is mostly caused by enzymatic browning reaction which known as an oxidative reaction of phenolic compounds stimulated by polyphenoloxidase (PPO) activity under aerobic condition (Ayaz et al. 2008; Wessels et al. 2014). The derived complex polymers from the reaction are responsible for browning of fresh-cut fruit and vegetables (McEvily et al. 1992). Among fresh-cut fruits, fresh-cut apple is recognised as one of popular fresh-cut products. In market, the recommend storage condition of fresh-cut fruits including fresh-cut apples is at 4 °C for 7 days. However, merely refrigerated storage is insufficient to prevent browning incidence which is the main factor limiting acceptability of fresh-cut fruits (Wessels et al. 2014). Our previous work found that browning incidence in fresh-cut ‘Gala’ apple appeared within a few minute after cut and the severity of browning during storage was obviously accompanied with the increments of PPO activity and total phenolic compounds concentration (Supapvanich et al. 2018). Similar results also reported by Lee et al. (2003) and Wessels et al. (2014) in fresh-cut apples.
Several approaches such as ascorbic acid and its derivatives treatments, edible coatings, modified atmosphere, salt solutions dips and sulphating (McEvily et al. 1992; Lee et al. 2003; Guan and Fan 2010) have been commercially used to prevent browning in fresh-cut products. Ascorbic acid and it derivatives immersions are commonly used to control browning due to their antioxidant properties however the effects of these chemicals are temporary. The browning incidence can be recovered during storage. Sulfiting is recognised as an industrial use for inhibiting browning incidence in fruit and vegetable products, including fresh-cut apples (Wessels et al. 2014). The treatments of sulphur dioxide and sulphites can achieve the long-term antibrowning effects due to the inhibition of PPO activity (McEvily et al. 1992). According to food safety, the use of sulfiting agents for food is limited. The application of natural agents can be alternative methods responding the demand of consumers. Many natural agents such as honey (Wen et al. 2018 and Supapvanich and Boonyaritthongchai 2016), pineapple fruit extract (Supapvanich et al. 2017), Aloe vera gel coating (Supapvanich et al. 2016) and the plant extracts from pumpkin, hibiscus flower and pelargonium root (Wessels et al. 2014) had been reported for browning control in fresh-cut fruits. These natural agents can serve as reducing agents because of the high contents of secondary metabolites and antioxidants (Supapvanich et al. 2016 and Wessels et al. 2014). According to our previous work, liquid endosperm, coconut water (CW), of aromatic young coconut has ability to prevent browning incidence of fresh-cut apples due to the retardation of PPO activity (Supapvanich et al. 2018). CW is recognised as a traditional drink for refreshing and hydration in tropical countries and oceanic islands (Prades et al. 2012). It contains approximately 3–7% of sugars, high potassium (K), estrogen-like compounds, ascorbic acid and phenolic compounds such as catechin and salicylic acid (Mahayothee et al. 2016). Mantena et al. (2003) reported the presence of reducing properties of CW was associated with ascorbic acid concentration. They also suggested that CW is an effective antioxidant agent by scavenging superoxide. Santos et al. (2013) also reported that CW from green dwarf, yellow dwarf, red dwarf and yellow Malaysian coconuts contained high antioxidant activity and reflected a reduction of reactive oxygen species (ROS) generation. These suggest that CW can reduce oxidative reactions including enzymatic browning reaction (Supapvanich et al. 2018). However, the demand of CW from young coconut as healthy and refreshing drink had been recently increased and high. Compared to CW from aromatic young coconut, CW from mature coconuts is waste from coconut milk and copra industries. Thus, we were interested in the investigation of antibrowning efficiency of CW from mature commercial coconuts, tall and yellow-dwarf coconuts, by using fresh-cut ‘Gala’ apples as the fresh-cut fruit model.
Materials and methods
Raw materials preparations and experiment
The mature tall coconut cv. ‘Sawi Hybrid No.1′ (cooking coconut) and yellow dwarf coconut were derived from a coconut orchard at Ladkrabang District, Bangkok, Thailand. The cooking and yellow dwarf coconuts were selected at full maturity stage, one year after anthesis. The fruit were peeled and the liquid endosperm (CW) was taken. CW was pasteurized at 80 °C for 30 s and then immediately cooled down to 4 °C by placing an ice bath.
Apple (Malus domestic) cv. ‘Gala’ fruit were purchased from “Talaad Thai”, a fruit market in Pathumthani Province, Thailand. The fruit were cleaned with tap water and then dipped in 200 µL L−1 of sodium hypochlorite for 2 min to eliminate contaminated microorganisms on peel. The fruit were then vertically cut into eight equal wedges and the seeds and core were then removed by using a sharp knife. The apple wedges were immediately immersed into sterilised water (control), C-CW or Y-CW for 1 min. The six apple wedges were packed in a polyethylene (PE) plastic box and then stored at 4 ± 1 °C for 9 days. Triplicates of each treatment were taken in every 3 days of storage period in order to determine visual appearance, colour attributes, browning index, browning pigment concentration, total phenols concentration and polyphenol oxidase (PPO) activity.
Visual appearance and superficial colour attributes measurement
The visual appearance was expressed using the photograph of apple wedges during storage period. The superficial colour attributes were measured using a Minolta CR 300 colorimeter (Minolta Co. Ltd., Japan). The L*, a* and b* values of hypanthium (flesh) and mesocarp (core) sections of apple wedges were monitored. The whiteness index (WI) and browning values were calculated according the equation of Bolin and Huxsoll (1999) (1) and the equation of Palou et al. (1991) (2), respectively, as shown below.
| 1 |
| 2 |
where .
Browning pigment concentration measurement
A 2 g of cutting surface tissue of apple wedges was homogenised with 60% (v/v) of ethanol. The homogenate was stirred at room temperature for 30 min and then filtered through a Whatman no. 1 filter paper. The absorbance at 420 nm wavelength (OD420) of filtrate was determined. Browning pigment concentration was expressed as OD420 per gram fresh weight (OD420 g−1).
Total phenols concentration assay
Total phenol concentration of cut surface tissue of apple wedges was determined using the method of Slinkard and Singleton (1997). A 2 g of sample was homogenised with 60% (v/v) of ethanol for 3 min and then filtered through a Whatman no.1 filter paper. One mL of filtrate was mixed with 1 mL of 50% (v/v) Folin–Ciocalteu reagent and then 2 mL of saturated Na2CO3 solution was added. The mixture was mixed with a vortex mixer and then left at ambient temperature for at least 30 min. After that, the absorbance of the mixture was measured at 750 nm wavelength. The total phenols concentration was reckoned using a linear equation of gallic acid standard curve. The data was expressed as mg gallic acid equivalent per kg fresh weight (mg kg−1).
PPO activity assay
A 2 g of sample was homogenised in 30 mL of the mixture of cold 0.2 M sodium phosphate buffer of pH 6.5, 0.5% (v/v) Trition X-100 and 1% (w/v) of polyvinylpyrolidone. The homogenate was stirred at 4 °C before centrifuged at 12,000 g and 4 °C for 20 min. The supernatant was collected and stored in an ice bath until the enzyme activity was determined. PPO activity was assayed according to the method of Galeazzi et al. (1981) with slight modification. One mL of enzyme extract was placed in a water bath at 30 °C and then 2 mL of substrate solution, 0.05 M catechol in 0.2 M sodium phosphate buffer pH 6.5, was added. PPO activity was determined as the increase in absorbance at 420 nm wavelength per 15 s. The initiate linear of the activity cure was used to present the enzyme activity. Unit (U) of PPO activity was defined as the increased absorbance by 0.001 min−1. The data was expressed as U per gram fresh weight (U g−1).
Antioxidant activity assay
A 2 g of sample was homogenized in 60% (v/v) ethanol and then filtrated through a Whatman no. 1 filter paper. The filtrate was collected to assay ferric reducing antioxidant power (FRAP) according to the method of Benzie and Strain (1996). A 0.1 mL of filtrate was added into 2.9 mL of FRAP reagent, consisting of acetate buffer pH 3, 10 mM 2,4,6-tripyridyl-1,3,5-triazine (TPTZ) and 20 mM ferric chloride hexahydrate in the ratio of 10:1:1 and was then mixed using a vortex mixer. The mixture was left at ambient temperature for at least 30 min before the absorbance at 630 nm wavelength was measured. The antioxidant activity was calculated using a linear equation of Trolox standard curve. The data was expressed as mole Trolox equivalents per kg fresh weight (mole kg−1).
Statistical analysis
The experiment was performed using completely randomized design (CRD) and data analysis was carried out using analysis of variance (ANOVA). Means comparison was performed using the Duncan’s multiple range test (DMRT) at the 5% level of significant differences (p ≤ 0.05).
Results and discussions
Visual appearance
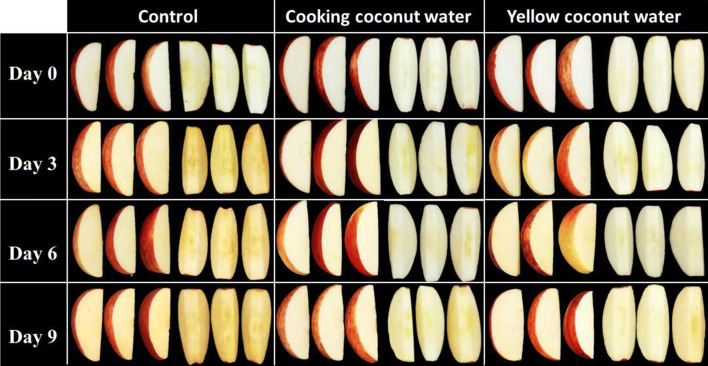
The visual appearance of apple wedges immersed in C-CW and Y-CW was showed in Fig. 1. Both of the CW immersions inhibited browning incidence of apple wedges throughout the storage period when compared to control treatment. After treatment (day 0), control samples turned to brown obviously in a short time (approximately 15 min after cut) whilst C-CW and Y-CW immersed apple wedges did not catch sight of browning incidence. The browning incidence of mesocarp (core) was evidently noticeable compared to hypanthium (flesh). It is widely acknowledged that the core of apple turns to brown rapidly compared to the flesh after cut. This suggests that the mesocarp of apple is more sensitive to enzymatic browning reaction than hypanthium. Likewise, a physiological disorder called heart-brown or internal browning of apples commonly manifests around the core. Murata et al. (1995) addressed that PPO activity was mainly found in the core tissue of apple and this was concomitant with the quick browning of the core compared to the flesh. During storage period, the increased browning incidence of both CW immersed apple wedges was not evident; however, that of C-CW immersed apple wedges was imperceptibly higher than that of Y-CW immersed apple wedges. Thus, this result revealed the efficiency of both CW immersions maintaining visual appearance and eliminating browning incidence of apple wedges during cold storage.
Fig. 1.
Visual appearance of fresh-cut ‘Gala’ apple wedges treated with C-CW and Y-CW during storage at 4 ± 1 °C for 9 days
Superficial colour attributes
The effects of both C-CW and Y-CW immersions on superficial colour changes of apple wedges were presented in Fig. 2. A declined L* value of both hypanthium and mesocarp of control samples was observed over the storage period. Both CW immersions obviously maintained the high L* value of hypanthium and mesocarp of apple wedges and was significantly higher than those of control samples (p ≤ 0.05). There were no significant difference in L* value between hypanthium and mesocarp of the both C-CW and Y-CW treated apple wedges throughout storage. An increased a* value was found in the both hypanthium and mesocarp of apple wedges during storage (Fig. 2b1, b2). It was also found that a* value of the mesocarp was higher than that of hypanthium. However, a* value of apple wedges in each treatment was not significantly different over storage period. Likewise, increased b* value was also observed in all treatments throughout storage (Fig. 2c1, c2). The b* value of mesocarp was higher than that of hypanthium. Both CW immersions obviously delayed the increase in b* value of apple wedges compared to control samples. We also found that the b* value of both hypanthium and mesocrap of control apple wedges were significantly higher than those of C-CW and Y-CW immersed apple wedges (p ≤ 0.05). The significant lower L* and higher b* values of control apple wedges compared to both CW treated apple wedges indicated the increment of browning incidence of apple wedges during storage. The changes of L* and b* values were consistent with the browning appearance of apple wedges as shown in Fig. 1. Likewise our previous work, young aromatic CW immersion maintained desirable visual appearance of fresh-cut ‘Gala’ apples due to the retention of high L* value and delayed the increased total colour difference (∆E*) value during storage at 4 °C for 7 days (Supapvanich et al. 2018). Therefore, the recent work indicated that the immersions of C-CW and Y-CW potentially maintained colour attributes of apple wedges by interception of decreased L* value and increased b* value during storage.
Fig. 2.
L* (a), a* (b) and b* (c) values of hypanthium (1) and mesocarp (2) of ‘Gala’ apple wedges treated with C-CW and Y-CW compared with control sample during storage at 4 ± 1 °C for 9 days. Data presented the means of triplicates with S.D. (as vertical bar). Asterisks indicate the differences between untreated and treated samples **(p ≤ 0.01), *(p ≤ 0.05)
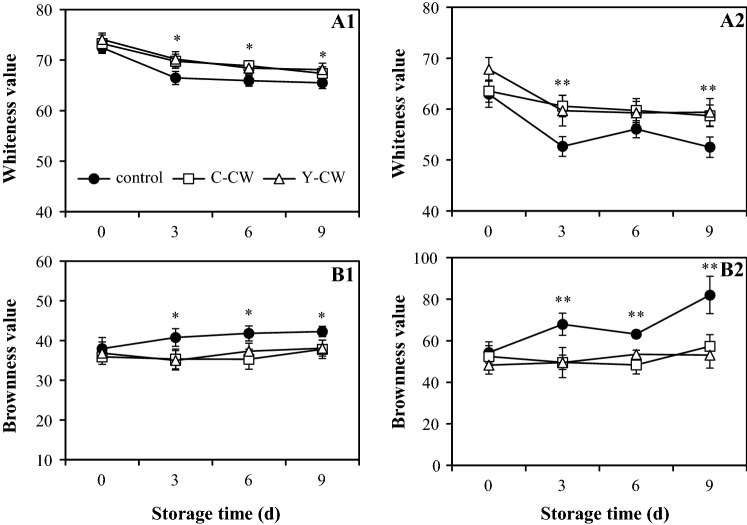
Whiteness and brownness values
A decreased whiteness and increased browning values were noticeable in the apple wedges throughout storage period (Fig. 3). The decreased whiteness value of both hypanthium and mesocarp of apple wedges was retarded by the immersion of C-CW or Y-CW. A marked decrease in whiteness value of both hypanthium and mesocarp of control apple wedges was observed throughout storage period when compared to both of the treated samples. The statistical difference in whiteness value between C-CW and Y-CW immersed apple wedges was not found throughout storage. Whereas, browning value of control apple wedges marked increased during storage period and was significantly higher than that of both CW immersed apple wedges (p ≤ 0.05). Both C-CW and Y-CW immersions could inhibit the increased browning value of both hypanthium and mesocarp of apple wedges. Likewise to whiteness value, no significant difference in browning value between C-CW and Y-CW immersed apple wedges was observed over storage period. These suggested that the immersion of C-CW or Y-CW retained whiteness value and inhibited the increment of browning value of apple wedges during storage. The changes in whiteness and browning values of apple wedges were consistent with the visual appearance (Fig. 1) and superficial colour attributes (Fig. 2). The preserved whiteness value was positively related to higher L* value and lower b* as well as browning values of both CW immersed apple wedges. These were also in agreement with previous work that the whiteness of fresh-cut ‘Gala’ apples was maintained and browning index was inhibited by the immersion of CW from young aromatic coconut during cold storage at 4 °C for 7 days (Supapvanich et al. 2018).
Fig. 3.
Whiteness (a) and browning (b) values of hypanthium (1) and mesocarp (2) of ‘Gala’ apple wedges treated with C-CW and Y-CW compared with control sample during storage at 4 ± 1 °C for 9 days. Data presented the means of triplicates with S.D. (as vertical bar). Asterisks indicate the differences between untreated and treated samples **(p ≤ 0.01), *(p ≤ 0.05)
Browning pigment, total phenols and PPO activity
Figure 4 shows factors involving enzymatic browning reaction such as the concentrations of browning pigment and total phenols and the activity of PPO of C-CW and Y-CW immersed apple wedges during storage. It was found that browning pigment concentration of control apple wedges was significantly higher than that of C-CW and Y-CW immersed apple wedges since the initial day of storage and then markedly increased during storage period (p ≤ 0.05). Both of the CW immersions delayed the increased browning pigment concentration during storage for 6 days when compared to control treatment. However, on day 9 of storage, no significant difference in browning pigment concentration between treatments was found. After immersions (day 0), the total phenols concentration of Y-CW immersed apple wedges was higher than that of control and C-CW immersed apple wedges, respectively, and then decreased afterwards. On day 3 and 6 of storage, the total phenols concentration of C-CW and Y-CW immersed apple wedges was significantly higher than that of control apple wedges (p ≤ 0.05). At the end of storage (day 9), total phenols concentration of control and C-CW immersed apple wedges was close whilst that of Y-CW immersed apple wedges was significantly higher than other samples (p ≤ 0.05). The PPO activity of both CW immersed apple wedges was inhibited and remained constant over storage compared to control samples. The PPO activity of control apple wedges was obviously higher than that of CW and Y-CW immersed apple wedges throughout storage period (p ≤ 0.05). The PPO activity of both CW immersed apple wedges was obviously low and they were not different over the storage. It is commonly acknowledged that the main three factors inducing enzymatic browning reaction are oxygen exposure, PPO activity and phenolic compounds (McEvily et al. 1992; Wessels et al. 2014). We also noticed that total phenols content of both CW immersed apple wedges trended to be higher than that of control samples. The high concentration of total phenols content in both of CW immersed apple wedges seemed to be not related to the increased browning pigment and PPO activity. Regarding to the report of Mahayothee et al. (2016), CW contained high phenolic compounds, especially catechin and salicylic acid. Thus, the high total phenols concentration in cut-surfaces of both CW immersed apple wedges might be derived from coconut waters. It is recognised that not all phenolic compounds are substrates of PPO activity which certain phenolic substances having antioxidant activity could retard oxidative enzymatic browning reaction. Previous works suggested that the high antioxidant activity in CW was accompanied with the content of phenolic compounds such as catechin and salicylic acid (Mantena et al. 2003; Mahayothee et al. 2016). Moreover, Zhou et al. (2015) suggested that salicylic acid is a potentially competitive inhibitor of PPO which it prevented browning incidence and PPO activity in fresh-cut Chinese chestnut. These previous works supported the recent results that the immersion of CW could retard enzymatic browning reaction due to the inhibition of PPO activity.
Fig. 4.

Browning pigment (a), total phenols (b) and PPO activity (c) of ‘Gala’ apple wedges treated with C-CW and Y-CW compared with control sample during storage at 4 ± 1 °C for 9 days. Data presented the means of triplicates with S.D. (as vertical bar). Asterisks indicate the differences between untreated and treated samples **(p ≤ 0.01), *(p ≤ 0.05)
Antioxidant activity in cut-surface of apple wedges and coconut waters
The antioxidant activity on cut-surface of apple wedges and in both C-CW and Y-CW were shown in Fig. 5a, and b, respectively. We found that the antioxidant activity of Y-CW immersed apple wedges after treatment (day 0) was significantly higher than other samples after immersion (Fig. 5a). Whereas, the antioxidant activity of C-CW immersed apple wedges was slightly higher than that of control samples. During storage, the antioxidant activity of control apple wedges was evidently lower than that of C-CW and Y-CW immersed apple wedges. The higher antioxidant activity of Y-CW immersed apple wedges after treatment was associated with the higher antioxidant activity of Y-CW when compared to that of C-CW as shown in Fig. 5b. These suggested that the higher antioxidant activity on cut-surface of apple wedges was from the immersion of CW. Thus, the increased antioxidant activity on cut-surface of both C-CW and Y-CW treated apple wedges was related to the retardation of oxidative enzymatic browning reaction of apple wedges during storage.
Fig. 5.
Antioxidant activity of ‘Gala’ apple wedges during storage at 4 ± 1 °C for 9 days. (a) and antioxidant activity of C-CW and Y-CW (b). Data presented the means of triplicates with S.D. (as vertical bar). Asterisks indicates the differences between untreated and treated samples **(p ≤ 0.01), *(p ≤ 0.05)
Conclusion
Both of the CW immersions could maintain desirable visual appearance due to retain L* and whiteness values of hypanthium and mesocarp of apple wedges during storage. The increased brownness and b* values of both hypanthium and mesocarp of apple wedges were inhibited by both of the CW immersions. Both C-CW and Y-CW potentially inhibited enzymatic browning reaction due to the retardation of PPO activity. The total phenols concentration in cut-surface of apple wedges was increased by CW immersions and seemed to be not related to the increased browning of apple wedges. The antioxidant activity in cut-surface of apple wedges was increased by CW immersions which Y-CW immersion increased antioxidant activity rather than C-CW immersion. In conclusion, CW from mature coconuts could be an effective alternative to use as natural antibrowning agent for fresh-cut fruits.
Acknowledgments
Authors would like to thank Department of Agricultural Education, Faculty of Industrial Education and Technology, KMITL for supporting laboratory facilities.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ayaz FA, Demira O, Torun H, Kolcuoglu Y, Colak A. Characterization of polyphenoloxidase (PPO) and total phenolic contents in medlar (Mespilus germanica L.) fruit during ripening and over ripening. Food Chem. 2008;106:291–298. doi: 10.1016/j.foodchem.2007.05.096. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bolin HR, Huxsoll CC. Control of minimally process carrot (Caucus carota) surface discoloration caused by abrasion peeling. J Food Sci. 1991;56:416–418. doi: 10.1111/j.1365-2621.1991.tb07975.x. [DOI] [Google Scholar]
- Galeazzi MAM, Sgarbierri VC, Costantinides SM. Isolation, purification and physic-chemical characterization of polyphenoloxidase (PPO) form a dwarf variety of banana (Musa cavedishii L.) J Sci Food Agric. 1981;46:150–155. [Google Scholar]
- Guan W, Fan X. Combination of sodium chlorite and calcium propionate reduces enzymatic browning and microbial population of fresh-cut “Granny Smith” apples. J Food Sci. 2010;75:M72–M77. doi: 10.1111/j.1750-3841.2009.01470.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Park HJ, Lee CY, Choi WY. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT Food Sci Technol. 2003;36:323–329. doi: 10.1016/S0023-6438(03)00014-8. [DOI] [Google Scholar]
- Mahayothee B, Koomyart I, Khuwijitaru P, Siriwongwilaichat P, Nagle M, Müller J. Phenolic compounds, antioxidant activity, and medium chain fatty acids profiles of coconut water and meat at different maturity stages. Int J Food Prop. 2016;19:2041–2051. doi: 10.1080/10942912.2015.1099042. [DOI] [Google Scholar]
- Mantena SK, Badduri SR, Siripurapu KB, Unnikrishnan MK. In vitro evaluation of antioxidant properties of Cocos nucifera Linn. Water Food/Nahrung. 2003;47:126–131. doi: 10.1002/food.200390023. [DOI] [PubMed] [Google Scholar]
- McEvily AJ, Iyengar R, Otwell WS. Inhibition of enzymatic browning in foods and beverages. Crit Rev Food Sci Nutr. 1992;32:253–273. doi: 10.1080/10408399209527599. [DOI] [PubMed] [Google Scholar]
- Murata M, Hiraide A, Murata C, Homma S, Sakuta M, Shimizu S, Kakiuchi N. Expression and induction of polyphenol oxidase in apple (Malus pumila) cell culture. Biosci Biotech Biochem. 1995;59(8):1472–1476. doi: 10.1271/bbb.59.1472. [DOI] [Google Scholar]
- Palou E, Lopez-Malo A, Barbosa-Canovas GV, Welti-Chanes J, Swanson GB. Polyphenoloxidase activity and colour of balanced and high hydrostatic pressure treated banana puree. J Food Sci. 1999;64:42–45. doi: 10.1111/j.1365-2621.1999.tb09857.x. [DOI] [Google Scholar]
- Prades A, Dornier M, Diop N, Pain JP. Coconut water preservation and processing; a review. Fruits. 2012;67:157–171. doi: 10.1051/fruits/2012009. [DOI] [Google Scholar]
- Saba MK, Sogvar OB. Combination of carboxymethyl cellulose-based coatings with calcium and ascorbic acid impacts in browning and quality of fresh-cut apples. LWT Food Sci Technol. 2016;66:165–171. doi: 10.1016/j.lwt.2015.10.022. [DOI] [Google Scholar]
- Santos JLA, Bispo VS, Filho ABC, Pinto IFD, Dantas LD, Vasconcelos DF, et al. Evaluation of chemical constituents and antioxidant activity of coconut water (Cocos nucifera L.) and caffeic acid in cell culture. An Acad Bras Ciênc. 2013;85:1235–1246. doi: 10.1590/0001-37652013105312. [DOI] [PubMed] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: Automation and comparison with manual methods. Am J Enol Viticult. 1997;28:49–55. [Google Scholar]
- Supapvanich S, Anartnet D, Krungpree C (2018) Efficiency of coconut water immersion inhibiting browning incidence on cut-surface of fresh-cut ‘Gala’ apples during storage. In: MATEC web of conferences. vol 192, 10.1051/matecconf/201819203004
- Supapvanich S, Boonyaritthongchai P. Visual appearance maintenance of fresh-cut 'Nam Dok Mai' mango fruit by honey dip. Int Food Res J. 2016;23(1):389–394. [Google Scholar]
- Supapvanich S, Mitrsang P, Srinorkham P, Boonyaritthongchai P, Wongs-Aree C. Effects of fresh Aloe vera gel coating on browning alleviation of fresh cut wax apple (Syzygium samarangenese) fruit cv. Taaptimjaan. J Food Sci Technol. 2016;53(6):2844–2850. doi: 10.1007/s13197-016-2262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supapvanich S, Mitrsang P, Srinorkham P. Effects of ‘Queen’ and ‘Smooth cayenne’ pineapple fruit core extracts on browning inhibition of fresh-cut wax apple fruit during storage. Int Food Res J. 2017;24(2):559–564. [Google Scholar]
- Wen B, Wu X, Boon-Ek Y, Xu L, Pan H, Xu P, et al. Effect of honey and calcium dips on quality of fresh-cut nectarine (Prunus persica L. Batsch) Agric Nat Res. 2018;52:140–145. [Google Scholar]
- Wessels B, Damm S, Kunz B, Schulze-Kaysers N. Effect of selected plant extracts on the inhibition of enzymatic browning in fresh-cut apple. J Appl Bot Food Qual. 2014;87:16–23. [Google Scholar]
- Zhou D, Li L, Wu Y, Fan J, Ouyang J. Salicylic acid inhibits enzymatic browning of fresh-cut Chinese chestnut (Castanea mollissima) by competitively inhibiting polyphenol oxidase. Food Chem. 2015;171:19–25. doi: 10.1016/j.foodchem.2014.08.115. [DOI] [PubMed] [Google Scholar]