Abstract
Nattokinase activity (NK), biogenic amine content and sensory properties of natto are of great significance to consumers, which are affected by strains and fermentation methods. In this study, changes in the pH, biogenic amine and free amino nitrogen (FAN) contents, NK and protease activities, and sensory characteristics of natto prepared using Bacillus subtilis GUTU09 combined with different strains (Lactobacillus, Bifidobacterium and Mucor) and fermentation methods were investigated. The combination of two strains showed the best fermentation performance among all samples. The NK and protease activity and FAN content in double-strain fermentation increased by 10.33 FU/g, 88.78 U/g, and 2.34 g/kg, respectively, compared with those in single-strain fermentation. Sensory evaluation demonstrated that mixed fermentation primarily affected the sensory acceptance. This method also reduced the contents of various biogenic amines in natto compared with single-strain fermentation. Tyramine, cadaverine, spermine, and spermidine were significantly reduced, whereas histamine was slightly increased. The total biogenic amines decreased from 390.76 mg/kg to a minimum of 16.16 mg/kg. Some Mucor strains also reduced the contents of various biogenic amines. In the dual-bacteria fermentation of Mucor and GUTU09, co-fermentation has advantages over stage-fermentation, with higher NK and protease activity and higher sensory scores. Correlation analysis showed that the formation and accumulation of some biogenic amines in natto prepared using different microbial combinations were related to NK activity and pH. All these results showed that the quality of natto was improved by mixed fermentation and suitable fermentation methods, which laid a foundation for its potential industrial application.
Keywords: Nattokinase, Bacillus subtilis GUTU09, Combination fermentation, Biogenic amines, Sensory evaluation
Introduction
Natto is a traditional fermented soy product prepared by fermenting soybeans with Bacillus subtilis. It has unique flavor and stickiness and contains health-promoting ingredients, such as plant sterols, fibers, daidzein, genistein, and some bioactive peptides from raw beans (Ahmad et al. 2014). The concentrations of various beneficial ingredients, such as isoflavones, vitamin K2, and antioxidants, increase during fermentation (Xu et al. 2015; Ali et al. 2018); thus, fermented soybean products can be used as functional food for preventing many diseases, such as cancer, obesity, and hypercholesterolemia (Kushida et al. 2018; Shin et al. 2016). Nattokinase (Sumi et al. 1986), an important active substance that can dissolve fibrin, is produced during the fermentation of natto. It is an effective thrombolytic enzyme in vitro and in vivo (Huang et al. 2013; Xu et al. 2014) and safer and more efficient in treating various thrombotic vascular diseases than clinical thrombolytic drugs. In addition, it has great potential to treat hypertension, Alzheimer's disease, and vitreoretinal diseases (Dabbagh et al. 2014). However, natto has a distinct smell and taste that are considered undesirable by most people. Therefore, adjusting the formula of the starter recipe of natto can enhance the sensory characteristics and general acceptability of the product and improve the content and activity of nattokinase, which prevents and relieves heart and brain thrombosis.
However, natto has some safety risks, including the considerable amounts of biogenic amines (BAs) (Tsai et al. 2007), which are common ingredients in high-protein fermented food, have low molecular weights, and form nonvolatile nitrogen-containing organic bases through the decarboxylation of free amino acids by microorganisms (Priyadarshani and Rakshit 2011). Furthermore, BAs are potential precursors of carcinogen nitrosamines. In humans, the excessive intake of BAs from food may cause adverse effects, such as migraine, hypertension, hypotension, rash, digestive problems, and even death in severe cases (Lu et al. 2015). Different BAs have been detected in cheese, alcoholic beverages, fish products, fermented meat products and fermented soy products (Benito 2019). Small amounts of putrescine and cadaverine are present in in nonfermented soy products, and spermine, and spermidine, which are considered naturally occurring amines, are present in raw soybeans; meanwhile, bacterium-related bioamines, such as histamine, tyramine, tryptophan, and beta-phenylethylamine, have not been detected from non-fermented soybean products (Toro-Funes et al. 2015; Regubalan and Ananthanarayan 2019). Given that natto contains abundant amino acids that are the precursors of biogenic amines, BA levels in natto products must be monitored. The strain of microorganism used in fermentation is another important factor affecting biogenic amines in natto, and thus strain selection is also crucial.
Natto is usually made by inoculating Bacillus in steamed soybeans. Natto products from single strain-fermented soybeans have not rich in function and a dominant natto taste, and their probiotic effects are limited. By contrast, many traditionally fermented food products are delicious and nutritious owing to the interactions of various microorganisms. In China, Sufu or Mucor-type Douchi (Fan et al. 2020), which is mainly fermented by Mucor, is a rich source of nutrition, has pleasant tastes, and is fresh and fragrant. In Korea, molds are often used in making fermented bean paste, which has been widely used in the country due to its diversified nutritional and health benefits, as well as unique aroma components (Shukla et al. 2015). Lactic acid bacteria and Bifidobacterium are often used as probiotics in food; they have beneficial effects on human health, particularly on nutrition, immune system, and gastrointestinal functions, and exert antitumor, anti-aging properties, and other important physiological functions (Karimi et al. 2018). Bacillus subtilis, often used in natto production, is also used as a probiotic because it improves intestinal microbial balance. When fermented with Lactobacillus plantarum, beans and grains in infant weaning food significantly improve nutrition (Adeyemo and Onilude 2013). When B. subtilis natto and Bif. animalis subsp. lactis v9 in a whole soybean solid-state co-fermentation, their gene expression levels affect each other, resulting in the overexpression of genes involved in substrate uptake and B. animalis subsp. Lactis metabolism and increase in cell concentration in the soybean medium (Wang et al. 2015). Similarly, red bean was fermented with co-culture of Bacillus subtilis and L. bulgaricus for the production of a novel natto product with multiple biological activities (Jhan et al. 2015). The fermentation mechanism of a microbial strain affects the natto production process.
A new strain with high nattokinase concentration, Bacillus subtilis GUTU09 (B9), was isolated from Chinese traditional fermented soybeans and used in natto preparation. Currently, how B9 single bacteria, multiple strains, and different fermentation methods affect the fermentation characteristics of natto is unclear. Determining such effect is helpful in understanding the role of strains in soybean fermentation for the preparation of high-quality natto. In this work, the effects of the new strain B9, different strain combinations, and fermentation methods on nattokinase activity, biogenic amine content, and natto sensory characteristics were investigated.
Materials and methods
Samples and chemicals
Putrescine dihydrochloride (≥ 98%, PU), 2-phenethylamine (≥ 99.5%, PHE), cadaverine dihydrochloride (98%, CA), tyramine (99%, TR), histamine dihydrochloride (99%, HI), tyramine hydrochloride (98%, TY), spermidine trihydrochloride (98%, SPD), and spermine (98%, SPM) were obtained from Sigma-Aldrich (Switzerland). Dansyl chloride (98%), thrombin (1000 U), and fibrinogen were purchased from Solarbio (Beijing, China). All the reagents used were of analytical grade.
Microorganisms
Mucor racemosus 40491 (M1) and Mucor wutungkiao 40854 (M4) were from China Center of Industrial Culture Collection. Actinomucor elegans 3118 (A8; Guangdong Culture Collection Center), B. animalis subsp. lactis BZ11 (China General Microbiological Culture Collection Centre [CGMCC] NO. 10224), B. animalis subsp. lactis BZ25 (CGMCC NO.10225), L. plantaroid SQ4 (M 2016002), and B. subtilis GUTU09 (B9) were isolated by our laboratory.
Inoculum preparation
For inoculum preparation, SQ4 were grown at 37 °C for 24 h, and BZ11 and BZ25 were grown at 37 °C for 48 h. Both bacteria were grown in an MRS broth (Zhang et al. 2014). B9 was grown in a liquid seed medium containing 10 g/L glucose, 5 g/L yeast extract, 10 g/L beef extract, and 5 g/L NaCl with pH of 7.0–7.5 at 37 °C and 180 rpm for 18 h. The cells were then harvested and re-suspended in sterilized physiological saline, and the cell concentrations were adjusted to 108 colony forming units (CFU)/mL. The culture solution of Mucor was prepared as follows: Mucor mycelium was inoculated on potato dextrose agar plate, cultured at 28 °C for 72 h, and washed with sterile normal saline, and the cell concentrations were adjusted to 108 CFU/mL. The spore solution was stored at 4 °C for storage.
Natto preparation
Small soybeans with particle diameters of 3–4.5 mm (Heilongjiang, China) were cleaned and soaked in water overnight at 20 °C. A total of 50 g of wet beans was weighed and put in a 250 mL conical flask, sterilized at 121 °C for 20 min, and cooled to 25 °C. The beans were then inoculated with 108 CFU/mL of SQ4, BZ11, BZ25, B9, and spore solution. The fermentation process using BZ11 and BZ25 mixed with B9 was carried out at 37 °C for 24 h under anaerobic conditions. The single-strain fermented natto by B9, double-strain fermented natto by B9 + probiotics (SQ4, BZ11 or BZ25) and B9 + Mucor (M1, M4 or A8), and triple-strain fermented natto by (B9 + probiotics + Mucor).
The stage-fermentation (SF) and co-fermentation (CF) of soybeans using the two strains of Mucor and B9 were each conducted in two different conditions for each technique. In stage fermentation, the spore solution of Mucor was added to steamed soybeans and cultured at 28 °C for 24 h (SF-1) or 48 h (SF-2), followed by the inoculation of B9 at 37 °C for 24 h. In co-fermentation, the spore solution of Mucor and B9 were simultaneously added to steamed soybeans and co-cultured at 30 °C for 24 h (CF-1) or 48 h (CF-2). All fermented natto beans were ripened at 4 °C for 24 h and then used for tasting and analysis.
Extraction of crude enzyme solution and determination of pH
Natto (10 g wet weight) was homogenized in a blender with 90 mL of distilled water for 30 s, and the pH of the suspension was measured with a pH meter. After 24 h of extraction at 4 °C, the mixture was centrifuged at 12,000 rpm at 4 °C for 10 min, and the supernatant was used for nattokinase and protease activity assays.
Free amino nitrogen contents (FANs)
After 24 h of extraction at 4 °C, the natto suspension was centrifuged at 4000 rpm for 10 min, and the supernatant was stored at 4 °C for use. Free amino nitrogen content was determined according to the method described by Lu et al. (2009).
Nattokinase (NK) activity assay
Fibrinolytic activity was evaluated through the fibrin degradation method, according to the method described by Deepak et al. (2008)
Protease activity assay
Protease activity was determined by the Folin–Ciocalteu method as described by Dong-gu Oh et al. (2016) with slight modifications. Equal volumes of 1 mL of the culture supernatants and 50 mM phosphate buffer (pH 7.5) containing 1% casein were mixed and incubated at 40 °C for 10 min. Then, 2 mL of 0.4 M trichloroacetic acid was added to terminate the reaction. The solution was mixed and left at room temperature for 20 min. After 20 min, the mixture was centrifuged at 12,000 rpm for 10 min; the supernatant was then collected. Approximately 1 mL of the supernatant was mixed with 5 mL of 0.4 M sodium carbonate and 1 mL of Folin solution (threefold dilution of 2 N Folin stock solution with water). After mixing, the solutions were incubated at 40 °C for 20 min, and the optical density was measured at 680 nm with a UV spectrometer. Protease activity is defined as hydrolyzing casein to produce 1 μg of tyrosine per unit time.
Biogenic amine analysis
Preparation of extracts from natto samples, chromatographic separation and derivatization of biogenic amines was carried out according to the method described by Kim et al. (2012).
Chromatographic separation of biogenic amines with minor modifications. HPLC unit equipped with a UV–Vis detector and Varian Star Workstation software was employed. The column used was 4 μm Nova-Pak C18 (150 mm × 3.9 mm, Waters), with acetonitrile (solvent A) and deionized water (solvent B) as the mobile phases at the flow rate of 0.8 mL/min. The program was set for a linear gradient starting from 60 to 90% solvent A at 28 min. The sample volume injected was 20 μL, and the sample was monitored at an ultraviolet detection wavelength of 254 nm. The detection limits were approximately 0.1 mg/kg for all the standard amine solutions analyzed.
Sensory properties
The sensory characteristics of natto was evaluated using the procedure described by Feng et al. (2015) with some modifications. A sensory team consisting of 15 teachers and graduate students majoring in food-related courses with sensory evaluation experience was recruited. The sensory evaluation was conducted in a ventilated food laboratory with sufficient light and space. The products were labeled with different numbers, then randomly disrupted, and finally replicated for three consecutive days under the same conditions.
The sensory characteristics of natto were mainly determined by its appearance, stickiness, flavor, taste, and chewiness. The appearance of the product was evaluated by color intensity, color uniformity, brightness, and glossiness. Stickiness was measured by the length, density, and adhesiveness of natto sticking to chopsticks. The flavor of natto was evaluated by olfaction, ammonia odor, and presence of bean odor, which are undesirable because natto should have a light or slightly fragrant smell. Taste was expected to be slightly acidic or mellow. A strong bitter aftertaste was considered undesirable. Chewiness is the feedback of the teeth on the softness, hardness, stickiness, and smoothness of natto. For all the sensory traits, rating scores from 1 to 5 were used, where 5 = excellent, 4 = good, 3 = moderate, 2 = poor, and 1 = inferior. Finally, an index score was obtained by multiplying each sensory property rating by a factor of 4. A high index score indicated good natto quality.
Statistics and analysis
Data were evaluated using descriptive statistics including mean, standard deviation (SD), and relative standard deviation (RSD). All statistical analyses were carried out with a significance level (a) of 0.05 using SPSS 12.0 (SPSS Inc., Chicago, Il. USA), and Origin 9.0 was used in drawing.
Results and discussion
Analysis of characteristics of natto product
The pH, FAN content, and NK and protease activity, and sensory index of steamed soybean and natto samples prepared using B9 or by two strains are shown in Table 1. The pH value of natto prepared using B9 increased from 6.79 ± 0.03 to 7.92 ± 0.14. Its FANs increased from 0.88 ± 0.03 g/kg to 4.72 ± 0.12 g/kg, and NK activity was 129.78 ± 2.42 FU/g. The natto produced was yellow, the beans were smooth and shiny, and the sticky silks were long and fine and generally has good appearance; however, it has an unpleasant ammonia taste, which makes the sensory score (76.4) not high enough. In natto prepared by two strains, the pH values of natto cultured by SQ4, BZ11, and BZ25 were slightly lower than that of natto prepared by B9 (P < 0.05), and the sensory index score was high (82.2). Lactic acid bacteria and Bifidobacteria produce organic acids, such as lactic acid, acetic acid, propionic acid, and butyric acid, which affect the flavor and taste of fermented food. Therefore, lactic acid bacteria and Bifidobacterium effectively improve the sensory characteristics of natto.
Table 1.
Determination of pH, FANs, NK, protease activity and sensory index in double-fermented natto samples
| Strains | Types | pH | FAN (g/kg) | NK (FU/g) | Protease (U/g) | Index |
|---|---|---|---|---|---|---|
| Steamed soybean | – | 6.79 ± 0.03f | 0.88 ± 0.03k | – | – | – |
| B9 | 4% | 7.92 ± 0.14a | 4.72 ± 0.12fg | 129.78 ± 2.42c | 71.43 ± 1.04f | 76.4 |
| B9 + SQ4 | 1:1 | 7.12 ± 0.03e | 4.38 ± 0.16h | 124.50 ± 2.32d | 47.47 ± 2.42j | 82.2 |
| B9 + BZ11 | 1:1 | 7.24 ± 0.10d | 4.77 ± 0.17fg | 134.56 ± 6.63b | 59.09 ± 1.63hi | 82.0 |
| B9 + BZ25 | 1:1 | 7.35 ± 0.13cd | 4.94 ± 0.13ef | 136.00 ± 1.74ab | 74.40 ± 2.57f | 81.6 |
| B9 + M1 | SF-1 | 7.68 ± 0.11bc | 4.57 ± 0.21gh | 123.61 ± 1.92de | 64.63 ± 3.17g | 73.0 |
| SF-2 | 7.98 ± 0.18a | 5.12 ± 0.11de | 125.56 ± 1.84d | 88.66 ± 1.65e | 75.2 | |
| CF-1 | 7.29 ± 0.05d | 3.09 ± 0.14j | 110.95 ± 0.63g | 105.03 ± 0.67c | 80.1 | |
| CF-2 | 7.86 ± 0.07ab | 6.77 ± 0.04ab | 136.34 ± 1.42ab | 114.27 ± 1.21b | 82.0 | |
| B9 + M4 | SF-1 | 7.63 ± 0.08bc | 4.57 ± 0.19gh | 117.00 ± 0.58f | 63.58 ± 3.57gh | 72.1 |
| SF-2 | 7.99 ± 0.26a | 5.41 ± 0.25d | 133.11 ± 2.96bc | 55.39 ± 1.21i | 76.8 | |
| CF-1 | 7.25 ± 0.06d | 2.82 ± 0.23j | 116.00 ± 0.60f | 98.69 ± 4.19d | 79.2 | |
| CF-2 | 7.77 ± 0.07b | 6.58 ± 0.14b | 140.11 ± 2.87a | 160.21 ± 2.55a | 80.2 | |
| SF-1 | 7.51 ± 0.09c | 5.93 ± 0.32c | 102.95 ± 2.80h | 62.26 ± 4.41gh | 68.3 | |
| B9 + A8 | SF-2 | 7.88 ± 0.18ab | 7.06 ± 0.26a | 119.95 ± 0.82ef | 113.47 ± 3.96b | 72.2 |
| CF-1 | 7.30 ± 0.05cd | 3.73 ± 0.17i | 117.61 ± 0.67f | 30.30 ± 2.62k | 75.6 | |
| CF-2 | 7.79 ± 0.06b | 6.62 ± 0.16b | 123.11 ± 1.36de | 62.64 ± 2.06gh | 76.4 |
FAN free amino nitrogen content, NK Nattokinase, CF co-fermentation, co-fermentation for 24 h (CF-1) or 48 h (CF-2); SF: stage-fermentation, M1, M4 or A8 were inoculated for 24 h (SF-1) or 48 h (SF-2), and then co-fermentation for 24 h with other strains; the same below. Different letter superscripts in the same column indicate statistically significant (P < 0.05), the same below
The pH, FAN content, and NK and protease activities of natto drastically changed owing to different Mucor strains and fermentation conditions. Table 1 shows the double-strain combination (B9 + M1, B9 + M4) surprisingly had similar trends of pH, FAN contents, sensory index, NK and protease activities, in different fermentation methods. The sensory index increased from SF-1 to CF-2, and the best score in CF-2 (82.0). The highest values of FAN and NK and protease activities appeared in CF-2 at 7.06 ± 0.26 g/kg, 140.11 ± 2.87 FU/g, and 160.21 ± 2.55 U/g, respectively. Various indicators and the quality of double strain-fermented natto were obviously improved by the co-inoculation of Mucor and B9 (bacteria) compared with those of single-bacteria natto. Similarly, it has been reported that the amino acid content, organic acid content and sensory quality of soybean paste fermented with a mixture of fungi and bacillus subtilis have been significantly improved (Shukla et al. 2015). Table 1 further shows that different fermentation methods hugely affect the quality of natto. Compared with SF-1, the protease activity and sensory index of CF-1 were significantly improved in the double-strain combination (B9 + M1, B9 + M4), but the NK activity and FAN content did not exceed SF-1. Therefore, the effect of CF is better and more suitable for natto production than that of SF. However, high protease activity was not detected in A8. Previous studies showed that steamed soybean fermented using A8 at 28 °C for 3 days resulted in high protease production (data not shown). In the present study, the results of double-strain fermentation showed that M4 had the highest protease activity. Some other double-strain combinations demonstrated good characters of their respective strains.
Natto from soybean fermented by multiple strains
Table 2 shows the changes in pH, FAN content, sensory index, and NK and protease activities in natto samples from triple strain-fermented soybean. The results showed that only two samples (2/9) had increased protease activity (highest at 93.66 ± 3.57 U/g), and the FAN contents in three combinations (3/9) exceeded those in single-bacteria samples (highest at 5.75 ± 0.19 g/kg). However, the NK activity and sensory index of all triple strain-fermented samples were significantly lower than that of single-bacteria samples (P < 0.05). In addition, the comprehensive quality of all triple strain-fermented samples was not as good as that of single-bacteria samples, indicating that the former could not use the advantages of the strains. On the contrary, some double-strain combinations can effectively improve the quality of natto (Table 1), indicating that the combination of two strains is more suitable for natto production than that of three strains.
Table 2.
Determination of pH, FAN content, NK and protease activity and sensory index in mixed-fermented natto samples
| Strains | pH | FAN (g/kg) | NK (FU/g) | Protease (U/g) | Index |
|---|---|---|---|---|---|
| B9 + M1 + SQ4 | 6.91 ± 0.05bc | 3.93 ± 0.10d | 112.61 ± 2.50d | 55.05 ± 4.32b | 69.6 |
| B9 + M1 + BZ11 | 7.51 ± 0.09a | 4.46 ± 0.19c | 120.89 ± 1.99a | 38.55 ± 2.49d | 70.4 |
| B9 + M1 + BZ25 | 7.25 ± 0.14ab | 4.89 ± 0.27b | 119.50 ± 1.42ab | 52.41 ± 3.48b | 73.6 |
| B9 + M4 + SQ4 | 6.89 ± 0.49c | 3.43 ± 0.19e | 110.17 ± 2.68d | 30.96 ± 2.06e | 66.8 |
| B9 + M4 + BZ11 | 7.42 ± 0.04a | 4.31 ± 0.19c | 116.89 ± 1.29bc | 44.16 ± 3.96c | 69.6 |
| B9 + M4 + BZ25 | 7.18 ± 0.07abc | 3.57 ± 0.14e | 116.22 ± 0.67c | 46.14 ± 2.62c | 73.2 |
| B9 + A8 + SQ4 | 5.08 ± 0.07d | 3.07 ± 0.20f | 65.28 ± 1.29f | 27.33 ± 1.71e | 64.1 |
| B9 + A8 + BZ11 | 7.36 ± 0.08a | 5.75 ± 0.19a | 104.95 ± 0.95e | 93.66 ± 3.57a | 67.2 |
| B9 + A8 + BZ25 | 7.26 ± 0.11ab | 4.98 ± 0.24b | 110.89 ± 2.11d | 89.04 ± 2.06a | 66.8 |
Sensory properties
The sensory profiles of the 25 natto samples were evaluated in terms of appearance, stringiness, flavor, taste, and chewiness. On the basis of the results, a spider plot was created to provide a graphical representation for the easy identification of differences and similarities, as shown in Fig. 1. Various culture combinations affected the sensory properties of natto (Fig. 1a); first, the sensory scores of single-strain-fermented natto by B9 showed no significant difference in the appearance, taste, and chewiness with the those of double-strain-fermented natto (B9 + probiotics or Mucor) and triple-strain-fermented natto (B9 + probiotics + Mucor), while the differences in flavor and stringiness were observed. In particular, the flavor score of double-strain fermented natto (B9 + probiotics), which used the strains B9 and SQ4, increased from 2.9 to 4.3. The increase was attributed to the production of lactic acid by SQ4, reduce the concentrations of odor substances and the subsequent decrease in pH from 7.92 to 7.12 (Table 1). Fermented soybeans can be used as carriers of probiotics, such as Lactobacillus, which can improve sensory properties when used in the co-fermentation of natto (Zhang et al. 2014). The ranking of natto in various culture combinations according to sensory attributes was as follows: (B9 + probiotics) > (B9 + Mucor) > B9 > (B9 + probiotics + Mucor).
Fig. 1.
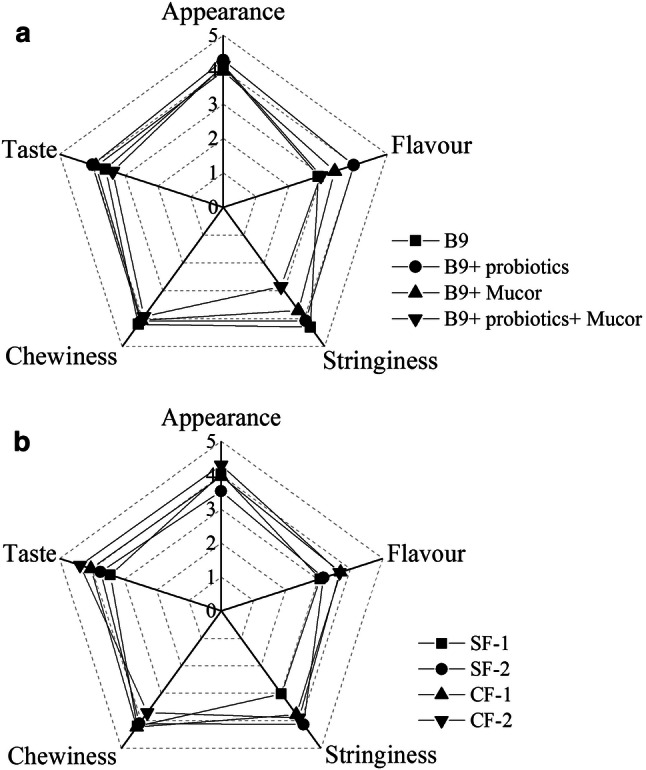
Net chart of the sensory attributes of the different fermentation natto in different fermentation strain combinations (a) and fermentation methods of Mucor (b)
In double-strain fermented natto (B9 + Mucor), the sensory properties of natto were affected by different fermentation methods of Mucor (Fig. 1b). The natto prepared by M1 and B9 had higher sensory scores of 80.1 and 82.0 in CF (Table 1), which had the main contribution in alleviating the ammonia taste and post-chewing bitterness of natto. Soybean fermentation products were influenced by various microorganisms, and various microorganisms and microbial enzymes subsequently affect the characteristic taste and flavor of the products (Kum et al. 2015). Soybean protein can be hydrolyzed by protease to produce polypeptides and amino acids. The bitterness of natto during chewing may be related to bitter peptides and amino acids such as leucine, histidine, proline, and pyroglutamic acid, especially the polypeptide and tyrosine produced by casein hydrolysis (Murray et al. 2018).
Effect of combined fermentation of soybean on biogenic amines of natto
The biogenic amine contents of 25 different natto prepared by various culture combinations and various fermentation methods were measured. As presented in Table 3, only five biogenic amines namely, PU, CA, TY, SPD, and SPM were detected in single-strain-fermented natto by B9, whereas TR, PHE and HI were not detected; the total biogenic amines reached 390.76 ± 11.98 mg/kg. Values of 100–800 mg/kg and 30 mg/kg were obtained for TY and PHE, respectively. These values are toxic doses in foods. Meanwhile, 1000 mg/kg (wt/wt) total amine in food is considered dangerous to health (Santos 1996). The total biogenic amines in 25 natto samples were lower than 390.76 ± 11.98 mg/kg, indicating that all the products were within the safe range. Low levels of both PU and CA amines were detected in some two-strain combination fermentation samples, except for combinations involving A8; CA was not detected in 17 natto samples, while samples with detected CA had concentrations of less than 10 mg/kg; moreover, PU concentrations detected were all lower than 50 mg/kg. Particularly, TR and PHE with low toxicity dose (30 mg/kg) were not detected in all products. High levels of HI are often the main causes of biogenic amine poisoning in foods. HI contents of 0–3.97 mg/kg were detected in all 25 natto varieties, which was much lower than the threshold value of 50 mg/kg set by United States Food and Drug Administration (USFDA 2001). In contrast, some studies have found that the average HI content in some Japanese natto is 35.4 mg/kg, while the average HI content of Taiwan natto is 45.1 mg/kg (Tsai et al. 2007).
Table 3.
Contents of biogenic amines in tested natto samples
| Strains | Inoculation method | Contents of biogenic amines (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| PU | CA | HI | TY | SPD | SPM | TBA | ||
| B9 | – | 4.39 ± 0.64fgh | 7.78 ± 0.52a | ND | 76.21 ± 3.45a | 84.14 ± 3.50a | 218.24 ± 9.76a | 390.76 ± 17.87 |
| B9 + SQ4 | CF-1 | 3.58 ± 0.69gh | 0.29 ± 0.26c | ND | 71.91 ± 4.34ab | 80.83 ± 1.19b | 208.5 ± 8.97a | 365.44 ± 15.45 |
| B9 + BZ11 | 4.09 ± 0.55gh | 0.37 ± 0.23c | ND | 70.26 ± 3.59abc | 84.31 ± 2.40a | 215.54 ± 8.03a | 374.79 ± 14.80 | |
| B9 + BZ25 | 5.04 ± 0.77fgh | 0.54 ± 0.15c | ND | 70.77 ± 2.52abc | 77.40 ± 1.72c | 206.09 ± 5.05a | 359.84 ± 10.21 | |
| B9 + M1 | SF-1 | 4.33 ± 0.48fgh | ND | ND | 64.03 ± 0.60bcd | 28.84 ± 0.56gh | 179.60 ± 1.44bc | 276.80 ± 3.08 |
| SF-2 | ND | ND | 1.94 ± 0.18bc | 53.24 ± 1.02dfe | 27.65 ± 0.42gh | 153.02 ± 1.69efg | 235.86 ± 3.31 | |
| CF-1 | 1.40 ± 0.51h | ND | 1.82 ± 0.48bc | 54.04 ± 0.87def | 71.56 ± 0.81d | 166.46 ± 0.05cde | 295.28 ± 2.72 | |
| CF-2 | 1.74 ± 0.14h | 0.35 ± 0.07c | 1.89 ± 0.75bc | 39.63 ± 1.85hij | 72.14 ± 1.07d | 154.04 ± 2.15defg | 268.15 ± 6.03 | |
| B9 + M4 | SF-1 | 3.89 ± 0.38gh | 0.31 ± 0.04c | ND | 70.12 ± 0.73abc | 29.423 ± 1.62g | 187.67 ± 0.85b | 291.41 ± 3.62 |
| SF-2 | ND | ND | 2.12 ± 0.42bc | 52.10 ± 2.70defg | 26.72 ± 1.53h | 156.45 ± 1.28def | 237.39 ± 5.93 | |
| CF-1 | 1.75 ± 0.29h | ND | 1.81 ± 0.59bc | 51.53 ± 2.13defg | 68.33 ± 0.84e | 140.86 ± 1.81fg | 264.28 ± 5.66 | |
| CF-2 | 4.73 ± 0.58fgh | ND | 2.04 ± 0.47bc | 45.82 ± 1.10fghi | 80.38 ± 1.28b | 166.45 ± 1.20cde | 299.41 ± 4.63 | |
| B9 + A8 | SF-1 | 17.55 ± 2.85c | ND | ND | 41.03 ± 0.62ghi | 0.28 ± 0.01n | 116.32 ± 0.96h | 175.18 ± 4.44 |
| SF-2 | 31.19 ± 0.66b | ND | 1.89 ± 0.36bc | 60.64 ± 1.82bcde | 12.19 ± 1.28k | 171.66 ± 1.01bcd | 277.58 ± 5.13 | |
| CF-1 | 41.57 ± 1.23a | ND | 2.39 ± 0.58ab | 57.55 ± 1.96cdef | 28.94 ± 0.99gh | 171.54 ± 1.62bcd | 301.41 ± 6.38 | |
| CF-2 | 30.68 ± 1.45b | ND | 2.48 ± 0.29ab | 53.53 ± 0.60def | 38.95 ± 1.32f | 182.78 ± 4.67bc | 308.95 ± 8.33 | |
| B9 + M1 + SQ4 | CF-1 | 7.87 ± 0.95efg | ND | 2.63 ± 0.12ab | 51.25 ± 2.89defg | 15.69 ± 0.38j | 137.47 ± 5.97g | 214.90 ± 10.31 |
| B9 + M1 + BZ11 | 13.63 ± 1.83d | 0.35 ± 0.08c | ND | 51.08 ± 0.57defg | 13.93 ± 0.18jk | 140.72 ± 1.04fg | 219.70 ± 3.71 | |
| B9 + M1 + BZ25 | 19.25 ± 2.73c | 1.64 ± 0.84b | 3.11 ± 0.83ab | 46.70 ± 2.23efgh | 20.49 ± 0.53i | 140.57 ± 9.59fg | 231.77 ± 16.75 | |
| B9 + M4 + SQ4 | 13.19 ± 0.65d | ND | 3.97 ± 0.90a | 39.90 ± 1.88ghi | 9.22 ± 1.03 l | 118.81 ± 8.83h | 185.10 ± 13.27 | |
| B9 + M4 + BZ11 | 8.50 ± 1.32ef | ND | 3.54 ± 1.44ab | 26.59 ± 1.21j | 4.45 ± 0.22 m | 81.18 ± 5.98i | 124.26 ± 10.17 | |
| B9 + M4 + BZ25 | 5.70 ± 0.63fgh | ND | 3.17 ± 0.04ab | 34.91 ± 0.58ij | 3.37 ± 0.07 m | 86.49 ± 1.07i | 133.65 ± 2.39 | |
| B9 + A8 + SQ4 | 7.12 ± 0.51efg | ND | 2.37 ± 0.57ab | 5.23 ± 1.17k | ND | 30.91 ± 1.66j | 46.33 ± 3.91 | |
| B9 + A8 + BZ11 | 10.83 ± 1.65de | ND | 0.55 ± 0.03c | ND | ND | 5.99 ± 1.12k | 17.37 ± 2.80 | |
| B9 + A8 + BZ25 | 5.24 ± 0.46fgh | ND | 2.76 ± 0.66ab | ND | ND | 8.15 ± 1.84k | 16.16 ± 2.96 | |
PU putrescine, CA cadaverine, HI histamine, TY tyramine, SPD spermidine, SPM spermine, TBA Total biogenic amines, ND Not detected (amine level less than 0.1 mg/kg). Tryptamine and 2-phenylethylamine were not detected in all samples, so they were not listed in the table
Interestingly, most samples showed relatively high levels of SPD and SPM compared with other amines, and the highest concentrations of SPD and SPM were 84.14 ± 3.50 and 218.24 ± 6.76 mg/kg, respectively. High levels of polyamines were found in 21 different natto products distributed in Korea (Kim et al. 2012). Similarly, considerable amounts of polyamines have been detected in non-fermented and fermented soybean products, and polyamines-rich foods are recommended for the elderly to prevent age-related chronic diseases, especially cardiovascular diseases (Toro-Funes et al. 2015). Meanwhile, we detected SPM content of 66.13 ± 2.32 mg/kg and 29.71 ± 1.27 mg/kg in the sterilized steamed soybeans. These values indicated that the high content of polyamines was not only caused by microorganisms but also related to soybean as a fermentation raw material.
The mechanism of the reduction of biogenic amines content by triple-strain fermentation can be ascertained by analyzing the contribution of different strains to the formation of biogenic amines. In natto prepared by two strains, L. plantaroid (SQ4) and anaerobic bacteria (BZ11 and BZ25) caused a significant decrease in CA (P < 0.05), Mucor significantly decreased the content of each biogenic amine and slightly increased HI content, except for A8, which had increased PU content. Mixing different strains for the fermentation of soybeans reduced the formation of biogenic amines (Shukla et al. 2015). Although the content of PU and HI in natto samples prepared by three strains had increased, other biogenic amines, particularly SPD and SPM contents, were reduced, causing a sharp decrease in total biogenic amines. The microbial consumption of SPD as an alternative source of nitrogen leads to significant decreases in SPD levels (Liu et al. 2011). A total of 38 mold-fermented Sufu samples from retail market and factories were found to mostly contain PU and CA, and only three samples contained a small amount of SPD and SPM (Guan et al. 2013). The three Mucor strains used, namely, M1, M4, and A8, were originally derived from fermented Sufu. Therefore, the growth of Mucor may consume SPD and SPM as nitrogen sources.
Correlation analysis
The significant correlations among biogenic amines and FANs, pH, NK and protease activity, sensory indicators in natto are shown in Table 4. The most abundant bioamines detected in this study were TY, SPD, and SPM, which were positively correlated to NK activity (P < 0.01), with R2 values of 0.61, 0.617, and 0.674, respectively. TBA followed the same correlation trend, with R2 of 0.679 (P < 0.01), whereas the other bioamines showed no correlation. NK is secreted by B9, and its correlation with BA somewhat reflects the bacterial quantity and activity status of B9 in mixed fermentation samples.
Table 4.
Regression correlation coefficients among biogenic amines, FANs, NK, protease activity, and sensory indicators
| PU | CA | HI | TY | SPD | SPM | TBA | |
|---|---|---|---|---|---|---|---|
| NK | − 0.136 | 0.196 | − 0.335 | 0.61** | 0.617** | 0.674** | 0.679** |
| protease | − 0.233 | − 0.02 | − 0.206 | − 0.009 | 0.416* | 0.09 | 0.149 |
| FAN | 0.229 | 0.004 | − 0.166 | 0.032 | 0.064 | 0.145 | 0.13 |
| pH | 0.035 | 0.192 | − 0.187 | 0.432* | 0.256 | 0.455* | 0.427* |
| Appearance | 0.033 | 0.091 | − 0.048 | − 0.053 | 0.199 | 0.013 | 0.061 |
| Stringiness | − 0.322 | 0.276 | − 0.521* | 0.586** | 0.812** | 0.671** | 0.713** |
| Flavour | 0.009 | − 0.148 | − 0.38 | 0.558** | 0.729** | 0.629** | 0.687** |
| Taste | 0.002 | 0.004 | − 0.21 | 0.446* | 0.686** | 0.57** | 0.621** |
| Chewiness | − 0.189 | 0.18 | − 0.338 | − 0.521* | 0.225 | 0.413* | 0.388 |
*Significance at P < 0.05; ** Significance at P < 0.01
Table 4 shows a significant correlation between SPD and protease activity, with R2 of 0.416 (P < 0.05). The remaining bioamines were not significantly correlated with PT activity and FANs. In addition, TY and SPM were correlated with pH value (P < 0.05), TBA followed the same correlation trend, with R2 of 0.427 (P < 0.05), whereas other biogenic amines showed no correlation. pH influences some important factors affecting BA formation, namely, the available free amino acids produced by microbial strains with high proteolytic enzyme activity, bacterial growth, and decarboxylase synthesis and activity (Swelam and Mehanna 2017).
Table 4 reveals the correlation between BA and the sensory indicators, namely, appearance, stringiness, flavor, taste, and chewiness. TY, SPD, and SPM showed a significant positive correlation with stringiness, flavor, and taste (P < 0.01), TBA followed the same correlation trend, with R2 values of 0.713, 0.687, and 0.621 respectively. The samples used for analysis were all mixed fermentation, except for one sample fermented using B9. Therefore, the correlation between BA and sensory indicators indicated that mixed fermentation primarily affects three sensory characteristics of natto, namely, stringiness, flavor, and taste, but has no significant effect on appearance and chewiness. SPM was positively correlated with chewiness, with R2 value of 0.413 (P < 0.05), whereas TBA showed no correlation with chewiness. The precursor of BA is the free amino acid in food; it can greatly affect food senses. Accordingly, we speculate that the intrinsic relation between BA and sensory properties was the content and type of free amino acids in the samples, which explains why there is a positive correlation between TBA and sensory properties. However, the content of biogenic amines beyond the range will not only endanger health, but also increase the adverse flavor of natto.
PU and CA are often used to reflect the degree of food spoilage and had no significant correlation with all test items (Table 4), indicating that all natto samples were in a relatively fresh state. Table 3 shows that HI (highest value of 3.97 ± 0.90 mg/kg) can be detected only in samples where Mucor was involved in fermentation. Thus, HI was only relevant to Mucor. A negative correlation was observed between HI and viscosity (Table 4), with R2 = 0.521 (P < 0.05), revealing that the state of Mucor greatly influenced the viscosity of the natto samples. High levels of HI are often known to be a major cause of biogenic amine poisoning in foods. HI showed negative and mostly non-significant correlations with all the items (Table 4), indicating that the combination of these strains was not conducive to HI production, but it was beneficial to its application in fermented food.
Determining the statistical correlation between BAs and some food characteristics was the objective of some studies, but contradictory results were obtained possibly because of the different chemical compositions of the studied food types, microbial conditions, and preparation processes. In silver carp fillets, Shi et al. (2012) found that TR, PHE, and PU were positively correlated with total volatile base nitrogen during storage, FU and TY were significantly correlated with microbial quality, and TR, PHE, PU and TY contents were negatively correlated with sensory score; the R2 of all correlations were between 0.840 and 0.992. Furthermore, Swelam and Mehanna ( 2017) found that TR and TY were positively correlated with protein in Ras cheese (P < 0.05), PHE and HI were positively correlated with soluble nitrogen/total nitrogen, and the remaining bioamines showed negligible correlation with protein and soluble nitrogen/total nitrogen, while acidity and pH have no effective effect on BA of cheese. Guan et al. (2013) found that CA and HI were positively correlated with pH in white Sufu (P < 0.05), and TBA also showed positive correlation with free amino acid, with R2 of 0.455 (P < 0.05). However, PU, TR, and SPD were negatively correlated with pH in red Sufu, BA was also negatively correlated with free amino acids, with R2 value of − 0.801 (P < 0.05), while gray Sufu showed no correlation with any food characteristics.
Conclusion
This research developed a novel starter culture involving the combined inoculation of probiotics (SQ4, BZ11, and BZ25), Mucor (M1, M4, and A8), and bacteria (B9) to produce fermented natto products with enhanced quality. A total of 25 kinds of natto products were produced via different strain combinations and fermentation methods, and their NK and protease activities, FAN and biogenic amine contents, and sensory characteristics were analyzed and compared. The results showed that the natto prepared by Bifidobacterium BZ25 or Mucor (M4, CF-2) with B9 had better quality, particularly having significantly improved natto flavor and showing higher enzyme activity. The fermentation method also greatly affected the quality of natto. Compared with the stage-fermentation, the co-fermented samples showed higher biological activity and sensory quality. The biogenic amines content was greatly reduced by the combination of multiple strains, tyramine was reduced by beneficial probiotics, whereas various biogenic amines were reduced by Mucor, which seemed to consumed some polyamines as a nitrogen source. Moreover, some biogenic amines have positive correlation with sensory characteristics. The fermentation methods involving combined strains influenced natto characteristics. This work lays the foundation for the development of novel starter cultures and fermentation methods for producing natto products with improved sensory quality, high contents of physiologically active substances beneficial to the human body, such as nattokinase and protease, and decreased contents of biological amines.
Acknowledgements
This work was financially supported by the Key Agricultural Project of Guizhou Province (QKHZC-[2019]2382 and QKHZC-[2016]2580), National Natural Science Foundation of China (31660010 and 31870002), Qiankehe talents project ([2018]5781 and [2017]5788–11).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adeyemo SM, Onilude AA. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger Food J. 2013;31(2):84–90. doi: 10.1016/s0189-7241(15)30080-1. [DOI] [Google Scholar]
- Ahmad A, Hayat I, Arif S, Masud T, Khalid N, Ahmed A. Mechanisms involved in the therapeutic effects of soybean (glycine max) Int J Food Prop. 2014;17(6):1332–1354. doi: 10.1080/10942912.2012.714828. [DOI] [Google Scholar]
- Ali MW, Shahzad R, Bilal S, Adhikari B, Kim I-D, Lee J-D, Lee I-J, Kim BO, Shin D-H. Comparison of antioxidants potential, metabolites, and nutritional profiles of Korean fermented soybean (Cheonggukjang) with Bacillus subtilis KCTC 13241. J Food Sci Technol. 2018;55(8):2871–2880. doi: 10.1007/s13197-018-3202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito S. The impacts of Schizosaccharomyces on winemaking. Appl Microbiol Biot. 2019;103(11):4291–4312. doi: 10.1007/s00253-019-09827-7. [DOI] [PubMed] [Google Scholar]
- Dabbagh F, Negahdaripour M, Berenjian A, Behfar A, Mohammadi F, Zamani M, Irajie C, Ghasemi Y. Nattokinase: production and application. Appl Microbiol Biot. 2014;98(22):9199–9206. doi: 10.1007/s00253-014-6135-3. [DOI] [PubMed] [Google Scholar]
- Deepak V, Kalishwaralal K, Ramkumarpandian S, Babu SV, Senthilkumar SR, Sangiliyandi G. Optimization of media composition for Nattokinase production by Bacillus subtilis using response surface methodology. Bioresour Technol. 2008;99(17):8170–8174. doi: 10.1016/j.biortech.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Fan X, Lv X, Meng L, Ai M, Li C, Teng F, Feng Z. Effect of microwave sterilization on maturation time and quality of low-salt sufu. Food Sci Nutr. 2020;8(1):584–593. doi: 10.1002/fsn3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Jin S, Luo M, Wang W, Xia XX, Zu YG, Li LP, Fu YJ. Optimization of production parameters for preparation of natto-pigeon pea with immobilized Bacillus natto and sensory evaluations of the product. Innov Food Sci Emerg. 2015;31:160–169. doi: 10.1016/j.ifset.2015.08.002. [DOI] [Google Scholar]
- Guan RF, Liu ZF, Zhang JJ, Wei YX, Wahab S, Liu DH, Ye X-Q. Investigation of biogenic amines in sufu (furu): a Chinese traditional fermented soybean food product. Food Control. 2013;31(2):345–352. doi: 10.1016/j.foodcont.2012.10.033. [DOI] [Google Scholar]
- Huang Y, Ding S, Liu M, Gao C, Yang J, Zhang X, Ding B. Ultra-small and anionic starch nanospheres: formation and vitro thrombolytic behavior study. Carbohydr Polym. 2013;96(2):426–434. doi: 10.1016/j.carbpol.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Jhan JK, Chang WF, Wang PM, Chou ST, Chung YC. Production of fermented red beans with multiple bioactivities using co-cultures of Bacillus subtilis and Lactobacillus delbrueckii subsp. bulgaricus. LWT Food Sci Technol. 2015;63(2):1281–1287. doi: 10.1016/j.lwt.2015.03.107. [DOI] [Google Scholar]
- Karimi S, Rashidian E, Birjandi M, Mahmoodnia L. Antagonistic effect of isolated probiotic bacteria from natural sources against intestinal Escherichia coli pathotypes. Electron Phys. 2018;10(3):6534–6539. doi: 10.19082/6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Byun BY, Mah JH. Biogenic amine formation and bacterial contribution in natto products. Food Chem. 2012;135(3):2005–2011. doi: 10.1016/j.foodchem.2012.06.091. [DOI] [PubMed] [Google Scholar]
- Kum SJ, Yang SO, Lee SM, Chang PS, Choi YH, Lee JJ, Hurh BS, Kim YS. Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang) J Agric Food Chem. 2015;63(5):1401–1418. doi: 10.1021/jf5056002. [DOI] [PubMed] [Google Scholar]
- Kushida M, Okouchi R, Iwagaki Y, Asano M, Du MX, Yamamoto K, Tsuduki T. Fermented soybean suppresses visceral fat accumulation in mice. Mol Nutr Food Res. 2018;62(17):1701054. doi: 10.1002/mnfr.201701054. [DOI] [PubMed] [Google Scholar]
- Liu ZF, Wei YX, Zhang JJ, Liu DH, Hu YQ, Ye XQ. Changes in biogenic amines during the conventional production of stinky tofu. Int J Food Sci Technol. 2011;46(4):687–694. doi: 10.1111/j.1365-2621.2011.02545.x. [DOI] [Google Scholar]
- Lu YM, Chen XH, Mei J, Xin L, Rahman N, Dong MS, Yan G. Biogenic amines in Chinese soy sauce. Food Control. 2009;20(6):593–597. doi: 10.1016/j.foodcont.2008.08.020. [DOI] [Google Scholar]
- Lu S, Ji H, Wang Q, Li B, Li K, Xu C, Jiang C. The effects of starter cultures and plant extracts on the biogenic amine accumulation in traditional Chinese smoked horsemeat sausages. Food Control. 2015;50:869–875. doi: 10.1016/j.foodcont.2014.08.015. [DOI] [Google Scholar]
- Murray NM, O'Riordan D, Jacquier JC, O'Sullivan M, Holton TA, Wynne K, Robinson RC, Barile D, Nielsen SD, Dallas DC. Peptidomic screening of bitter and nonbitter casein hydrolysate fractions for insulinogenic peptides. J Dairy Sci. 2018;101(4):2826–2837. doi: 10.3168/jds.2017-13853. [DOI] [PubMed] [Google Scholar]
- Oh D-G, Jang YK, Woo JE, Kim JS, Lee CH. Metabolomics reveals the effect of garlic on antioxidant- and protease-activities during Cheonggukjang (fermented soybean paste) fermentation. Food Res Int. 2016;82:86–94. doi: 10.1016/j.foodres.2016.01.019. [DOI] [Google Scholar]
- Priyadarshani WMD, Rakshit SK. Screening selected strains of probiotic lactic acid bacteria for their ability to produce biogenic amines (histamine and tyramine) Int J Food Sci Technol. 2011;46(10):2062–2069. doi: 10.1111/j.1365-2621.2011.02717.x. [DOI] [Google Scholar]
- Regubalan B, Ananthanarayan L. Investigation of biogenic amines content in fermented idli batter during storage. J Food Sci Technol. 2019;56(4):1775–1784. doi: 10.1007/s13197-019-03609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MHS. Biogenic amines: their importance in foods. Int J Food Microbiol. 1996;29(3):213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Shi C, Cui J, Lu H, Shen H, Luo Y. Changes in biogenic amines of silver carp (Hypophthalmichthys molitrix) fillets stored at different temperatures and their relation to total volatile base nitrogen, microbiological and sensory score. J Sci Food Agric. 2012;92(15):3079–3084. doi: 10.1002/jsfa.5729. [DOI] [PubMed] [Google Scholar]
- Shin HW, Jang ES, Moon BS, Lee JJ, Lee DE, Lee CH, Shin CS. Anti-obesity effects of gochujang products prepared using rice koji and soybean meju in rats. J Food Sci Technol. 2016;53(2):1004–1013. doi: 10.1007/s13197-015-2162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Lee JS, Park HK, Yoo JA, Hong SY, Kim JK, Kim M. Effect of novel starter culture on reduction of biogenic amines, quality improvement, and sensory properties of doenjang, a traditional Korean soybean fermented sauce variety. J Food Sci. 2015;80(8):1794–1803. doi: 10.1111/1750-3841.12942. [DOI] [PubMed] [Google Scholar]
- Sumi H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto; a typical and popular soybean food in the Japanese diet. Experientia. 1986;43(10):1110–1111. doi: 10.1007/BF01956052. [DOI] [PubMed] [Google Scholar]
- Swelam S, Mehanna NM. Profile of biogenic amines and their correlations with chemical constituents and some properties of Egyptian Ras cheese. Indian J Dairy Sci. 2017;70(5):556–562. [Google Scholar]
- Toro-Funes N, Bosch-Fuste J, Latorre-Moratalla ML, Veciana-Nogues MT, Vidal-Carou MC. Biologically active amines in fermented and non-fermented commercial soybean products from the Spanish market. Food Chem. 2015;173:1119–1124. doi: 10.1016/j.foodchem.2014.10.118. [DOI] [PubMed] [Google Scholar]
- Tsai Y-H, Chang S-C, Kung HF. Histamine contents and histamine-forming bacteria in natto products in Taiwan. Food Control. 2007;18(9):1026–1030. doi: 10.1016/j.foodcont.2006.06.007. [DOI] [Google Scholar]
- USFDA (2001) Fish and fishery products hazards and controls guide (3rd ed.). Washington, DC: Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Seafood
- Wang HK, Ng YK, Koh E, Yao L, Chien AS, Lin HX, Lee YK. RNA-Seq reveals transcriptomic interactions of Bacillus subtilis natto and Bifidobacterium animalis subsp. lactis in whole soybean solid-state co-fermentation. Food Microbiol. 2015;51:25–32. doi: 10.1016/j.fm.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Xu J, Du M, Yang X, Chen Q, Chen H, Lin DH. Thrombolytic effects in vivo of nattokinase in a carrageenan-induced rat model of thrombosis. Acta Haematol-Basel. 2014;132(2):247–253. doi: 10.1159/000360360. [DOI] [PubMed] [Google Scholar]
- Xu L, Du B, Xu B. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015;174:202–213. doi: 10.1016/j.foodchem.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang ST, Shi Y, Zhang SL, Shang W, Gao XQ, Wang HK. Whole soybean as probiotic lactic acid bacteria carrier food in solid-state fermentation. Food Control. 2014;41(1):1–6. doi: 10.1016/j.foodcont.2013.12.026. [DOI] [Google Scholar]


