Abstract
The comparative phytochemicals, antioxidative and antidiabetic activities of Camellia sinensis (black tea) and Aspalathus linearis (rooibos tea) were studied in vitro and ex vivo. Concentrated infusions of the teas showed significant free radical scavenging activities in vitro. They significantly increased the glutathione level, superoxide dismutase and catalase enzyme activities in oxidative hepatic injury, while concomitantly depleting malondialdehyde level. The teas significantly inhibited intestinal glucose absorption and α-amylase activities, and elevated muscle glucose uptake. LCMS phytochemical profiling revealed the presence of hydroxycaffeic acid, l-threonate, caffeine, vanillic acid, n-acetylvaline, and spinacetin 3-glucoside in C. sinensis. While quinolinic acid, coumestrol, phloroglucinol, 8-hydroxyquercetagetin, umbelliferone, and ajoene were identified in A. linearis. These results portray the antioxidant and antidiabetic potencies of both teas, with A. linearis showed better activity compared to C. sinensis. These teas may thus be used as functional foods in the management of diabetes and other oxidative stress related metabolic disorders.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04471-w) contains supplementary material, which is available to authorized users.
Keywords: Antioxidants, Antidiabetics, Black tea, Rooibos tea, Type 2 diabetes
Introduction
Diabetes mellitus (DM) is the most endemic of all metabolic diseases, as it was reported to affect over 425 million people in 2017 (IDF 2018). This depicts a 2.4% rise in prevalence from 415 million in 2015 (IDF 2016) and it is expected to increase by 48% to 629 million in 2045, with an upsurge of 156% expected for Africa (IDF 2018).
Diabetes mellitus is characterized by increased blood glucose level (hyperglycemia) owing to disorder in the metabolism of carbohydrate, protein and lipids (Erukainure et al. 2013), which is caused by failure of the pancreatic β-cell to secrete insulin, and/or failure of the cells to use the secreted insulin (Erukainure et al. 2018a, b, c). The former is referred to as type 1 diabetes (T1D), while the latter is often referred to as type 2 diabetes (T2D) and the most prevalent of all diabetes types as it is responsible for over 90% of morbidity and mortality due to DM (IDF 2016, 2018). Hyperglycemia leading to oxidative stress is the major trigger of T2D pathogenesis, that leads to micro- and macro-vascular complications such as retinopathy and neuropathy (Chukwuma and Islam 2017; Constantino et al. 2013; Erukainure et al. 2017a, b; Tiwari et al. 2013). Oxidative stress occurs in T2D as a result of increased generation of reactive oxygen species (ROS) from increased glucose oxidation, which overwhelms the cell’s endogenous antioxidative system (Maritim et al. 2003; Sanni et al. 2018). Increased activities of carbohydrate hydrolyzing enzymes particularly α-glucosidase and α-amylase have also been reported to contribute to hyperglycemia owing to rapid breakdown of dietary carbohydrate leading to postprandial rise in blood glucose level (Oyebode et al. 2018).
Camellia sinensis is a well known medicinal plant commonly referred to as tea and has been consumed as beverage from time immemorial. Its origin has been ascribed to Asia and it has been described as the most globally consumed beverage second to water (Macfarlane and Macfarlane 2004). Camellia sinensis is commercially available in most countries as black, green and white teas. Several studies have reported its antidiabetic and antioxidant properties (Bhatt et al. 2010; Dufresne and Farnworth, 2001; Fu et al. 2017; Kumar and Rizvi 2015), which has been attributed to its phytochemical constituents particularly the catechins and alkaloids (Frei and Higdon 2003; Han et al. 2016; Williamson et al. 2011).
Aspalathus linearis is a medicinal plant native to South Africa, belonging to the Fabaceae family and the Aspalathus genus. Its leaves are utilized in the production of the herbal tea, rooibos or bush tea which is widely consumed globally (Joubert et al. 2008). Its medicinal properties have been widely studied and has been reported for antidiabetic and antioxidant activities (Joubert et al. 2008; Marnewick et al. 2003; Patel et al. 2016). These medicinal properties have been attributed to its reported high ascorbic acid content as well as polyphenols such as the flavones and dihydrochalcones particularly aspalathin and nothofagin (Iswaldi et al. 2011; Lee and Bae 2015).
Camellia sinensis and A. linearis constitute the most common teas consumed in Southern Africa, and often used singly or combined in the management of various ailments including DM. However, there is a dearth in their comparative studies. This study thus aims at comparing the phytochemical, antidiabetic and antioxidative properties of C. sinensis (black tea) and A. linearis (rooibos tea) by investigating their ability to promote hypoglycaemic processes vis-à-vis muscle stimulation of glucose uptake, inhibiting intestinal glucose absorption and activities of major carbohydrate digestive enzymes, as well as improving antioxidant enzymes activities.
Materials and methods
Tea bags
Commercial C. sinensis and A. linearis tea bags were purchased from local malls at Fuzhou, China and Durban, South Africa respectively. Five bags (10 g) of each product were infused in 100 mL of boiled water and allowed to stand for 2 h. The infusions were decanted into a weighed beaker and concentrated at <50 °C in a water bath. After concentrating, the beaker was reweighed, and the yielded concentration was calculated to be 4.2 and 3.6 g of C. sinensis and A. linearis samples respectively. They were stored in airtight vials until further analysis.
A stock solution (1 mg/mL) was prepared from each sample using distilled water, from which different working concentrations (15, 30, 60, 120 and 240 µg/mL) were prepared for further studies.
Total phenolic content
The total phenolic content of the samples was analyzed via the Folin–Ciocalteu reagent assay and expressed as milligrams of gallic acid equivalents (GAE) per gram of dry weight (Liu and Yao 2007).
In vitro antioxidant activity
In vitro antioxidant activities were determined for the teas using the 2,2′-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity (Braca et al. 2002) and the ferric reducing antioxidant power (FRAP) assay (Benzie and Strain 1996).
For DPPH activity, 100 μL of each sample concentrations were incubated with 50 µL of 0.3 mM DPPH solution (in methanol) for 30 min in the dark. Absorbance was read at 517 nm.
For FRAP, 100 μL of each sample concentration was incubated with equal volumes of sodium phosphate buffer (0.2 M, pH 6.6) and 1% potassium ferricyanide at 50 °C for 30 min. 100 μL of 10% trichloroacetic acid was used in acidifying the reaction mixture, 100 μL of distilled water and 200 µL of 0.1% FeCl3 were then added. Absorbance was read at 700 nm.
Ascorbic acid was used as standard drugs for both activities.
Enzyme inhibitory activity
The teas were assayed for their antidiabetic activities by determining their inhibitory effect on pancreatic α-amylase (Shai et al. 2010). Briefly, 50 µL of each tea concentration or acarbose was incubated with equal volume of porcine pancreatic amylase (2 U/mL; in 100 mM phosphate buffer, pH 6.8) for 10 min at 37 °C. After which, an equal volume of 50 µL of 1% starch solution in 100 mM phosphate buffer (pH 6.8) was added to the reaction mixture and further incubated for 10 min at 37 °C. A 100 µL of the dinitrosalicylate (DNS) color reagent was added to the mixture and boiled for 10 min. Absorbance was read at 540 nm.
Animals
Five male albino rats of Sprague Dawley strain and weighing about 200–250 g were procured for the experiment from the Biomedical Research Unit (BRU), University of KwaZulu-Natal, Durban, South Africa. The rats were sacrificed by euthanizing with halothane, after overnight fasting (12 h). Their small intestines (jejunum), muscles and liver were harvested, rinsed in 0.9% NaCl solution to remove blood stains and used immediately for ex vivo studies comprising of glucose absorption and uptake, and anti-oxidative stress activities.
The animals were maintained under the guidelines approved by the Animal Ethics Committee of the University of KwaZulu-Natal, Durban, South Africa (Protocol approval number: AREC/067/017D).
Ex vivo anti-oxidative activity
After homogenizing in 50 mM sodium phosphate buffer (pH 7.5; with 10% Triton X-100), the harvested livers were centrifuged at 15,000 rpm at 4 °C for 10 min. The supernatants were decanted and stored in 2 mL Eppendorf tubes.
A 100 µL of each tea concentration was incubated with a reaction mixture containing 100 µL of the liver homogenates and 30 µL of 0.1 mM FeSO4 for 30 min in 5% CO2 incubator. A reaction mixture without any tea sample or standard drug served as negative control (untreated). Ascorbic acid was used as the standard drug.
The incubated samples were then analyzed for reduced glutathione (GSH) level (Ellman 1959), catalase (Chance and Maehly 1955) and superoxide dismutase (SOD) (Kakkar et al. 1984) activities, and malondialdehyde (MDA) level (Chowdhury and Soulsby 2002).
Glucose absorption in isolated rat jejunum
The inhibitory activity of the teas on intestinal glucose absorption was assayed by incubating the different concentrations of the teas with 5 cm of the isolated rat jejunum in Krebs buffer (118 mM NaCl, 5 mM KCl, 1.328 mM CaCl2·2H2O, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3 and 11.1 mM glucose) (Chukwuma and Islam 2015). Glucose concentration were determined before and after the incubation with an Automated Chemistry Analyzer (Labmax Plenno, Labtest Inc., Costa Brava, Brazil). Glucose absorption was calculated with the following formula:
where GC1 and GC2 are glucose concentrations (mg/dL) before and after incubation respectively.
Glucose uptake in isolated rat psoas muscles
The ability of the teas to exacerbate glucose uptake in the isolated rat psoas muscles was assayed by determining the glucose concentration after a 2 h incubation of 0.5 g of isolated rat psoas muscle with different concentrations of the test samples in 8 mL of Krebs buffer (Chukwuma and Islam 2015). Metformin (Sigma Aldrich, South Africa) was used as a positive control. Glucose concentration were measured before and after the incubation with an Automated Chemistry Analyzer (Labmax Plenno, Labtest Inc., Lagoa Santa, Brazil). The intestinal glucose absorption was calculated with the following formula:
where GC1 and GC2 are glucose concentrations (mg/dL) before and after the incubation respectively.
Liquid Chromatography-Mass Spectrometric (LC–MS) Analysis
The tea samples were subjected to LC–MS analysis using Shimadzu LCMS-2020 Single Quadrupole Liquid Chromatograph Mass Spectrometer (LC–MS). A HP-5MS capillary column was used (30 m × 0.25 mm ID, 0.25 μm film thickness, 5% phenylmethylsiloxane). The LC stop time was set at 4.00 min. The PDA sampling frequency was set at 1.5625 Hz and the operating mode was on low pressure gradient. Other operating parameters were as follows: Pump A: LC-2030 Pump, Flow rate: 0.2000 mL/min, Mobile Phase B Conc.: 95.0%; C Conc.: 0.0%; D Conc.: 0.0%; A: Water; Mobile Phase B: Methanol; Start Wavelength: 190 nm; End Wavelength: 800 nm; Cell Temp.: 40 °C; Start Time: 0.00 min; End Time: 4.00 min; Acquisition Mode: Scan Polarity: Positive; Event Time: 1.00 s; Detector Voltage: + 1.00 kV; Threshold: 0; Start m/z: 50.00; End m/z: 1700.00; Scan Speed: 1667 u/sec. Compounds were identified by direct comparison of mass spectral data with those in the https://foodb.ca/spectra/ms/search.
Statistics
Data were presented as mean ± SD, and significance of difference was established at p < 0.05 using one-way analysis of variance (ANOVA). Statistical analyses were carried out using IBM Statistical Package for the Social Sciences (SPSS) for Windows, version 23.0 (IBM Corp, Armonk, NY, USA). The difference between the treated and untreated samples was used in calculating the IC50 values for each tea using their respective regression lines, where x = 50 (Erukainure et al. 2017a, b).
Results
As shown in Fig. 1, both tea samples had moderate phenolic contents. The phenolic content of A. linearis was significantly (p < 0.05) higher than that of C. sinensis.
Fig. 1.

Total phenolic contents of C. sinensis and A. linearis. Data are presented as mean ± SD; n = 3. ZSXZ: C. sinensis; RRBT: A. linearis
Both teas significantly (p < 0.05) scavenged DPPH, with A. linearis displaying the best scavenging activity as shown in Fig. 2a and Table 1. Both teas showed a dose-dependent FRAP activity as shown in Fig. 2b, with the highest activity observed at the highest concentration (240 µg/mL).
Fig. 2.

a DPPH scavenging and b Ferric reducing antioxidant power (FRAP) activities of C. sinensis and A. linearis. Data are presented as mean ± SD. abcValues with different letter above the bars for a given concentration are significantly (p < 0.05) different from each other. ZSXZ: C. sinensis; RRBT: A. linearis
Table 1.
IC50 values of C. sinensis and A. linearis activities
| Activities | C. sinensis | A. linearis | Ascorbic acid | Acarbose |
|---|---|---|---|---|
| DPPH | 0.03 µg/mL | 0.01 µg/mL | 0.05 µg/mL | – |
| FRAP | > 1000 µg/mL | > 1000 µg/mL | > 1000 µg/mL | – |
| α-amylase | > 1000 µg/mL | > 1000 µg/mL | > 1000 µg/mL | > 1000 µg/mL |
| GSH | 112.02 µg/mL | 84.10 µg/mL | 90.34 µg/mL | – |
| SOD | > 1000 µg/mL | 87.27 µg/mL | 797.56 µg/mL | – |
| Catalase | 1.71 µg/mL | 1.62 µg/mL | 2.21 µg/mL | – |
| MDA | 1.55 µg/mL | 2.6 µg/mL | 3.52 µg/mL | – |
| Glucose absorption | 162.22 µg/mL | 85.82 µg/mL | – | – |
| Glucose uptake | 242.64 µg/mL | 383.63 µg/mL | – | – |
Both teas moderately inhibited the activities of α-amylase but were significantly (p < 0.05) lower when compared to the standard drug, acarbose as depicted in Fig. 3.
Fig. 3.

Inhibitory effect of C. sinensis and A. linearis on α-amylase activity. Data are presented as mean ± SD. abcValues with different letter above the bars for a given concentration are significantly (p < 0.05) different from each other. ZSXZ: C. sinensis; RRBT: A. linearis
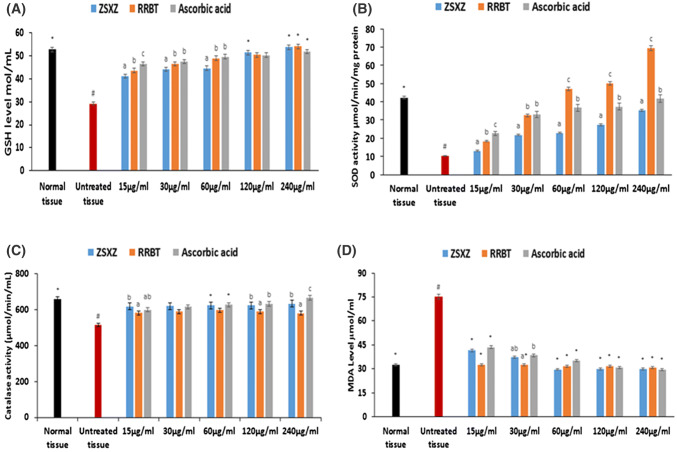
Incubation of hepatic tissue homogenates with FeSO4 led to significant (p < 0.05) depletion of GSH level, SOD and catalase activities, while significantly (p < 0.05) increasing MDA level as depicted in Fig. 4a–d. Incubation with the teas significantly (p < 0.05) increased the GSH level, SOD and catalase activities, and concomitantly depleted MDA level. The ability of both teas to increase the SOD activities were dose-dependent, with A. linearis showing the best activity (Fig. 4b). Based on the IC50 values (Table 1), A. linearis had the best activities except for MDA depletion.
Fig. 4.
Effect of C. sinensis and A. linearis on (a) GSH level, (b) SOD activity, (c) catalase activity, and (d) MDA level in oxidative hepatic injury. Data are presented as mean ± SD; n = 3. *Significantly different from untreated sample and #Significantly (p < 0.05) different from normal sample. ZSXZ: C. sinensis; RRBT: A. linearis
Incubation of isolated rat jejunum with the teas significantly (p < 0.05) inhibited intestinal glucose absorption as depicted in Fig. 5a, with C. sinensis showing a dose dependent activity. The low IC50 value of A. linearis, indicates a better activity compared to C. sinensis.
Fig. 5.
Effects of C. sinensis and A. linearis on glucose (a) absorption in isolated rat jejunum and (b) uptake in isolated psoas muscle. Data are presented as mean ± SD; n = 3. *Significantly different from untreated sample and #Significantly (p < 0.05) different from normal sample. ZSXZ: C. sinensis; RRBT: A. linearis
Incubation of isolated psoas muscle with the teas led to significant (p < 0.05) increase in muscle glucose uptake as shown in Fig. 5b. Both teas showed does-dependent activities, with C. sinesnis having the best activity.
LCMS analysis led to the identification of hydroxycaffeic acid, l-threonate, caffeine, vanillic acid, n-acetylvaline, and spinacetin 3-glucoside in C. sinensis as depicted in Fig. 6a, while quinolinic acid, coumestrol, phloroglucinol, 8-hydroxyquercetagetin, umbelliferone, and ajoene were identified in A. linearis as shown in Fig. 6b.
Fig. 6.
LCMS identified compounds in (a) C. sinensis and (b) A. linearis
Discussion
Tea drinking culture has been in practice from time immemorial, with Yunnan in western China said to be the birthplace of this culture (Kumakura 2002). Aside C. sinensis, there have been in increase in other types of tea notably herbal teas which also enjoy worldwide consumption (Joubert et al. 2008). Though often taken as recreational beverages and food, teas have been reported for their medicinal properties (Sharma et al. 2007; Siddiqui et al. 2004). This study reports the ability of C. sinensis and A. linearis teas to scavenge free radicals and to inhibit the activities of major carbohydrate catabolic enzymes linked to type 2 diabetes as well as their phytoconstituents.
The total phenolic contents of both teas were rather very low (Fig. 1) which corroborates previous studies by Anesini et al. (2008), Pal et al. (2012) and Bhebhe et al. (2015) which reported low total phenolic content for C. sinensis and A. linearis. This however contradicts previous reports that both teas were had rich contents of phenolics. Pereira et al. (2014) reported high phenolic contents for black, green and white C. sinensis and correlated them with the antioxidant properties of the studied teas. Damiani et al. (2019) also reported high phenolic properties for A. linearis and also correlated the antioxidant activity of the tea to the phenolic content. Although the present study reports low phenolic contents for both teas, they however contribute to the antioxidant properties of the teas as depicted by their ability to scavenge DPPH and reduce Fe3+ (Fig. 2a, b).
The influence of oxidative stress in the pathogenesis of type 2 diabetes and its complications due to hyperglycemia induced increased production of free radicals are well documented (Erukainure et al. 2012; King and Loeken 2004; Tiwari et al. 2013). These free radicals have been shown to attack cellular proteins, DNAs, membrane lipids which may subsequently lead to cell death (Maritim et al. 2003). Increased lipid peroxidation owing to suppressed GSH level, SOD and catalase activities is a major oxidative mechanism. The high DPPH scavenging and FRAP activities (Fig. 2a, b) of the teas indicates their free radical and reducing power properties. This corroborates previous reports on the potent antioxidant properties of C. sinensis and A. linearis (Pereira et al. 2014; Damiani et al. 2019). This is further depicted by the ability of both teas to elevate the levels of GSH, SOD and catalase activities, while suppressing lipid peroxidation in oxidative hepatic injury (Fig. 4a–d). These potencies may be attributed to the LC–MS identified compounds of the teas (Fig. 6), particularly the phenolics which are well known antioxidants (Heleno et al. 2015; Zhao et al. 2014).
Inhibition of major carbohydrate digestive enzymes has been reported in several studies to be effective in the treatment and management of type 2 diabetes (Van 2006). The inhibition of α – amylase by the teas C. sinensis and A. linearis (Fig. 3) corroborates previous studies (Gao et al. 2013; Mikami et al. 2015; Muller et al. 2012; Ramírez et al. 2012; Vinholes and Vizzotto 2017) and further portrays their antidiabetic properties. These studies attributed the enzyme inhibitory properties to the phytoconstituents of both teas (Dludla et al 2017; Gao et al. 2013; Muller et al. 2012; Wang, et al. 2012), thereby implying that the total phenol content (Fig. 1) and identified compounds (Fig. 6a, b) may play a synergetic role regarding this activity.
Inhibition of and/or delayed intestinal glucose absorption can also lead to decreased postprandial elevation of blood glucose level, thus can be employed in the treatment and management of T2D (Chukwuma and Islam 2015). Studies have reported the ability of plant extracts to suppress glucose absorption in the intestine mostly at the first quarter jejunal and duodenal regions (Erukainure et al. 2018a, b, c, 2019; Oyebode et al. 2018). The inhibitory effects of the studied teas (Fig. 5a) demonstrates their ability to delay the intestinal absorption of dietary glucose, thus preventing postprandial elevation of blood glucose level. This also corroborates with their ability to inhibit α-amylase activity (Fig. 3).
The role of skeletal muscle in carbohydrate metabolism have been well documented (Oyebode et al. 2018; Sinacore and Gulve 1993). This can be attributed to their richness in the glucose transporter, GLUT-4 which facilitates glucose uptake (Oyebode et al. 2018; Satoh 2014). Some commercial antidiabetic drugs such as metformin exhibit their antidiabetic activity by triggering muscle glucose uptake (Natali and Ferrannini 2006). Thus, the ability of the studied teas to stimulate muscle glucose uptake (Fig. 5b) further insinuates their antidiabetic potentials. This may also portray an improved insulin sensitivity, as insulin resistance has been implicated in the defects in muscle glucose uptake (Satoh 2014; Sinacore and Gulve 1993).
Phytochemicals have been reported for their antioxidant and antidiabetic activities (Alasalvar and Bolling 2015; Chukwuma et al. 2019). The studied biological activities of C. sinensis and A. linearis maybe attributed to the identified phytochemicals (Fig. 6a, b), thus depicting a synergistic effect. The presence of the phenolics, hydroxycaffeic acid, vanillic acid, and n-acetylvaline as well as the phenolic glycoside, spinacetin 3-glucoside in C sinensis portrays a strong antioxidant potency as phenolics are well known for their antioxidant and antidiabetic properties (Chukwuma et al. 2019; Erukainure et al. 2018a, b, c). The presence of caffeine may also contribute to the antidiabetic activity of C. sinensis, as the hypoglycemic activity of caffeine has been reported in non-diabetics, pre-diabetics and diabetics (Bhaktha et al. 2015; Lane 2011; Lee et al. 2016). Similarly, the presence of phloroglucinol, 8-hydroxyquercetagetin, and umbelliferone in A. linearis (Fig. 6b) may also contribute to its antioxidant and antidiabetic activities. Phytoestrogen, coumestrol and ajoene have also been shown to possess antioxidant and antidiabetic properties (Bhathena and Velasquez 2002; Hattori et al. 2005; Yuk et al. 2011), and may also contribute to the biological activities of A. linearis.
Conclusion
These results depict the antioxidative and antidiabetic potencies of C. sinensis and A. linearis as demonstrated by their ability to scavenge free radicals, suppress lipid peroxidation, inhibit α-amylase enzyme activity and intestinal glucose absorption, and concomitant increase in antioxidant enzymes activities and muscle glucose uptake. Thus, further affirming the utilization of these teas for managing T2D and its complications, with A. linearis being more potent compared to C. sinensis. Hence, they may be employed as functional foods in the management of diabetes and other oxidative stress related metabolic disorders.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by a competitive research grant from the Research Office, University of KwaZulu-Natal (UKZN), Durban; an incentive grant for rated researchers and a grant support for women and young researchers from the National Research Foundation (NRF), Pretoria, South Africa.
Compliance with ethical standards
Conflict of interest
The authors report no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alasalvar C, Bolling BW. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Brit J Nutr. 2015;113(S2):S68–S78. doi: 10.1017/S0007114514003729. [DOI] [PubMed] [Google Scholar]
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem. 2008;56(19):9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhaktha G, Nayak BS, Mayya S, Shantaram M. Relationship of caffeine with adiponectin and blood sugar levels in subjects with and without diabetes. J Clin Diag Res. 2015;9(1):BC01–BC03. doi: 10.7860/JCDR/2015/10587.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76(6):1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- Bhatt PR, Pandya KB, Sheth NR. Camellia sinensis (L): the medicinal beverage: a review. Inter J Pharm Sci Rev Res. 2010;3(2):3–6. [Google Scholar]
- Bhebhe M, Chipurura B, Muchuweti M. Determination and comparison of phenolic compound content and antioxidant activity of selected local Zimbabwean herbal. South Afri J Bot. 2015;100:213–218. [Google Scholar]
- Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharm. 2002;79(3):379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly A. Assay of catalases and peroxidases. Method Enzymol. 1955;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Chowdhury P, Soulsby M. Lipid peroxidation in rat brain is increased by simulated weightlessness and decreased by a soy-protein diet. Ann Clin Lab Sci. 2002;32(2):188–192. [PubMed] [Google Scholar]
- Chukwuma CI, Islam MS. Effects of xylitol on carbohydrate digesting enzymes activity, intestinal glucose absorption and muscle glucose uptake: a multi-mode study. Food Func. 2015;6(3):955–962. doi: 10.1039/c4fo00994k. [DOI] [PubMed] [Google Scholar]
- Chukwuma CI, Islam MS. Xylitol improves anti-oxidative defense system in serum, liver, heart, kidney and pancreas of normal and type 2 diabetes model of rats. Acta Pol Pharm. 2017;74(3):817. [PubMed] [Google Scholar]
- Chukwuma CI, Matsabisa MG, Ibrahim MA, Erukainure OL, Chabalala MH, Islam MS. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: a review. J Ethnopharm. 2019 doi: 10.1016/j.jep.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes. Diab Care. 2013;36(12):3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Carloni P, Rocchetti G, Senizza B, Tiano L, Joubert E, Beer E, Lucini L. Impact of cold versus hot brewing on the phenolic profile and antioxidant capacity of rooibos (Aspalathus linearis) herbal tea. Antioxidants. 2019;8(10):499. doi: 10.3390/antiox8100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dludla PV, Joubert E, Muller CJ, Louw J, Johnson R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-D-glucoside. Nutr Metab. 2017;14(1):45. doi: 10.1186/s12986-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12(7):404–421. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erukainure OL, Chukwuma CI, Sanni O, Matsabisa MG, Islam MS. Histochemistry, phenolic content, antioxidant, and anti-diabetic activities of Vernonia amygdalina leaf extract. J Food Biochem. 2019;43(2):e12737. doi: 10.1111/jfbc.12737. [DOI] [PubMed] [Google Scholar]
- Erukainure OL, Ebuehi OA, Adeboyejo FO, Okafor EN, Muhammad A, Elemo GN. Fiber-enriched biscuit enhances insulin secretion, modulates β-cell function, improves insulin sensitivity, and attenuates hyperlipidemia in diabetic rats. PharmaNutrition. 2013;1(2):58–64. [Google Scholar]
- Erukainure OL, Hafizur R, Kabir N, et al. Suppressive effects of clerodendrum volubile P beauv.[labiatae] methanolic extract and its fractions on type 2 diabetes and its complications. Front Pharm. 2018;9:8. doi: 10.3389/fphar.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erukainure OL, Hafizur RM, Choudhary MI, et al. Anti-diabetic effect of the ethyl acetate fraction of Clerodendrum volubile: protocatechuic acid suppresses phagocytic oxidative burst and modulates inflammatory cytokines. Biomed Pharmacother. 2017;86:307–315. doi: 10.1016/j.biopha.2016.12.035. [DOI] [PubMed] [Google Scholar]
- Erukainure OL, Mopuri R, Chukwuma CI, Koorbanally NA, Islam MS. Phaseolus lunatus (lima beans) abates Fe2+-induced hepatic redox imbalance; inhibits intestinal glucose absorption and major carbohydrate catabolic enzymes; and modulates muscle glucose uptake. J Food Biochem. 2018;42(6):e12655. [Google Scholar]
- Erukainure OL, Mopuri R, Oyebode OA, Koorbanally NA, Islam MS. Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed Pharmacother. 2017;96:37–47. doi: 10.1016/j.biopha.2017.09.106. [DOI] [PubMed] [Google Scholar]
- Erukainure OL, Okafor O, Oke OV, et al. Protective potentials of blends of selected fruit extracts used for diabetes management against lipid peroxidation in hepatic cells and free radicals in vitro. Int J Trad Nat Med. 2012;1:41–53. [Google Scholar]
- Erukainure OL, Sanni O, Islam MS. Clerodendrum volubile: phenolics and applications to health. In: Watson R, Preedy V, Zibadi S, editors. Polyphenols: mechanisms of action in human health and disease. 2. Amsterdam: Elsevier; 2018. [Google Scholar]
- Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133(10):3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- Fu Q-Y, Li Q-S, Lin X-M, et al. Antidiabetic effects of tea. Molecules. 2017;22(5):849. doi: 10.3390/molecules22050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Xu P, Wang Y, Wang Y, Hochstetter D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against α-amylase and α-glucosidase in vitro. Molecules. 2013;18(9):11614–11623. doi: 10.3390/molecules180911614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Bai X, Zhang N, et al. Activities constituents from yaowang tea (Potentilla glabra Lodd.) Food Sci Tech Res. 2016;22(3):371–376. [Google Scholar]
- Hattori A, Yamada N, Nishikawa T, Fukuda H, Fujino T. Antidiabetic effects of Ajoene in genetically diabetic KK-Ay mice. J Nutr Sci Vit. 2005;51(5):382–384. doi: 10.3177/jnsv.51.382. [DOI] [PubMed] [Google Scholar]
- Heleno SA, Martins A, Queiroz MJR, Ferreira IC. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chem. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation (IDF) IDF diabetes atlas. 7. Brussels: International Diabetes Federation; 2016. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation (IDF) IDF diabetes atlas. 8. Brussels: International Diabetes Federation; 2018. [Google Scholar]
- Iswaldi I, Arráez-Román D, Rodríguez-Medina I, et al. Identification of phenolic compounds in aqueous and ethanolic rooibos extracts (Aspalathus linearis) by HPLC-ESI-MS (TOF/IT) Anal Bioanal Chem. 2011;400(10):3643–3654. doi: 10.1007/s00216-011-4998-z. [DOI] [PubMed] [Google Scholar]
- Joubert E, Gelderblom W, Louw A, de Beer D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides—a review. J Ethnopharm. 2008;119(3):376–412. doi: 10.1016/j.jep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122(4):333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- Kumakura I. Tea drinking culture in the world. Food Food Ingred J Jap. 2002;204:204–209. [Google Scholar]
- Kumar D, Rizvi SI. Black tea extract improves anti-oxidant profile in experimental diabetic rats. Arch Physiol Biochem. 2015;121(3):109–115. doi: 10.3109/13813455.2015.1043308. [DOI] [PubMed] [Google Scholar]
- Lane JD. Caffeine, glucose metabolism, and type 2 diabetes. J Caff Res. 2011;1(1):23–28. [Google Scholar]
- Lee J-H, Oh M-K, Lim J-T, Kim H-G, Lee W-J. Effect of coffee consumption on the progression of type 2 diabetes mellitus among prediabetic individuals. Korean J Fam Med. 2016;37(1):7. doi: 10.4082/kjfm.2016.37.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Bae J-S. Anti-inflammatory effects of aspalathin and nothofagin from rooibos (Aspalathus linearis) Inflammation. 2015;38(4):1502–1516. doi: 10.1007/s10753-015-0125-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yao H. Antioxidant activities of barley seeds extracts. Food Chem. 2007;102(3):732–737. [Google Scholar]
- Macfarlane A, Macfarlane I. The empire of tea. New York: The Overlook Press; 2004. [Google Scholar]
- Maritim A, Sanders R, Watkins J., III Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Marnewick JL, Joubert E, Swart P, van der Westhuizen F, Gelderblom WC. Modulation of hepatic drug metabolizing enzymes and oxidative status by rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), green and black (Camellia sinensis) teas in rats. J Agric Food Chem. 2003;51(27):8113–8119. doi: 10.1021/jf0344643. [DOI] [PubMed] [Google Scholar]
- Mikami N, Tsujimura J, Sato A, et al. Green rooibos extract from Aspalathus linearis, and its component, aspalathin, suppress elevation of blood glucose levels in mice and Inhibit α-amylase and α-glucosidase activities in vitro. Food Sci Technol Res. 2015;21(2):231–240. [Google Scholar]
- Muller C, Joubert E, De Beer D, et al. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine. 2012;20(1):32–39. doi: 10.1016/j.phymed.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49(3):434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- Oyebode OA, Erukainure OL, Chukwuma CI, Ibeji CU, Koorbanally NA, Islam S. Boerhaavia diffusa inhibits key enzymes linked to type 2 diabetes in vitro and in silico; and modulates abdominal glucose absorption and muscle glucose uptake ex vivo. Biomed Pharmacother. 2018;106:1116–1125. doi: 10.1016/j.biopha.2018.07.053. [DOI] [PubMed] [Google Scholar]
- Pal S, Ghosh D, Saha C, Chakrabarti AK, Datta SC, Dey SK. Total polyphenol content, antioxidant activity and lipid peroxidation inhibition efficacy of branded tea (Camellia sinensis) available in India. Int J Tea Sci. 2012;8:13–20. [Google Scholar]
- Patel O, Muller C, Joubert E, Louw J, Rosenkranz B, Awortwe C. Inhibitory interactions of Aspalathus linearis (Rooibos) extracts and compounds, aspalathin and z-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid, on cytochromes metabolizing hypoglycemic and hypolipidemic drugs. Molecules. 2016;21(11):1515. doi: 10.3390/molecules21111515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira VP, Knor FJ, Vellosa JCR, Beltrame FL. Determination of phenolic compounds and antioxidant activity of green, black and white teas of Camellia sinensis (L.) Kuntze, Theaceae. Rev Bras Pl Med. 2014;16(3):490–498. [Google Scholar]
- Ramírez G, Zavala M, Pérez J, Zamilpa A. In vitro screening of medicinal plants used in Mexico as antidiabetics with glucosidase and lipase inhibitory activities. Evid-Base Compl Altern Med. 2012 doi: 10.1155/2012/701261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanni O, Erukainure OL, Oyebode OA, Koorbanally NA, Islam MS. Concentrated hot water-infusion of phragmanthera incana improves muscle glucose uptake, inhibits carbohydrate digesting enzymes and abates Fe2+-induced oxidative stress in hepatic tissues. Biomed Pharmacother. 2018;108:417–423. doi: 10.1016/j.biopha.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Satoh T. Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Inter J Mol Sci. 2014;15(10):18677–18692. doi: 10.3390/ijms151018677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai LJ, Masoko P, Mokgotho MP, et al. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa. South Africa South Afri J Bot. 2010;76(3):465–470. [Google Scholar]
- Sharma V, Bhattacharya A, Kumar A, Sharma H. Health benefits of tea consumption. Trop J Pharm Res. 2007;6(3):785–792. [Google Scholar]
- Siddiqui IA, Afaq F, Adhami VM, Ahmad N, Mukhtar H. Antioxidants of the beverage tea in promotion of human health. Antioxid Redox Signal. 2004;6(3):571–582. doi: 10.1089/152308604773934323. [DOI] [PubMed] [Google Scholar]
- Sinacore DR, Gulve EA. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: implications for physical therapy. Phys Ther. 1993;73(12):878–891. doi: 10.1093/ptj/73.12.878. [DOI] [PubMed] [Google Scholar]
- Tiwari BK, Pandey KB, Abidi A, Rizvi SI (2013) Markers of oxidative stress during diabetes mellitus. J Biomarker 2013 [DOI] [PMC free article] [PubMed]
- Van de Laar F, Lucassen PLBJ, Akkermans RP, Van de Lisdonk EH, Rutten G, van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus: a systematic review. Chin J Evidence-Based Med. 2006;6(5):335–351. [Google Scholar]
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C (2005) Alpha‐glucosidase inhibitors for type 2 diabetes mellitus. The Cochrane Library [DOI] [PMC free article] [PubMed]
- Vinholes J, Vizzotto M. Synergisms in alpha-glucosidase inhibition and antioxidant activity of Camellia sinensis L. Kuntze and Eugenia uniflora L. ethanolic extracts. Pharmacog Res. 2017;9(1):101. doi: 10.4103/0974-8490.197797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang S, Shao S, Qian L, Xu P. Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Indust Crop Product. 2012;37(1):520–526. [Google Scholar]
- Williamson G, Dionisi F, Renouf M. Flavanols from green tea and phenolic acids from coffee: critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol Nutr Food Res. 2011;55(6):864–873. doi: 10.1002/mnfr.201000631. [DOI] [PubMed] [Google Scholar]
- Yuk HJ, Lee JH, Curtis-Long MJ, et al. The most abundant polyphenol of soy leaves, coumestrol, displays potent α-glucosidase inhibitory activity. Food Chem. 2011;126(3):1057–1063. [Google Scholar]
- Zhao H-X, Zhang H-S, Yang S-F. Phenolic compounds and its antioxidant activities in ethanolic extracts from seven cultivars of Chinese jujube. Food Sci Hum Well. 2014;3(3):183–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.