Abstract
Purpose
To investigate the potential etiologies of premature ovarian insufficiency (POI) and diminished ovarian reserve (DOR).
Methods
Fourteen women with sporadic POI and 6 women with DOR were enrolled. We used whole-exome sequencing (WES) and bioinformatics analysis to identify variants in a subset of 599 selected POI candidate genes. The identified genes were subjected to gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment and protein-protein interaction (PPI) network analyses to uncover key genes and pathways.
Results
Among the 20 patients, 79 heterozygous variants were detected in 49 genes, which were classified as “likely pathogenic” or “variants of uncertain significance” according to the guidelines of the American College of Medical Genetics and Genomics. Most patients (17/20) carried two or more variants. Monoacylglycerol O-acyltransferase 1 mutations were found in six patients, and cytochrome P450 family 26 subfamily B member 1 and Bardet-Biedl syndrome 9 mutations were each found in four patients. Some variants were shared between DOR and POI. Enrichment analyses showed that the identified genes participate in key ovarian processes, such as follicular development, gonadal development, meiosis, Fanconi anemia, homologous recombination, and transforming growth factor β signaling. A PPI network revealed interactions between these proteins.
Conclusion
Premature ovarian function decline may be polygenic, and overlap exists between the genetic backgrounds of DOR and POI. WES and in silico analyses may be a useful clinical tool for etiological diagnosis and risk prediction for high-risk women in the future.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01919-y) contains supplementary material, which is available to authorized users.
Keywords: Premature ovarian insufficiency, Diminished ovarian reserve, Whole-exome sequencing, Enrichment analysis
Introduction
The age of menopause depends on the complex balance of forces governing the underlying formation and progressive loss of ovarian follicles. Premature ovarian insufficiency (POI) is characterized by loss of ovarian function before the age of 40, and is a major cause of female infertility [1]. POI is common, affecting approximately 1–3% of women [2]. In fact, a very recent meta-analysis calculated a global POI prevalence of 3.7% [3]. Young women with POI live a relatively longer portion of their lives in estrogen deficiency, significantly increasing their risk of cardiovascular disease and osteoporosis [1]. Therefore, accurate risk prediction and early diagnosis would greatly benefit these women.
Ovarian reserve decline occurs gradually [4] and the concept of diminished ovarian reserve (DOR) has been explored [5]. Women with DOR typically have regular menses; however, they have reduced numbers of ovarian follicles and reduced fecundity compared with unaffected women of similar age [6]. DOR may or may not progress into POI (depending on whether amenorrhea occurs before the age of 40).
A small pool of primordial follicles, premature follicle depletion due to accelerated atresia, and disturbances in follicle function are major factors in the development of ovarian reserve decline [4, 7]. The disorder is clinically and etiologically heterogeneous. Known causes of POI include genetic aberrations, autoimmune ovarian damage, and iatrogenic and environmental factors; however, the etiology of most cases remains unknown. Over 75 POI-associated genes have been identified, and genetic factors account for approximately 20–25% of cases [7–9]. Changes in fragile X mental retardation 1 (FMR1) are a leading cause of POI, with FMR1 premutations occurring in an estimated 11–14% and 2–6% of familial and sporadic POI cases, respectively, in western countries [10]. Women with DOR may also harbor premutation-containing alleles [10]. In addition, mutations in genes related with gonadal development, hormonal signaling, meiosis, and DNA repair have been associated with POI [11]. However, the genetic causes of up to 90% of POI cases are undetermined [9], suggesting that additional causative genes remain to be discovered. Few studies have examined gene mutations associated with DOR, and the relationship between POI and DOR requires further evaluation.
Currently, only karyotyping and FMR1 screening are recommended during clinical genetic testing for POI [12], with no recommendations to examine any other specific genes. Recently, studies have shown that next-generation sequencing (NGS) can efficiently map novel variants participating in POI etiology, which could be used in clinical diagnosis in the near future [13]. Many new genes associated with POI etiology have been identified via whole-genome sequencing or whole-exome sequencing (WES), especially in consanguineous and large POI pedigrees [7, 8]. Meanwhile, only a few studies have performed NGS on women with sporadic POI (i.e., affected women without a family history of POI) [14–16], and NGS have not been used to analyze women with DOR.
In this pilot study, WES was performed on women affected by sporadic POI or DOR. The analysis focused on 599 candidate POI-related genes, and bioinformatics analysis was used to identify promising candidate variants for future study. These analyses provide insight on the biological mechanisms associated with POI development, which may inform the development of tools for the early diagnosis of premature ovarian aging.
Materials and methods
Participants
Fourteen women affected by idiopathic POI and 6 women diagnosed with DOR were recruited from the Clinic of Gynecological Endocrinology and Reproductive Medicine of Peking Union Medical College Hospital (PUMCH) in the period between May 2018 and April 2019. All women with POI were amenorrhea before 40 years old and had elevated serum follicle-stimulating hormone (FSH) levels (> 25 IU/L on two occasions one month apart). The criteria for DOR included regular menstrual cycles, FSH values > 10 IU/L on days 2–4 of the spontaneous menstrual cycle and/or anti-Müllerian hormone (AMH) values ≤ 1.1 ng/ml before the age of 40 years. All women in both groups have no family history of POI or DOR, and had a normal 46,XX karyotype. FMR1 premutations were excluded. Women having a background of pelvic surgery, polycystic ovarian syndrome, endometriosis, ovarian infections, chemotherapy, radiotherapy, endocrine disorders, and/or autoimmune disease were excluded from the study.
This study protocol was granted by the Institutional Review Board (IRB) of PUMCH (No. JS-1604) and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All individuals signed a written informed consent form.
Whole-exome sequencing and variant analysis
After searching public databases, such as PubMed, HGMD, Highwire, Genecards, and Geoprofiles, a 599 genes list (Supplementary Table S1) was selected. When searching the literature, the combinations of the following keywords were included: “primary ovarian insufficiency,” “premature ovarian insufficiency,” “premature ovarian failure,” “POI/POF genetics,” “mouse models of premature ovarian failure,” “diminished ovarian reserve,” “early menopause,” “gene expression and ovary,” “hypergonadotropic hypogonadism,” “genetics of sex determination,” “genetics of gametogenesis,” “molecular regulation of meiosis,” “genetics of folliculogenesis,” “ovulation genetics,” and “ovary and transcriptomics.” These genes were considered candidates as they had been reported as having expression/function during processes of sex determination, meiosis, folliculogenesis, and ovulation, or were identified in patients with POI.
Blood samples were obtained from each participant for molecular studies. Genomic DNA was extracted from peripheral blood leukocytes using the TIANamp Blood DNA Kit (TIANGEN Biotech, Beijing, China) following standard procedures. WES of the genomic DNA was performed, and all samples were sequenced using the Illumina HiSeq X Ten with 150 bp paired-end reads. All sequenced data were mapped to the human reference genome (human genome 19, hg19) using Burrows-Wheeler Alignment tool (BWA) version 0.7.12 (http://bio-bwa.sourceforge.net/). GATK software was used for the realignment correction nearby the insertions/deletions (InDels) and statistical analysis of the genome coverage depth. Based on the calibration results, GATK version 3.5.0 software was used for single nucleotide variations (SNVs)/InDel identification. The results were stored in Variant Call Format (VCF) files. The ANNOVAR tool was adopted to annotate single nucleotide polymorphisms (SNPs) and InDels and to produce statistical analyses of genome-wide SNVs/InDels distribution. The VCF file was annotated using ANNOVAR software. Only variants located on the 599 genes list were extracted for further analysis.
The annotated SNVs fulfilling the following criteria were retained: (1) sequence variants reported as having minor allele frequencies (MAF) of 5% or less in the 1000 genomes project (http://www.internationalgenome.org/), gnomAD (http://gnomad.broadinstitute.org/), and ExAC (http://exac.broadinstitute.org/); (2) variants with potential effects at the protein sequence level (e.g., missense, nonsense, splice site, and frameshift mutations) for downstream analysis; (3) SNVs/InDels with average sequencing depths > 40; and (4) nonsynonymous SNVs predicted to be deleterious or damaging by analysis of amino acid substitutions in silico using three prediction algorithms (SIFT, PolyPhen-2, and PROVEAN) based on scores of < 0.05 (SIFT), > 0.85 (PolyPhen-2), and ≤ − 2.5 (PROVEAN).
All filtered candidate sequence variants obtained by WES were validated by Sanger sequencing of amplified genomic DNA from the original samples. Target regions containing candidate variations were amplified by polymerase chain reaction (PCR) using corresponding primers (the sequences of the primers are available upon request). The PCR products were purified by polyethylene glycol precipitation followed by labeling with an ABI-PRISM BigDye Terminator Ready Reaction Cycle Sequencing Kit (Applied Biosystems, USA) and sequencing on a 3730xl DNA Analyzer (Applied Biosystems) according to the manufacturer’s instructions. The sequencing data were analyzed using CHROMAS software version 1.62. Variants confirmed by Sanger sequencing were retained.
Since our population was exclusively Chinese, we used the EAS (East Asian) population variant frequencies in the gnomAD database for comparisons. Associations between identified variants and POI/DOR phenotypes were assessed by comparing the frequency of the variants in our study and in the EAS population. Allele frequencies were compared using Fisher’s exact test, and P < 0.05 was considered statistically significant. Statistical analysis was performed in SPSS version 24 (IBM, USA).
Each variant was evaluated using the most recent guidelines from the American College of Medical Genetics and Genomics (ACMG) [17]. Variants were sorted into five classes: pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, or benign. We considered pathogenic and likely pathogenic variants to be causative. Variants with MAFs > 5% in the public databases were considered benign and excluded. Variants not present in the 1000 Genomes Project, ExAC, gnomAD, or Single Nucleotide Polymorphism database were considered novel. Novelty and very low population frequency were considered moderate evidence for pathogenicity (PM2). Nonsense, frameshift, canonical ± 1 or 2 splice sites, and initiation codon variants in genes where loss of function are known mechanisms of disease were recognized as evidence for pathogenicity (PVS1). Significantly increased prevalence in our study compared with that in the general population (P < 0.05) was recognized as pathogenic (PS4). Nonsynonymous variants predicted to be deleterious or damaging in in silico analysis were assigned as pathogenic (PP3). If a variant could not be unanimously identified as damaging or benign, it was designated as a VUS.
Functional enrichment analysis and biological signaling pathway analysis
The Gene Ontology (GO, www.geneontology.org) [18] analysis is common genes and gene product annotating method. GO database can provide a functional classification for genomic data from three aspects: biological processes (BP), cellular component (CC), and molecular function (MF). The Kyoto Encyclopedia of Genes and Genomes (KEGG, www.genome.ad.jp/kegg/) [19] database is a knowledge base for systematic analysis, annotation, and visualization of gene functions and relationships between genes. We used the R package clusterProfiler [20] to conduct the GO enrichment and KEGG pathway analysis of the gene list. The enrichment score was assessed by the P value, indicating the significance of the pathway associated with the conditions.
The Search Tool for the Retrieval of Interacting Genes (STRING, stringdb.org) is a biological database designed to predict protein-protein interaction (PPI) information [21]. We used STRING for constructing a PPI network. The selected genes were mapped to STRING to evaluate the interactive relationships, with a confidence score > 0.4 defined as significant. The predicted interaction includes both the physical interaction between proteins and the indirect functional interaction of proteins.
Results
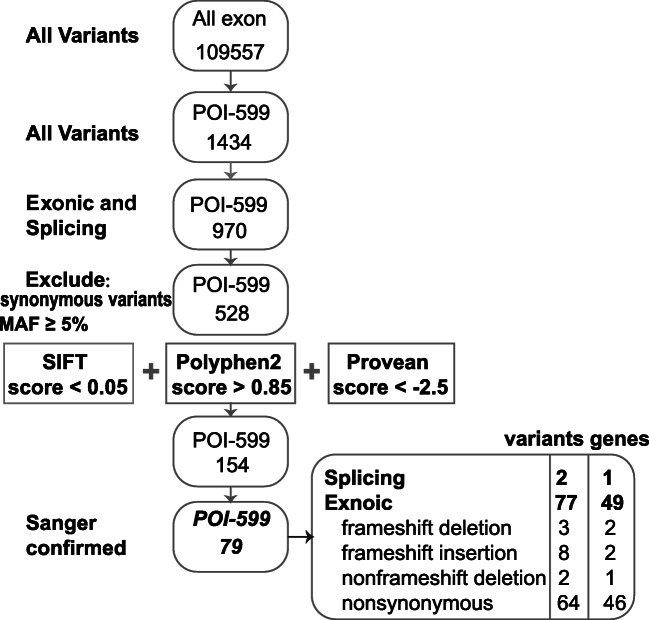
We identified 109,557 sequence variants in the exomes of the 20 samples, including 1434 variants in the 599 POI genes (Fig. 1). After excluding synonymous variants and retaining variants in exon regions and splicing sites with MAFs < 0.05, 154 variants predicted to have deleterious effects by SIFT, PolyPhen-2, and PROVEAN were retained. After verification by Sanger sequencing, 79 variants in 49 genes were confirmed (Fig. 1). The 49 genes and the occurrence of each variant are listed in Table 1. These variants included 2 alternative splice sites, 11 frameshifts, 2 non-frameshift deletions, and 65 nonsynonymous variants. All variants were heterozygous.
Fig. 1.
Screening of variants. Rectangle 1 with rounded edges encloses the 109,557 variants found throughout the exomes of 20 samples. The subset of premature ovarian insufficiency (POI) genes included 599 candidate genes. Rectangle 2 with rounded edges highlights the 1434 variants found among the 599 genes of the POI subset. Rectangle 3 with rounded edges represents the 970 variants detected in the exons and splicing sites, while rectangle 4 with rounded edges shows the 528 variants retained after excluding the synonymous variants and the variants having MAF ≥ 0.05. In rectangle 5 with rounded edges, 154 variants were retained, while rectangle 6 includes 79 variants confirmed after verification using Sanger sequencing, and the last rectangle with rounded edges lists the numbers and types of identified variants. MAF: minor allele frequency
Table 1.
Identified genes selected in the 20 patients with ovarian function decline
| Gene | Mutation/total | Gene | Mutation/total | Gene | Mutation/total |
|---|---|---|---|---|---|
| ABCC5 | 1/20 | GATA6 | 1/20 | PGRMC1 | 2/20 |
| ADAMTSL3 | 1/20 | GRIP1 | 1/20 | PIWIL1 | 1/20 |
| AIRE | 1/20 | HK3 | 1/20 | PRDM1 | 2/20 |
| ALMS1 | 1/20 | HSD17B4 | 1/20 | RAD54L | 1/20 |
| AOX1 | 1/20 | KNTC1 | 2/20 | SOHLH1 | 2/20 |
| ARFGAP3 | 1/20 | LATS1 | 1/20 | SPIDR | 2/20 |
| BBS9 | 4/20 | LEPR | 1/20 | SPO11 | 1/20 |
| BMP8B | 3/20 | LHX9 | 1/20 | STRA8 | 1/20 |
| BMPR2 | 1/20 | LMNA | 1/20 | TBP | 1/20 |
| CDC25B | 1/20 | MAP3K4 | 1/20 | TCF21 | 1/20 |
| CNOT6 | 1/20 | MOGAT1 | 6/20 | THBS1 | 1/20 |
| CYP26B1 | 4/20 | MSH6 | 2/20 | TOP3B | 1/20 |
| DHRS11 | 1/20 | MYO19 | 2/20 | UMODL1 | 3/20 |
| ERCC2 | 1/20 | NLRP11 | 2/20 | VCX | 4/20 |
| ERCC6 | 1/20 | NOS3 | 1/20 | ZNF729 | 3/20 |
| FANCA | 3/20 | NOTCH2 | 1/20 | ||
| FANCL | 1/20 | PGAP3 | 2/20 |
Patient clinical characteristics and identified variants are shown in Table 2. Two women with POI displayed hypergonadotropic primary amenorrhea, and 12 exhibited secondary amenorrhea (mean menopausal age: 24.6 ± 9.3 years). All patients in the POI group had markedly elevated FSH (81.4 ± 24.6 IU/L) and luteinizing hormone (LH; 42.1 ± 15.4 IU/L) levels and low levels of estradiol (22.9 ± 13.3 ng/mL). The six patients diagnosed with DOR had slightly increased FSH (16.4 ± 3.3 IU/L), normal LH (4.6 ± 1.3 IU/L), and relatively low estradiol (39.0 ± 28.8 ng/mL) levels. Monoacylglycerol O-acyltransferase 1 mutations were found in six patients, while cytochrome P450 family 26 subfamily B member 1 and Bardet-Biedl syndrome 9 mutations were each identified in four patients. Some variants were found in both POI and DOR patients. Most patients (17/20) carried two or more genetic variants.
Table 2.
Clinical and molecular findings of POI and DOR patients studied via whole-exome sequencing
| Patient ID | Phenotype | Age of menarche | Age at diagnosis/menopause | FSH (IU/L) | LH (IU/L) | E2 (pg/ml) | AMH (ng/ml) | Gene | Accession number | Exon | Sequence variation | Protein position | 1000G | gnomAD | ExAC | S/Po/Pr* | dbSNP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D15 | DOR | 14 | 32 | 19.89 | 4.39 | 68.42 | 0.28 | ABCC5 | NM_001320032 | 20 | c.1478C>T | p.T493M | 0.0002 | 0.00009 | 0.00008 | D/D/D | rs564676660 |
| ADAMTSL3 | NM_207517 | 16 | c.1852C>T | p.R618W | 0.0016 | 0.0008 | 0.0007 | D/D/D | rs139253965 | ||||||||
| ALMS1 | NM_015120 | 8 | c.2351A>G | p.E784G | 0.00679 | 0.0023 | 0.0023 | -/D/D | rs17848880 | ||||||||
| CYP26B1 | NM_019885 | 5 | c.967C>T | p.R323W | 0.0026 | 0.0014 | 0.0018 | D/D/D | rs138478634 | ||||||||
| ZNF729 | NM_001242680 | 4 | c.2326G>C | p.A776P | D/D/D | - | |||||||||||
| D18 | DOR | 14 | 30 | 15.17 | 6.05 | 78.15 | 0.25 | AOX1 | NM_001159 | 21 | c.2264C>T | p.T755I | 0.002 | 0.0009 | 0.0009 | D/D/D | rs35217482 |
| BBS9 | NM_001348041 | 21 | c.2470C>T | p.R824C | 0.00739 | 0.0026 | 0.0026 | D/D/D | rs146752751 | ||||||||
| GATA6 | NM_005257 | 2 | c.43G>C | p.G15R | 0.03355 | 0.0138 | 0.0137 | D/D/D | rs116262672 | ||||||||
| TCF21 | NM_198392 | 1 | c.10G>A | p.G4S | D/D/D | - | |||||||||||
| UMODL1 | NM_001199527 | 16 | c.3169_3170insCCAT | p.A1057fs | 0.002 | 0.0021 | -/-/- | rs201855268 | |||||||||
| D23 | DOR | 14 | 30 | 17.07 | 3.95 | 38.06 | 0.25 | BMP8B | NM_001720 | 4 | c.778C>T | p.R260C | 0.0025 | 0.0032 | D/D/D | rs199806017 | |
| GRIP1 | NM_021150 | 22 | c.2846A>G | p.E949G | D/D/D | - | |||||||||||
| HSD17B4 | NM_001199291 | 24 | c.2135C>T | p.T712I | 0.01338 | 0.0067 | 0.0067 | D/P/D | rs28943592 | ||||||||
| MAP3K4 | NM_001291958 | 12 | c.1301C>T | p.P434L | 0.0012 | 0.0003 | 0.0002 | D/D/D | rs202168620 | ||||||||
| MOGAT1 | NM_058165 | 3 | c.415_416insATCTTCAC | p.Y139fs | 0.0033 | 0.0032 | -/-/- | rs147393916 | |||||||||
| MYO19 | NM_001163735 | 6 | c.203C>G | p.A68G | 0.0002 | 0.0002 | -/D/D | rs768388466 | |||||||||
| SPIDR | NM_001080394 | 7 | c.815G>A | p.R272Q | 0.0002 | 0.00004 | 0.00004 | D/D/D | rs182613962 | ||||||||
| VCX | NM_013452 | 3 | c.388_445del | p.V130fs | 0.0064 | 0.0029 | -/-/- | - | |||||||||
| VCX | NM_013452 | 3 | c.385_386del | p.Q129fs | 0.008 | 0.0027 | -/-/- | rs752893781 | |||||||||
| D29 | DOR | 12 | 32 | 12.33 | 2.44 | 25.07 | 0.14 | LHX9 | NM_020204 | 1 | c.48delC | p.R16fs | -/-/- | - | |||
| MOGAT1 | NM_058165 | 3 | c.415_416insATCTTCAC | p.Y139fs | 0.0033 | 0.0032 | -/-/- | rs147393916 | |||||||||
| SPO11 | NM_012444 | 1 | c.38T>C | p.F13S | 0.0008 | 0.0002 | 0.0002 | D/D/D | rs185741379 | ||||||||
| UMODL1 | NM_001199527 | 16 | c.3169_3170insCCAT | p.A1057fs | 0.002 | 0.0021 | -/-/- | rs201855268 | |||||||||
| D35 | DOR | 13 | 29 | 20.33 | 5.8 | 19.15 | 0.49 | BMP8B | NM_001720 | 4 | c.778C>T | p.R260C | 0.0025 | 0.0032 | D/D/D | rs199806017 | |
| MOGAT1 | NM_058165 | 3 | c.415_416insATCTTCAC | p.Y139fs | 0.0033 | 0.0032 | -/-/- | rs147393916 | |||||||||
| PIWIL1 | NM_004764 | 15 | c.1691G>C | p.R564P | D/D/D | - | |||||||||||
| SPIDR | NM_001080394 | 19 | c.2569T>C | p.S857P | 0.0004 | 0.0003 | 0.0006 | D/D/D | rs376975557 | ||||||||
| D36 | DOR | 14 | 32 | 13.58 | 4.99 | 5 | 0.24 | CDC25B | NM_021873 | 10 | c.945C>G | p.C315W | 0.0014 | 0.0005 | 0.0006 | D/D/D | rs200104998 |
| MYO19 | NM_001163735 | 6 | c.203C>G | p.A68G | 0.0002 | 0.0002 | -/D/D | rs768388466 | |||||||||
| PRDM1 | NM_001198 | 2 | c.239A>T | p.E80V | 0.0018 | 0.0003 | 0.0002 | D/D/D | rs202200155 | ||||||||
| ZNF729 | NM_001242680 | 4 | c.1007-1009GAA>CTG | p.336_337ArgLys>ThrGlu | |||||||||||||
| ZNF729 | NM_001242680 | 4 | c.1027A>G | p.T343A | 0.0079 | 0.0002 | D/D/D | rs764887966 | |||||||||
| P39 | PA | NA | 14 | 75.57 | 28.97 | 11.8 | 0.07 | LATS1 | NM_001350339 | 6 | c.1766G>A | p.R589H | 0.00001 | D/D/D | - | ||
| P40 | PA | NA | 72.24 | 38.11 | 24.48 | < 0.01 | AIRE | NM_000383 | 9 | c.1051C>T | p.R351W | 0.0004 | 0.00005 | 0.0002 | D/D/D | rs149078622 | |
| BBS9 | NM_001348041 | 21 | c.2470C>T | p.R824C | 0.00739 | 0.0026 | 0.0026 | D/D/D | rs146752751 | ||||||||
| CYP26B1 | NM_019885 | 5 | c.967C>T | p.R323W | 0.0026 | 0.0014 | 0.0018 | D/D/D | rs138478634 | ||||||||
| P24 | SA | 15 | 16 | 110.7 | 41.48 | 10.1 | < 0.01 | CNOT6 | NM_001303241 | 4 | c.26C>T | p.P9L | 0.00399 | 0.0019 | 0.0019 | D/D/D | rs75964385 |
| MOGAT1 | NM_058165 | 3 | c.415_416insATCTTCAC | p.Y139fs | 0.0033 | 0.0032 | -/-/- | rs147393916 | |||||||||
| PGAP3 | NM_033419 | 6 | c.683A>G | p.N228S | 0.0024 | 0.0021 | 0.0021 | D/D/D | rs142596676 | ||||||||
| RAD54L | NM_003579 | 5 | c.354G>C | p.L118F | 0.00002 | 0.00002 | D/D/D | rs778622356 | |||||||||
| SOHLH1 | 4 | c.346-1G>A | 0.003 | 0.003 | rs140132974 | ||||||||||||
| P27 | SA | 11 | 15 | 64.6 | 47.87 | 18.86 | < 0.01 | FANCA | NM_001286167 | 33 | c.3263C>T | p.S1088F | 0.02336 | 0.0509 | 0.051 | D/P/D | rs17233497 |
| P28 | SA | 16 | 18 | 69.63 | 50.63 | 44.5 | 0.01 | BBS9 | NM_001348041 | 21 | c.2470C>T | p.R824C | 0.00739 | 0.0026 | 0.0026 | D/D/D | rs146752751 |
| FANCA | NM_001286167 | 33 | c.3263C>T | p.S1088F | 0.02336 | 0.0509 | 0.051 | D/P/D | rs17233497 | ||||||||
| FANCL | NM_001114636 | 8 | c.637G>A | p.D213N | 0.0002 | 0.0001 | 0.0001 | D/D/D | rs199564543 | ||||||||
| TOP3B | NM_001349852 | 19 | c.1597G>C | p.D533H | D/D/D | - | |||||||||||
| P35 | SA | 10 | 13 | 107.1 | 64.01 | 8 | < 0.01 | CYP26B1 | NM_019885 | 5 | c.967C>T | p.R323W | 0.0026 | 0.0014 | 0.0018 | D/D/D | rs138478634 |
| KNTC1 | NM_014708 | 44 | c.4535T>G | p.V1512G | 0.0016 | 0.0005 | 0.0005 | D/D/D | rs75696429 | ||||||||
| MOGAT1 | NM_058165 | 3 | c.415_416insATCTTCAC | p.Y139fs | 0.0033 | 0.0032 | -/-/- | rs147393916 | |||||||||
| NLRP11 | NM_145007 | 4 | c.94C>T | p.R32C | 0.01777 | 0.0107 | 0.0104 | -/D/D | rs76935241 | ||||||||
| P37 | SA | 14 | 17 | 85.61 | 30.53 | 20.94 | < 0.01 | BBS9 | NM_001348041 | 21 | c.2470C>T | p.R824C | 0.00739 | 0.0026 | 0.0026 | D/D/D | rs146752751 |
| ERCC6 | NM_000124 | 7 | c.1639G>A | p.A547T | 0.000004 | 0.000008 | D/D/D | rs768810263 | |||||||||
| FANCA | NM_001286167 | 33 | c.3263C>T | p.S1088F | 0.02336 | 0.0509 | 0.051 | D/P/D | rs17233497 | ||||||||
| PGRMC1 | NM_006667 | 3 | c.533C>T | p.T178I | 0.00026 | 0.0003 | 0.0003 | D/D/D | rs201254642 | ||||||||
| VCX | NM_013452 | 3 | c.522_581del | p.174_194del | 0.0023 | 0.0062 | -/-/- | - | |||||||||
| P61 | SA | 13 | 18 | 125.8 | 76.66 | 32.00 | < 0.01 | BMP8B | NM_001720 | 7 | c.1165C>T | p.R389C | 0.0008 | 0.0002 | 0.0003 | D/D/D | rs138043926 |
| NOTCH2 | NM_024408 | 31 | c.5683C>T | p.R1895C | D/D/D | - | |||||||||||
| PGAP3 | NM_033419 | 6 | c.683A>G | p.N228S | 0.0024 | 0.0021 | 0.0021 | D/D/D | rs142596676 | ||||||||
| PGRMC1 | NM_006667 | 3 | c.533C>T | p.T178I | 0.00026 | 0.0003 | 0.0003 | D/D/D | rs201254642 | ||||||||
| P10 | SA | 13 | 30 | 55.34 | 28.50 | 34.00 | 0.11 | MOGAT1 | NM_058165 | 3 | c.415_416insATCTTCAC | p.Y139fs | 0.0033 | 0.0032 | -/-/- | rs147393916 | |
| MSH6 | NM_000179 | 9 | c.3832C>G | p.P1278A | D/D/D | - | |||||||||||
| STRA8 | NM_182489 | 2 | c.164G>C | p.R55P | 3E-05 | 0.00002 | D/D/D | rs373793185 | |||||||||
| TBP | NM_003194 | 6 | c.767A>G | p.Q256R | D/D/D | - | |||||||||||
| P15 | SA | 12 | 33 | 51.15 | 48.16 | 195 | 0.01 | DHRS11 | NM_024308 | 3 | c.395C>G | p.A132G | -/D/D | - | |||
| ERCC2 | NM_000400 | 8 | c.691G>A | p.V231M | 0.0006 | 0.0001 | 0.0001 | D/D/D | rs200895828 | ||||||||
| HK3 | NM_002115 | 17 | c.2282G>A | p.R761H | 0.0002 | 0.00007 | D/D/D | rs143713331 | |||||||||
| LMNA | NM_170707 | 4 | c.659G>A | p.R220H | 0.00002 | 0.00002 | D/D/D | rs780066296 | |||||||||
| NOS3 | NM_000603 | 4 | c.382C>T | p.R128W | 0.0004 | 0.0003 | 0.0003 | D/D/D | rs143324164 | ||||||||
| VCX | NM_013452 | 3 | c.522_581del | p.174_194del | 0.0023 | 0.0062 | -/-/- | - | |||||||||
| P1 | SA | 14 | 39 | 68.5 | 25.50 | 42 | 0.1 | KNTC1 | NM_014708 | 44 | c.4535T>G | p.V1512G | 0.0016 | 0.0005 | 0.0005 | D/D/D | rs75696429 |
| LEPR | NM_001003679 | 13 | c.1820A>G | p.Y607C | 0.00002 | 0.00002 | D/D/D | rs777224298 | |||||||||
| MSH6 | NM_000179 | 9 | c.3819T>G | p.N1273K | 0.00001 | 0.00001 | D/D/D | rs759642651 | |||||||||
| P29 | SA | 12 | 34 | 70.8 | 20.79 | 11.04 | < 0.01 | ARFGAP3 | NM_014570 | 15 | c.1469A>G | p.Q490R | 0.00619 | 0.0116 | 0.0115 | D/P/D | rs11551619 |
| SOHLH1 | 4 | c.346-1G>A | rs140132974 | ||||||||||||||
| THBS1 | NM_003246 | 11 | c.1690A>G | p.S564G | 0.0002 | 0.0002 | 0.0002 | D/P/D | rs200366954 | ||||||||
| P31 | SA | 13 | 32 | 121 | 45.47 | 11.8 | 0.01 | NLRP11 | NM_145007 | 4 | c.94C>T | p.R32C | 0.01777 | 0.0107 | 0.0104 | -/D/D | rs76935241 |
| P32 | SA | 12 | 27 | 61.38 | 43.04 | 11.80 | 0.01 | BMPR2 | NM_001204 | 11 | c.1481C>T | p.A494V | 6E-05 | 0.00004 | D/D/D | rs2229778 | |
| CYP26B1 | NM_019885 | 5 | c.967C>T | p.R323W | 0.0026 | 0.0014 | 0.0018 | D/D/D | rs138478634 | ||||||||
| PRDM1 | NM_001198 | 5 | c.1400C>T | p.P467L | 0.0012 | 0.0005 | 0.0005 | D/D/D | rs77256382 |
*The results of SIFT/Polyphen2/Provean score; SIFT score < 0.05: D (damaging); Polyphen2 score > 0.909: D (probably damaging), Polyphen2 score 0.85~0.909: P (possibly damaging); PROVEAN< − 2.5: D (deleterious); - indicated not available
Abbreviation: DOR, diminished ovarian reserve; PA, primary amenorrhea; SA, secondary amenorrhea
1000G: the frequency of each variation in the 1000G database; gnomAD: the frequency of each variation in the gnomAD database; ExAC: the frequency of each variation in the ExAC database of east Asian population
The identified variants are depicted in Table 3, and we compared their frequencies in our cohort and in the EAS population in the gnomAD database. Twenty variants were significantly associated with POI or DOR phenotypes compared with the reference values reported by the gnomAD database (P < 0.05). When the pathogenicity of each variant was evaluated according to ACMG guidelines, 19 were predicted to be likely pathogenic and 38 were considered VUS.
Table 3.
Frequency of candidate variants and their pathogenicity according to the ACMG guidelines
| Gene | Exon | Sequence variation | Protein position | dbSNP | MAF reported by gnomAD_EAS | Cases (no. alleles/no. total alleles) | Patient ID | Fisher’s exact test (P value) | ACMG evidence | Variant classification |
|---|---|---|---|---|---|---|---|---|---|---|
| ABCC5 | 20 | c.1478C>T | p.T493M | rs564676660 | 0.00006 (1/17972) | 0.025 (1/40) | D15 | 0.004 | PS4, PM2, PP3 | Likely pathogenic |
| ADAMTSL3 | 16 | c.1852C>T | p.R618W | rs139253965 | 0.00833 (153/18366) | 0.025 (1/40) | D15 | 0.286 | PM2, PP3 | VUS |
| AIRE | 9 | c.1051C>T | p.R351W | rs149078622 | 0.00035 (5/14172) | 0.025 (1/40) | P40 | 0.017 | PS4, PM2, PP3 | Likely pathogenic |
| ALMS1 | 8 | c.2351A>G | p.E784G | rs17848880 | 0.0321 (577/17976) | 0.025 (1/40) | D15 | 1.00 | PP3 | VUS |
| AOX1 | 21 | c.2264C>T | p.T755I | rs35217482 | 0.01187 (218/18364) | 0.025 (1/40) | D18 | 0.381 | PM2, PP3 | VUS |
| ARFGAP3 | 15 | c.1469A>G | p.Q490R | rs11551619 | 0.00011 (2/18394) | 0.025 (1/40) | P29 | 0.006 | PS4, PP3 | VUS |
| BBS9 | 21 | c.2470C>T | p.R824C | rs146752751 | 0.03366 (619/18392) | 0.1 (4/40) | D18, P28, P37, P40 | 0.045 | PS4, PP3 | VUS |
| BMP8B | 4 | c.778C>T | p.R260C | rs199806017 | 0.03302 (597/18078) | 0.05 (2/40) | D23, D35 | 0.383 | PM2, PP3 | VUS |
| BMP8B | 7 | c.1165C>T | p.R389C | rs138043926 | 0.0019 (35/18394) | 0.025 (1/40) | P61 | 0.075 | PM2, PP3 | VUS |
| BMPR2 | 11 | c.1481C>T | p.A494V | rs2229778 | 0.00092 (17/18394) | 0.025 (1/40) | P32 | 0.038 | PS4, PM2, PP3 | Likely pathogenic |
| CDC25B | 10 | c.945C>G | p.C315W | rs200104998 | 0.00705 (129/18310) | 0.025 (1/40) | D36 | 0.248 | PP3 | VUS |
| CNOT6 | 4 | c.26C>T | p.P9L | rs75964385 | 0.01594 (293/18380) | 0.025 (1/40) | P24 | 0.475 | PM2, PP3 | VUS |
| CYP26B1 | 5 | c.967C>T | p.R323W | rs138478634 | 0.01786 (324/18144) | 0.1 (4/40) | D15, P32, P35, P40 | 0.006 | PS4, PP3 | Likely pathogenic |
| DHRS11 | 3 | c.395C>G | p.A132G | - | 0.025 (1/40) | P15 | PP3 | VUS | ||
| ERCC2 | 8 | c.691G>A | p.V231M | rs200895828 | 0.00169 (31/18376) | 0.025 (1/40) | P15 | 0.067 | PM2, PP3 | VUS |
| ERCC6 | 7 | c.1639G>A | p.A547T | rs768810263 | 0 (0/3128) | 0.025 (1/40) | P37 | 0.013 | PS4, PM2, PP3 | Likely pathogenic |
| FANCA | 33 | c.3263C>T | p.S1088F | rs17233497 | 0.02361 (434/18384) | 0.075 (3/40) | P27, P28, P37 | 0.069 | PS3 ,PP3 | VUS |
| FANCL | 8 | c.637G>A | p.D213N | rs199564543 | 0.00103 (19/18392) | 0.025 (1/40) | P28 | 0.043 | PS4, PM2, PP3 | Likely pathogenic |
| GATA6 | 2 | c.43G>C | p.G15R | rs116262672 | 0.04629 (813/17564) | 0.025 (1/40) | D18 | 1.00 | PP3 | VUS |
| GRIP1 | 22 | c.2846A>G | p.E949G | – | 0.025 (1/40) | D23 | PM2, PP3 | VUS | ||
| HK3 | 17 | c.2282G>A | p.R761H | rs143713331 | 0.00087 (16/18392) | 0.025 (1/40) | P15 | 0.036 | PS4, PM2, PP3 | Likely pathogenic |
| HSD17B4 | 24 | c.2135C>T | p.T712I | rs28943592 | 0.07997 (1470/18382) | 0.025 (1/40) | D23 | 0.372 | PP3 | VUS |
| KNTC1 | 44 | c.4535T>G | p.V1512G | rs75696429 | 0.00695 (125/17976) | 0.05 (2/40) | P1, P35 | 0.032 | PS4, PM2, PP3 | Likely pathogenic |
| LATS1 | 6 | c.1766G>A | p.R589H | - | 0.025 (1/40) | P39 | PM2, PP3 | VUS | ||
| LEPR | 13 | c.1820A>G | p.Y607C | rs777224298 | 0 (0/18394) | 0.025 (1/40) | P1 | 0.002 | PS4, PM2, PP3 | Likely pathogenic |
| LHX9 | 1 | c.48delC | p.R16fs | - | 0.025 (1/40) | D29 | PVS1, PM2 | Likely pathogenic | ||
| LMNA | 4 | c.659G>A | p.R220H | rs780066296 | 0 (0/18394) | 0.025 (1/40) | P15 | 0.002 | PS4, PM2, PP3 | Likely pathogenic |
| MAP3K4 | 12 | c.1301C>T | p.P434L | rs202168620 | 0.00375 (69/18394) | 0.025 (1/40) | D23 | 0.141 | PM2, PP3 | VUS |
| MOGAT1 | 3 | c.415_416insATCTTCAC | p.Y139fs | rs147393916 | 0.03627 (652/17976) | 0.15 (6/40) | D23, D29, D35, P10, P24, P35 | 0.003 | PS4, PM2 | VUS |
| MSH6 | 9 | c.3832C>G | p.P1278A | - | 0.025 (1/40) | P10 | PM2, PP3 | VUS | ||
| MSH6 | 9 | c.3819T>G | p.N1273K | rs759642651 | 0.00011 (2/18394) | 0.025 (1/40) | P1 | 0.006 | PS4, PM2, PP3 | Likely pathogenic |
| MYO19 | 6 | c.203C>G | p.A68G | rs768388466 | 0.00245 (44/17968) | 0.05 (2/40) | D23, D36 | 0.005 | PS4, PM2, PP3 | Likely pathogenic |
| NLRP11 | 4 | c.94C>T | p.R32C | rs76935241 | 0.08296 (1526/18394) | 0.05 (2/40) | P31, P35 | 0.771 | PP3 | VUS |
| NOS3 | 4 | c.382C>T | p.R128W | rs143324164 | 0.00197 (36/18238) | 0.025 (1/40) | P15 | 0.078 | PM2, PP3 | VUS |
| NOTCH2 | 31 | c.5683C>T | p.R1895C | - | 0.025 (1/40) | P61 | PM2, PP3 | VUS | ||
| PGAP3 | 6 | c.683A>G | p.N228S | rs142596676 | 0.02007 (369/18390) | 0.05 (2/40) | P24, P61 | 0.192 | PM2, PP3 | VUS |
| PGRMC1 | 3 | c.533C>T | p.T178I | rs201254642 | 0.0039 (54/13860) | 0.05 (2/40) | P36, P61 | 0.011 | PS4, PP3 | Likely pathogenic |
| PIWIL1 | 15 | c.1691G>C | p.R564P | - | 0.025 (1/40) | D35 | PM2, PP3 | VUS | ||
| PRDM1 | 2 | c.239A>T | p.E80V | rs202200155 | 0.00381 (70/18394) | 0.025 (1/40) | D36 | 0.143 | PP3 | VUS |
| PRDM1 | 5 | c.1400C>T | p.P467L | rs77256382 | 0.00626 (115/18372) | 0.025 (1/40) | P32 | 0.224 | PP3 | VUS |
| RAD54L | 5 | c.354G>C | p.L118F | rs778622356 | 0.00027 (5/18394) | 0.025 (1/40) | P24 | 0.013 | PS4, PM2, PP3 | Likely pathogenic |
| SOHLH1 | 4 | c.346-1G>A | rs140132974 | 0.00975 (179/18362) | 0.05 (2/40) | P24,P29 | 0.059 | PVS1, PM2 | Likely pathogenic | |
| SPIDR | 7 | c.815G>A | p.R272Q | rs182613962 | 0.00034 (6/17804) | 0.025 (1/40) | D23 | 0.016 | PS4, PM2, PP3 | Likely pathogenic |
| SPIDR | 19 | c.2569T>C | p.S857P | rs376975557 | 0.00421 (70/16630) | 0.025 (1/40) | D35 | 0.157 | PM2, PP3 | VUS |
| SPO11 | 1 | c.38T>C | p.F13S | rs185741379 | 0.00261 (48/18368) | 0.025 (1/40) | D29 | 0.101 | PM2, PP3 | VUS |
| STRA8 | 2 | c.164G>C | p.R55P | rs373793185 | 0.00044 (8/18382) | 0.025 (1/40) | P10 | 0.019 | PS4, PM2, PP3 | Likely pathogenic |
| TBP | 6 | c.767A>G | p.Q256R | - | 0.025 (1/40) | P10 | PM2, PP3 | VUS | ||
| TCF21 | 1 | c.10G>A | p.G4S | - | 0.025 (1/40) | D18 | PM2, PP3 | VUS | ||
| THBS1 | 11 | c.1690A>G | p.S564G | rs200366954 | 0.00332 (61/18394) | 0.025 (1/40) | P29 | 0.126 | PM2, PP3 | VUS |
| TOP3B | 19 | c.1597G>C | p.D533H | - | 0.025 (1/40) | P28 | PM2, PP3 | VUS | ||
| UMODL1 | 16 | c.3169_3170insCCAT | p.A1057fs | rs201855268 | 0.02297 (72/3134) | 0.05 (2/40) | D18, D29 | 0.239 | PVS1, PM2 | Likely pathogenic |
| VCX | 3 | c.388_445del | p.V130fs | rs1569092849 | 0.00324 (75/23181) | 0.025 (1/40) | D23 | 0.122 | PVS1 | VUS |
| VCX | 3 | c.385_386del | p.Q129fs | rs752893781 | 0.00395 (93/23559) | 0.025 (1/40) | D23 | 0.147 | PVS1 | VUS |
| VCX | 3 | c.522_581del | p.174_194del | - | 0.0019 (60/31593) | 0.05 (2/40) | P15, P37 | 0.003 | PS4 | VUS |
| ZNF729 | 4 | c.2326G>C | p.A776P | - | 0.025 (1/40) | D15 | PM2, PP3 | VUS | ||
| ZNF729 | 4 | c.1007-1009GAA>CTG | p.336_337ArgLys>ThrGlu | - | 0.025 (1/40) | D36 | VUS | |||
| ZNF729 | 4 | c.1027A>G | p.T343A | rs764887966 | 0.00764 (135/17670) | 0.025 (1/40) | D36 | 0.266 | PP3 | VUS |
Abbreviation: MAF, minor allele frequencies; ACMG, the American College of Medical Genetics and Genomics; EAS, East Asian population; VUS, variant of unknown significance. Significant P values (P < 0.05) are presented in italic
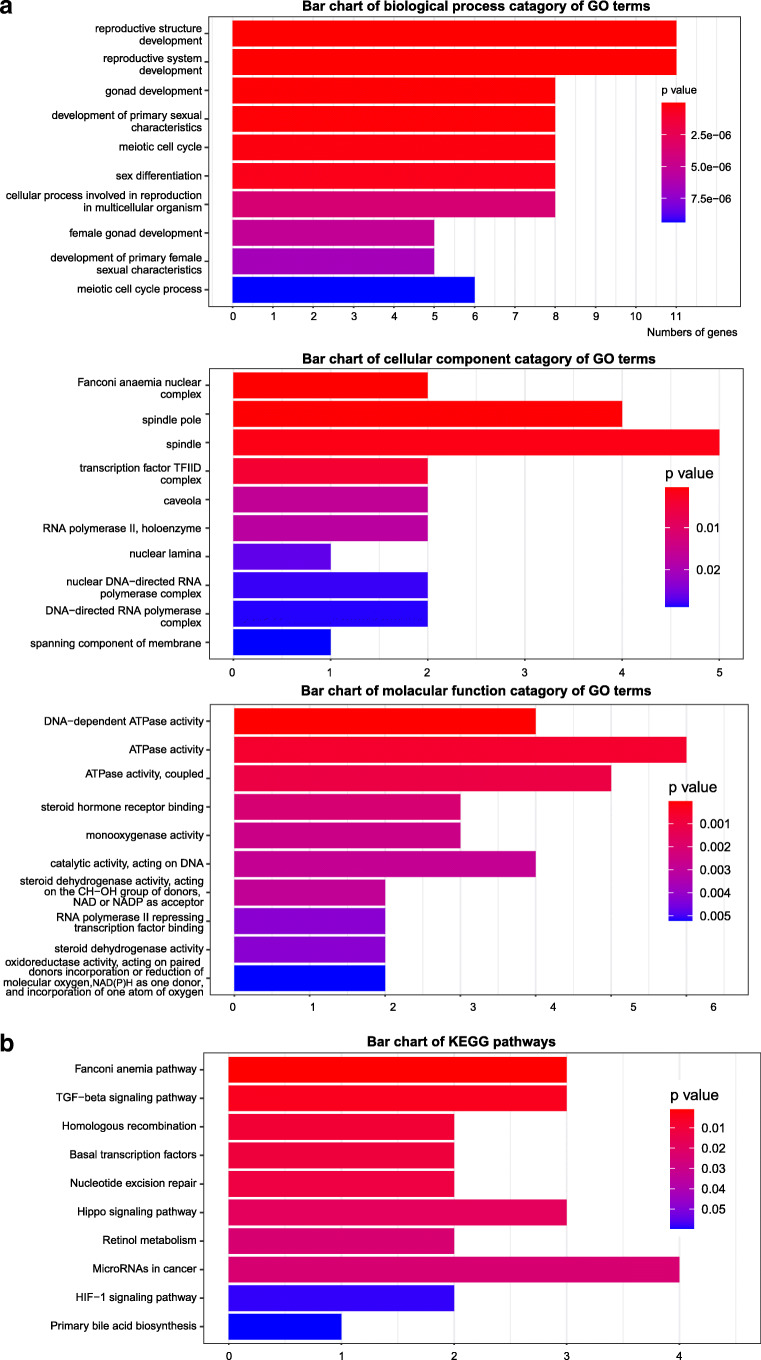
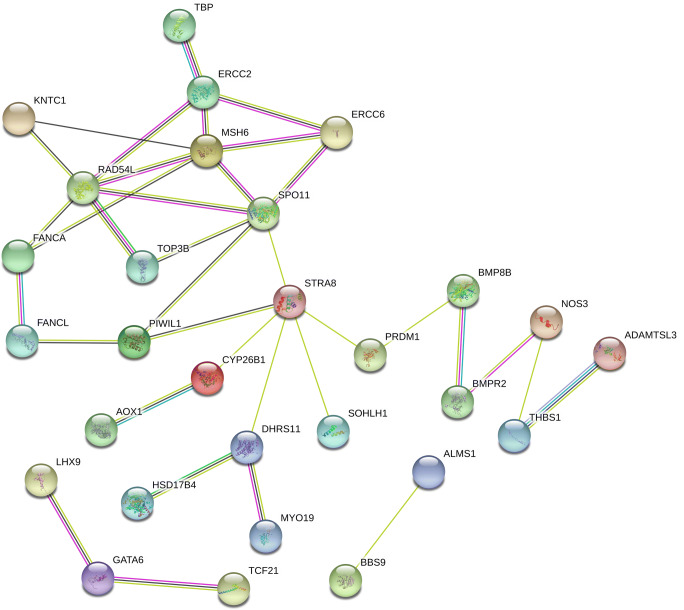
To acquire further understanding of the biological implications of the identified genes, and identify the most important genes and processes to focus gene list in future studies, we conducted enrichment analysis to identify significant GO categories and KEGG pathways. The top ten terms and pathways are shown in Fig. 2 and Supplementary Table S2. Enriched GO terms included the biological processes reproductive structure development (P = 1.16E−08), reproductive system development (P = 1.24E−08), gonad development (P = 8.77E−08), development of primary sexual characteristics (P = 1.08E−07), meiotic cell cycle (P = 2.52E−07), sex differentiation (P = 4.66E−07), cellular process involved in reproduction in multicellular organism (P = 3.76E−06), and female gonad development (P = 5.16E−06). The cellular components Fanconi anemia (FA) nuclear complex (P = 0.00044498) and spindle pole (P = 0.00067514) were enriched, as were the molecular functions DNA-dependent ATPase activity (P = 3.93E−05) and ATPase activity (P = 0.00063824). The most enriched KEGG pathways were the FA pathway (P = 0.00092661), the transforming growth factor (TGF)β signaling pathway (P = 0.00454567), and homologous recombination (P = 0.00945354). To identify core regulatory genes for further study, we entered the 49 variant-containing genes into the STRING database to obtain a PPI network. Proteins such as ERCC excision repair 2, TFIIH core complex helicase subunit; ERCC excision repair 6, chromatin remodeling factor; mutS homolog (MSH)6; RAD54 like (RAD54L); SPO11 initiator of meiotic double-stranded breaks; stimulated by retinoic acid 8; and dehydrogenase/reductase 11 were important central nodes in the PPI network (Fig. 3).
Fig. 2.
GO terms and KEGG pathway analyses of candidate genes. The top ten pathways were retained in the bar plot. a GO analysis show enrichment in the following three terms: biological process (BP), cellular component (CC), and molecular function (MF). b Enrichment of KEGG pathway
Fig. 3.
Protein-protein interaction network generated by STRING software for selected proteins. Each node in a PPI network represents a protein, and an edge between two nodes represents an interactional relationship between them. Colored lines display known and predicted interactions. Light blue: curated databases; pink: experimentally determined; green: gene neighborhood; red: gene fusions; blue: gene co-occurrence; yellow: textmining; black: co-expression; purple: protein homology
Discussion
This study is the first to report the results of WES of Chinese women affected by POI or DOR, and describes 79 variants in 49 genes that are potentially related to premature ovarian aging. These variants can be considered strong etiological candidates for ovarian aging, as they have been selected using innovative bioinformatics analysis and stringent filtering. These genes participate in key biological processes in the ovaries, such as meiosis, follicular development, and DNA repair.
Although it is unclear whether the onset of puberty, normal reproductive aging, POI, and menopause share the same genetic mechanisms, they may have a somewhat shared genetic etiology, with overlap between the genes involved [22, 23]. Additionally, it is highly plausible that DOR and POI represent different stages of the same pathological phenomenon and have similar underlying genetic backgrounds [24]. The additive effects of environmental factors also cannot be ruled out [7]. Over the past decades, many genes have emerged as POI candidates, including those involved in primordial germ cell migration and proliferation (such as nanos C2HC-type zinc finger 3 (NANOS3)) [25], oocyte specific transcription factors (such as spermatogenesis and oogenesis specific basic helix-loop-helix (SOHLH)1, SOHLH2, folliculogenesis specific bHLH transcription factor (FIGLA), and NOBOX oogenesis homeobox (NOBOX)) [26–29], folliculogenesis (nuclear receptor subfamily 5 group A member 1 (NR5A1), WT1 transcription factor (WT1), and forkhead box L2 (FOXL2)) [30–32], meiosis (stromal antigen 3 (STAG3), helicase for meiosis 1 (HFM1), MSH4, MSH5, and synaptonemal complex central element protein 1 (SYCE1)) [33–37], DNA damage repair (BRCA1 DNA repair associated (BRCA1); BRCA2 DNA repair associated (BRCA2); ERCC6; abraxas 1, BRCA1 A complex subunit (ABRAXAS1); scaffold protein involved in DNA repair (SPIDR); minichromosome maintenance 8 homologous recombination repair factor (MCM8); and minichromosome maintenance 9 homologous recombination repair factor (MCM9)) [8, 23, 38–41], members of the TGFβ superfamily (bone morphogenetic protein 15 (BMP15), growth differentiation factor 2 (GDF9), and AMH) [42, 43], and hormones and their receptors (follicle-stimulating hormone receptor (FSHR) and anti-Müllerian hormone receptor type 2 (AMHR2)) [43, 44]. However, each of these genes is affected in only a few women, and most patients lack a clear genetic diagnosis [8]. There are still candidate genes to be discovered and further genetic studies of POI are warranted.
Although DOR is more common than POI, fewer studies have been conducted on it. Some recent studies support a role for genetic factors in DOR, and many candidate genes are known genetic causes of POI, including FMR1 [45], FSHR [46], GDF9 [47], and NR5A1 [48]. Women with BRCA1/2 mutations also present with DOR and accelerated primordial follicle loss [49]. Several genes, including AMH, luteinizing hormone/choriogonadotropin receptor (LHCGR), insulin-like growth factor (IGF)1, IGF2, IGF1 receptor (IGF1R), IGF2 receptor (IGF2R), and gremlin 1, DAN family BMP antagonist (GREM1) [50–52] are differentially expressed between women with DOR and controls, representing candidates for further research. In mouse studies, a number of genes, including autoimmune regulator (Aire) [53]; forkhead box L2 (Foxl2) [54]; Gdf9 [55]; wingless-type MMTV integration site family, member 4 (Wnt4) [56]; G protein–coupled receptor 3 (Gpr3) [57]; and Bmp15 [58], result in a DOR-like phenotype. The previous studies demonstrate the overlap between the known DOR- and POI-associated genes [47, 48]. In this study, some variants were shared between women with POI and DOR, providing further evidence of this overlap.
Some researchers have proposed the polygenicity of POI [15, 16]. Bouilly et al. reported that 36% of patients in their cohort harbored mutations in two different loci, suggesting a digenic nature for POI pathogenesis [15]. A WES study of a cohort of Caucasian patients with POI showed similar oligogenicity/polygenicity [16]. In the present study, 17 patients (85%) carried more than two variants, supporting a polygenic origin for ovarian aging. It is possible that multiple genetic variants influence ovarian function, and while individually, each may have a relatively low effect, their combinations may contribute substantially to the wide variation in reproductive aging observed in healthy populations and, thus, determine the age of menopause. Multifactorial heredity may partly explain the variable phenotypes observed in patients with POI, such as different ages at amenorrhea onset [22].
In recent decades, women have been increasingly postponing childbirth due to work or study. This tendency has consequently led to an increase in age-related infertility and unwanted childlessness. Declined ovarian reserve is one of the main causes of female infertility. Patients diagnosed with POI have only a 5–10% chance of natural pregnancy [12], while women with DOR tend to have decreased fertility and a higher risk of spontaneous abortion [59]. However, these diseases are often diagnosed too late, with severe and irreversible consequences for fertility. Therefore, it would be of great significance to be able to screen women at high risk for these diseases in the early stages of ovarian function decline and accurately predict the end of their reproductive lifespans. This would enable such individuals to acquire timely guidance regarding family planning and fertility treatment as early as possible, improving their quality of life.
The etiology of ovarian insufficiency remains poorly understood in most cases, and genetic factors appear to play an important role [7]. However, identifying precise causative genes has been challenging. Recently, through new technologies, a prodigious leap has occurred in our understanding of POI genetics [13]. NGS is a powerful tool for the study of genetic diseases and has great potential in future clinical applications [13]. However, previously, NGS has been mainly used in POI pedigrees [7], and only a few studies have used it to analyze women with sporadic POI to identify new putative candidate genes [14–16]. In this study, WES was performed in Chinese women with sporadic ovarian function decline, including both POI and DOR samples.
It was interesting to note that 19 variants were statistically associated with the POI or DOR phenotype. However, functional studies and additional reports on affected women will be required to confirm the predicted deleterious effects of the variants on ovarian function. Pathway analyses of groups of candidate genes can implicate etiological mechanisms. In this study, GO and KEGG enrichment revealed that the identified genes play important roles in meiosis, DNA damage repair, and reproductive system development, which may influence folliculogenesis and ovarian function, promoting POI or DOR.
Meiosis is a universal cellular process in eukaryotes that is required for gametogenesis. Mutations in meiotic genes usually impair meiotic progression and trigger oocyte death, resulting in POI [60]. During meiosis, large amounts of programmed DNA double-strand breaks are introduced and repaired through homologous recombination [61]. The homologous recombination pathway is required during physiological cell cycle progression to repair replication-associated DNA damage [61]. Variants in RAD54L and DNA topoisomerase III beta (TOP3B), which encode components of the homologous recombination pathway, were selected as candidates in this study. The structure of RAD54L is similar to that of RAD54, which is a core component of the homologous recombination machinery and plays a key role in maintaining the genomic integrity of developing germ cells [62]. TOP3B also plays a role in maintaining genome stability. In vitro, disruption of TOP3B causes genomic instability, including increased DNA damage and chromosome bridging [63]. In addition, male Top3b knockout mice display chromosome instability in their spermatocytes as well as infertility [64].
In the present study, we also found variants in FA complementation group A (FANCA) and FA complementation group L (FANCL) in women with POI. The FA pathway is another important DNA repair pathway, which is mainly involved in repairing interstrand crosslinks [65]. This pathway involves at least 20 proteins, including those encoded by FANCA and FANCL. Mutations in both genes have been associated with POI [66, 67]; however, the variants at these loci differed from those identified in this study. A recent study identified the FANCA heterozygous missense variants c.1772G > A (p.R591Q) and c.3887A > G (p.E1296G) in patients with POI, and in vitro functional studies and mice models indicated that the two heterozygous variants may be pathogenic [66]. Another study found that FANCL mutations can potentially cause POI by disrupting DNA damage repair. They identified two novel heterozygous frameshift mutations, c.1048_1051delGTCT (p.Gln350Valfs*18) and c.739dupA (p.Met247Asnfs*4) in the FANCL genes of patients with POI, and verified the deleterious effect of the variants via in vitro studies [67]. Both studies were conducted in Chinese women, indicating that the FA pathway plays an important role in the pathogenesis of POI in Han Chinese women.
There are several strengths to this study. First, this study is the first to conduct WES in patients with DOR and evaluate variants in women with POI and DOR simultaneously to confirm the overlap between their genetic backgrounds. Second, our study performed WES in women with sporadic premature ovarian decline and used innovative bioinformatics analysis. Considering the strong genetic background of this disorder, with a standardized selective approach, NGS may become a useful tool for the etiological diagnosis and risk prediction of high-risk women in the future.
Our study’s limitations also need to be considered. First, all variants found in this study were evaluated using in silico tools, and some would have to be reported clinically as VUS [17]. Therefore, the findings of this study are unverified and should not be directly used in clinical practice until additional studies have been performed. Further validation will be necessary to understand the functional significance of identified variants. Second, this study only examined the coding regions and exon-intron boundaries of genes, and the functions of noncoding regulatory regions were not studied. Third, we could not determine the presence of compound heterozygous variants in our participants, or whether variants occurred in cis or in trans, as their parents’ DNA samples were not available for genotype analysis. Fourth, the prevalence and genetic heterogeneity of POI may be related to ethnicity [3]. Therefore, the pathophysiology of POI in a Chinese population might differ from that in other ethnicities. For example, fewer patients with POI in China and other Asian countries carry FMR1 premutations compared with patients in western countries [68]. These variations may or may not extend to other genes/variants, and the results of this study may or may not apply to other ethnic groups.
In summary, we used WES and in silico analyses to identify variants and genes involved in sporadic POI and DOR. The results indicate that ovarian function decline is polygenic and that there is overlap between the genetic backgrounds of DOR and POI. In the future, WES and in silico analyses may become useful tools in the genetic diagnosis of patients with ovarian dysfunction and in risk prediction for high-risk women.
Electronic supplementary material
(DOCX 68 kb)
Acknowledgments
The authors are deeply grateful to all participants involved in this study and all the doctors and researchers who participated in the study.
Authors’ contributions
All authors contributed to the study conception and design, material preparation, data collection, and analysis. The first draft of the manuscript was written by R. T, and Q. Y revised it. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding information
This study was supported by the National Key Research and Development Program [grant number 2018YFC1002105]; CAMS Innovation Fund for Medical Sciences (CIFMS) [grant number 2017-I2M-1-002].
Compliance with ethical standards
This study was approved by the Ethics Committee of our hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee of Peking Union Medical College Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 3.Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22(4):403–411. doi: 10.1080/13697137.2019.1574738. [DOI] [PubMed] [Google Scholar]
- 4.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol. 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J Assist Reprod Genet. 2015;32(12):1709–12. doi: 10.1007/s10815-015-0595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medicine ASR. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9–e17. doi: 10.1016/j.fertnstert.2014.12.093. [DOI] [PubMed] [Google Scholar]
- 7.Jiao X, Ke H, Qin Y, Chen ZJ. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29(11):795–807. doi: 10.1016/j.tem.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Franca MM, Mendonca BB. Genetics of primary ovarian insufficiency in the next-generation sequencing era. J Endocr Soc. 2020;4(2):bvz037. doi: 10.1210/jendso/bvz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Y, Jiao X, Simpson J, Chen Z. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808. doi: 10.1093/humupd/dmv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man L, Lekovich J, Rosenwaks Z, Gerhardt J. Fragile X-associated diminished ovarian reserve and primary ovarian insufficiency from molecular mechanisms to clinical manifestations. Front Mol Neurosci. 2017;10:290. doi: 10.3389/fnmol.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic Spectrum. Endocr Rev. 2016;37(6):609–635. doi: 10.1210/er.2016-1047. [DOI] [PubMed] [Google Scholar]
- 12.Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol 2014;124(1):193–197. doi:10.1097/01.AOG.0000451757.51964.98. [DOI] [PubMed]
- 13.Laissue P. The molecular complexity of primary ovarian insufficiency aetiology and the use of massively parallel sequencing. Mol Cell Endocrinol. 2018;460:170–180. doi: 10.1016/j.mce.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca DJ, Patino LC, Suarez YC, de Jesus Rodriguez A, Mateus HE, Jimenez KM et al. Next generation sequencing in women affected by nonsyndromic premature ovarian failure displays new potential causative genes and mutations. Fertil Steril. 2015;104(1):154–62.e2. doi:10.1016/j.fertnstert.2015.04.016. [DOI] [PubMed]
- 15.Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, et al. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab. 2016;101(12):4541–4550. doi: 10.1210/jc.2016-2152. [DOI] [PubMed] [Google Scholar]
- 16.Patino LC, Beau I, Carlosama C, Buitrago JC, Gonzalez R, Suarez CF, et al. New mutations in non-syndromic primary ovarian insufficiency patients identified via whole-exome sequencing. Hum Reprod. 2017;32(7):1512–1520. doi: 10.1093/humrep/dex089. [DOI] [PubMed] [Google Scholar]
- 17.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 19.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D3D8. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossetti R, Ferrari I, Bonomi M, Persani L. Genetics of primary ovarian insufficiency. Clin Genet. 2017;91(2):183–198. doi: 10.1111/cge.12921. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Zhang Y, Zhao S, Bian Y, Ning Y, Qin Y. Mutational analysis of theFAM175A gene in patients with premature ovarian insufficiency. Reprod BioMed Online. 2019;38(6):943–950. doi: 10.1016/j.rbmo.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Feng F, Chu C, Yue W, Li L. A novel EIF4ENIF1 mutation associated with a diminished ovarian reserve and premature ovarian insufficiency identified by whole-exome sequencing. J Ovarian Res. 2019;12(1):119. doi: 10.1186/s13048-019-0595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin Y, Zhao H, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Mutation analysis of NANOS3 in 80 Chinese and 88 Caucasian women with premature ovarian failure. Fertil Steril. 2007;88(5):1465–1467. doi: 10.1016/j.fertnstert.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao S, Li G, Dalgleish R, Vujovic S, Jiao X, Li J, et al. Transcription factor SOHLH1 potentially associated with primary ovarian insufficiency. Fertil Steril. 2015;103(2):548–53.e5. doi: 10.1016/j.fertnstert.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Qin Y, Jiao X, Dalgleish R, Vujovic S, Li J, Simpson JL, et al. Novel variants in the SOHLH2 gene are implicated in human premature ovarian failure. Fertil Steril. 2014;101(4):1104–9.e6. doi: 10.1016/j.fertnstert.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Tosh D, Rani HS, Murty US, Deenadayal A, Grover P. Mutational analysis of the FIGLA gene in women with idiopathic premature ovarian failure. Menopause. 2015;22(5):520–526. doi: 10.1097/gme.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Wang B, Zhang W, Chen B, Luo M, Wang J, et al. A homozygous NOBOX truncating variant causes defective transcriptional activation and leads to primary ovarian insufficiency. Hum Reprod. 2017;32(1):248–255. doi: 10.1093/humrep/dew271. [DOI] [PubMed] [Google Scholar]
- 30.Philibert P, Paris F, Lakhal B, Audran F, Gaspari L, Saad A, et al. NR5A1 (SF-1) gene variants in a group of 26 young women with XX primary ovarian insufficiency. Fertil Steril. 2013;99(2):484–489. doi: 10.1016/j.fertnstert.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Settas N, Anapliotou M, Kanavakis E, Fryssira H, Sofocleous C, Dacou-Voutetakis C, et al. A novel FOXL2 gene mutation and BMP15 variants in a woman with primary ovarian insufficiency and blepharophimosis-ptosis-epicanthus inversus syndrome. Menopause. 2015;22(11):1264–1268. doi: 10.1097/gme.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 32.Laissue P. Aetiological coding sequence variants in non-syndromic premature ovarian failure: from genetic linkage analysis to next generation sequencing. Mol Cell Endocrinol. 2015;411:243–257. doi: 10.1016/j.mce.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 33.He WB, Banerjee S, Meng LL, Du J, Gong F, Huang H, et al. Whole-exome sequencing identifies a homozygous donor splice-site mutation in STAG3 that causes primary ovarian insufficiency. Clin Genet. 2018;93(2):340–344. doi: 10.1111/cge.13034. [DOI] [PubMed] [Google Scholar]
- 34.de Vries L, Behar DM, Smirin-Yosef P, Lagovsky I, Tzur S, Basel-Vanagaite L. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J Clin Endocrinol Metab. 2014;99(10):E2129–E2132. doi: 10.1210/jc.2014-1268. [DOI] [PubMed] [Google Scholar]
- 35.Carlosama C, Elzaiat M, Patino LC, Mateus HE, Veitia RA, Laissue P. A homozygous donor splice-site mutation in the meiotic gene MSH4 causes primary ovarian insufficiency. Hum Mol Genet. 2017;26(16):3161–3166. doi: 10.1093/hmg/ddx199. [DOI] [PubMed] [Google Scholar]
- 36.Guo T, Zhao S, Zhao S, Chen M, Li G, Jiao X, et al. Mutations in MSH5 in primary ovarian insufficiency. Hum Mol Genet. 2017;26(8):1452–1457. doi: 10.1093/hmg/ddx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Zhang W, Jiang H, Wu BL. Mutations in HFM1 in recessive primary ovarian insufficiency. N Engl J Med. 2014;370(10):972–974. doi: 10.1056/NEJMc1310150. [DOI] [PubMed] [Google Scholar]
- 38.Qin Y, Guo T, Li G, Tang TS, Zhao S, Jiao X, et al. CSB-PGBD3 mutations cause premature ovarian failure. PLoS Genet. 2015;11(7):e1005419. doi: 10.1371/journal.pgen.1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz NK, Karagin PH, Terzi YK, Kahyaoglu I, Yilmaz S, Erkaya S, et al. BRCA1 and BRCA2 sequence variations detected with next-generation sequencing in patients with premature ovarian insufficiency. J Turk Ger Gynecol Assoc. 2016;17(2):77–82. doi: 10.5152/jtgga.2016.16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg-Shukron A, Rachmiel M, Renbaum P, Gulsuner S, Walsh T, Lobel O, et al. Essential role of BRCA2 in ovarian development and function. N Engl J Med. 2018;379(11):1042–1049. doi: 10.1056/NEJMoa1800024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai S, Wood-Trageser M, Matic J, Chipkin J, Jiang H, Bachelot A, et al. MCM8 and MCM9 nucleotide variants in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2017;102(2):576–582. doi: 10.1210/jc.2016-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renault L, Patino LC, Magnin F, Delemer B, Young J, Laissue P, et al. BMPR1A and BMPR1B missense mutations cause primary ovarian insufficiency. J Clin Endocrinol Metab. 2019. 10.1210/clinem/dgz226. [DOI] [PubMed]
- 43.Alvaro Mercadal B, Imbert R, Demeestere I, Gervy C, De Leener A, Englert Y, et al. AMH mutations with reduced in vitro bioactivity are related to premature ovarian insufficiency. Hum Reprod. 2015;30(5):1196–1202. doi: 10.1093/humrep/dev042. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Xu X, Han T, Yan L, Cheng L, Qin Y, et al. A novel homozygous mutation in the FSHR gene is causative for primary ovarian insufficiency. Fertil Steril. 2017;108(6):1050–5.e2. doi: 10.1016/j.fertnstert.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod BioMed Online. 2010;20(6):768–775. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Ghezelayagh Z, Totonchi M, Zarei-Moradi S, Asadpour O, Maroufizadeh S, Eftekhari-Yazdi P, et al. The impact of genetic variation and gene expression level of the follicle-stimulating hormone eceptor on ovarian reserve. Cell J. 2018;19(4):620–626. doi: 10.22074/cellj.2018.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang TT, Ke ZH, Song Y, Chen LT, Chen XJ, Feng C, et al. Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod. 2013;28(9):2473–2481. doi: 10.1093/humrep/det291. [DOI] [PubMed] [Google Scholar]
- 48.Warman DM, Costanzo M, Marino R, Berensztein E, Galeano J, Ramirez PC, et al. Three new SF-1 (NR5A1) gene mutations in two unrelated families with multiple affected members: within-family variability in 46,XY subjects and low ovarian reserve in fertile 46,XX subjects. Horm Res Paediatr. 2011;75(1):70–77. doi: 10.1159/000320029. [DOI] [PubMed] [Google Scholar]
- 49.Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839–3847. doi: 10.1210/jc.2017-00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skiadas CC, Duan S, Correll M, Rubio R, Karaca N, Ginsburg ES, et al. Ovarian reserve status in young women is associated with altered gene expression in membrana granulosa cells. Mol Hum Reprod. 2012;18(7):362–371. doi: 10.1093/molehr/gas008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenseid K, Jindal S, Hurwitz J, Santoro N, Pal L. Differential granulosa cell gene expression in young women with diminished ovarian reserve. Reprod Sci. 2011;18(9):892–899. doi: 10.1177/1933719111398502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jindal S, Greenseid K, Berger D, Santoro N, Pal L. Impaired gremlin 1 (GREM1) expression in cumulus cells in young women with diminished ovarian reserve (DOR) J Assist Reprod Genet. 2012;29(2):159–162. doi: 10.1007/s10815-011-9684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jasti S, Warren BD, McGinnis LK, Kinsey WH, Petroff BK, Petroff MG. The autoimmune regulator prevents premature reproductive senescence in female mice. Biol Reprod. 2012;86(4):110. doi: 10.1095/biolreprod.111.097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, et al. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407–421. doi: 10.1210/me.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 56.Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24(8):3010–3025. doi: 10.1096/fj.09-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ledent C, Demeestere I, Blum D, Petermans J, Hämäläinen T, Smits G, et al. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci U S A. 2005;102(25):8922–8926. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMahon HE, Hashimoto O, Mellon PL, Shimasaki S. Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology. 2008;149(6):2807–2815. doi: 10.1210/en.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyttle Schumacher BM, Jukic AMZ, Steiner AZ. Antimullerian hormone as a risk factor for miscarriage in naturally conceived pregnancies. Fertil Steril. 2018;109(6):1065–71.e1. doi: 10.1016/j.fertnstert.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dou X, Guo T, Li G, Zhou L, Qin Y, Chen ZJ. Minichromosome maintenance complex component 8 mutations cause primary ovarian insufficiency. Fertil Steril. 2016;106(6):1485–9.e2. doi: 10.1016/j.fertnstert.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Ranjha L, Howard SM, Cejka P. Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes. Chromosoma. 2018;127(2):187–214. doi: 10.1007/s00412-017-0658-1. [DOI] [PubMed] [Google Scholar]
- 62.Ceccaldi R, Sarangi P, D’Andrea AD. The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol. 2016;17(6):337–349. doi: 10.1038/nrm.2016.48. [DOI] [PubMed] [Google Scholar]
- 63.Zhang T, Wallis M, Petrovic V, Challis J, Kalitsis P, Hudson DF. Loss of TOP3B leads to increased R-loop formation and genome instability. Open Biol. 2019;9(12):190222. doi: 10.1098/rsob.190222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwan KY, Moens PB, Wang JC. Infertility and aneuploidy in mice lacking a type IA DNA topoisomerase III. beta Proc Natl Acad Sci U S A. 2003;100(5):2526–2531. doi: 10.1073/pnas.0437998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Räschle M, Knipscheer P, Knipsheer P, Enoiu M, Angelov T, Sun J, et al. Mechanism of replication-coupled DNA interstrand crosslink repair. Cell. 2008;134(6):969–980. doi: 10.1016/j.cell.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X, Zhang X, Jiao J, Zhang F, Pan Y, Wang Q, et al. Rare variants in FANCA induce premature ovarian insufficiency. Hum Genet. 2019;138(11–12):1227–1236. doi: 10.1007/s00439-019-02059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Guo T, Liu R, Ke H, Xu W, Zhao S, et al. FANCL gene mutations in premature ovarian insufficiency. Hum Mutat. 2020;41(5):1033–1041. doi: 10.1002/humu.23997. [DOI] [PubMed] [Google Scholar]
- 68.Pastore L, Manichaikul A, Wang X, Finkelstein J. FMR1 CGG repeats: reference levels and race-ethnic variation in women with normal fertility (study of women’s health across the nation) Reprod Sci. 2016;23(9):1225–1233. doi: 10.1177/1933719116632927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 68 kb)