Abstract
Enzymatic extraction of arabinoxylans (AXs) is an attractive and environmentally friendly extraction option, in which technical considerations (yield and purity) have been coupled with environmental concerns. Amano HC 90 and Cellulase were combined to evaluate their interactive effects on AX extraction from destarched, deproteinised bran (DSDPB). A response surface methodology was used to obtain the optimal extraction conditions. The experimental data fit well with the predicted values and the model adequately represented the actual relationship among the measured parameters. The extraction yield and AX content in the extract under optimal conditions (double-enzyme dose of 920 U/g, pH of 3.0, extraction temperature of 35.0 °C; extraction time of 6 h; and DSDPB to liquid ratio of 1:30) were 40.73 ± 0.09% and 75.88 ± 0.11%, respectively. The double-enzymatic extraction method of AX from fresh corn fibre was more efficient than the chemical method.
Keywords: Arabinoxylan, Central composite rotatable design, Enzymatic, Fresh corn fibre
Introduction
Lignocellulosic materials currently represent the largest biomass resource in the world. If separated into cellulose, xylan and lignin, these materials could be used for the production of food and bioenergy, which may be important given the limitations in available agricultural area and the future scarcity of fossil fuels (de Figueiredo et al. 2017).
Corn fibre is one of the most abundantly produced low-value lignocellulosic materials in the world, and is composed mainly of testa, pericarp, the aleurone layer and residual sclerenchyma of the endosperm (Yadav et al. 2008; Rose et al. 2010; Ayala-Soto et al. 2014; Sárossy et al. 2013). Thus, comprehensive utilisation of corn bran is very important.
The major components of corn fibre are hemicellulose, cellulose and lignin. Arabinoxylans (AXs) are mainly localised in endosperm cell walls, the aleurone layer and the pericarp of cereal grains. Corn fibre contains higher quantities of AXs than other cereal grains (Fadel et al. 2017). AXs are comprised of a linear ß-(1,4)-D-xylopyranose backbone and L-arabinofuranose residues as side chains on O-2 and/or O-3. Some of the arabinose moieties are ester-linked to the O-5 positions of hydroxycinnamic acids, such as ferulic acid, p-coumaric acid, and dimers and trimers of ferulic acid (Ayala-Soto et al. 2014; Bai et al. 2017; Ayala-Soto et al. 2016; Xiang and Runge 2016).
For its structure features, AXs gel can be used as a viscosity regulator, thickener, adsorbent of flavour substances and edible membrane material. AXs provide ideal aqueous conditions for bioactive drugs or texturing molecules (Rose et al. 2010; Izydorczyk et al. 1991; Vansteenkiste et al. 2004). AXs reportedly exert various biological effects, such as antioxidant, immune enhancement, prebiotic potential, serum cholesterol-lowering and blood sugar level-modifying properties (Malunga and Beta 2015; Zhang et al. 2016; Zhou et al. 2010; Monteagudomera et al. 2018; Wang et al. 2014).
Evidence suggests that corn AX polymers in intact corn kernels are cross-linked to each other and/or to other cell wall polymers through dehydrodiferulate ester bridges, making it difficult to extract AXs from corn fibre (Zhang et al. 2014).
The production of AXs from cereal grains and their by-products has been investigated for more than two decades. The most common chemical method to isolate AXs is alkaline extraction. Alkaline solvents include aqueous alkaline media, and combinations such as alkali and hydrogen peroxide, and alkali and chlorite solution or dimethyl sulfoxide (Wang et al. 2014). Alkaline solvents efficiently solubilise AXs from cell wall materials, as they disrupt hydrogen and covalent bonds and loosen up the cell wall matrix. However, alkaline solvents may also break down functional groups of AXs, such as ferulic acid, reducing their functional properties (Bender et al. 2017; Mathew et al. 2017; Zhang et al. 2015; Aguedo et al. 2014), and producing a great deal of wastewater; thus, alkaline extraction of AXs has been limited to the laboratory.
Enzymatic extraction of AXs is an attractive extraction alternative due to its environmental friendliness, lack of any change to functional groups, and efficient and controlled degradation of AX molecules (Zhang et al. 2016; Maes et al. 2004; Beaugrand et al. 2004; Ogawa et al. 2005; Höije et al. 2005). Enzymatic methods using endoxylanases to extract water-unextractable AXs are as efficient as chemical methods (Wang et al. 2014). The combination of cellulase and endoxylanases provides higher AX extraction yields than those of endoxylanases alone (Escarnot et al. 2012).
In the present study, enzymatic extraction with xylanase combined with cellulase was performed on fresh corn fibre. The effects of a double-enzyme on the extraction yield and AX contents in the extract were studied, and extraction parameters, such as extraction pH, liquid to solid material ratio, double-enzyme dose, extraction temperature and extraction time were optimised using a response surface methodology (RSM) by employing a five-level, five-variable central composite design (CCD). These extracts could serve as a basis for producing healthier foods.
Materials and methods
Materials
Fresh corn fibre was kindly provided by Sky Scenery Food Co., Ltd. (Jilin Province, China) as a juicing by-product. Birchwood xylan was purchased from Sigma-Aldrich (Beijing, China). The internal standard D-( +)-allose was purchased from Miragen Inc. (Boulder, CO, USA), and the neutral monosaccharides D-( +)-galactose, D-( +)-xylose and D-( +)-glucose were purchased from Sigma-Aldrich. L-( +)-Arabinose, D-( +)-mannose and L-( +)-rhamnose were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). All other chemicals used were of at least analytical grade.
Amano HC 90 from Aspergillus niger was purchased from Amano Enzyme. Co., Ltd. (Shanghai, China) and Cellulasec from A. niger was purchased from Merck & Co., Inc. (Darmstadt, Germany).
Destarching and deproteinising
The fresh maize bran was washed with water until the water was clear, and the residue was dried in an oven at 70 °C. The dried fresh maize bran was ground to a 20-mesh particle size, deoiled by hexane extraction, dried, autoclaved for 20 min at 121 °C and stored at − 20 °C.
The destarched, deproteinised fresh corn bran (DSDPB) was produced as described by Escarnot et al. (2011a, b).
Enzyme assay
The activity of Amano HC 90 xylanase was determined in at least triplicate, as described previously with slight modification (Escarnot et al. 2012; Hu et al. 2008). One gram of Amano HC 90 was dissolved in 100 mL of 50 mmol/L NaAc buffer (pH 4.5) with continuous stirring for 30 min at 25 °C. The supernatant was obtained as the enzyme solution by centrifugation at 10,000 g for 20 min. Then, 150 µL of 1% Birchwood xylan, 75 µL of 50 mM NaAc buffer (pH 4.5) and 45 µL of deionised water were added to a tube, mixed and warmed for 3 min, 30 µL of Amano HC 90 was added and mixed. After a 20-min incubation at 50 °C, 900 µL of DNS solution was added and boiled in water for 5 min, cooled and assayed at 540 nm with D-xylose as the standard. All activity measurements were performed in triplicate.
Cellulase activity was determined in triplicate as described by Zhang et al. (2008) with slight modifications. Cellulase was appropriately diluted in 50 mM NaAc buffer (pH 4.8). Then, 150 µL of 1% CMC-Na, 75 µL of 50 mM NaAc buffer (pH 4.8) and 45 µL of deionised water were added to a tube, mixed and warmed for 3 min. Following this, 30 µL of Cellulase was added and mixed. After a 20-min incubation at 40 °C, 900 µL of DNS solution was added and boiled in water for 5 min, and cooled and assayed at 540 nm with D-glucose as the standard. All activity measurements were performed in triplicate, and means were calculated.
Double-enzymatic extraction of AX from corn fibre
DSDPB was suspended in 50 mM NaAc buffer at a particular liquid to solid material ratio (w/v) and warmed up in a parallel fermentation system (Hui Neng Da Bio-Engineering Equipment Co., Ltd, Zhenjiang, China). A particular amount of the combined enzymes was added. Each suspension was set at a particular temperature with continuous mixing, incubated for a particular time, heated in boiling water for 10 min to inactivate the enzyme, cooled to room temperature and then centrifuged (5 min, 3000 rpm). The supernatant was filtered through a 0.45 mm membrane filter, concentrated, and freeze-dried into a double-enzymatic extract of corn fibre AXs. All hydrolyses were performed in duplicate (Escarnot et al. 2012).
Experimental design
The xylanase extractions combined with the cellulase stage were optimised by RSM according to the CCD. The effects of extraction pH, liquid to solid material ratio, double-enzyme dose, extraction temperature and extraction time at five different levels were evaluated. The parameters and levels chosen were based on the results of preliminary experiments, and two responses were measured: extraction yield (Y1) and the AX contents in the extract (Y2). The actual and coded levels of the independent variables and results are shown in Table 1. The experimental protocol was designed, and the results statistically analysed, using Design-Expert software (ver. 10.0; Stat-Ease, Minneapolis, MN, USA).
Table 1.
Central composite design factors levels
| Coded levels | Factors | ||||
|---|---|---|---|---|---|
| X1 pH | X2 liquid to solid material ratio | X3 extraction time/h | X4 extraction temperature/°C | X5 double enzyme dose/U/g | |
| − 2 | 3.0 | 1:20 | 4 | 35 | 500 |
| − 1 | 3.3 | 1:25 | 5 | 40 | 700 |
| 0 | 3.6 | 1:30 | 6 | 45 | 900 |
| 1 | 3.9 | 1:35 | 7 | 50 | 1100 |
| 2 | 4.2 | 1:40 | 8 | 55 | 1300 |
Determination of AX contents in the extract
Monosaccharide content was analysed as described previously (Craeyveld et al. 2009; Delcour et al. 1999) with slight modification. Hydrolysis of the extract (15 mg) was with 4.0 M TFA (2.5 ml) at 110 °C for 60 min. To 1.5 mL of the hydrolysates, 0.5 mL of internal standard solution (100 mg of‚-D-allose in 100 mL of a 1:1 diluted saturated benzoic acid solution) was added. The tubes were placed in ice-water, and 0.5 mL of NH3 (25%), 1 drop of octan-2-ol, and 100 µL of 2.0 M NH3 containing sodium borohydride (200 mg/mL) were added. After incubation (30 min, 40 °C), the solutions were mixed with 200 µL of glacial acetic acid. To 500 µL of the resulting mixture were added 500 µL of N-methylimidazole and 5.0 mL of acetic anhydride. After 10 min, the solutions were mixed with 900 µL of ethanol and left for 5 min. Water (10.0 mL) was added. After addition of 500 µL (0.04%) bofromophenol blue, the tubes were placed in ice-water and 5.0 mL of 7.5 M potassium hydroxide was added twice within a 5-min period. The tubes were mixed, and after phase separa-tion, the upper phase was isolated and dried over anhydroussodium sulfate.
Four microlitres of sample was injected and separated on a Supelco SP-2380 column (30 m 0.25 mm ID, 0.2 μm film thickness; Sigma-Aldrich Co. LLC., Shanghai, China) with nitrogen as the carrier gas in a GC-14C series chromatograph (Shimadzu, Beijing, China) equipped with a flame-ionisation detector. Separation occurred at 225 °C with an injection and detection temperature of 280 °C. Beta-D-allose was used as the internal standard. AX content was defined as the sum of the contents of arabinose and xylose multiplied by 0.88 to correct for hydration water, as follows.
Statistical analysis
Analysis of variance (ANOVA) was performed using SPSS software (ver. 17.0; SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered significant.
Results and discussion
Effect of double-enzyme dose, pH, extraction time, extraction temperature and solid–liquid ratio on the yield and contents of AXs in the extract
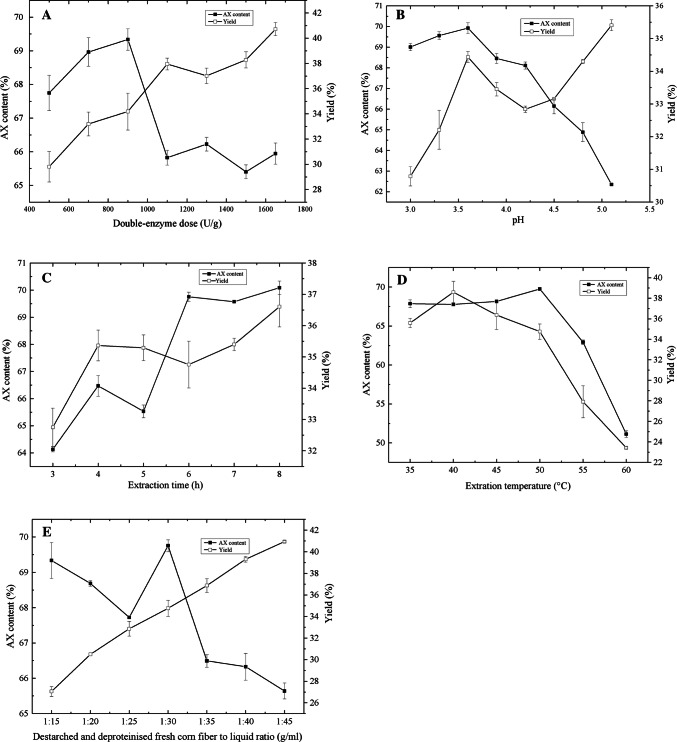
The effects of a double-enzyme dose in the range of 500–1700 U/g DSDPB on the yield of AX and the AX content of the extract are shown in Fig. 1a The AX content of the extract increased with the increase in double-enzyme dose, reached a maximum when the double-enzyme dose was 900 U/g, and then declined. The AX yield increased consistently with the increase in double-enzyme dose. A higher double-enzyme dose may provide more cellulase, causing hydrolysis of the cellulose and release of glucose. The effects of pH on the yield of AX and AX content of the extract are shown in Fig. 1b. The increase in AX content of the extract was sharp when the pH was increased from 3.3 to 3.9; the AX content reached a maximum at about 68.5%, and then decreased significantly. The AX yield increased to the maximum value with an increase in pH from 3.3 to 3.9 and then decreased slightly, but sharply increased as the pH was increased from 3.9 to 5.4. This result indicates that the optimum pH of Amano HC 90 and Cellulase was 3.9 when DSDPB was used as the substrate. Figure 1c shows the effect of extraction time on the yield of AX and the AX content of the extract. The AX content of the extract increased with the increase in extraction time, and reached a plateau of about 69.5% at an extraction time of 6 h. The AX yield increased consistently with the increase in extraction time. The effect of extraction temperature on the AX yield and content of the extract is illustrated in Fig. 1d. A significant increase in AX content was observed at 35–50 °C, beyond which the AX content decreased with a further increase in temperature. A marked increase in yield was observed at 40 °C, but the yield decreased with the increase in extraction temperature. These results indicate that higher temperatures lead to decreased enzyme activity.
Fig. 1.
Effect of Double-enzyme dose, pH, extraction time, extraction 1 temperature and DSDPB-liquid ratio on the yield of AX and the AX content of the extract a Effect of double-enzyme dose on the yield of AX and the AX content of the extract. pH, extraction temperature, DSDPB-liquid ratio and extraction time were constant at 4.8, 50 °C, 1:30 and 4 h, respectively. b Effect of pH on the yield of AX and the AX content of the extract. Extraction temperature, extraction time, DSDPB-liquid ratio and double-enzyme dose were constant at 50 °C, 4 h, 1:30 and 900 U/g, respectively. c Effect of extraction time on the yield of AX and the AX content of the extract. Extraction temperature, DSDPB-liquid ratio, double-enzyme dose and pH were constant at 50 °C, 1:30, 900 U/g and 3.6, respectively. d Effect of extraction temperature on the yield of AX and the AX content of the extract. pH, DSDPB-liquid ratio, extraction time and double-enzyme dose were constant at 3.6, 1:30, 4 h and 900 U/g, respectively. e Effect of destarched and deproteinised fresh corn fiber to liquid ratio on yield and AX content. pH, extraction temperature, extraction time and double-enzyme dose and were constant at 3.6, 50 °C, 4 h and 900 U/g, respectively. Error bars represent standard deviation of the means (n = 3)
The effects of the solid (DSDPB) to liquid ratio on AX content and yield were also determined, and the results are shown in Fig. 1e. AX yield increased consistently with the increase in the solid (DSDPB) to liquid ratio. The AX content of the extract reached its maximum value when the solid (DSDPB) to liquid ratio was 1:30.
Statistical analysis and model fit
Amano HC 90 and Cellulase were combined to evaluate their cooperative effects on AX extraction from DSDPB. To optimise the AX double-enzymatic extraction process, the central conditions were: double-enzyme dose of 900 U/g, pH of 3.9, extraction temperature of 50 °C, extraction time of 6 h and solid (DSDPB) to liquid ratio of 1:30. Table 2 shows the experimental conditions and the results of the double-enzymatic extraction obtained after RSM optimisation. The values of Y1 (AX yield) and Y2 (AX content of the extract) ranged from 23.37% to 46.21%, and from 71.98% to 76.98%, respectively. The optimum conditions predicted by Design Expert software (Stat-Ease, Inc, Minneapolis, MN, USA) were double-enzyme dose of 924.74 U/g, pH of 3.0, extraction temperature of 35.0 °C, extraction time of 6 h and solid (DSDPB) to liquid ratio of 1:30. Under these conditions, the AX content of the extract was 76.94%, and the AX yield was 41.85%.
Table 2.
Response surface design and test results of corn sheath AX extracted by complex enzyme
| Run | X1 pH | X2 DSDPB-liquid ratio | X3 extraction time/h | X4 extraction temperature/°C | X5 double-enzyme dose/U/g | AX context/% | Yield of AX/% |
|---|---|---|---|---|---|---|---|
| 1 | − 1 | 1 | 1 | − 1 | 1 | 75.23 | |
| 2 | 1 | 1 | − 1 | − 1 | − 1 | 74.76 | 38.09 |
| 3 | − 1 | 1 | − 1 | 1 | − 1 | 74.75 | 29.93 |
| 4 | 0 | 0 | 0 | 0 | 0 | 76.13 | 39.55 |
| 5 | 1 | − 1 | 1 | 1 | − 1 | 73.72 | 33.65 |
| 6 | − 2 | 0 | 0 | 0 | 0 | 76.13 | 38.52 |
| 7 | 1 | − 1 | − 1 | 1 | 1 | 72.59 | 35.84 |
| 8 | 1 | − 1 | 1 | − 1 | − 1 | 75.82 | 36.99 |
| 9 | − 1 | − 1 | − 1 | 1 | − 1 | 75.40 | 30.61 |
| 10 | 0 | 0 | 0 | 0 | 0 | 76.06 | 40.52 |
| 11 | 0 | 0 | 0 | 0 | 0 | 76.19 | 40.18 |
| 12 | − 1 | − 1 | 1 | 1 | − 1 | 76.02 | 31.93 |
| 13 | 0 | 0 | 2 | 0 | 0 | 75.53 | 41.48 |
| 14 | 0 | 0 | 0 | 0 | 0 | 75.77 | 40.51 |
| 15 | 0 | 0 | 0 | 0 | 0 | 76.23 | 39.46 |
| 16 | 1 | 1 | − 1 | 1 | − 1 | 74.00 | 33.30 |
| 17 | 0 | 0 | 0 | 0 | 0 | 76.98 | 38.90 |
| 18 | − 1 | 1 | 1 | 1 | 1 | 73.82 | 35.77 |
| 19 | − 1 | − 1 | − 1 | − 1 | 1 | 76.09 | 40.03 |
| 20 | 1 | − 1 | 1 | − 1 | 1 | 74.44 | 40.86 |
| 21 | 0 | 0 | 0 | 2 | 0 | 71.98 | 23.37 |
| 22 | − 1 | − 1 | 1 | 1 | 1 | 75.21 | 34.74 |
| 23 | 0 | 0 | 0 | 0 | 2 | 74.77 | 44.53 |
| 24 | 0 | 0 | 0 | − 2 | 0 | 76.32 | 39.55 |
| 25 | 1 | 1 | 1 | − 1 | 1 | 73.91 | 43.78 |
| 26 | − 1 | − 1 | − 1 | 1 | 1 | 75.57 | 35.44 |
| 27 | − 1 | 1 | − 1 | − 1 | − 1 | 76.91 | 40.34 |
| 28 | 1 | 1 | 1 | 1 | 1 | 73.86 | 40.05 |
| 29 | 1 | − 1 | 1 | 1 | 1 | 74.14 | 36.79 |
| 30 | 2 | 0 | 0 | 0 | 0 | 73.55 | 38.19 |
| 31 | 0 | 0 | 0 | 0 | 0 | 76.43 | 39.56 |
| 32 | 0 | 0 | 0 | 0 | 0 | 76.31 | 39.79 |
| 33 | 0 | 0 | 0 | 0 | − 2 | 76.53 | 36.23 |
| 34 | 1 | 1 | 1 | 1 | − 1 | 73.83 | 35.14 |
| 35 | 0 | − 2 | 0 | 0 | 0 | 75.80 | 36.33 |
| 36 | 1 | − 1 | − 1 | − 1 | − 1 | 75.56 | 34.40 |
| 37 | − 1 | 1 | 1 | − 1 | − 1 | 76.61 | 39.78 |
| 38 | 0 | 0 | − 2 | 0 | 0 | 75.42 | 37.99 |
| 39 | − 1 | 1 | 1 | 1 | − 1 | 74.25 | 29.63 |
| 40 | 0 | 2 | 0 | 0 | 0 | 75.34 | 41.47 |
| 41 | 1 | 1 | 1 | − 1 | − 1 | 74.90 | 40.07 |
| 42 | 1 | − 1 | − 1 | 1 | − 1 | 73.07 | 32.08 |
| 43 | 1 | − 1 | − 1 | − 1 | 1 | 74.71 | 38.16 |
| 44 | − 1 | 1 | − 1 | 1 | 1 | 74.32 | 35.74 |
| 45 | − 1 | 1 | − 1 | − 1 | 1 | 75.82 | 44.17 |
| 46 | − 1 | − 1 | 1 | − 1 | − 1 | 76.86 | 38.45 |
| 47 | 1 | 1 | − 1 | 1 | 1 | 72.99 | 38.40 |
| 48 | 1 | 1 | − 1 | − 1 | 1 | 74.06 | 42.06 |
| 49 | − 1 | − 1 | − 1 | − 1 | − 1 | 77.00 | 36.94 |
| 50 | − 1 | − 1 | 1 | − 1 | 1 | 76.03 | 42.54 |
The data in Table 2 were analysed by multiple regression to develop response surface models. The coefficients of the Y1 values were evaluated for significance using an F-test at the 95% confidence level. The linear coefficients include four significant terms: the solid (DSDPB) to liquid ratio, extraction time, extraction temperature and double-enzyme dose; six significant quadratic terms were detected (P < 0.05). The following second-order models were obtained by fitting the data and eliminating the insignificant terms: Y1 is the predicted value for AX yield (%), and X1, X2, X3, X4 and X5 are the variables described in Table 2.
| 1 |
The results of the ANOVA for AX yield are shown in Table 3. Table 4 shows that the second-order model was highly significant (P < 0.001) but the lack-of-fit term was not (P > 0.05). The coefficient of determination (R2) of the model was 0.9779, and the adjusted R2 (R2Adj) was 0.9627, indicating that the experimental data have minor errors and the obtained values fit well with the predicted values. Thus, the model adequately represents the relationship among the parameters.
Table 3.
Yield regression model analysis of variance
| Source | Sum of square | df | Mean squares | F value | P value (Pr > F) | Significance |
|---|---|---|---|---|---|---|
| Model | 860.75 | 20 | 43.04 | 64.29 | < 0.0001 | ** |
| X1 | 1.01 | 1 | 1.01 | 1.51 | 0.2284 | |
| X2 | 47.24 | 1 | 47.24 | 70.57 | < 0.0001 | ** |
| X3 | 19.62 | 1 | 19.62 | 29.31 | < 0.0001 | ** |
| X4 | 404.18 | 1 | 404.18 | 603.81 | < 0.0001 | ** |
| X5 | 182.84 | 1 | 182.84 | 273.14 | < 0.0001 | ** |
| X1X2 | 3.94 | 1 | 3.94 | 5.89 | 0.0217 | * |
| X1X3 | 2.62 | 1 | 2.62 | 3.91 | 0.0576 | |
| X1X4 | 39.41 | 1 | 39.41 | 58.87 | < 0.0001 | ** |
| X1X5 | 0.72 | 1 | 0.72 | 1.08 | 0.3073 | |
| X2X3 | 0.51 | 1 | 0.51 | 0.77 | 0.3887 | |
| X2X4 | 11.58 | 1 | 11.58 | 17.30 | 0.0003 | ** |
| X2X5 | 3.48 | 1 | 3.48 | 5.20 | 0.0302 | |
| X3X4 | 2.07 | 1 | 2.07 | 3.09 | 0.0895 | |
| X3X5 | 0.028 | 1 | 0.028 | 0.042 | 0.8388 | |
| X4X5 | 0.44 | 1 | 0.44 | 0.66 | 0.4244 | |
| X12 | 6.65 | 1 | 6.65 | 9.93 | 0.0038 | ** |
| X22 | 3.46 | 1 | 3.46 | 5.17 | 0.0306 | * |
| X32 | 0.58 | 1 | 0.58 | 0.86 | 0.3607 | |
| X42 | 136.06 | 1 | 136.06 | 203.36 | < 0.0001 | ** |
| X52 | 8.12 × 10–3 | 1 | 8.12 × 10–3 | 0.012 | 0.9131 | |
| Residual | 19.41 | 29 | 0.67 | |||
| Lack of fit | 17.20 | 22 | 0.78 | 2.47 | 0.1106 | |
| Error | 2.21 | 7 | 0.32 | |||
| Cor total | 880.16 | 49 |
**Extremely significantly (P < 0.01), *Significant (P < 0.05)
Table 4.
AX content regression model analysis of variance
| Source | Sum of square | df | Mean squares | F value | P value (Pr > F) | Significance |
|---|---|---|---|---|---|---|
| Model | 67.66 | 20 | 3.38 | 15.17 | < 0.0001 | ** |
| X1 | 20.32 | 1 | 20.32 | 91.11 | < 0.0001 | ** |
| X2 | 2.00 | 1 | 2.00 | 8.96 | 0.0056 | ** |
| X3 | 0.040 | 1 | 0.040 | 0.18 | 0.6761 | |
| X4 | 22.90 | 1 | 22.90 | 102.67 | < 0.0001 | ** |
| X5 | 5.10 | 1 | 5.10 | 22.85 | < 0.0001 | ** |
| X1X2 | 0.70 | 1 | 0.70 | 3.14 | 0.0871 | |
| X1X3 | 0.69 | 1 | 0.69 | 3.11 | 0.0884 | |
| X1X4 | 0.049 | 1 | 0.049 | 0.22 | 0.6433 | |
| X1X5 | 0.018 | 1 | 0.018 | 0.079 | 0.7809 | |
| X2X3 | 0.37 | 1 | 0.37 | 1.67 | 0.2067 | |
| X2X4 | 5.25 × 10−3 | 1 | 5.25 × 10−3 | 0.024 | 0.8791 | |
| X2X5 | 0.055 | 1 | 0.055 | 0.25 | 0.6223 | |
| X3X4 | 0.33 | 1 | 0.33 | 1.50 | 0.2308 | |
| X3X5 | 1.53 × 10−4 | 1 | 1.53 × 10−4 | 6.8 × 10−4 | 0.9793 | |
| X4X5 | 0.98 | 1 | 0.98 | 4.38 | 0.0453 | * |
| X12 | 4.89 | 1 | 4.89 | 21.94 | < 0.0001 | ** |
| X22 | 1.56 | 1 | 1.56 | 7.01 | 0.0130 | * |
| X32 | 1.89 | 1 | 1.89 | 8.48 | 0.0068 | ** |
| X42 | 9.74 | 1 | 9.74 | 43.68 | < 0.0001 | ** |
| X52 | 1.31 | 1 | 1.31 | 5.87 | 0.0218 | * |
| Residual | 6.47 | 29 | 0.22 | |||
| Lack of fit | 5.61 | 22 | 0.26 | 2.10 | 0.1593 | |
| Error | 0.85 | 7 | 0.12 | |||
| Cor total | 74.13 | 49 |
**Extremely significantly (P < 0.01), *Significant (P < 0.05)
The coefficients of the Y2 values were also evaluated using an F-test at the 95% confidence level. The linear coefficients included four significant terms, i.e. the pH, solid (DSDPB) to liquid ratio, extraction temperature and double-enzyme dose (P < 0.05). There were six significant quadratic terms (P < 0.05). The following second-order model was obtained by fitting the data and eliminating the insignificant terms. Y2 was the predicted value for the AX content of the extract (%), and X1, X2, X3, X4 and X5 were the variables described in Table 2.
| 2 |
The ANOVA for AX yield is shown in Table 4: it can be seen that the second-order model was highly significant (P < 0.001) but the lack-of-fit term was not. The coefficient of determination (R2) of the model was 0.9128, and the R2Adj was 0.8526, indicating that the experimental data have minor errors, the observed values fit well with the predicted values, and the model adequately represented the relationship among the parameters.
Analysis of response surface
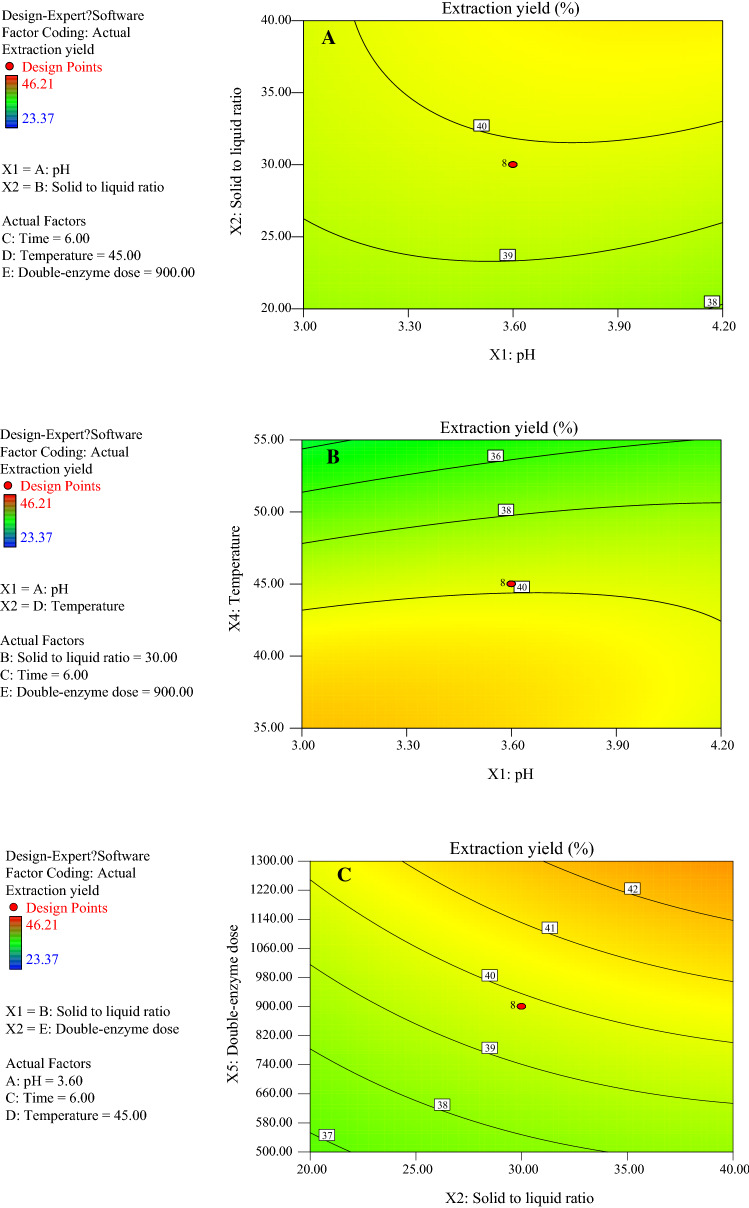
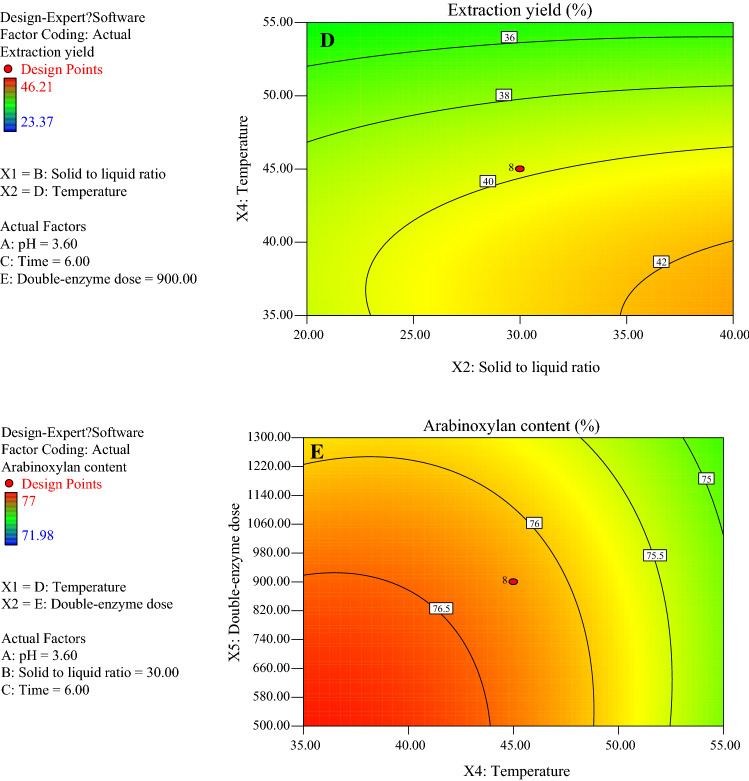
The plane figures were obtained using the second-order model with a response value of Y1 (Fig. 2). Figure 2 shows that the contour lines of the response surface were relatively dense and precipitous, indicating that the response value of the extraction yield was significantly affected by the interaction terms. Figure 2a shows that when pH ranged from 3.0 to 4.2 and the DSDPB to liquid ratio ranged from 25 to 40, the extraction yield was ranged from 39 to 40%; Fig. 2b shows that when the pH was 3.0–4.2, and temperature was 40–55 °C, the extraction yield was 36–40%. Figure 2c indicates that when the solid (DSDPB) to liquid ratio was 20–40, and the double-enzyme dose was 500–1300 U/g, the extraction yield was 37–42%. Figure 2d illustrates that when the solid (DSDPB) to liquid ratio was 20–40 and temperature increased from 35 to 55 °C, the extraction yield was 36–42%.
Fig. 2.
Contour map of AX content of enzymatic extract and extraction yield of fresh corn bran. (a) Contour map of extraction yield of fresh corn bran influenced by pH and solid to liquid ratio. (b) Contour map of extraction yield of fresh corn bran influenced by pH and temperature. (c) Contour map of extraction yield of fresh corn bran influenced 23 by solid to liquid ratio and double-enzyme dose. (d) Contour map of extraction yield of fresh corn bran influenced by solid to liquid ratio and temperature. (e) Contour map of AX content of enzymatic extract of fresh corn bran
Plane figures were also obtained using the second-order model with a response value of Y2 (Fig. 2e). Figure 2e shows that when temperature was increased from 35 to 55 °C, and the double-enzyme dose was 500–1300 U/g, the AX content of the extract was 75–76.3%.
Verification of the optimum conditions
The optimum conditions predicted by Design Expert were: pH of 3.0, extraction temperature of 35.0 °C, extraction time of 5.8 h and solid (DSDPB) to liquid ratio of 1:30. Under these conditions, the AX content of the extract was 76.94% and the AX yield was 41.85%. The optimum conditions were modified slightly, as follows: double-enzyme dose of 920 U/g, pH of 3.0, extraction temperature of 35.0 °C, extraction time of 6 h andsolid (DSDPB) to liquid ratio of 1:30. To ensure that the predicted result was not biased relative to the actual value, a verification experiment was performed under these modified predicted optimal conditions. The AX content of the extract was 75.88 ± 0.11%, and AX yield was 40.73 ± 0.09%; this validated the RSM model. The strong correlation between these results confirmed that the response model adequately reflected the expected optimisation. Wang et al. (2014) reported a yield of about 14.26% of AX from wheat bran using ultrasound-assisted enzymatic extraction technology. Escarnot et al. (2011a, b) obtained their highest yield of AXs, of 68.8%, from spelt bran with Ultraflo L. (endo-1,3-4-glucanase activity and xylanase and cellulase as side activities), while the AX content of the extract after 24 h was 46.8%. Zhang et al. (2008) extracted AX from wheat bran using Pentopan Mono BG (Novozymes, Bagsværd, Denmark) and the maximum yield was 16.38%. Maes et al. (2004) also solubilised water unextractable AX from wheat bran with endoxylanases and observed a maximum AX content of 16.8% using DSDPB dry matter; the yield was not reported. Beaugrand et al. (2004) extracted AX from peripheral tissues of developing wheat grains using purified (1 → 4)-α-endo-xylanase (EC3.2.1.8), and the maximum AX content was 85.6% Escarnot et al. (2011a, b.) extracted AX from spelt hull by alkli, and the AX content ranged from 26.7 to 33.3% using NaOH; the extraction yield varied between 45.6 and 49.6% with NaOH de Figueiredo et al. (2017). extracted AX from sugarcane bagasse using KOH and NaBH2 solutions and obtained a yield ranging from 24.8–27%, with 72.1–76.3% AX content.
The extraction yield of AX and the AX was affected by the cereal grain species, and also by the extraction method, as well as the extraction time and temperature. Double-enzymatic extraction of AX from fresh corn fibre was more efficient than the chemical method, and the purity of the extract was higher.
Conclusion
Amano HC 90 and Cellulase were combined to evaluate their cooperative effects to extract AX from DSDPB. RSM was used to obtain the optimal extraction conditions. The experimental data fit well with the predicted values, and the model adequately represented the relationship among the parameters. The extraction yield and AX content in the extract under optimal conditions (double-enzyme dose of 920 U/g, pH of 3.0, extraction temperature of 35.0 °C, extraction time of 6 h and solid (DSDPB) to liquid ratio of 1:30) were 40.73 ± 0.09% and 75.88 ± 0.11%, respectively. The double-enzymatic extraction of AX from fresh corn fibre was more efficient than the chemical method.
Acknowledgements
Special thanks are extended to Mrs Xiaolin Li, of Sky Scenery Food Co., Ltd., Jilin Province, for kindly providing the fresh maize bran. This study was supported by the National Natural Science Foundation of China (No. 31701611) and the Education Department of Jilin Province of China (No. JJKH20200579KJ).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguedo M, Fougnies C, Dermience M, Richel A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohydr Polym. 2014;105:317–324. doi: 10.1016/j.carbpol.2014.01.096. [DOI] [PubMed] [Google Scholar]
- Ayala-Soto FE, Campanell OH, Serna-Saldívar SO, Welti-Chanes J. Changes in the structure and gelling properties of maize fiber arabinoxylans after their pilot scale extraction and spray-drying. J Cereal Sci. 2016;70:275–281. doi: 10.1016/j.jcs.2016.07.005. [DOI] [Google Scholar]
- Ayala-Soto FE, Serna-Saldívar SO, García-Lara S, Pérez-Carrillo E. Hydroxycinnamic acids, sugar composition and antioxidant capacity of arabinoxylans extracted from different maize fiber sources. Food Hydrocoll. 2014;35:471–475. doi: 10.1016/j.foodhyd.2013.07.004. [DOI] [Google Scholar]
- Bai L, Huan S, Li Zh, McClements DJ. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017;66:144–153. doi: 10.1016/j.foodhyd.2016.12.019. [DOI] [Google Scholar]
- Beaugrand J, Chambat G, Wong VWK, Goubet F, Rémond C, Paës G, Benamrouche S, Debeire P, O’Donohue M, Chabbert B. Impact and efficiency of GH10 and GH11 thermostable endoxylanases on wheat bran and alkali-extractable arabinoxylans. Carbohydr Res. 2004;339:2529–2540. doi: 10.1016/j.carres.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bender D, Nemeth R, Wimmer M, Götschhofer S, Biolchi M, Török K, Tömösközi S, D’Amico S, Schoenlechner R. Optimization of arabinoxylan isolation from rye bran by adapting extraction solvent and use of enzymes. J Food Sci. 2017;82(11):2562–2568. doi: 10.1111/1750-3841.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craeyveld VV, Delcour JA, Courtin CM. Extractability and chemical and enzymic degradation of psyllium (Plantago ovata Forsk) seed husk arabinoxylans. Food Chem. 2009;112:812–819. doi: 10.1016/j.foodchem.2008.06.035. [DOI] [Google Scholar]
- Delcour JA, Win HV, Grobet PJ. Distribution and structural variation of arabinoxylans in common wheat mill streams. J Agric Food Chem. 1999;47:271–275. doi: 10.1021/jf9805294. [DOI] [PubMed] [Google Scholar]
- de Figueiredo FC, Carvalho AFA, Brienzo M, Campioni TS, de Oliva-Neto P. Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction and xylooligosaccharides production. Bioresour Technol. 2017;228:164–170. doi: 10.1016/j.biortech.2016.12.097. [DOI] [PubMed] [Google Scholar]
- Escarnot E, Aguedo M, Paquot M. Enzymatic hydrolysis of arabinoxylans from spelt bran and hull. J Cereal Sci. 2012;55:243–253. doi: 10.1016/j.jcs.2011.12.009. [DOI] [Google Scholar]
- Escarnot E, Aguedo M, Agneessens R, Wathelet B, Paquot M. Extraction and characterization of water-extractable and water-unextractable arabinoxylans from spelt bran: Study of the hydrolysis conditions for monosaccharides analysis. J Cereal Sci. 2011;53:45–52. doi: 10.1016/j.jcs.2010.09.002. [DOI] [Google Scholar]
- Escarnot E, Aguedo M, Paquot M. Characterization of hemicellulosic fractions from spelt hull extracted by different methods. Carbohydr Polym. 2011;85:419–428. doi: 10.1016/j.carbpol.2011.03.005. [DOI] [Google Scholar]
- Fadel A, Mahmoud AM, Ashworth JJ, Li W, Ng YL, Plunkett A. Health-related effects and improving extractability of cereal arabinoxylans. Int J Biol Macromol. 2017 doi: 10.1016/j.ijbiomac.2017.11.055. [DOI] [PubMed] [Google Scholar]
- Höije A, Gröndahl M, Tommeraas K, Gatenholm P. Isolation and characterization of physicochemical and material properties of arabinoxylans from barley husks. Carbohydr Polym. 2005;61:266–275. doi: 10.1016/j.carbpol.2005.02.009. [DOI] [Google Scholar]
- Hu B, Wang Zh, Xu S-Y. Treatment of corn bran dietary fiber with xylanase increases its ability to bind bile salts, in vitro. Food Chem. 2008;106:113–121. doi: 10.1016/j.foodchem.2007.05.054. [DOI] [Google Scholar]
- Izydorczyk M, Biliaderis CG, Bushuk W. Physical properties of water-soluble pentosans from different wheat varieties. Cereal Chem. 1991;68(2):145–150. doi: 10.1021/bp00008a015. [DOI] [Google Scholar]
- Maes C, Vangeneugden B, Delcour JA. Relative activity of two endoxylanases towards water-unextractable arabinoxylans in wheat bran. J Cereal Sci. 2004;39:181–186. doi: 10.1016/j.jcs.2003.08.001. [DOI] [Google Scholar]
- Malunga LN, Beta T. Antioxidant capacity of water-extractable arabinoxylan from commercial barley, wheat, and wheat fractions. Cereal Chem. 2015;92(1):29–36. doi: 10.1094/cchem-11-13-0247-r. [DOI] [Google Scholar]
- Mathew S, Karlsson EN, Adlercreutz P. Extraction of soluble arabinoxylan from enzymatically pretreated wheat bran and production of short xylo-oligosaccharides and arabinoxylooligosaccharides from arabinoxylan by glycoside hydrolase family 10 and 11endoxylanases. J Biotechnol. 2017;260:53–61. doi: 10.1016/j.jbiotec.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Monteagudomera A, Chatzifragkou A, Kosik O, Gibson G, Lovegrove A, Shewry PR, Dimitris C. Evaluation of the prebiotic potential of arabinoxylans extracted from wheat distillers' dried grains with solubles (DDGS) and in-process samples. Appl Microbiol Biot. 2018;102:7577–7587. doi: 10.1007/s00253-018-9171-6. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Takeuchi M, Nakamura N. Immunological effects of partially hydrolyzed arabinoxylan from corn husk in mice. Biosci Biotech Biochem. 2005;69(1):19–25. doi: 10.1271/bbb.69.19. [DOI] [PubMed] [Google Scholar]
- Rose DJ, Inglett GE, Liu SX. Utilisation of corn (Zea mays) bran and corn fiber in the production of food components. J Sci Food Agr. 2010;90:915–924. doi: 10.1002/jsfa.3915. [DOI] [PubMed] [Google Scholar]
- Sárossy Z, Tenkanen M, Pitkänen L, Bjerre A-B, Plackett D. Extraction and chemical characterization of rye arabinoxylan and the effect of β-glucan on the mechanical and barrier properties of cast arabinoxylan films. Food Hydrocoll. 2013;30:206–216. doi: 10.1016/J.foodhyd.2012.05.022. [DOI] [Google Scholar]
- Vansteenkiste E, Babot C, Rouau X, Micard V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004;18:557–564. doi: 10.1016/j.foodhyd.2003.09.004. [DOI] [Google Scholar]
- Wang J, Sun B, Liu Y, Zhang H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014;150:482–488. doi: 10.1016/j.foodchem.2013.10.121. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Runge T. Emulsifying properties of succinylated arabinoxylan-protein gum produced from corn ethanol residuals. Food Hydrocoll. 2016;52:423–430. doi: 10.1016/j.foodhyd.2015.07.018. [DOI] [Google Scholar]
- Yadav MP, Parris N, Johnston DB, Hicks KB. Fractionation, characterization, and study of the emulsifying properties of corn fiber gum. J Agric Food Chem. 2008;56:4181–4187. doi: 10.1021/jf703672d. [DOI] [PubMed] [Google Scholar]
- Zhang Sh, Li W, Smith CJ, Musa H. Cereal-derived arabinoxylans as biological response modifiers: extraction, molecular features, and immune-stimulating properties. Crit Rev Food Sci Nutr. 2015;55:1035–1052. doi: 10.1080/10408398.2012.705188. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou S, Wang S. Optimizing enzymatic hydrolysis conditions of arabinoxylan in wheat bran through quadratic orthogonal rotation combination design. Food Sci. 2008;28:141–145. [Google Scholar]
- Zhang Zh, Smith C, Li WL. Extraction and modification technology of arabinoxylans from cereal by-products: a critical review. Food Res Int. 2014;65:423–436. doi: 10.1016/j.foodres.2014.05.068. [DOI] [Google Scholar]
- Zhang Zh, Smith C, Li WL, Ashworth J. Characterization of nitric oxide modulatory activities of alkaline-extracted and enzymatic-modified arabinoxylans from corn bran in cultured human monocytes. J Agric Food Chem. 2016;64(43):8128–8137. doi: 10.1021/acs.jafc.6b02896. [DOI] [PubMed] [Google Scholar]
- Zhou S, Liu X, Yan G, Wang Q, Peng D, Cao L. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr Polym. 2010;81:784–789. doi: 10.1016/j.carbpol.2010.03.040. [DOI] [Google Scholar]