Abstract
Objective
Increased oxidative stress has been identified as a pathogenetic mechanism in female infertility. However, the effect of specific antioxidants, such as coenzyme Q10 (CoQ10), on the outcomes after assisted reproductive technologies (ART) has not been clarified. The aim of this study was to systematically review and meta-analyze the best available evidence regarding the effect of CoQ10 supplementation on clinical pregnancy (CPR), live birth (LBR), and miscarriage rates (MR) compared with placebo or no-treatment in women with infertility undergoing ART.
Methods
A comprehensive literature search was conducted in PubMed (MEDLINE), Cochrane, and Scopus, from inception to March 2020. Data were expressed as odds ratio (OR) with 95% confidence intervals (CI). The I2 index was employed for heterogeneity.
Results
Five randomized-controlled trials fulfilled eligibility criteria (449 infertile women; 215 in CoQ10 group and 234 in placebo/no treatment group). Oral supplementation of CoQ10 resulted in an increase of CPR when compared with placebo or no-treatment (28.8% vs. 14.1%, respectively; OR 2.44, 95% CI 1.30–4.59, p = 0.006; I2 32%). This effect remained significant when women with poor ovarian response and polycystic ovarian syndrome were analyzed separately. No difference between groups was observed regarding LBR (OR 1.67, 95% CI 0.66–4.25, p = 0.28; I2 34%) and MR (OR 0.61, 95% CI 0.13–2.81, p = 0.52; I2 0%).
Conclusions
Oral supplementation of CoQ10 may increase CPR when compared with placebo or no-treatment, in women with infertility undergoing ART procedures, without an effect on LBR or MR.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01906-3) contains supplementary material, which is available to authorized users.
Keywords: Coenzyme Q10, CoQ10, Female infertility, Assisted reproduction, Pregnancy outcomes, Meta-analysis
Introduction
Infertility is characterized by failure to achieve a clinical pregnancy after ≥ 12 months of regular, unprotected sexual intercourse [1]; it is currently affecting one out of six couples worldwide [2]. Increasingly, infertile couples seek assisted reproductive technologies (ART) for conceiving and achieving pregnancy. ART includes all in vitro procedures handling both human oocytes and sperm and/or embryos for reproduction [1], referring to ovarian stimulation, ovulation induction, in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI) [2]. An estimated 40–45% of infertility cases are attributed to female factors [3]. The pathophysiology of female infertility is multifactorial and still not fully elucidated. Maternal aging and diminished ovarian reserve are among the most investigated pathogenetic mechanisms [4]. Both of them are related to oxidative stress, the effect of which on female infertility remains unclear [4, 5]. Oxidative stress is defined as the imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses [6]. Although in physiological states, ROS play an essential regulatory role in the female reproductive system [7–9], they may also exert unfavorable effects on fertility when overabundant [10].
Antioxidants are biological and chemical compounds, synthesized endogenously or exogenously, which mitigate oxidative stress and its negative impact on reproductive procedures, acting as free radical scavengers [11]. Coenzyme Q10 (CoQ10) is a lipid-soluble quinone, acting as an effective antioxidant, which prevents lipid peroxidation and DNA oxidation, as well as a bioenergetic molecule, empowering the body’s energy production cycle through adenosine triphosphate (ATP) synthesis [12]. CoQ10 supplementation has long been used to ameliorate infertility outcomes, associated with an increased clinical pregnancy rate (CPR), although the evidence is still of low quality [3]. There are also insufficient data regarding its effect on live birth (LBR) and miscarriage rate (MR), as well as its impact on ART clinical outcomes [3].
The aim of this study was to systematically investigate and meta-analyze the best available evidence from randomized-controlled trials (RCTs) regarding the effect of CoQ10 supplementation on CPR, LBR, and MR, compared with placebo or no-treatment, in infertile women of reproductive age undergoing any ART.
Materials and methods
Guidelines followed
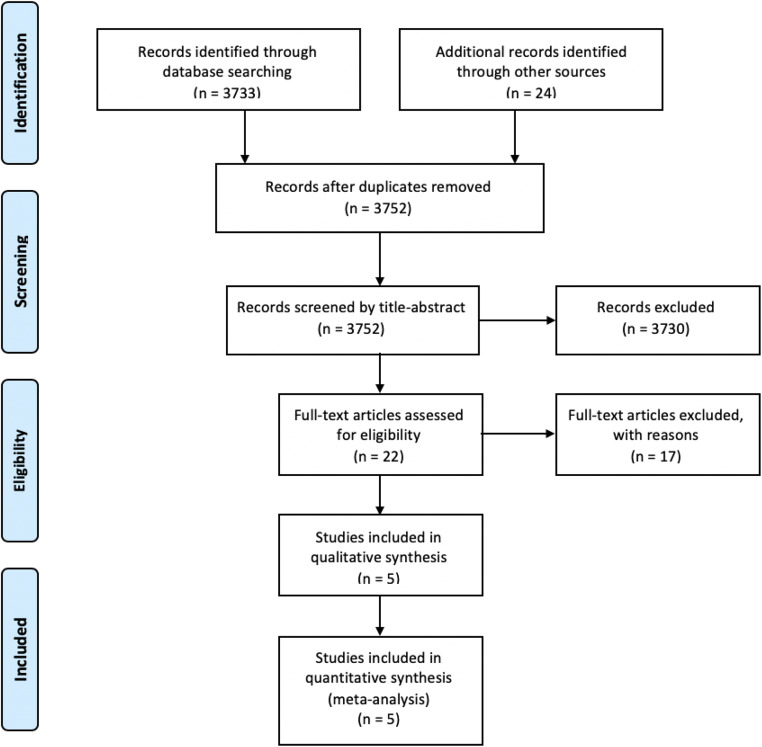
This systematic review followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [13]. A flow diagram is presented in Fig. 1. A completed PRISMA checklist is provided in Online Resource 1.
Fig. 1.
Flow chart diagram
Search strategy
The following PICO (Population, Intervention, Comparison, Outcome) elements were applied as inclusion criteria for this systematic review: (i) Population: women of reproductive age with infertility, undergoing ART after the intervention; (ii) Intervention: oral supplementation of CoQ10; (iii) Comparison: placebo or no-treatment; (iv) Outcomes: CPR, LBR, MR. To identify eligible studies, the main search was conducted in the electronic databases PubMed, Scopus, and CENTRAL covering the period from inception to March 9, 2020, and using the following search strings: “(((((((((((((((((((“coq10”[All Fields] OR “coenzymeq10”[All Fields]) OR “coenzyme q10”[All Fields]) OR “ubiquinone”[All Fields]) OR “ubiquinone”[MeSH Terms]) OR “mitoquinone”[All Fields]) OR “ubidecarenone”[All Fields]) OR “vitamin q10”[All Fields]) AND “in vitro fertilization”[All Fields]) OR “in vitro fertilization”[All Fields]) OR “reproductive techniques, assisted”[MeSH Terms]) OR “reproductive techniques assisted”[All Fields]) AND “ovarian response”[All Fields]) OR “ovarian reserve”[All Fields]) OR “ovarian reserve”[MeSH Terms]) OR “pregnancy rate”[All Fields]) OR “pregnancy rate”[MeSH Terms]) OR “live birth rate”[All Fields]) OR “fertilization rate”[All Fields]) OR “clinical pregnancy”[All Fields] AND “infertility, female/therapy”[MeSH Terms]) OR “Oocyte aneuploidy”[All Fields]) OR (“Ovarian Diseases/drug therapy”[MeSH Terms] AND “Ovarian Diseases/physiopathology”[MeSH Terms]) NOT (“animals”[MeSH Terms] NOT “humans”[MeSH Terms]) NOT “murinae”[MeSH Terms] NOT (letter[pt] OR comment[pt] OR editorial[pt] OR Review[pt] OR “practice guideline”[ptyp] OR “case reports”[ptyp]).” Manual literature search has also taken place. The main search was completed independently by two investigators (PF and PT). Any discrepancy was solved by consultation of an investigator not involved in the initial procedure (PA).
Study selection
Inclusion criteria were set as follows: (i) women of reproductive age with infertility, with or without a history of previous treatment (ART or other fertility treatment), undergoing ART after treatment; (ii) oral supplementation of CoQ10; (iii) absence of additional antioxidant therapy; and (iv) RCTs providing extractable data for at least one of the primary outcomes (CPR, LBR, MR). Medical or other treatment could be given as long as it was equally administered in both groups (intervention and control). Secondary outcomes included (i) ovarian stimulation parameters (number of mature follicles, oocytes retrieved, oocytes fertilized), (ii) embryological parameters [day 3 high-quality embryos, number of embryos per embryo transfer, proportion of cryopreserved embryos, frozen-thawed embryo transfers (ET)], (iii) ART parameters [canceled treatment cycles, total dose of gonadotropins, peak estradiol (E2) serum concentration], and (iv) ovarian reserve markers [anti-Müllerian Hormone (AMH), antral follicle count (AFC), day 3 follicle-stimulating hormone (FSH)].
Exclusion criteria were as follows: (i) non-RCTs, (ii) women of non-reproductive age or without infertility disorders, (iii) comparison of CoQ10 with another antioxidant, (iv) clinical trials prematurely terminated without providing data on any of the primary outcomes, (v) Non-English language, and (vi) studies conducted in animals.
Data extraction
Two researchers (PF and PT) independently reviewed all eligible studies. The following data were extracted and recorded: (i) first author, (ii) year of publication, (iii) country in which the study was conducted, (iv) study duration, (v) total number of participants, (vi) etiology of infertility, (vii) number of women in each group (intervention and control groups), (viii) fertility treatment, (x) daily dose and duration of CoQ10 supplementation, (xi) type of comparison (placebo or no-treatment), and (xii) primary and secondary outcomes. Parameters such as mean age of the participants at study entry, mean BMI, ovarian reserve markers [AMH, AFC, cycle day 3 FSH, luteinizing hormone (LH)], duration of infertility, and number of stimulation days were also recorded when available.
Risk of bias and quality assessment
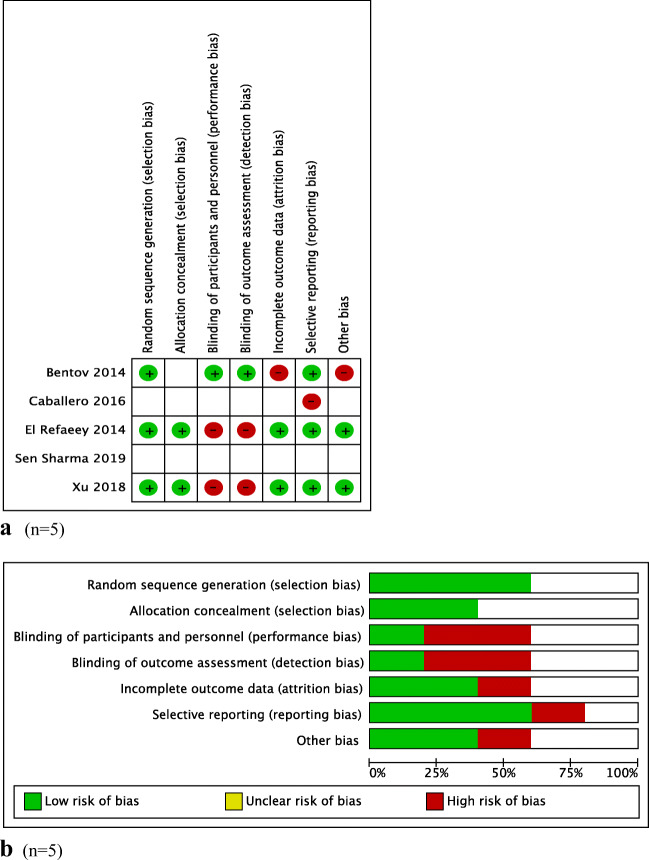
“Cochrane’s Collaboration tool for assessing the risk of bias” (RevMan) was used for assessing the quality of each study. Briefly, this system evaluates studies based upon the following criteria: randomization generation, allocation concealment, blinding of participants and outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. The final five RCTs were assessed as “low risk” (+), “high risk” (−), or “unclear,” when there was insufficient data [14] (Fig. 2a and b).
Fig. 2.
a Risk of bias of the included studies. b Summary of risk of bias of the meta-analysis
Statistical analysis
Heterogeneity was tested with the Cochrane chi-square test (χ2) and the degree of heterogeneity was quantified by the I-squared statistics (I2). An I2 of 30–60% was considered as moderate, whereas values > 60% were considered as high degree of heterogeneity. Random effects model was used for data synthesis when I2 > 30% and fixed effects model was used when I2 < 30% [15]. Associations were reported as odds ratio (OR) with their 95% confidence intervals (CIs). A p value of < 0.05 was considered statistically significant. Publication bias was formally tested with Begg-Mazumdar test (presented in funnel plot diagram, with p values > 0.1 indicating absence of publication bias) and the Egger’s test (p values > 0.1 indicating absence of publication bias). Outcomes were expressed as percentages (%). All analyses were done with the software RevMan 5.3 (Cochrane Collaboration). Additional analyses including subgroup and sensitivity analyses were performed to find the source of heterogeneity by potential moderator variables and to determine the impact of one-by-one included RCTs on reliability of the pooled ORs, respectively.
Results
Study selection and descriptive data
The initial database and manual search provided 3757 results. After excluding five duplicates, 3752 records were screened, 22 of which were assessed as full-text papers for eligibility (Fig. 1). Of those, only five were included in the qualitative and quantitative analysis [5, 16–19]. The excluded studies and the reasons for their exclusion are available in Online Resource 2. The studies were published between 2014 and 2019. The countries in which they were conducted were Egypt, Canada, Argentina, China, and India. The number of participants ranged from 39 to 169, yielding a total number of 449 infertile women, 286 of whom were in the poor ovarian response (POR) group and 163 were in the polycystic ovarian syndrome (PCOS) group.
Three studies included women with infertility and POR following ovarian stimulation [5, 17, 18]. The diagnosis of POR was based upon Bologna criteria (at least two of the following three should be present): advanced maternal age (or any other risk factor for POR), a previous POR, and/or an abnormal ovarian reserve test [20]. Two studies included women with infertility and PCOS [16, 19]. The diagnosis of PCOS was based upon the American Society for Reproductive Medicine (ASRM) and the European Society of Human Reproduction and Embryology (ESHRE) (Rotterdam) criteria (at least two of the following three should be present): οligo- and/or anovulation, clinical and/or biochemical signs of hyperandrogenism and/or polycystic ovarian morphology (≥ 12 follicles, 2–9 mm in diameter), or increased ovarian volume (> 10 ml), after exclusion of other conditions associated with oligo-/amenorrhea and/or hyperandrogenism, such as congenital adrenal hyperplasias, androgen-secreting tumors, and/or Cushing’s syndrome [21].
With respect to the dosage and duration of CoQ10 supplementation, in women with POR the dosage varied from 600 mg once a day for 8 weeks [17], 600 mg twice a day for 12 weeks [18] or 200 mg three times a day for 8 weeks [5]. ART was commenced in the first menstrual cycle upon CoQ10 treatment completion. In women with PCOS, the dosage was 60 mg three times a day starting on the first [19] or second cycle day [16] until the hCG administration day. HCG was administered when at least one follicle ≥ 18 mm was found via transvaginal ultrasound. No local or systemic side effects related to the use of CoQ10 were mentioned. Moreover, any CoQ10 discontinuation was based on personal choice or compliance issues.
Categorization of patients according to their infertility etiology and the ART procedure that they underwent are presented in Table 1. Quality assessment of included studies is presented in Fig. 2a and b.
Table 1.
Studies including women with infertility and poor ovarian response or polycystic ovarian syndrome
| Study/publication year | Study duration (months) | Study population (n) | Mean age/range (years) | City, Country | Intervention group (CoQ10 daily dose/duration/ART commencement or hCG administration) (n) | Comparison group (n) |
|---|---|---|---|---|---|---|
| Women with infertility and poor ovarian response | ||||||
| Bentov/2014 | 30 | 39 women with POR undergoing IVF-ICSI [Intervention (17)/Control (22)] | 39/35–43 | Toronto, Canada | 600 mg/8 weeks/day 3 of the following menstrual cycle (17) | Placebo (22) |
| Caballero/2016 | Not mentioned |
78 women with POR undergoing IVF-ICSI [Intervention (39)/Control (39)] |
38/36–40 | Buenos Aires, Argentine | 1200 mg/12 weeks/beginning of the following menstrual cycle (39) | No-treatment (39) |
| Xu/2018 | 13 | 169 women with POR undergoing IVF-ICSI [Intervention (76)/Control (93)] | 32/28–36 | Beijing, China | 600 mg/8 weeks/beginning of the following menstrual cycle (76) | No-treatment (93) |
| Women with infertility and polycystic ovarian syndrome | ||||||
| El Refaeey /2014 | 36 | 101 women with PCOS, resistant to clomiphene citrate, undergoing ovarian stimulation and ovulation induction [Intervention (51)/Control (50)] | 21–35 /28 | Mansura, Egypt | 180 mg/2nd cycle day until hCG administration day/hCG given when ≥ 1 follicle of ≥ 18 mm (51) | No-treatment (50) |
| Sen Sharma/2017 | Not mentioned | 62 women with PCOS, resistant to clomiphene citrate, undergoing ovarian stimulation and ovulation induction [Intervention (32)/Control (30)] | 21–40 /30 | West Bengal, India | 180 mg/1st cycle day until hCG administration day/hCG given when ≥ 1 follicle of ≥ 18 mm (32) | No-treatment (30) |
Effect of CoQ10 supplementation on CPR, LBR, and MR compared with placebo or no-treatment
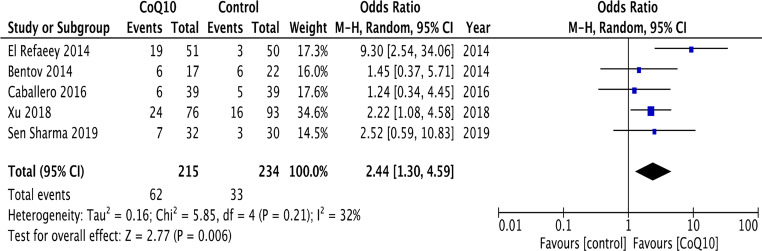
According to per-protocol analysis, oral supplementation of CoQ10 in infertile women undergoing ART resulted in an increase of CPR when compared with placebo or no-treatment (five RCTs) [5, 16–19] (28.8% vs. 14.1%; OR 2.44, 95% CI 1.30–4.59, p = 0.006; I2 32%) (Fig. 3). No evidence of publication bias was detected (Online Resource 3). These results remained significant, after performing an intention-to-treat analysis (25.2% vs. 13.5%; OR 2.08, 95% CI 1.03—4.23, p = 0.004; I2 46%).
Fig. 3.
Forest plot of the effect of CoQ10 supplementation on clinical pregnancy rate in women with infertility undergoing assisted reproductive technology in comparison with placebo or no-treatment (control)
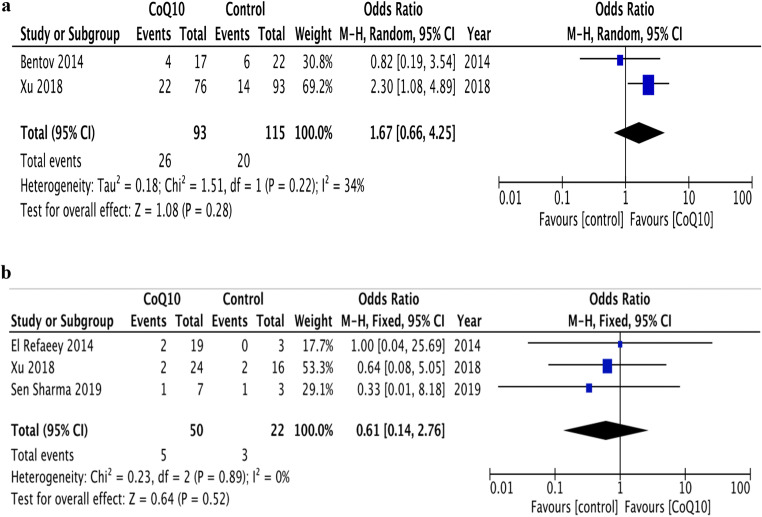
According to per-protocol analysis, the effect of CoQ10 on LBR (28% vs. 17.4%; OR 1.67, 95% CI 0.66—4.25, p = 0.28; I2 34%) [5, 17] and MR (10% vs. 13.6%; OR 0.61, 95% CI 0.14–2.76, p = 0.52; I2 0%) [5, 16, 19] was similar between the groups (Fig. 4a and b). According to intention-to-treat analysis, the effect of CoQ10 on LBR (21.7% vs. 16.7%; OR 1.23, 95% CI 0.46–3.26, p = 0.68; I2 42%) and MR (2.8% vs. 1.7%; OR 1.41, 95% CI 0.34–5.9, p = 0.64; I2 0%) was again similar to placebo.
Fig. 4.
a Forest plot of the effect of CoQ10 supplementation on live birth rate in women with infertility undergoing assisted reproductive technology compared with placebo or no-treatment (control). b Forest plot of the effect of CoQ10 supplementation on miscarriage rate in women with infertility undergoing assisted reproductive technology compared with placebo or no-treatment (control)
Effect of CoQ10 supplementation on the number of mature follicles, retrieved oocytes, and fertilized oocytes after ovarian stimulation compared with no-treatment
Oral CoQ10 supplementation in PCOS women increased the mean number of mature follicles (> 18 mm) (1.85 ± 0.27 vs. 1.3 ± 0.32, p < 0.001) and the ovulation rate per cycle (65.9% vs. 15.5%, p < 0.001) compared with no-treatment (one study) [16]. In addition, the study by Sen Sharma also reported a significant increase in the number of mature follicles by CoQ10, however, without providing detailed data [19].
Oral CoQ10 supplementation in women with POR undergoing IVF-ICSI resulted in no increase in the number of oocytes retrieved when compared with no-treatment (1.82 ± 0.82 vs. 1.87 ± 0.76, respectively, p = 0.77) (one study) [18]. In another study in POR women, CoQ10 increased the median number of oocytes retrieved [4 (range 2, 5) vs. 2 (range 1, 4), p = 0.002] and fertilized when compared with no-treatment (67.5% vs. 45.1% fertilization rate, p = 0.001) [5].
Effect of CoQ10 supplementation on embryological parameters compared with placebo or no-treatment
Oral CoQ10 supplementation in women with POR increased the median number of day 3 high-quality embryos compared with no-treatment [1 (0, 2) vs. 0 (0, 1.75) embryos, p = 0.03], the median number of embryos per embryo transfer (ET) [2 (1, 2) vs. 1 (1, 2), p=0.04], as well as the proportion of cryopreserved embryos (18.4% vs. 4.3%, p = 0.012) and frozen-thawed ET (15.8% vs. 3.2%, p = 0.01) [5]. A study found similar rates of high-quality embryos at 48 and 72 h between CoQ10 and placebo group (81.4% vs. 64.7%, p > 0.05 and 66% vs. 42%, p > 0.05), respectively) [17]. Similarly, another study in women with POR found no difference in implantation rates per ET (26.2% vs. 21.4%, p = 0.75) [18].
Comparison of the effect of CoQ10 supplementation on the number of canceled treatment cycles with no-treatment
Oral supplementation of CoQ10 in women with POR resulted in similar rates of canceled treatment cycles, including cases of no-response to stimulation and no oocyte retrieval, when compared to no-treatment (5.2% vs. 10.8%, p = 0.27), but decreased rates of retrieval not followed by ET (8.3% vs. 22.9%, p = 0.04) [5].
Comparison of the effect of CoQ10 supplementation on ART cycle stimulation parameters with placebo or no-treatment
Median total dose of gonadotropin (Gn) needed for ovarian stimulation was decreased when POR women of CoQ10 group were compared to those of no-treatment group [2000 (1200, 4275) vs. 3075 (1900, 4275) IU, p = 0.03] [5]. In the same study, peak E2 serum concentration was higher in the CoQ10 group [2349 (892, 4784) vs. 1685 (1125, 3042) pmol/l, p = 0.02] [5]. In a study in women with POR, mean E2 and progesterone serum concentrations were similar between CoQ10 and placebo group (7569 ± 1871 vs. 6875 ± 973 pmol/l, p > 0.05 and 6.33 ± 0.7 vs. 6 ± 0.7 nmol/l, p > 0.05), respectively) [17]. Mean E2 and progesterone serum concentrations were higher in PCOS women supplemented with CoQ10 in comparison with no-treatment (168.9 ± 75 vs. 138.3 ± 70.2 pg/ml, p < 0.05 and 10.2 ± 1.03 vs. 8.9 ± 0.9 pg/ml, p < 0.001, respectively) [16].
The day of hCG administration the value of mean endometrium size in women with POR was similar between CoQ10 and no-treatment group (10.1 ± 1.9 vs. 10.3 ± 1.5 mm, p = 0.13) [5], whereas in women with PCOS its value was higher (8.8 ± 1.5 vs. 7 ± 0.7 mm, p < 0.001 [16] and 9.4 vs. 7.8 mm, p < 0.05) [19].
Comparison of the effect of CoQ10 supplementation on laboratory biomarkers before and after CoQ10 treatment with placebo or no-treatment or before and after CoQ10 treatment
In women with POR, basal concentration of day-3 FSH was lower after 60 days of CoQ10 supplementation [12.3 (9.4, 15.5) vs. 10.5 (9.2, 12.6) IU/ml, p = 0.006]. In contrast, AMH and AFC values were similar before and after CoQ10 treatment [0.6 (0.4. 0.8) vs. 0.6 (0.4, 0.8) ng/ml, p = 0.91 and 5 (3, 6) vs. 5 (3, 7) (n), p = 0.94], respectively] [5].
Subgroup analysis
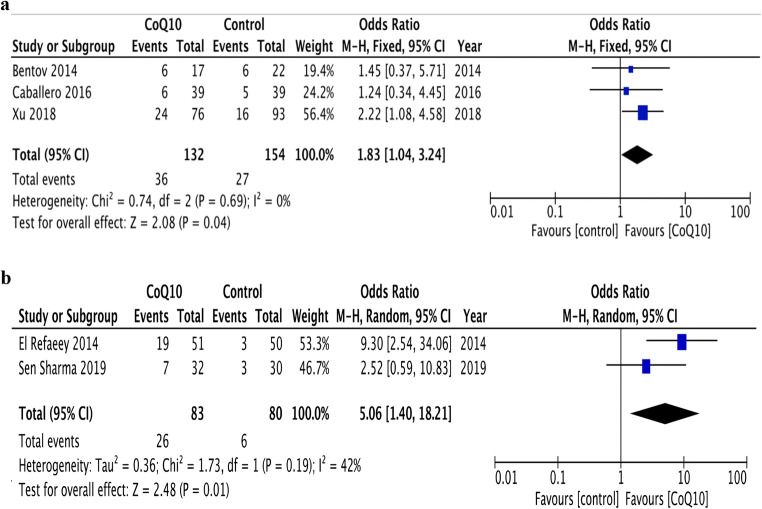
Subgroup analysis was performed concerning infertility cause. According to per-protocol analysis, oral CoQ10 supplementation in women with POR resulted in an increase in CPR compared with placebo or no-treatment (three RCTs) [5, 17, 18] (27.3% vs.17.5%; OR 1.83, 95% CI 1.04–3.24, p = 0.04; I2 0%) (Fig. 5a). Also, an increase in CPR was detected when analysis was restricted to PCOS women (two studies) [16, 19] (31.3% vs.7.5%; OR 5.06, 95% CI 1.40–18.21, p = 0.01; I2 42%) (Fig. 5b). According to intention-to-treat analysis, the effect of CoQ10 on CPR was not significant in women with POR (22.6% vs. 17%; OR 1.43, 95% CI 0.82–2.51, p = 0.21; I2 0%), whereas CoQ10 did increase CPR in women with PCOS (29.9% vs. 7.1%; OR 5.03, 95% CI 1.42–17.82, p = 0.01; I2 0%).
Fig. 5.
a Forest plot of the effect of CoQ10 supplementation on clinical pregnancy rate in poor ovarian responders, undergoing assisted reproductive technology compared with placebo or no-treatment (control). b Forest plot of the effect of CoQ10 supplementation on clinical pregnancy rate in women with polycystic ovarian syndrome, undergoing assisted reproductive technology compared with placebo or no-treatment (control)
Sensitivity analysis
Sensitivity analysis was performed by excluding the study of El Refaeey and colleagues [16] to assess whether its high OR 9.3 (95% CI 2.54–34.06, p < 0.001) played a determinant role on significant increase of CPR. The increase of CPR after CoQ10 supplementation remained significant (four studies) [5, 17–19] (OR 1.91, 95% CI 1.12–3.26, p = 0.02; I2 0%) (Online Resource 4). Moreover, a sensitivity analysis was conducted by excluding the studies of Caballero and Sen Sharma [18, 19] because they were qualitatively characterized as “unclear.” The increase of CPR after CoQ10 supplementation remained significant (three studies) [5, 16, 17] (OR 2.98, 95% CI 1.13–7.18, p = 0.03; I2 57%) (Online Resource 5). The effect on MR remained similar between the groups (two studies) [5, 16] (OR 0.73, 95% CI 0.13–4.16, p = 0.72; I2 0%).
Discussion
To the best of our knowledge, this systematic review and meta-analysis including 449 infertile women is the first regarding the effect of CoQ10 supplementation on CPR, LBR, and MR in comparison with placebo or no-treatment, in women of reproductive age with infertility undergoing ART. Oral supplementation of CoQ10 increased CPR, without difference between women with POR or PCOS. There was no effect of CoQ10 on LBR and MR in women with infertility undergoing ART when compared with placebo or no-treatment. This could partially be explained by insufficient data for clinical parameters, such as LBR and MR, which were only provided by two and three studies, respectively.
Research on CoQ10’s impact on female infertility is still at an early stage. Animal studies on female mice suggest that CoQ10 supplementation may reduce cumulus cells apoptosis, resulting in an increase of oocyte quantity and quality [22]. It may also contribute to the increase of IVF success rates by inhibiting DNA oxidation and, thus, oocyte apoptosis [23]. Furthermore, CoQ10 could act as a protector of ovarian reserve from the adverse effects of aging [24].
As far as CoQ10’s impact on male infertility is concerned, three systematic reviews and meta-analyses argue for its benefits on semen quality, quantity, and mobility [25–27]; however, CoQ10 failed to have any effect on CPR and LBR [27].
Oxidative stress exerts deleterious effects on in vivo and in vitro reproductive procedures [6, 28]. In the last decades, the potential benefits of oral antioxidants on female infertility treatment are being increasingly investigated, suggesting conflicting results. In terms of vitamin supplementation, vitamin C seems to increase placental steroidogenesis favoring pregnancy preservation [29], whereas its low serum concentrations have been associated with pregnancy loss [30]. Vitamin A promotes high-quality oocytes and blastogenesis, whereas vitamin E, pentoxifylline, and ʟ-arginine contribute to angiogenesis and achievement of an optimum endometrium size for implantation [31, 32]. Moreover, vitamin D, as well as myoinositol, seems to increase fertility rate through the downregulation of hyperandrogenism in PCOS women [33, 34]. Higher serum concentrations of folic acid and B12 before ART have been associated with higher LBR after folate fortification [35].
In 2017, a meta-analysis [3] failed to show any difference on CPR between intervention and control group, when vitamin C, D, E, B complex, N-acetylcysteine, ʟ-arginine, or myoinositol were supplemented individually. However, an increase in CPR was observed when they were administered as an antioxidant combination. Interestingly, only CoQ10 (OR 4.28, 95% CI 1.79–10.26, I2 73%) and ʟ-carnitine (OR 82.1, 95% CI 11.0–616.6) achieved an increase in CPR. Similar associations emerged on LBR and MR between intervention and control group due to insufficient data [3]. In 2018, a systematic review on CoQ10’s effect in PCOS was published, including three RCTs, one of which suggested that clomiphene citrate with CoQ10 resulted in ovulation increase CoQ10 [43]. In 2019, an RCT in women with PCOS found no difference in ovulation rate and CPR when CoQ10 was compared with vitamin D [36]. Oral supplementation of melatonin with CoQ10 achieved an increase in oocyte quality in comparison with melatonin alone [37]. Similarly, a combination of dehydroepiandrosterone (DHEA) with CoQ10 reduced the total dose of gonadotropins needed for ovarian stimulation and increased the number of AFC and mature follicles in comparison to DHEA alone [38]. Assessment of dietary intake of prenatal multivitamin and/or other antioxidant consumption, such as DHEA or omega-3, was outside the scope of the current meta-analysis, thus the consumption of any or all of these antioxidants in either control or CoQ10 groups may confound the results observed attributed to CoQ10 supplementation.
The main strength of the present meta-analysis is that, within the broad heterogenous group of women with infertility, it focused specifically on those undergoing ART. All participants were of reproductive age (mean age 33 years) attending a reproductive clinic, either with POR or PCOS. In addition, randomization and selective outcome reporting were the most unbiased procedures of qualitative analysis. In general, CoQ10 supplementation was well-tolerated, as all studies included in the present meta-analysis did not report any local or systemic adverse effects related to its use. Also, the studies mention that discontinuation of CoQ10 treatment was due to personal choice or compliance issues rather than any adverse effects.
In the study by El Refaeey and colleagues, 110 women were initially enrolled, equally distributed in each group, four of whom from the CoQ10 and five from the no-treatment group dropped out, resulting in a final total of 101 women [16]. Similarly, Xu and colleagues enrolled 186 women, 17 of whom were on the CoQ10 group and dropped out, resulting in a final total of 169 women [5]. Studies by Caballero and Sen Sharma were qualitatively characterized as “unclear” because their protocols were not available [18, 19]. The study by Caballero and colleagues [18] had high “reporting bias,” since, although LBR was considered as the primary outcome, there were no data for LBR [18]. No details were available after correspondence via e-mail.
The present meta-analysis has certain limitations. First, the lack of an effect of CoQ10 on LBR and MR may be attributed to the small number of studies provided data on these outcomes (two and three studies, respectively), whereas CPR data were extracted from all included studies (five studies). However, a trend for a beneficial effect of CoQ10 on LBR and MR was evident, despite the lack of statistical significance. Demonstrating an effect on CPR, but not on MR or LBR is not uncommon in the field of Reproductive Medicine; obviously, on top of the intervention, additional parameters affect the outcome of pregnancy (i.e., LBR), which takes place more than 30 weeks after its confirmation (i.e., CPR). Second, the dosage and duration of CoQ10’s supplementation varied among studies as the optimal timing, duration, and dose of CoQ10 remains unclear. Thus, data provided from this meta-analysis are insufficient to guide on the appropriate CoQ10 dose, frequency, duration, and exposure relative to ART. However, it must be stated that, as a lipid-soluble nutrient, CoQ10’s absorption can be enhanced when combined with fatty meal [39]. Studies related to its pharmacokinetics report a Tmax of 6.5 h and that solubilized formulations yield higher bioavailability [40]. As far as its pharmacodynamics is concerned, it has an excellent safety record, except for mild gastrointestinal symptoms [41, 42].
Conclusions
This systematic review and meta-analysis in infertile women undergoing ART indicates that CoQ10 supplementation increases CPR both in total and in infertility subgroups (POR and PCOS) compared with placebo or no-treatment. However, there is a lack of effect on LBR and MR by CoQ10 supplementation. Although the available data are insufficient to conclude a beneficial or detrimental effect on fertility outcomes with regard to CoQ10 supplementation and ART, one could consider this as non-pharmaceutical, inexpensive, and safe therapy to enhance infertility treatment in women of reproductive age undergoing any ART. In any case, well-designed, interventional studies, with a larger number of participants, mainly emphasizing on clinical outcomes, will further elucidate these issues.
Electronic supplementary material
(DOCX 256 kb).
Abbreviations
- IVF
In vitro fertilization
- ICSI
Intracytoplasmic sperm injection
- hCG
Human chorionic gonadotropin
- PCOS
Polycystic ovary syndrome
- POR
Poor ovarian response
Author’s contributions
PF designed the research, searched the literature, extracted and analyzed the data, and wrote the first draft of the paper. PT searched the literature, extracted the data, and was responsible for the statistical analysis. PA and MC reviewed the manuscript and provided critical scientific input. DGG resolved discrepancies regarding the quality of the studies included in the meta-analysis, provided critical scientific input, and had the primary responsibility for the paper’s final content.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farquhar C, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2018;8:CD010537. doi: 10.1002/14651858.CD010537.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. 2017;7:Cd007807. doi: 10.1002/14651858.CD007807.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki H, Hamatani T, Kamijo S, Iwai M, Kobanawa M, Ogawa S, et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front Endocrinol. 2019;10:811. doi: 10.3389/fendo.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Nisenblat V, Lu C, Li R, Qiao J, Zhen X, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol. 2018;16(1):29. doi: 10.1186/s12958-018-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee P, Bhattacharya J. Impact of oxidative stress on infertility, with emphasis on infertility management strategies. Glob J Fertil Res. 2019;4(1):10–18. doi: 10.17352/gjfr.000012. [DOI] [Google Scholar]
- 7.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108(4):1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol. 2005;3:43. doi: 10.1186/1477-7827-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):80. doi: 10.1186/s12958-018-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15(1):71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodick T, Seibels D, Jeganathan R, Huggins K, Ren G, Mathews S. Potential role of coenzyme Q10 in health and disease conditions. Nutr Diet Suppl. 2018;10:1–11. doi: 10.2147/NDS.S112119. [DOI] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13:196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 16.El Refaeey A, Selem A, Badawy A. Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene-citrate-resistant polycystic ovary syndrome. Reprod BioMed Online. 2014;29(1):119–124. doi: 10.1016/j.rbmo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Bentov Y, Hannam T, Jurisicova A, Esfandiari N, Casper RF. Coenzyme Q10 Supplementation and Oocyte Aneuploidy in Women Undergoing IVF-ICSI Treatment. Clin Med Insights Reprod Health. 2014;8:31–36. doi: 10.4137/cmrh.S14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caballero T, Fiameni F, Valcarcel A, Buzzi J. Dietary supplementation with coenzyme Q10 in poor responder patients undergoing IVF-ICSI Treatment. Fertil Steril. 2016;106(3):e58. doi: 10.1016/j.fertnstert.2016.07.177. [DOI] [Google Scholar]
- 19.Sen SD. Co-enzyme q10-a mitochondrial antioxidant -a new hope for success in infertility in clomiphene-citrate-resistant polycystic ovary syndrome. CENTRAL. 2019;2019(3). 10.1111/1471-0528.2_14571.
- 20.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 21.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Meir A, Kim K, McQuaid R, Esfandiari N, Bentov Y, Casper RF, Jurisicova A. Co-enzyme Q10 supplementation rescues cumulus cells dysfunction in a maternal aging model. Antioxidants. 2019;8(3):58. doi: 10.3390/antiox8030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, ShiYang X, Zhang Y, Miao Y, Chen Y, Cui Z, Xiong B. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic Biol Med. 2019;143:84–94. doi: 10.1016/j.freeradbiomed.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Ozcan P, Ficicioglu C, Kizilkale O, Yesiladali M, Tok OE, Ozkan F, et al. Can coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. 2016;33(9):1223–1230. doi: 10.1007/s10815-016-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lafuente R, González-Comadrán M, Solà I, López G, Brassesco M, Carreras R, Checa MA. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet. 2013;30(9):1147–1156. doi: 10.1007/s10815-013-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal A, Sharma A, Master K, Sharma R, Henkel R. Meta-analysis of double-blind placebo control trials evaluating the role of coenzyme Q10 on semen parameters. Fertil Steril. 2018;110(4):e167–e1e8. doi: 10.1016/j.fertnstert.2018.07.497. [DOI] [Google Scholar]
- 27.Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;(3). 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed]
- 28.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Iguchi T, Itoh N, Okamoto K, Takagi T, Tanaka K, Nakanishi T. Ascorbic acid transported by sodium-dependent vitamin C transporter 2 stimulates steroidogenesis in human choriocarcinoma cells. Endocrinol. 2008;149(1):73–83. doi: 10.1210/en.2007-0262. [DOI] [PubMed] [Google Scholar]
- 30.Vural P, Akgul C, Yildirim A, Canbaz M. Antioxidant defence in recurrent abortion. Clin Chim Acta. 2000;295(1-2):169–177. doi: 10.1016/s0009-8981(99)00255-7. [DOI] [PubMed] [Google Scholar]
- 31.Ledee-Bataille N, Olivennes F, Lefaix JL, Chaouat G, Frydman R, Delanian S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum Reprod. 2002;17(5):1249–1253. doi: 10.1093/humrep/17.5.1249. [DOI] [PubMed] [Google Scholar]
- 32.Takasaki A, Tamura H, Taniguchi K, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Sugino N. Luteal blood flow and luteal function. J Ovarian Res. 2009;2:1. doi: 10.1186/1757-2215-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson RL, Spedding S, Buckley JD. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol. 2012;77(3):343–350. doi: 10.1111/j.1365-2265.2012.04434.x. [DOI] [PubMed] [Google Scholar]
- 34.Nestler JE. Inositolphosphoglycans (IPGs) as Mediators of Insulin's Steroidogenic Actions. J Basic Clin Physiol Pharmacol. 2011;9(2-4):197–204. doi: 10.1515/JBCPP.1998.9.2-4.197. [DOI] [PubMed] [Google Scholar]
- 35.Gaskins AJ, Chiu YH, Williams PL, Ford JB, Toth TL, Hauser R, Chavarro JE, for the EARTH Study Team Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. Am J Clin Nutr. 2015;102(4):943–950. doi: 10.3945/ajcn.115.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AbdulameerYahya A, Abdulridha MK, Al-Rubuyae BJ, Al-Atar HA. The effect of vitamin D and co-enzyme Q10 replacement therapy on hormonal profile and ovulation statusin women with clomiphene citrate resistant polycystic ovary syndrome. J Pharm Sci Res. 2019;11(1):208–215. [Google Scholar]
- 37.Pritchard N, Healey M, Sorby KL, Sivapalan S, Osianlis T, Jatkar S, et al. A case control study of melatonin with or without coenzyme Q10 in improving oocyte quality and outcomes in in vitro fertilization. Reprod Biol Insights. 2015;8:1–7. doi: 10.4137/RBI.S27776. [DOI] [Google Scholar]
- 38.Gat I, Blanco Mejia S, Balakier H, Librach CL, Claessens A, Ryan EA. The use of coenzyme Q10 and DHEA during IUI and IVF cycles in patients with decreased ovarian reserve. Gynecol Endocrinol. 2016;32(7):534–537. doi: 10.3109/09513590.2015.1137095. [DOI] [PubMed] [Google Scholar]
- 39.Bhagavan HN, Chopra RK. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40(5):445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 40.Miles MV, Horn P, Miles L, Tang P, Steele P, DeGrauw T. Bioequivalence of coenzyme Q10 from over-the-counter supplements. Nutr Res. 2002;22(8):919–929. doi: 10.1016/S0271-5317(02)00402-5. [DOI] [Google Scholar]
- 41.Jones K, Hughes K, Mischley L, McKenna DJ. Coenzyme Q-10: Efficacy, safety, and use. Altern Ther Health Med. 2002;8(3):42–55. [PubMed] [Google Scholar]
- 42.Langsjoen PH, Langsjoen PH, Folkers K. Long-term efficacy and safety of coenzyme Q10 therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1990;65(7):521–523. doi: 10.1016/0002-9149(90)90824-K. [DOI] [PubMed] [Google Scholar]
- 43.Gouveia F, Costa R, Lemos T. Coenzyme Q10 and polycitic ovary syndrome: systematic review. CMI. 2018;3. 10.15761/CMI.1000168.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 256 kb).
Data Availability Statement
Not applicable.