Abstract
Purpose
It is known that delivery rates from spontaneous conception vary according to season which may be due to cultural or environmental factors; however, conflicting data exist regarding whether outcomes from IVF are also seasonally dependent. The present study was designed to test the hypothesis that the season at oocyte retrieval is associated with livebirth after fresh transfer.
Methods
Dates of oocyte retrieval for all autologous cycles in our IVF program between January 2012 and December 2017 were categorized by season. Dates were linked to local temperature (min, max, average) and day length obtained from meteorological records. Average maximum temperature and day length were categorized into tertiles. Multivariable logistic regression, adjusted for age and quadratic age, were used to model odds (aOR) of implantation, clinical pregnancy, spontaneous abortion, and livebirth.
Results
Patient characteristics were similar across seasons. As expected, temperature and day length varied by season. When compared with cycles started during winter, there was no difference in the age-adjusted odds of livebirth for the other three seasons (spring: aOR: 0.97, 95% CI: 0.82–1.13; summer: aOR: 1.05, 0.90–1.23; fall: aOR: 0.98, 0.84–1.15). There was a positive linear trend between temperature and odds of implantation, and clinical pregnancy (p value, test for linear trend (implantation, p = 0.02; clinical pregnancy, p = 0.01)) but no association with livebirth for temperature or day length.
Conclusions
We found that season at oocyte retrieval was not associated with livebirth, contrary to patterns seen in naturally conceived populations. However, our data did suggest modestly higher odds of clinical pregnancy for retrievals in June and July, and that higher temperature at time of retrieval was associated with higher odds of clinical pregnancy but not livebirth.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01915-2) contains supplementary material, which is available to authorized users.
Keywords: Livebirth, IVF, Season, Temperature, Day length, Seasonal variation, Heat, Pregnancy, ART
Background
Seasonal variation in natural conception and therefore birth has been established in human populations [1–7]. For example, in naturally conceiving populations in the USA, the highest birth rates occur in the summer and early fall (July to September) and are at their lowest in the spring (April and May) [8, 9]; by contrast, in Northern Europe, birth rates are highest in spring (March and April) and lowest in fall (October and November) [3, 8, 10].
Natural conception may be influenced by a number of factors including cultural and sociologic factors (e.g., annual work cycles, time of year vacations occur, national holidays, preferred marriage season) as well as environmental and biological factors (e.g., temperature, sunlight, weather, pollution) and may change over time [6]. With regard to cultural factors, prior research in the USA has suggested that the timing of the US Thanksgiving holiday and Christmas may contribute to the peak in conceptions in November and December and subsequently higher birth rates in the summer and early fall [3]. Among environmental exposures, other research has suggested that temperature and sunlight seem to be the most important factors that influence natural conception [11].
It is well established that heat can suppress spermatogenesis and possibly testicular steroidogenesis in the male [11]. In humans, scrotal warming can temporarily diminish the number of spermatozoa ejaculated [12]. In regions where a strong seasonal contrast in number of daylight hours exists, it has been shown that the conception rate is decreased during the dark winter months and increased during summer months which, in turn, leads to a peak in birth rate in spring [13]. Both ovulation rates and endometrial receptivity were demonstrated to decrease during long, dark winters in northern European countries, where a summer peak in conception and multiple pregnancy rates were observed [13].
While cultural and behavioral factors may influence natural birth patterns, they would not influence overall biologic fecundity. Thus, by investigating the seasonal variation in births following assisted reproductive technology (ART), we reduce the influence of cultural and behavioral factors unrelated to fecundity, thereby allowing further clarification of the relationship between seasonality and conception in humans. A demonstrated link between season and IVF success rates would aid programs in maximizing success rates by avoiding seasons for oocyte retrieval that lead to lower livebirth rates.
Available evidence for seasonal variation and IVF success is conflicting possibly due to small sample sizes (≤ 1000 cycles) and varying environmental exposures and geographic populations. Some studies on season and IVF outcomes have found no association [14–18], while some have suggested higher implantation and livebirth rates for IVF cycles started in the spring and summer [19–21]. Other studies have suggested higher positive IVF outcomes for cycles started in the fall and winter [22, 23]. To improve understanding of the relationship between seasonal variation and IVF success, we investigated the relationship between season, temperature, and day length at time of oocyte retrieval in a large cohort of more than 6000 IVF cycles performed at one IVF center in Massachusetts over a 6-year period.
Materials and methods
We conducted a retrospective cohort study of all fresh IVF cycles having an oocyte retrieval between January 1, 2012, and December 31, 2017, at Brigham and Women’s Hospital, Boston, MA. Donor oocyte and gestational carrier cycles were excluded. This study was approved by the Partners’ Healthcare Institutional Review Board (no. 2018P000389).
Seasonal parameters
Seasonality was defined using multiple measures, including meteorological season, day length, and temperature at oocyte retrieval (primary analysis) and at cycle start (sensitivity analysis). Season of oocyte retrieval and season of cycle start were defined based on meteorological season: spring (March 1–May 31), summer (June 1–August 31), fall (September 1–November 30), and winter (December 1–February 28, or 29 if a leap year). Information on day length for the Boston, MA, area for each day within the study period was accessed via the Time and Date website (timeanddate.com). Information on temperature for each day within the study period was accessed from historical data for Boston, MA, on the Weather Underground website (www.wunderground.com). For the analysis, temperature and day length were categorized into tertiles (day length (hours): < 10.81, 10.81–13.65, > 13.65; temperature (°F): < 44, 44–63, > 63).
Clinical outcomes
Clinical IVF outcomes of interest included implantation rate, clinical pregnancy, spontaneous abortion, and livebirth. Implantation rate was defined as the maximum number of intrauterine gestational sacs observed on ultrasound divided by the total number of embryos transferred. Clinical pregnancy was defined as the presence of at least one intrauterine gestational sac ever observed. Spontaneous abortion refers to the loss of a clinical pregnancy before 20 weeks’ gestation. Livebirth was defined as delivery of at least one newborn.
Statistical analysis
Generalized estimating equations (GEE) with a binary distribution and logit link were used to estimate odds ratios and 95% confidence intervals for the clinical outcomes of interest. Because some patients contributed multiple cycles, an exchangeable correlation structure was used to account for multiple cycles from the same patient. All models were adjusted for age and quadratic age. Tests for linear trend were conducted by setting each tertile category to its median value and testing for linear trend of the new variable. All statistical analysis was conducted in R version 4.0.1 [24]. GEE models were fit using the gee package [25].
Sensitivity analyses
Due to known differences in clinical IVF outcomes based on day of transfer, we conducted a variety of sensitivity analyses to test the robustness of our findings. We restricted our analyses to cycles with embryos transferred (Supplemental Table 1). We also conducted analyses stratified by day of embryo transfer (day 3, Supplemental Table 2; day 5, Supplemental Table 3).
Results
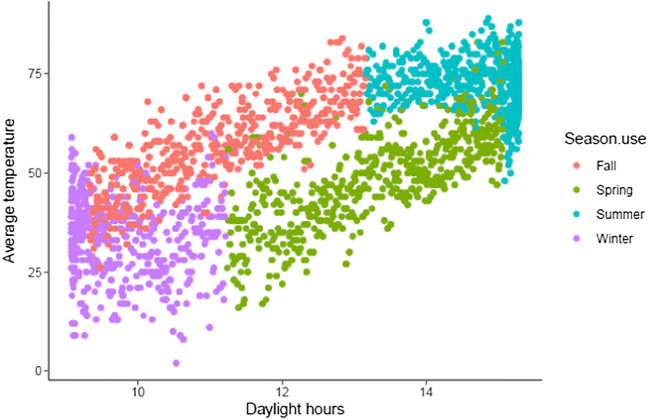
A total of 6669 fresh cycles met inclusion criteria. Among these, the most common season for oocyte retrieval was fall (n = 1758) and the least common season was winter (n = 1516) (Table 1). Patient age, parity, day 3 FSH, number of oocytes retrieved, number of mature oocytes, and number of oocytes fertilized were similar across season of oocyte retrieval. Likewise, the incidence of a retrieval resulting in a fresh embryo transfer, and the number of embryos transferred, or the day of transfer were all similar among seasons. As expected, average temperature and daylight hours were highly correlated across all seasons (correlation = 0.72) (Fig. 1).
Table 1.
Demographic characteristics by season at oocyte retrieval among 6669 fresh IVF cycles
| Winter | Spring | Summer | Fall | |
|---|---|---|---|---|
| n = 1516 | n = 1704 | n = 1691 | n = 1758 | |
| Median (IQR) | ||||
| n (%) | ||||
| Patient age (years) | 37.0 (33.7–40.2) | 37.2 (33.8–40.3) | 37.1 (33.4–40.1) | 37.2 (33.7–40.3) |
| Parity | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) |
| Gravidity | 1 (0–2) | 1 (0–2) | 0 (0–2) | 1 (0–2) |
| Day 3 FSH (IU) | 7.5 (6.1–9.3) | 7.6 (6.2–9.4) | 7.5 (6.2–9.3) | 7.6 (6.2–9.4) |
| Total number of follicles | 11 (7–16) | 12 (8–16) | 12 (8–17) | 12 (8–17) |
| Number of oocytes retrieved | 11 (7–16) | 11 (7–17) | 11 (7–17) | 11 (7–17) |
| Number of mature oocytes | 7 (4–12) | 8 (4–13) | 8 (4–12) | 7 (4–13) |
| Number of oocytes fertilized | 6 (3–10) | 6 (3–10) | 6 (3–10) | 6 (3–10) |
| Number of cycles with ET | 1263 (83%) | 1449 (85%) | 1385 (82%) | 1464 (83%) |
| Number of cycles that resulted in cryopreservation among cycles with no embryos transferred | 239 (94.5%) | 240 (94.1%) | 279 (91.2%) | 278 (94.6%) |
| Number of embryos transferred | 2 (1–2) | 2 (1–2) | 2 (1–2) | 2 (1–2) |
| Day of transfer | ||||
| Day 3 | 872 (69%) | 1006 (69%) | 937 (68%) | 948 (65%) |
| Day 5 | 391 (31%) | 443 (31%) | 449 (32%) | 514 (35%) |
Fig. 1.
Correlation between day length and temperature across seasons
No statistically significant difference in livebirth by season of oocyte retrieval was identified (Table 2; Fig. 2). While the dichotomized threshold of statistical significance was not met, a consistent modest trend was observed for some clinical IVF outcomes, where cycles with oocyte retrieval in the summer had a higher incidence of implantation (OR: 1.09 (95% CI: 0.95–1.24)), clinical pregnancy (OR: 1.11 (95% CI: 0.96–1.29)), and livebirth (OR: 1.05 (95% CI: 0.90–1.23)) compared with cycles with oocyte retrievals in the winter. Likewise, there was no statistically significant association between day length and livebirth. The month of oocyte retrieval with the highest probability of livebirth was July (27.5%) and the month of oocyte retrieval with the lowest probability of livebirth was December (23.6%) (Fig. 2). We saw no association between season, day length, or temperature and risk of spontaneous abortion (Table 2).
Table 2.
Clinical IVF outcomes among 6669 fresh IVF cycles, by day at oocyte retrieval
| Implantation | Clinical pregnancy | Spontaneous abortion | Livebirth | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | OR1 (95% CI) | n (%) | OR1 (95% CI) | n (%) | OR1 (95% CI) | n (%) | OR1 (95% CI) | |
| Season2 | ||||||||
| Winter | 591 (22%) | 1.00 (Ref) | 500 (33%) | 1.00 (Ref) | 102 (20%) | 1.00 (Ref) | 391 (26%) | 1.00 (Ref) |
| Spring | 671 (21%) | 0.96 (0.84–1.09) | 557 (33%) | 1.01 (0.87–1.17) | 124 (22%) | 1.11 (0.82–1.49) | 424 (25%) | 0.97 (0.82–1.13) |
| Summer | 695 (23%) | 1.09 (0.95–1.24) | 588 (35%) | 1.11 (0.96–1.29) | 129 (22%) | 1.11 (0.82–1.49) | 447 (26%) | 1.05 (0.89–1.23) |
| Fall | 681 (22%) | 1.04 (0.91–1.18) | 567 (32%) | 0.99 (0.86–1.15) | 115 (20%) | 1.02 (0.75–1.39) | 442 (25%) | 0.98 (0.84–1.15) |
| Average day length (h) | ||||||||
| < 10.81 | 833 (22%) | 1.00 (Ref) | 696 (32%) | 1.00 (Ref) | 142 (20%) | 1.00 (Ref) | 544 (25%) | 1.00 (Ref) |
| 10.81–13.65 | 875 (22%) | 1.03 (0.92–1.16) | 732 (34%) | 1.09 (0.96–1.24) | 158 (22%) | 1.10 (0.85–1.42) | 559 (26%) | 1.05 (0.91–1.20) |
| > 13.65 | 930 (22%) | 1.04 (0.93–1.16) | 784 (34%) | 1.15 (1.01–1.30) | 170 (22%) | 1.04 (0.81–1.35) | 601 (26%) | 1.10 (0.96–1.26) |
| p value, linear trend | 0.54 | 0.03 | 0.76 | 0.16 | ||||
| Average temperature (°F) | ||||||||
| < 44 | 839 (21%) | 1.00 (Ref) | 698 (32%) | 1.00 (Ref) | 142 (20%) | 1.00 (Ref) | 548 (25%) | 1.00 (Ref) |
| 44–63 | 909 (22%) | 1.04 (0.93–1.16) | 764 (33%) | 1.09 (0.97–1.24) | 165 (22%) | 1.05 (0.82–1.36) | 588 (26%) | 1.05 (0.91–1.20) |
| > 63 | 890 (23%) | 1.15 (1.03–1.29) | 750 (35%) | 1.17 (1.03–1.32) | 163 (22%) | 1.05 (0.81–1.36) | 568 (26%) | 1.10 (0.96–1.26) |
| p value, linear trend | 0.02 | 0.01 | 0.72 | 0.19 | ||||
1Generalized estimating equations with a binary distribution, logit link, and exchangeable correlation structure were used to estimate odds ratios (ORs) and 95% confidence intervals. All models were adjusted for age and quadratic age
2Spring (March 1–May 31), summer (June 1–August 31), fall (September 1–November 30), and winter (December 1–February 28, or 29 if a leap year)
Fig. 2.
Probability of livebirth by month at oocyte retrieval
Investigation of temperature at time of oocyte retrieval revealed a linear trend between increasing temperature and clinical IVF outcomes (Fig. 3). The highest tertile of temperature (> 63 °F) compared with the lowest tertile (< 44 °F) was associated with higher odds of implantation (OR: 1.15 (95% CI: 1.03–1.29); p value linear trend: 0.02) and higher odds of clinical pregnancy (OR: 1.17 (95% CI: 1.03–1.32); p value linear trend: 0.01). The relationship between temperature and livebirth followed a similar pattern, but did not meet the threshold of statistical significance (OR: 1.10 (95% CI: 0.96–1.26)); p value test for linear trend: 0.19).
Fig. 3.
Clinical pregnancy outcomes by tertiles of average day length (A) and average temperature (B)
In sensitivity analyses restricted to cycles with an embryo transfer (n = 5561), we observed a similar pattern of association, with the highest incidence of clinical IVF outcomes in the summer as compared with the winter. Both higher temperatures and increased day length suggested a similar pattern of association (Supplemental Table 1). Among day 3 embryo transfers (n = 3762), we also observed a similar pattern of association with the highest incidence of clinical pregnancy among oocyte retrievals in the summer compared with the winter, again at higher temperatures, and increased day length (Supplemental Table 2). Conversely, among day 5 embryo transfers (n = 1797) there was no discernable pattern of association between season, temperature, and day length in relation to any of clinical IVF outcomes assessed (Supplemental Table 3).
Discussion
Overall, we found that season at oocyte retrieval was not associated with livebirth. However, the data did suggest modest patterns of association, where clinical outcomes for cycles with oocyte retrieval during the summer had a higher incidence of success. The association was driven by a positive association with temperature on the day of oocyte retrieval.
Specifically, we found that oocyte retrievals performed in the summer had a slightly higher incidence of implantation, clinical pregnancy, and livebirth compared with the other seasons. When investigating solar and meteorological patterns on the day of oocyte retrieval, both longer day length and higher temperature revealed positive associations with the clinical outcomes assessed. These observations are consistent with prior studies in Israel and China reporting improved clinical IVF outcomes in the summer [14, 19, 21] as compared with spring and winter. Prior research has also suggested that longer day length [21, 26] may be associated with positive clinical outcomes.
Interestingly, we found that association with season, temperature, and day length attenuated when restricted to day 5 cycles. This may represent biological differences in embryo quality/susceptibility or be driven by clinical practice. It is current practice in many clinical IVF centers to only transfer embryos on day 3 if there is concern that the embryos are poor quality and may not continue development to day 5. Thus, the attenuation we see among day 5 transfers may indicate that the effect of seasonal variation is strongest among day 3 transfers and attenuates among embryos from higher-quality oocytes transferred on day 5. However, the possibility also exists that our inconsistent findings between day 3 and day 5 transfers are related to the smaller number of cycles having day 5 transfer in our dataset. Future research should continue to investigate this potential difference.
Seasonal parameters at oocyte retrieval may be correlated with other environmental exposures including air pollution and smog. Laboratory air quality is known to be associated with IVF outcomes [27] and some research has suggested that poor air quality prior to conception was associated with higher rates of miscarriage [28, 29] and lower rates of pregnancy [30]. However, our laboratory air is filtered through a five-stage filtration system comprised of three carbon-activated filters and two HEPA filters in sequence, which results in particulate and VOC values in line with recommendations for air quality in the IVF laboratory [31]. Nevertheless, concentrations of particulate matter (PM 2.5) in ambient air in the northeastern United States, in which our IVF center is located, have been found to be 2.5 times higher in January as compared with July [32], which would argue that higher levels of pollution, at least the concentrations in this region of the world, may have some unknown adverse effect on health, which may be a contributing factor to the present findings.
The suggestion of an association with clinical IVF outcomes during the summer may also be associated with vitamin D production. While diet contributes to circulating vitamin D levels, the majority is derived from sun exposure. Vitamin D deficiency is higher in the winter compared with the summer, particularly in the northeastern United States [33]. It has been suggested that vitamin D deficiency is associated with a lower incidence of livebirth among IVF practices [34], although this finding has not been consistent across all studies [35]. Thus, our findings may represent a crude proxy measure of circulating vitamin D status.
This study has many strengths including its large sample size and rigorous data collection of laboratory variables. We also must recognize its limitations. Our main exposure is related to the day of oocyte retrieval. This variable may not represent the most critical window of exposure for clinical IVF outcomes. Indeed, prior researchers have prioritized both the time of cycle start [18, 20, 21] and the time of oocyte retrieval [14, 15, 23]. However, given that the time of oocyte retrieval is highly correlated with the time at cycle start, we would expect any misclassification to be non-differential and cause an attenuation of effect estimates. Moreover, we use temperature and day length characteristics at the day of oocyte retrieval as a proxy for the environment during treatment although there may be the occurrence of unseasonable weather patterns that may not be representative of the period. We would expect any misclassification of temperature to be non-differential with respect to the outcomes and therefore cause an underestimation of the true association. Some cycles did not result in embryos being transferred. The proportion of cycles with embryos transferred did not vary across seasons. Unfortunately we did not have information on what proportion of cycles were cryopreserved to conduct PGT-A. Our data represent standard practice for fresh oocyte retrievals at our clinic from January 2012 until December 2017, which may not be representative of all clinical practice. To disentangle the influence of clinical practice, we restricted our study sample to fresh oocyte retrievals, and we presented results stratified by day of transfer. Our data were generated from a clinic in the northeastern United States; findings from our analysis may not be generalizable to locations without four seasons or with differing levels of temperature, day length, humidity, and particulate matter.
We found no difference in the incidence of livebirth after fresh transfer for season at oocyte retrieval. However, we found a suggestion of modestly increased odds of clinical pregnancy for retrievals in June and July, and that higher temperature at retrieval was associated with improved clinical outcomes. Whether this reflects a subtle influence of elevated temperatures on the uterus being more receptive to implantation, which subsequently fails, remains to be determined. Future research is warranted to better understand these associations in other climates and should emphasize research into the role of temperature, daylight, and related environmental exposures.
Electronic supplementary material
(DOCX 27 kb)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenberg HM. Seasonal variation of births, United States, 1933-63. Vital Health Stat 1. 1966;21(9):1–59. [PubMed] [Google Scholar]
- 2.Cowgill U. Season of birth in man, contemporary situation with special refernce to Europe and the Southern Hemisphere. Ecology. 1965;47(4):614–623. doi: 10.2307/1933939. [DOI] [Google Scholar]
- 3.Lam DA, Miron JA. Seasonality of births in human populations. Soc Biol. 1991;38(1-2):51–78. doi: 10.1080/19485565.1991.9988772. [DOI] [PubMed] [Google Scholar]
- 4.Becker S. Seasonal patterns of births and conception throughout the world. Adv Exp Med Biol. 1991;286:59–72. doi: 10.1007/978-1-4684-5913-5_6. [DOI] [PubMed] [Google Scholar]
- 5.Becker S. Seasonality of deaths in Matlab, Bangladesh. Int J Epidemiol. 1981;10(3):271–280. doi: 10.1093/ije/10.3.271. [DOI] [PubMed] [Google Scholar]
- 6.James WH. Seasonal variation in human births. J Biosoc Sci. 1990;22(1):113–119. doi: 10.1017/S0021932000018423. [DOI] [PubMed] [Google Scholar]
- 7.Wesselink AK, Wise LA, Hatch EE, Mikkelsen EM, Sørensen HT, Riis AH, et al. Seasonal patterns in fecundability in North America and Denmark: a preconception cohort study. Hum Reprod. 2020:dez265. 10.1093/humrep/dez265. [DOI] [PMC free article] [PubMed]
- 8.Lam DA, Miron JA. Global patterns of seasonal variation in human fertility. Ann N Y Acad Sci. 1994;709:9–28. doi: 10.1111/j.1749-6632.1994.tb30385.x. [DOI] [PubMed] [Google Scholar]
- 9.Seiver DA. Trend and variation in the seasonality of U.S. fertility, 1947-1976. Demography. 1985;22(1):89–100. doi: 10.2307/2060988. [DOI] [PubMed] [Google Scholar]
- 10.Russell D, Douglas AS, Allan TM. Changing seasonality of birth--a possible environmental effect. J Epidemiol Community Health. 1993;47(5):362–367. doi: 10.1136/jech.47.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronson FH. Seasonal variation in human reproduction: environmental factors. Q Rev Biol. 1995;70(2):141–164. doi: 10.1086/418980. [DOI] [PubMed] [Google Scholar]
- 12.Robinson D, Rock J. Intrascrotal hyperthermia induced by scrotal insulation: effect on spermatogenesis. Obstet Gynecol. 1967;29(2):217–223. [PubMed] [Google Scholar]
- 13.Rojansky N, Brzezinski A, Schenker JG. Seasonality in human reproduction: an update. Hum Reprod. 1992;7(6):735–745. doi: 10.1093/oxfordjournals.humrep.a137729. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Bai H, Mol BW, Shi W, Gao M, Shi J. Seasonal variability does not impact in vitro fertilization success. Sci Rep. 2019;9(1):17185. doi: 10.1038/s41598-019-53919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirshenbaum M, Ben-David A, Zilberberg E, Elkan-Miller T, Haas J, Orvieto R. Influence of seasonal variation on in vitro fertilization success. PLoS One. 2018;13(7):e0199210-e. doi: 10.1371/journal.pone.0199210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming C, Nice L, Hughes AO, Hull MG. Apparent lack of seasonal variation in implantation rates after in-vitro fertilization. Hum Reprod. 1994;9(11):2164–2166. doi: 10.1093/oxfordjournals.humrep.a138411. [DOI] [PubMed] [Google Scholar]
- 17.Dunphy BC, Anderson-Sykes S, Brant R, Pattinson HA, Greene CA. Human embryo implantation following in-vitro fertilization: is there a seasonal variation? Hum Reprod. 1995;10(7):1825–1827. doi: 10.1093/oxfordjournals.humrep.a136184. [DOI] [PubMed] [Google Scholar]
- 18.Wunder DM, Limoni C, Birkhäuser MH, Swiss F-G. Lack of seasonal variations in fertilization, pregnancy and implantation rates in women undergoing IVF. Hum Reprod. 2005;20(11):3122–3129. doi: 10.1093/humrep/dei177. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M, Zhang H, Waters THB, Chung JPW, Li TC, Chan DYL. The effects of daily meteorological perturbation on pregnancy outcome: follow-up of a cohort of young women undergoing IVF treatment. Environmental health: a global access science source. 2019;18(1):103. doi: 10.1186/s12940-019-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamoun D, Udoff L, Scott L, Magder L, Adashi EY, McClamrock HD. A seasonal effect on pregnancy rates in an in vitro fertilization program. J Assist Reprod Genet. 1995;12(9):585–589. doi: 10.1007/bf02212579. [DOI] [PubMed] [Google Scholar]
- 21.Rojansky N, Benshushan A, Meirsdorf S, Lewin A, Laufer N, Safran A. Seasonal variability in fertilization and embryo quality rates in women undergoing IVF. Fertil Steril. 2000;74(3):476–481. doi: 10.1016/S0015-0282(00)00669-5. [DOI] [PubMed] [Google Scholar]
- 22.Stolwijk AM, Reuvers MJ, Hamilton CJ, Jongbloet PH, Hollanders JM, Zielhuis GA. Seasonality in the results of in-vitro fertilization. Hum Reprod. 1994;9(12):2300–2305. doi: 10.1093/oxfordjournals.humrep.a138441. [DOI] [PubMed] [Google Scholar]
- 23.Weigert M, Feichtinger W, Kulin S, Kaali SG, Dorau P, Bauer P. Seasonal influences on in vitro fertilization and embryo transfer. J Assist Reprod Genet. 2001;18(11):598–602. doi: 10.1023/a:1013160905497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R: A language and environment for statistical computing. Vienna, Austria. R Core Team 2017.
- 25.gee: Generalized Estimation Equation Solver. In: Ripley. VJCPtRbTLaB, editor.2015.
- 26.Vandekerckhove F, Van der Veken H, Tilleman K, De Croo I, Van den Abbeel E, Gerris J, et al. Seasons in the sun: the impact on IVF results one month later. Facts Views Vis Obgyn. 2016;8(2):75–83. [PMC free article] [PubMed] [Google Scholar]
- 27.Khoudja RY, Xu Y, Li T, Zhou C. Better IVF outcomes following improvements in laboratory air quality. J Assist Reprod Genet. 2013;30(1):69–76. doi: 10.1007/s10815-012-9900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perin PM, Maluf M, Czeresnia CE, Januário DANF, Saldiva PHN. Impact of short-term preconceptional exposure to particulate air pollution on treatment outcome in couples undergoing in vitro fertilization and embryo transfer (IVF/ET) J Assist Reprod Genet. 2010;27(7):371–382. doi: 10.1007/s10815-010-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conforti A, Mascia M, Cioffi G, De Angelis C, Coppola G, De Rosa P, et al. Air pollution and female fertility: a systematic review of literature. Reprod Biol Endocrinol. 2018;16(1):117. doi: 10.1186/s12958-018-0433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choe SA, Jun YB, Lee WS, Yoon TK, Kim SY. Association between ambient air pollution and pregnancy rate in women who underwent IVF. Hum Reprod. 2018;33(6):1071–1078. doi: 10.1093/humrep/dey076. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer D, Cohen J, Mortimer ST, Fawzy M, McCulloh DH, Morbeck DE, et al. Cairo consensus on the IVF laboratory environment and air quality: report of an expert meeting. Reprod BioMed Online. 2018;36(6):658–674. doi: 10.1016/j.rbmo.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Dawson J, Adams P, Pandis S. Sensitivity of PM 2.5 to climate in the Eastern US: a modeling case study. Atmos Chem Phys. 2007;7(16):4295–4309. doi: 10.5194/acp-7-4295-2007. [DOI] [Google Scholar]
- 33.Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One. 2015;10(3):e0118108-e. doi: 10.1371/journal.pone.0118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod. 2012;27(11):3321–3327. doi: 10.1093/humrep/des280. [DOI] [PubMed] [Google Scholar]
- 35.Franasiak JM, Molinaro TA, Dubell EK, Scott KL, Ruiz AR, Forman EJ, et al. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol. 2015;212(3):315. doi: 10.1016/j.ajog.2014.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 27 kb)