Abstract
This study aimed to explore the antimicrobial effects of activated lactoferrin (ALF) and rosemary extract (RE) on Escherichia coli O157:H7, Salmonella Enteritidis and Listeria monocytogenes, and to investigate their application potential in the meat storage. Minimum inhibitory concentrations (MICs) of ALF, RE and ALF–RE combinations were determined via microtiter plate assay. MICs of ALF were 1% for E. coli O157:H7; 0.5% for S. Enteritidis and 0.1% for L. monocytogenes. While 15% RE inhibited L. monocytogenes; 30% RE partially inhibited E. coli O157:H7 and S. Enteritidis growth. Synergistic effect of ALF and RE on the inhibition of E. coli O157:H7 increased the activity of ALF two to three folds. Food application of antimicrobials was performed by dipping of meat samples. Both ALF and RE were found effective in the prevention of L. monocytogenes growth about two logs. According to the data, choice of natural antimicrobials may be promising in food preservation.
Keywords: Activated lactoferrin, Antimicrobial activity, Foodborne pathogens, Rosemary
Introduction
Large numbers of foodborne diseases originate from the presence of pathogenic bacteria in food that consequently cause serious health concerns and economic cost (Chen et al. 2015). Although the application of several food preservation methods, proliferation of bacteria and production of several toxins result in food poisonings (Gonelimali et al. 2018; Quinto et al. 2019). Among these disease-related bacteria, Salmonella spp., Listeria monocytogenes and Escherichia coli O157:H7 cause outbreaks and even results in deaths (Law et al. 2015; Braden 2006; Ye et al. 2008); Kieckens et al. 2018; Rybarczyk et al. 2017).
Use of natural preservatives to secure food safety is considered as an arising trend for consumers who want to escape the negative effects of the synthetic preservatives. These natural preservatives can be obtained from plants, animals, algae and even from bacteria and viruses (Quinto et al. 2019). Lactoferrin (LF)—a glycoprotein that belongs to the transferrin protein family—is able to bind iron within the cells (Giansanti et al. 2016). Ability of this 80 kDa protein to regulate free iron levels contributes its bacteriostatic and health beneficial properties, such as promoting bone growth, protecting intestinal epithelium and stimulating the immune system in animals (Niaz et al. 2019). Likewise, it has antiviral (Wakabayashi et al. 2014), antifungal (Fernandes and Carter 2017), antioxidant, anti-inflammatory and anticancer properties (Niaz et al. 2019). Antimicrobial activities of lactoferrin are commonly investigated in the literature on a wide range of bacteria (Acosta-Smith et al. 2018; Lizzi et al. 2016; Méndez et al. 2017). Immobilization of lactoferrin to a substrate by its N-terminus generates activated lactoferrin (ALF). To produce ALF, milk LF is immobilized on glycosaminoglycans and then it is solubilized in a citrate/bicarbonate buffer system containing sodium chloride and an excessive amount of unbound LF (Naidu 2001). Noting that the antimicrobial action mostly depends on the iron depletion in the microorganisms (Karav et al. 2017), immobilized form of lactoferrin has increased antibacterial activity (Naidu 2002) due to its capacity to increase binding capacity for iron (Fe3+) and other cellular targets. Additionally, cationic peptides on the N-terminus of lactoferrin contribute to disruption of permeability of the cell membrane and energy metabolism of the microorganism (Bellamy et al. 1992). Another attraction to lactoferrin origins from its status as ‘generally recognized as safe (GRAS)’ which contributes to its selection in food industry (Rybarczyk et al. 2017).
A Mediterranean-originated plant rosemary (Rosmarinus officinalis) is an aromatic plant which also serves medical purposes (Oliveira et al. 2017). It is a commonly investigated plant due to its antimicrobial (Elhariry et al. 2014; Gonelimali et al. 2018), antifungal (Türe et al. 2008), antioxidant (Nieto et al. 2018), anticancer, antidiabetic, antidepressant and anti-inflammatory properties. Essential oils and the extract of the rosemary are responsible for its preservative and therapeutic properties (Hamidpour et al. 2017). The antimicrobial activity of rosemary extract (RE) origins from its phenolic components such as rosmarinic acid, carnosic acid, rosmaridiphenol, carnosol and rosmanol. The presence of these phenolic compounds provide interaction with the cell membrane of bacteria results in the loss of membrane integrity and functionality. Safety of RE has been reviewed by EFSA, and in European Union, and its addition into the food and beverages are allowed up to 400 mg/kg (Nieto et al. 2018). Although there are studies showing the antimicrobial effects of LF and RE, to the best of our knowledge, no information was available in the literature showing the antibacterial activities of ALF in combination with RE on Escherichia coli O157:H7, Salmonella Enteritidis and Listeria monocytogenes. Therefore, the purposes of this study were to investigate the minimum inhibitory concentrations (MICs) of ALF and RE against the most dangerous foodborne pathogens both individually and synergistically, and also to assess their potential application in the meat storage. The use of two antimicrobial compounds with different origins and properties in combination may increase the observed antimicrobial activity. The significance of this study is the determination of the interaction (synergism or antagonism) between a natural peptide and a plant extract, and their food application due to their antimicrobial properties.
Materials and methods
Microorganisms and culture conditions
Escherichia coli O157:H7 NCTC 12900, Salmonella Enteritidis NCTC 12694 and Listeria monocytogenes NCTC 11994 were obtained from Natural Culture Type of Collection (NCTC, United Kingdom). E. coli O157:H7 were grown in Lauria broth (LB) containing 10% tryptone (Fluka), 5% sodium chloride (Riedel–deHaen) and 5% yeast extract (Acumedia) and on LB agar (Agar, Merck). S. Enteritidis were grown in Tryptic Soy Broth (TSB; Fluka) and on TSB agar. L. monocytogenes were grown in Brain Heart Infusion broth (BHI; Fluka) and on BHI agar. The following media were used for selections and identifications of corresponding pathogens from meat samples: Sorbitol MacConkey (SMAC; Fluka) agar supplemented with Cefixime Tellurite (CT) for E. coli O157:H7; Xylose Lysine Deoxycholate (XLD; Merck) agar for S. Enteritidis and PALCAM (Merck) agar for L. monocytogenes. All bacteria were maintained in appropriate media weekly and long-term maintenance was provided by storage at − 80 °C in 20% glycerol.
Preparation of bacterial cultures
For the propagation of bacteria, a single colony of all strains were transferred into corresponding broth media and incubated at 37 °C for 16 h (h). Following incubation, optical densities (OD) of bacteria were adjusted to intended values at 600 nm (OD600) by using a spectrophotometer (Thermo Multiscan Spectra Reader, Finland). Then, 2% inoculums from each bacterial culture were transferred into the fresh media and incubated at 37 °C to keep the bacteria at mid-logarithmic phase. Incubation periods after refreshing were as follows: 2.5 h for E. coli O157:H7; 3 h for S. Enteritidis and 5.5 h for L. monocytogenes. The bacterial load was adjusted to 1 × 104 cfu/mL for each strain and confirmed via viable count assay.
Preparation of natural compounds
ALF and RE were prepared freshly prior to use and the concentrations of both antimicrobials were prepared with sterilized deionized water (dH2O). Preparation of stock solution of ALF (DMV International Nutritionals, USA) was performed by following the recommended steps of the manufacturer. The concentration of the ALF stock solution was 4% (w/v). Rosemary samples were obtained from Izmir Institute of Technology campus area and subjected to ethanol extraction. Final concentrations for ALF were 2%, 1.5%, 1.25%, 1%, 0.5% and 0.25%; while for RE were 30%, 20%, 15%, 10% and 5%.
Extraction of rosemary
For the extraction of rosemary samples, the procedure carried out by Madsen et al. (1998) was used with slight modifications. After washing the collected plants with dH2O, the leaves were removed and homogenized (Heidolph Silent Crusher M Homogenizer, Germany) with 70 mL of absolute ethanol per 12 g leaves. Homogenization was carried out at 26,000 rpm for 5 min. Following a 30-min stirring process in dark and 5-min centrifuging process at 5000 rpm (Nuve NF 615, Turkey), the aqueous phase was precipitated with the addition of absolute ethanol. In total, this cycle was repeated three times by decreasing the ethanol volume to 30 mL and 20 mL. Then, the collected supernatant was mixed with 44 mL dH2O. Evaporation of the RE sample was carried out at 40 °C for 1 h under vacuum by a rotary evaporator (Heidolph Laborato 4000, Germany). The obtained solution was filtered through a cellulose nitrate filter (Sartorius) with the pore size of 5.0 µm under vacuum and filtrate was stored as RE extract (100%) in a dark glass bottle.
Antimicrobial activities of natural compounds
Antimicrobial experiments were performed with ALF and RE on E. coli O157:H7, S. Enteritidis and L. monocytogenes both individually and in combinations. All experiments were carried out as duplicates and with at least two independent repeats. For determination of antimicrobial effects of ALF and RE on bacteria, microtiter plate assay was performed by following the experimental procedure carried out by Dufour et al. (2003) with minor modifications. Briefly, ALF and RE stock solutions were serially diluted to the twofold of the final concentrations. By mixing 100 µL of each concentration (twofold of the final concentration) with 100 µL of the bacteria at the load of 1 × 104 cfu/mL in the wells of 96-well plate (Bio-Greiner, flat bottom), final antimicrobial concentration was halved. Bacterial control for each strain was prepared by mixing of the bacterial culture with the corresponding medium without addition of any antimicrobial compound. During incubation at 37 °C for 24 h, OD600 was measured at 2 h intervals.
For determination of the synergistic activities of ALF and RE, the aforementioned procedure was performed in combinations. This time each antimicrobial concentration was prepared as fourfold of the final concentration and 50 µL of two types of antimicrobial compounds was dispensed into the wells of the microtiter plate that contains 100 µL of 1 × 104 cfu/mL bacteria. Combined final concentrations of ALF and RE in wells were listed in Table 1. A control was included by mixing the bacterial culture with the appropriate medium without addition of any antimicrobial compound.
Table 1.
Concentrations of ALF and RE for determination of synergistic activity
| Final concentrations of natural compounds | ||
|---|---|---|
| ALF% | RE% | |
| E. coli O157:H7 | 0.5 | 20 |
| 15 | ||
| 10 | ||
| 0.3 | 20 | |
| 15 | ||
| 10 | ||
| 0.1 | 20 | |
| 15 | ||
| 10 | ||
| Salmonella Enteritidis | 0.25 | 20 |
| 15 | ||
| 10 | ||
| 0.15 | 20 | |
| 15 | ||
| 10 | ||
| 0.05 | 20 | |
| 15 | ||
| 10 | ||
| Listeria monocytogenes | 0.075 | 10 |
| 7.5 | ||
| 5 | ||
| 0.05 | 10 | |
| 7.5 | ||
| 5 | ||
| 0.025 | 10 | |
| 7.5 | ||
| 5 | ||
Food applications of antimicrobial agents
Fresh round-beef were purchased from Tanet slaughterhouse (Buca-Izmir, Turkey) and transported to the laboratory in an insulated box containing ice packs. All experimental steps were performed in BSL-2 cabinets (Esco, Singapure). For minimizing the microflora of purchased round-beef, a thin layer was skinned and the meat blocks were chopped into 2 cm-thick-pieces that weigh around 20 ± 1 g. Dipping applications were performed by immersing the meat samples into the 30 mL bacterial culture (1 × 105 cfu/mL) for 2 min. The attachment of bacteria onto the meat samples was allowed by a 30 min waiting process prior to dipping of inoculated meat samples into the antimicrobial solutions (30 mL) for 15 min. Control groups were non-treated meat samples (C1) and the bacteria inoculated meat samples (C2) which were dipped into 30 mL of sterilized dH2O instead of antimicrobial solutions. All samples were placed in sterile Petri dishes and then, these were stored in zipped plastic bags. Storage period was 9 days for E. coli O157:H7 and L. monocytogenes at 10 °C and 4 °C, respectively; and 8 days for S. Enteritidis at 10 °C.
Microbiological analyses for meat samples were performed by using the aforementioned selective media for each bacterium. Meat samples were taken at 0, 3, 6, 9th days for E. coli O157:H7 and L. monocytogenes; and at 0, 2, 4, 6, 8th days for S. Enteritidis. Each 20 g meat sample was subjected to two-minute-homogenization in 180 mL of 0.1% peptone water by a stomacher (BagMixer, France). Following serial dilution of homogenized meat samples in peptone water, 0.1 mL was spread onto corresponding selective agars and incubated at 37 °C for 24 h. Microbiological analyses were carried out as duplicates.
Statistical analyses
One-way analysis of variance (ANOVA) with MINITAB (Version 13.20) was used for the statistical analyses of data obtained from microtiter plate assays. For the data of meat applications, amounts of bacteria were transformed into log10. All the results given in the graphs were means and standard deviations of means. The statistical significance was of P < 0.05.
Results and discussion
Effects of antimicrobial compounds
The MICs of ALF and RE individually, and ALF-RE combination were noted as the lowest concentration of the antimicrobial compound which did not allow the growth of bacteria. The MIC values and the differences of means (indicated with superscript letters) at 12th, 18th and 24th h of incubation were listed in Table 2.
Table 2.
Means of concentrations of antimicrobials
| ALF% | RE% | |||||
|---|---|---|---|---|---|---|
| 12 h | 18 h | 24 h | 12 h | 18 h | 24 h | |
| E. coli O157:H7 | 1.5a | 1.5a | 1.5a | 30a | 30a | 30a |
| 1a | 1a | 1a* | 20b | 20ab | 20ab | |
| 0.75ab | 0.75ab | 0.75b | 15bc | 15ab | 15ab | |
| 0.5b | 0.5b | 0.5b | 10cd | 10bc | 10bc | |
| 0.25b | 0.25ab | 0.25b | 5d | 5c | 5c | |
| 0.1c | 0.1c | 0.1c | 0e | 0d | 0d | |
| 0d | 0d | 0d | – | – | – | |
| S. Enteritidis | 2a | 2a | 2a | 30a | 30a | 30a |
| 1.5a | 1.5a | 1.5a | 20b | 20a | 20ab | |
| 1.25a | 1.25a | 1.25a | 15bc | 15ab | 15ab | |
| 1a | 1a | 1a | 10cd | 10ab | 10bc | |
| 0.5a | 0.5a | 0.5a* | 5d | 5b | 5c | |
| 0.25b | 0.25b | 0.25b | 0e | 0c | 0d | |
| 0c | 0c | 0c | – | – | – | |
| L. monocytogenes | 0.5ab | 0.5a | 0.5a | 30a | 30a | 30a |
| 0.25a | 0.25a | 0.25a | 20a | 20a | 20a | |
| 0.1b | 0.1a | 0.1a* | 15a | 15a | 15a* | |
| 0.05c | 0.05b | 0.05b | 10a | 10a | 10b | |
| 0.025d | 0.025c | 0.025c | 5b | 5b | 5c | |
| 0e | 0c | 0d | 0c | 0c | 0c | |
a–dLetters indicate there is no statistical difference at P > 0.05. *MIC value for ALF and RE
The growth of the microorganisms in the presence of ALF concentrations were as shown in Fig. 1. Accordingly, MICs of ALF were determined as 1% for E. coli O157:H7; 0.5% for S. Enteritidis and 0.1% for L. monocytogenes. E. coli O157:H7 growth was significantly retarded when ALF concentrations were used between 0.25 and 0.75%. At the concentration of 0.25%, ALF retarded the growth of S. Enteritidis, while at 0.05% concentration it elongated the lag phase of L. monocytogenes (Fig. 1). The obtained MIC of ALF for E. coli O157:H7 in our study was consistent with the literature that reported by Naidu (2002) as 1%. The antimicrobial activity of ALF was not only limited to E. coli O157:H7, its inhibitory effect on the growth of several pathogens including Bacillus spp., Pseudomonas spp., drug-resistant Salmonella Typhimurium DT104, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium were also reported (Naidu et al. 2003). This multi-antimicrobial activity against pathogens makes ALF a promising candidate for usage in food decontamination and preservation processes. However, different studies may report varying concentrations of lactoferrin and this might be due to the purity of LF, iron saturation level of LF, temperature, water activity and pH of the environment and also variations in the food components and cations (Rybarczyk et al. 2017).
Fig. 1.
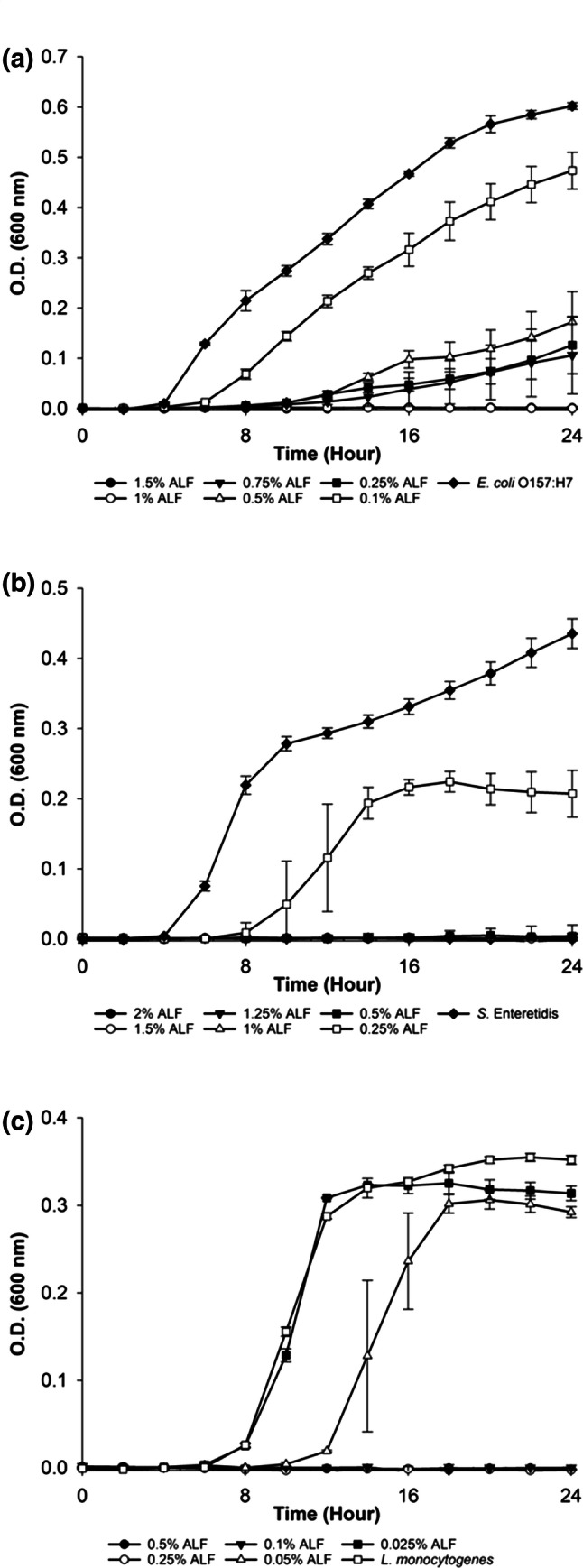
Antimicrobial activity of activated lactoferrin (ALF) on the growth of a E. coli O157:H7, b S. Enteritidis and c L. monocytogenes
The antimicrobial activity of RE was tested for the concentrations ranged between 5 and 30% (v/v) and none of these concentrations displayed total inhibitory effect for the growth of E. coli O157:H7 and S. Enteritidis and the MIC values could not be determined (Fig. 2). However, 30% RE decreased the growth and prolonged the lag phases of both bacteria. On the other hand, 15% RE resulted in total inhibition of L. monocytogenes growth and was determined as MIC. It could be speculated that Gram-negative bacteria were more resistant at the same concentrations of RE when compared to Gram-positive organisms. The literature supported the susceptibility of Gram-positive bacteria to antimicrobial agents and plant extracts when compared with Gram-negative bacteria (Bozin et al. 2007). Relatively high MIC value for RE could be explained by the extraction protocol. Antimicrobial activity of the extracts may change depending on the used extraction method (Pobiega et al. 2019). Differences among studies might be explained with the choose of solvent, extraction procedure, bacterial strains and bacterial load. Moreover, the same experimental procedure may give different results based on the composition of the plant collected from the different environmental conditions, climates and seasons (Oussalah et al. 2006).
Fig. 2.
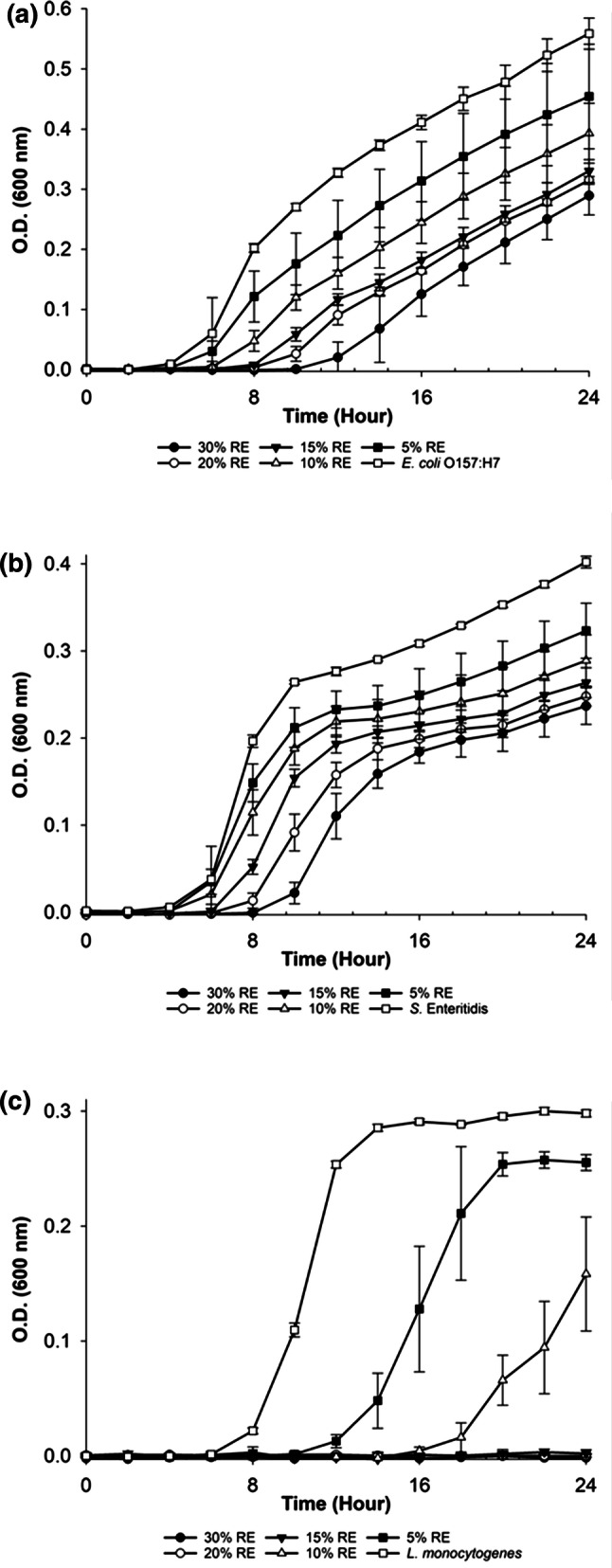
Antimicrobial activity of rosemary extract (RE) on the growth of a E. coli O157:H7, b S. Enteritidis and c L. monocytogenes
Synergistic activities of antimicrobial compounds
For the determination of synergistic activities of ALF and RE, the concentrations of each antimicrobial compound were chosen between MIC and sub-MIC. RE combinations (20-15-10%) with 0.5 and 0.3% ALF resulted in the growth inhibition in E. coli O157:H7, while ALF alone inhibited the growth at the concentration of 1% (Table 3). However, their synergistic activity did not allow the determination of MIC values for S. Enteritidis and L. monocytogenes at the tested concentrations. E. coli O157:H7 was found as the most susceptible strain against the antibacterial activity of ALF-RE combination. Therefore, it could be concluded that both antimicrobial compounds act synergistically for the inhibition of E. coli O157:H7 growth.
Table 3.
Percent growth inhibitions of combinations of activated lactoferrin (ALF) and rosemary extract (RE) on bacteria at the end of 24-h incubation
| Concentrations of compounds | Synergistic activity | ||
|---|---|---|---|
| ALF% | RE% | Percent inhibitions (%) | |
| E. coli O157:H7 | 0.5 | 20 | 99 |
| 15 | 98 | ||
| 10 | 98 | ||
| 0.3 | 20 | 99 | |
| 15 | 98 | ||
| 10 | 99 | ||
| 0.1 | 20 | 52 | |
| 15 | 39 | ||
| 10 | 19 | ||
| Salmonella Enteritidis | 0.25 | 20 | 45 |
| 15 | 46 | ||
| 10 | 40 | ||
| 0.15 | 20 | 44 | |
| 15 | 41 | ||
| 10 | 31 | ||
| 0.05 | 20 | 41 | |
| 15 | 33 | ||
| 10 | 17 | ||
| Listeria monocytogenes | 0.075 | 10 | 35 |
| 7.5 | 25 | ||
| 5 | 7 | ||
| 0.05 | 10 | 19 | |
| 7.5 | 17 | ||
| 5 | 4 | ||
| 0.025 | 10 | 18 | |
| 7.5 | 14 | ||
| 5 | 2 | ||
A recent study (Acosta-Smith et al. 2018) showed the synergistic activity of lactoferrin with antibiotics that resulted in the inhibition of antibiotic-resistant Vibrio strains with increased bactericidal effect. Another study similar to ours was performed by Abdallah et al. (2017), in which antibacterial activity of camel whey protein was tested against three pathogenic bacteria in combination with plant extracts. According to their results, whey and ethanol extracts of Ballota undulata and Ruta chalepensis showed synergistic activity in the growth inhibition of E. coli, Staphylococcus aureus and Pseudomonas aeruginosa (Abdallah et al. 2017). Our study was consistent with the literature in terms of showing a higher antimicrobial activity of peptide antimicrobials when combined with plant extracts. Considering the ability of the plant extracts to target the several sites in the bacterial cell due to their phenolic content, the combinational use of ALF with RE was reasonable to provide more effective bacterial growth inhibition.
Food applications of antimicrobials
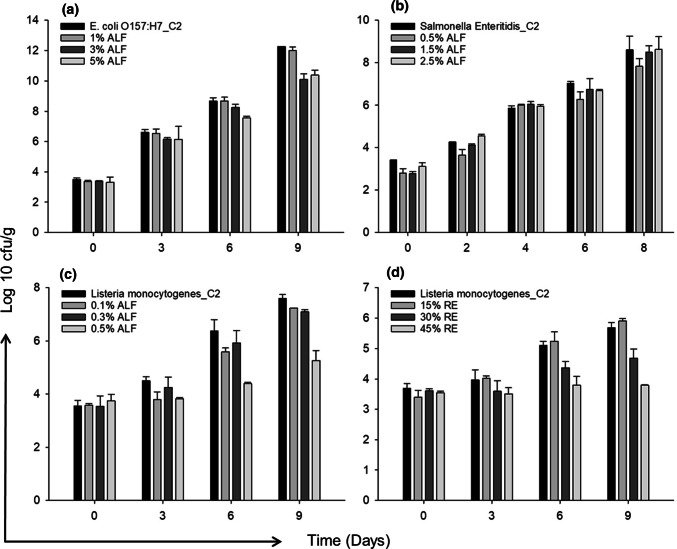
The application of ALF at the concentrations of 3% and 5% on the meat samples inoculated with E. coli O157:H7 resulted in 2 log reductions at the end of the 9-day growth period at 10 °C (Fig. 3). The meat samples that were not treated with bacteria (C1 control group) did not show any bacterial growth, therefore C1 was not included in Fig. 3. No growth reduction for S. Enteritidis on meat was observed for the ALF concentrations ranging between 0.5% and 1.5%. Only the 0.8 log reduction in S. Enteritidis growth was obtained when the ALF concentration was increased to 2.5%. Individual applications of 0.5% ALF and 45% RE for L. monocytogenes growth on meat resulted in 2 log reductions in the growth. When the growth characteristics of bacteria on meat were compared (Fig. 3), the faster growth of E. coli O157:H7 could be seen when compared to S. Enteritidis and L. monocytogenes. The slower growth of L. monocytogenes might be due to the lower incubation temperature of bacteria. Dependence of antimicrobial activity on the storage temperature and the tested strain was shown in other studies (Solomakos et al. 2008). Moreover, the lower concentrations of ALF resulted in the more log reduction in the growth of L. monocytogenes on the meat sample when compared with E. coli O157:H7 and S. Enteritidis. It was reported (Naidu 2002) that spray application of 1% ALF was effective in the sanitation of the contaminated beef surfaces form E. coli O157:H7. The application technique, purity or preparation of ALF and number of bacteria are crucial parameters leading to different results between studies.
Fig. 3.
Antimicrobial activities of tested compounds on meat samples treated with bacterial strains a ALF against E. coli O157:H7, b ALF against S. Enteritidis, c ALF against L. monocytogenes, and d RE against L. monocytogenes. C2 refers to bacterial strain inoculated meat samples
In the dipping application; MIC, three and five folds of MIC values were found as required concentrations for the reduction of the bacterial number on the meat samples. Similarly, in the study of Moreira et al. (2007), the reduction of the pathogens in food samples was provided by two, three and four folds of MIC values of the tea tree essential oil. The reason of decreased antibacterial activity of the natural antimicrobials in food applications might be related with (i) the interaction between the antimicrobials and the food components, (ii) the solubility of antimicrobial compounds on foods, or (iii) the prevention of pathogen and the antimicrobial interaction due to the rough surfaces of the foods (Moreira et al. 2007; Singh et al. 2003). To prevent this, the addition of a high amount of plant extracts into foods might be considered; however, this might result in the formation of undesired organoleptic properties. In other respects, the combinational use of the plant extracts with the antimicrobial peptides contribute to the prevention of bacterial growth in food without affecting the food quality. Moreover, the combinational usage of the plant extracts even in lower concentrations provides antioxidant activity due to their high antioxidant capacity (Singh et al. 2003).
Conclusion
To sum up, preservation of food and prevention of bacterial contamination are issues for both developing and developed countries. Use of natural components as food additives not only assure the food safety but also provides health beneficial properties. This study highlighted the individual and the synergistic antimicrobial activities of ALF and RE that could be promising for using in the food applications. Further studies might involve the determination of the effects of natural antimicrobials in vitro and in vivo on other pathogens. Toxicity levels should also be determined to confirm the safety of ALF and RE combination as food additives. Considering our encouraging results, the research on the natural compounds and the investigation of their synergistic activities might be expanded to other biotechnological applications such as development of drugs and the wound healing agents.
Acknowledgements
This study was financially supported by The Scientific and Technological Research Council of Turkey (TUBITAK-TOVAG Project No: 104O543) and Izmir Institute of Technology Research Fund Project No: 2006IYTE04.
Author’s contribution
FS, EE, and HT performed the experiments and contributed to the design of the study, to the analysis and interpretation of data. DK and EE provided the arrangement of data for the manuscript. DK and FS contributed to the writing of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ferda Soyer, Email: ferdasoyer@iyte.edu.tr, Email: ferdasoyer@gmail.com.
Deniz Keman, Email: denizkeman@iyte.edu.tr, Email: denizkeman@gmail.com.
Erdal Eroğlu, Email: erdal.eroglu@cbu.edu.tr, Email: erogluerdal@gmail.com.
Hasan Türe, Email: hasanture@odu.edu.tr.
References
- Abdallah L, Almanasrah M, Taradeh M, Khasib M, Haddad M, Jabir K. Antibacterial activity of camel whey in combination with various medicinal plant extracts. J Med Plant Stud. 2017;5:50–55. [Google Scholar]
- Acosta-Smith E, Viveros-Jiménez K, Canizalez-Román A, Reyes-Lopez M, Bolscher J, Nazmi K, Flores-Villasenor H, de la Garza M, Martinez-Garcia JJ, Valezquez-Roman J, Leon-Sicairos N. Bovine lactoferrin and lactoferrin-derived peptides inhibit the growth of Vibrio cholerae and other Vibrio species. Front Microbiol. 2018;8:2633. doi: 10.3389/fmicb.2017.02633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawaseand K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-F. [DOI] [PubMed] [Google Scholar]
- Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiace) essential oils. J Agric Food Chem. 2007;55:7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- Braden CR. Salmonella enterica serotype enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis. 2006;43:512–517. doi: 10.1086/505973. [DOI] [PubMed] [Google Scholar]
- Chen J, Tang J, Bhunia AK, Tang C, Wang C, Shi H. Development of a multi-pathogen enrichment broth for simultaneous growth of five common foodborne pathogens. J Gen Appl Microbiol. 2015;61:224–231. doi: 10.2323/jgam.61.224. [DOI] [PubMed] [Google Scholar]
- Dufour M, Simmonds RS, Bremer PJ. Development of method to quantify in vitro the synergistic activity of “natural” antimicrobials. Int J Food Sci. 2003;85:249–258. doi: 10.1016/S0168-1605(02)00544-5. [DOI] [PubMed] [Google Scholar]
- Elhariry H, Abuzaid AA, Khiralla GM, Gherbawy Y. Antibiofilm formation and anti-adhesive (to HEp-2 cells) effects of rosemary water extract against some food-related pathogens. Int J Food Sci Technol. 2014;49:1132–1141. doi: 10.1111/ijfs.12409. [DOI] [Google Scholar]
- Fernandes KE, Carter DA. The antifungal activity of lactoferrin and its derived peptides: mechanisms of action and synergy with drugs against fungal pathogens. Front Microbiol. 2017;8:2. doi: 10.3389/fmicb.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti F, Panella G, Leboffe L, Antonini G. Lactoferrin from milk: nutraceutical and pharmacological properties. Pharmaceuticals. 2016;9(4):61. doi: 10.3390/ph9040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol. 2018;9:1639. doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidpour R, Elias G, Hamidpour S. Rosmarinus officinalis (Rosemary): a novel therapeutic agent for antioxidant, antimicrobial, anticancer, antidiabetic, antidepressant, neuroprotective, anti-inflammatory, and anti-obesity treatment. Biomed Sci Tech Res. 2017;4:1098–1103. doi: 10.26717/bjstr.2017.01.000371. [DOI] [Google Scholar]
- Karav S, German JB, Rouquie C, Le Parc A, Barile D. Studying lactoferrin N-glycosylation. Int J Mol Sci. 2017;18:870. doi: 10.3390/ijms18040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieckens E, Rybarczyk J, Cox E, Vanrompay D. Antibacterial and immunomodulatory activities of bovine lactoferrin against Escherichia coli O157:H7 infections in cattle. Biometals. 2018;31:321–330. doi: 10.1007/s10534-018-0082-x. [DOI] [PubMed] [Google Scholar]
- Law JW-F, Mutalib N-SA, Chan K-G, Lee L-H. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2015;5(770):1–19. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizzi AR, Carnicelli V, Clarkson MM, Nazzicone C, Segatore B. Bovine lactoferrin and its tryptic peptides: antibacterial activity against different species. Appl Biochem Biotechnol. 2016;52:435–440. doi: 10.1134/S0003683816040116. [DOI] [Google Scholar]
- Madsen HL, Sorensen B, Skibsted LH, Bertelsen G. The antioxidative activity of summer Savory (Satureja hortensis L.) and Rosemary (Rosmarinus officinalis L.) in dressing stored exposed to light or in darkness. Food Chem. 1998;63:173–180. doi: 10.1016/S0308-8146(98)00038-7. [DOI] [Google Scholar]
- Méndez NDJH, Casanova YV, Chimbi AKG, Hernández E, Castro ALL, Diaz JMM, Monroy ZJR, Castaneda JEG. Synthetic peptides derived from bovine lactoferricin exhibit antimicrobial activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076. Molecules. 2017;22(452):1–10. doi: 10.3390/molecules22030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira MR, Ponce AG, Del Valle CE, Roura SI. Effects of clove and tea tree oils on Escherichia coli O157:H7 in blanched spinach and minced cooked beef. J Food Process Preserv. 2007;31:379–391. doi: 10.1111/j.1745-4549.2007.00135.x. [DOI] [Google Scholar]
- Naidu AS (2001) Immobilized lactoferrin antimicrobial agents and the use. U.S. patent 6,172,040 B1
- Naidu AS. Activated lactoferrin—a new approach to meat safety. Food Technol. 2002;56:40–45. [Google Scholar]
- Naidu AS, Tulpinski J, Gustilo K, Nimmagudda R, Morgan JB. Activated lactoferrin part 2: natural antimicrobial for food safety. Agro Food Ind Hi Tech. 2003;14:27–31. [Google Scholar]
- Niaz B, Saeed F, Ahmed A, Imran M, Maan AA, Khan MKI, Tufail T, Anjum FM, Hussain S, Suleria HAR. Lactoferrin (LF): a natural antimicrobial protein. Int J Food Prop. 2019;22:1626–1641. doi: 10.1080/10942912.2019.1666137. [DOI] [Google Scholar]
- Nieto G, Ros G, Castillo J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): a review. Medicines. 2018;5:1–13. doi: 10.3390/medicines5030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JR, Jesus D, Figueira LW, Oliveira FE, Soares CP, Camargo SEA, Jorge AOC, Oliveira LD. Biological activities of Rosmarinus officinalis L. (rosemary) extract as analyzed in microorganisms and cells. Exp Biol Med. 2017;242:1–10. doi: 10.1177/1535370216688571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oussalah M, Caillet S, Saucier L, Lacroix M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006;73:236–244. doi: 10.1016/j.meatsci.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Pobiega K, Krasnievska K, Derewiaka D, Gniewosz M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J Food Sci Technol. 2019;56(12):5386–5395. doi: 10.1007/s13197-019-04009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto EJ, Caro I, Villalobos-Delgado LH, Mateo J, De-Mateo-Silleras B, Redondo-Del-Rio MP. Food safety through natural antimicrobials. Antibiotics. 2019;8:1–30. doi: 10.3390/antibiotics8040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybarczyk J, Kieckens E, Vanrompay D, Cox E. In vitro and in vivo studies on the antimicrobial effect of lactoferrin against Escherichia coli O157:H7. Vet Microbiol. 2017;202:23–28. doi: 10.1016/j.vetmic.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Singh A, Singh RK, Bhunia AK, Singh N. Efficacy of plant essential oils as antimicrobial agents against Listeria monocytogenes in hotdogs. Swiss Soc Food Sci Technol. 2003;36:787–794. doi: 10.1016/S0023-6438(03)00112-9. [DOI] [Google Scholar]
- Solomakos N, Govaris A, Koidis P, Botsoglou N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008;25:120–127. doi: 10.1016/j.fm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Türe H, Eroğlu E, Soyer F, Özen B. Antifungal activity of biopolymers containing natamycin and rosemary extract against Aspergillus niger and Penicillium roquefortii. Int J Food Sci Technol. 2008;43:2026–2032. doi: 10.1111/j.1365-2621.2008.01816.x. [DOI] [Google Scholar]
- Wakabayashi H, Oda H, Yamauchi K, Abe F. Lactoferrin for prevention of common viral infections. J Infect Chemother. 2014;20:666–671. doi: 10.1016/j.jiac.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Ye M, Neetoo H, Chen H. Control of Listeria monocytogenes on ham steaks by antimicrobial incorporated into chitosan-coated plastic films. Food Microbiol. 2008;25:260–268. doi: 10.1016/j.fm.2007.10.014. [DOI] [PubMed] [Google Scholar]