Abstract
Black pepper (Piper nigrum L.), black cumin (Nigella sativa L.) and black cardamom (Amomum subulatum) are considered as important spices, seasoning and folk medicines. They have a diverse range of bioactive compounds, especially for polyphenolic compounds. These polyphenolic compounds contribute to the putative health benefits of these black spices. The purpose of this study was to identify, characterize and quantify the phenolic profile of these black spices using LC-ESI-QTOF/MS and HPLC–PDA and to access their antioxidant potential. The LC-ESI-QTOF/MS analysis led to the identification of 138 phenolic compounds in three black spices. In HPLC–PDA, the p-hydroxybenzoic acid was the most predominant phenolic acid in black pepper and black cumin while diosmin was the most abundant flavonoid in black cardamom (> 20 mg/g). Furthermore, black spices were systematically measured for their TPC, TFC and TTC followed by measurement of their antioxidant activities using DPPH, FRAP and ABTS assays. Black pepper showed the highest TPC, TFC, TTC, DPPH and ABTS activities as compared to other black spices while black cardamom exhibited the highest FRAP activity. The obtained results highlight the importance of these black spices as promising sources of phenolic compounds and they could be potentially utilized in food, feed and nutraceutical industries.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04504-4) contains supplementary material, which is available to authorized users.
Keywords: Black spices, Black pepper, Black cumin, Black cardamom, Polyphenols, LC-ESI-QTOF/MS, HPLC–PDA, Antioxidant activity
Introduction
Polyphenols are secondary metabolites originated from plants, who constitute the largest group of phytochemicals (Li et al. 2014). Phenolic compounds have an aromatic ring with one or more hydroxyl substituents. There are at least 10,000 polyphenol compounds have been identified. Among them, phenolic acids, flavonoids and tannins are regarded as the most important dietary phenolic compounds. They attracted attention due to their diverse nature of bioactivities, especially for antioxidative attributes, which could be accounted for by redox ability of phenolic compounds. Polyphenols demonstrated their antioxidant activity through two pathways, acting as radical scavengers to prevent the cellular damage which is produced by reactive oxygen species, and to prevent the generation of reactive oxygen species directly (Teodora et al. 2019). Considering safety health concerns, standards, regulations and approval of synthetic antioxidants, identification and characterization of natural polyphenols extracted from diverse food materials is a demand for researchers (Yuan-Yuan et al. 2018).
Herbs and spices were used in cooking to flavor cuisines and medicinal purposes, like treating coughs and colds for children (Carlsen et al. 2010). There has been dramatically increasing research for spices and herbs because of their strong antioxidant activity, which is crucial for reduce oxidative stress, thus preventing aging-related diseases, including heart and chronic degenerative diseases that resulted from poor eating habits and high-speed lifestyles. Apart from antioxidant property, herbs and spices possess lowering glucose activities and anti-inflammatory effect (Kaefer and Milner 2008). Spices and aromatic plants, like black pepper (Piper nigrum L.), black cardamom (Amomum subulatum) and black cumin (Nigella sativa L.) contain a wide range of bioactive compounds, including polyphenols, vitamins, and enzymes (Nazzaro et al. 2017). These bioactive compounds could be utilized in several industries for different purposes including developing functional foods, ingredients, additives in food and pharmaceutical industries to improve human health (Sagar et al. 2018).
Polyphenols constitutions and antioxidant activity in black spices can be estimated using different in vitro assays, TPC (total phenolic content), TFC (total flavonoid content), tannins assays, DPPH (2,2 diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis-3ethylbenzothiazoline-6-sulfonic acid) and FRAP (ferric reducing antioxidant power) assays. These assays are based on analyzing the ability of electron donation or free radical scavenging of samples with different mechanisms respectively (Kandi and Charles 2019). Black pepper contains ascorbic-acid, lauric-acid, linalyl-acetate, methyl-eugenol, piperine, ubiquinone flavonides, ferulic acid, piperine, phenolic amide feruperine (Suhaj 2006). The antioxidant effect of black cardamom was contributed mainly by α-terpinolene, γ-terpinene, sabinene, and thymol (Misharina 2016). Some of the phytochemicals were identified from black cumin including α-pinene, eucalyptol, linalyl anthranilate, geraniol, D-limonene and epoxy-α-terpenyl acetate (Kumar Kandikattu et al. 2017). The precise identification and quantitation of these phenolic compounds were complex because of structural diversity of polyphenols. Currently, liquid chromatography coupled with electrospray ionization—quadrupole time-of-flight and mass spectrometry (LC-ESI-QTOF/MS) is one of the latest techniques to identify and characterize polyphenols while high-performance liquid chromatography with photodiode array detector (HPLC–PDA) can be used for quantification purposes (Spinola et al. 2015).
The objective of this study was to (a) extract polyphenols from black pepper, black cardamom and black cumin (b) test whether they are anti-oxidative, and measure their antioxidant capacity, and (3) comprehensively characterize and quantify polyphenols from selected black spices by LC-ESI-QTOF/MS and HPLC. The results acquired from this study will be useful for food, feed and pharmaceutical industries.
Materials and methods
Chemicals and reagents
Most of the chemicals used for extraction and characterization were analytical grade and purchased from Sigma-Aldrich (St. Louis, MO, USA). Folin and Ciocalteu’s phenol, aluminum chloride, sodium acetate, vanillin, sulfuric acid, 2,2-diphenyl-1-picrylhy-drazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), potassium persulfate (Fe[III]Cl3•6H20), 3-ethylbenzothiazoline-6-sulphonic acid (ABTS), potassium persulfate and acetic acid solution were obtained from Sigma-Aldrich (St. Louis, MO, USA). The HPLC standards (kaempferol-3-O-glucoside, quercetin, kaempferol, diosmin, protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium carbonate (anhydrous) was obtained from Chem-Supply Pty Ltd (Mitcham, VIC, AU). Hydrated sodium acetate, methanol, hydrochloric acid, acetonitrile, anhydrous sodium acetate and glacial acetic acid were purchased from Thermo Fisher Scientific Inc (Scoresby, VIC, AU). A 98% sulfuric acid was bought from RCI Labscan (Melbourne, VIC, AU).
Sample preparation
Raw materials (black pepper, black cumin and black cardamom) used for the study were purchased from a local grocery store (Werribee Spice House, Melbourne, VIC, Australia). Samples were grounded into a fine powder by electric grinder (Sunbeam Multi Grinder—EM0405, Melbourne, VIC, AU) and stored at room temperature in dark area.
Extraction of phenolic compounds
Extracts were prepared using 30% ethanol and homogenizing with Ultra-Turrax T25 Homogenizer (IKA, Staufen, Germany) in 30% (v/v) ethanol at 10,000 rpm for 30 s followed by incubation in a ZWYR-240 incubator shaker (Labwit, Ashwood, VIC, Australia) at 120 rpm at 4 °C for 12 h. After incubation, extracts were centrifuged at 5000 rpm at 4 °C for 15 min (Hettich ROTINA 380R, Tuttlingen, Baden-Württemberg, Germany) and the supernatant was collected and stored at − 20 °C for further analysis. For HPLC analysis, the extracted samples were filtered through syringe filters (0.45 µm) bought from Sigma-Aldrich (St. Louis, MO, USA).
Estimation of polyphenols and antioxidant assays
For polyphenol estimation, TPC, TFC and TTC were measured while for antioxidant capacity, three different antioxidant assays, including DPPH, FRAP, and ABTS, were performed using the method of Gu et al. (2019). The data was obtained by the Multiskan® Go microplate photometer (Thermo Fisher Scientific, Waltham, MA, USA).
Determination of total phenolic content (TPC)
The TPC in the sample was determined by modifying the spectrophotometric method using Folin-Ciocalteu reagent (Yunfeng et al. 2018). 25 µL extract, 25 µL Folin reagent solution and 200 µL water were added in 96-well plate (Costar, Corning, NY, USA), the reaction mixture was incubated at room temperature in the dark for 5 min. Subsequently, 25 µL 10% (w:w) sodium carbonate was added and incubated the reaction mixture again for 60 min at 25 °C. The TPC was quantified from a calibration curve prepared with gallic acid standard, with concentrations ranged from 0 to 200 µg/mL. An increase in absorbance was measured at 765 nm against blank (methanol) using spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The TPC content was expressed as mg of gallic acid equivalents per gram of sample (mg GAE/g of sample).
Determination of total flavonoid content (TFC)
The aluminum chloride method (Rajurkar and Hande 2011) was used for quantification of the TFC with some modifications. 80 μL sample extract was added to a mixture solution of 2% aluminum chloride and 50 g/L sodium acetate solution, followed with 2.5 h’ incubation at 25 °C in 96-well plate in the dark. The total flavonoid content was calculated by linear regression after plotting the absorbance at 440 nm against quercetin concentration (0–50 µg/mL) and expressed as mg quercetin equivalents per gram dry material (mg QE/g of sample).
Determination of total tannins content (TTC)
Total tannins contents were determined by vanillin–sulfuric acid method with some modification (Mesfin and Won Hee 2019). 25 µL 32% sulfuric acid, 25 µL sample and 150 µL 4% vanillin solution was added to 96-well plate and incubated at room temperature for 15 min in darkness. Subsequently, the absorbance was measured at 500 nm against blank using plate reader. Catechin solution with concentration from 0 to 1 mg/mL were used for constitution of standard curve. The results were expressed as mg catechin equivalents (CE) per g of sample weight.
2,2 Diphenyl-1-picrylhydrazyl (DPPH) assay
The free-radical scavenging activity of extracts of black spices was assessed by modifying DPPH method of Ouyang et al. (2018). The DPPH radical solution was prepared by dissolving 4 mg DPPH in 100 ml methanol. 40 μL sample and 260 µL of DPPH solution were added to 96-well plate and kept at 25 °C for 30 min in the dark, absorbance of the mixture was measured at 517 nm against methanol. The calibration curve was plotted with different concentration of ascorbic acid ranging from 0 to 50 µg/mL. The results were reported as mg of ascorbic acid equivalent per gram (mg AAE/g) of sample.
Ferric reducing antioxidant power (FRAP) assay
FRAP assay is based on the reduction of Fe3+ tripyridyltriazine (TPTZ) complex (colorless complex) to Fe2+ TPTZ (blue colored complex) formed by the action of electron-donating antioxidants at low pH (Rajurkar and Hande 2011). The antioxidant capacity of different spices samples were estimated according to the previously reported method with slight modification (Rajurkar and Hande 2011). The FRAP reagent was prepared by mixing 300 mM sodium acetate solution, 10 mM TPTZ solution and 20 mM Fe[III] solution at 10:1:1. 280 μL prepared dye solution was transferred into a 96-well plate of which containing 20 μL sample, and absorbance was determined at 593 nm against blank solution after incubation at 37 °C for 10 min. From this assay, the standard curve was constructed with ascorbic acid; the concentration range was 0–50 µg/mL. The FRAP values were expressed as mg of ascorbic acid equivalent per gram of sample (mg AAE/g).
2,2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay
Free radical scavenging activity of samples was also determined by ABTS radical cation decolorization assay of Rajurkar and Hande (2011) with some modification. The ABTS+ radical stock solution was prepared by mixing 5 mL of 7 mM ABTS with 88 µL of 140 mM potassium persulfate in the dark at room temperature for 16 h. ABTS+ radical solution was then diluted with ethanol to obtain an absorbance of 0.700 at 734 nm to make dye. After adding 290 µL dye solution to 10 µL extract in a 96-well plate, the absorbance was measured after incubation at 25 °C for 6 min. The scavenging activity of spices was calculated using the calibration curve generated from ascorbic acid with concentration ranging from 0 to 2000 µg/mL. ABTS values were reported as ascorbic acid equivalents (AAE) in mg per gram of sample.
Characterization of phenolic compounds by LC-ESI-QTOF/MS analysis
Characterization of phenolic compounds of three spices was performed on an Agilent 1200 series HPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with an Agilent 6520 Accurate-Mass Q-TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA).
Analyses were conducted at 25 °C for column and 10 °C for sample, using a 250 × 4.6 mm i.d. and particle size of 4 µm reverse phase LC column (Synergi 4 µm Hydro-RP 80A Lane Cove, NSW, Australia), who is protected by a Phenomenex 4.0 × 2.0 mm i.d. C18 ODS guard column. The binary solvent system was composed of water and acetic acid solution (98:2, v/v; eluent A), acetonitrile, water and acetic acid solution (50:49.5:0.5, v/v/v; eluent B), at a flow rate of 0.8 mL/min with a sample injection volume 6 µL. Both mobile phases were degassed for 15 min at 21 °C. Elution conditions were as follows: 0 min with 10% B, 20 min with 25% B, 30 min with 35% B, 40 min with 40% B, 70 min with 55% B, 75 min with 80% B, 77 min with 100% B, 79 min with 100% B, 82–85 min with isocratic 10% B.
Peak identification was performed in both positive and negative ion modes with capillary and nozzle voltage of 3.5 kV and 500 V respectively. Nitrogen gas at a pressure of 45 psi was used as the nebulizing and drying gas, with a flow rate of 5 L/min at 300 °C, whereas sheath gas was set at 11 L/min with lower temperature, 250 °C. The mass spectra were obtained over the m/z range of 50–1300 amu. Data acquisition and processing were performed using MassHunter (Qualitative Analysis, version B.03.01, Agilent).
HPLC–PDA analysis
The quantification of targeted phenolic compounds present in spices was carried out by (Waters Alliance 2690, Chromatograph Separation Module) equipped with a photodiode array (PDA) detector. The same column and conditions described in LC-ESI-QTOF/MS analysis were remained, except for sample injection volume was 20 μL and wavelength of 280 nm, 320 nm, 370 nm were used for detection. Concentrations of individual compounds found in each sample were determined using the calibration curves generated from standards. Results were expressed as µg/g of sample. Instrument control, data acquisition and processing of the chromatographic information were accomplished by Empower Software (2010).
Statistical analysis
Results of total polyphenol content and antioxidant activity were presented as means ± standard deviation of three parallel experiments (n = 3). The significance of antioxidant properties differences between three spices was tested by the one-way analysis of variance (ANOVA), followed by Tukey’s honestly significant differences (HSD) multiple rank test at p < 0.05 using Minitab Statistical software for Windows Version 18.0 (Minitab Inc., USA).
Results and discussion
Polyphenol estimation (TPC, TFC and TTC)
The TPC of black spices were determined using the method of Folin-Ciocalteu, and TPC results were expressed as gallic acid equivalents (GAE)/g of the sample. Among spices, the TPC of black pepper was significantly higher than other two black spices (p < 0.05), with 5.46 ± 0.01 mg GAE/g, which was approximately two times higher than black cumin (2.79 ± 0.01 mg GAE/g) (Table 1). The total polyphenol contents of three black spices were in the order of black pepper > black cardamom > black cumin. Considerable differences in the TPC values among different spices had already been reported in twenty different spices, ranging from 12.03 to 22.88 mg GAE/g, which was much higher than our black spices (Soňa et al. 2017). It was widely accepted that the geographical environment and harvest time could influence the contents of spices polyphenols (Soňa et al. 2017).
Table 1.
Total polyphenols content and antioxidant activities of black pepper, black cumin and black cardamom
| Antioxidant assays | Black pepper | Black cumin | Black cardamom |
|---|---|---|---|
| TPC (mg GAE/g) | 5.46 ± 0.01a | 2.79 ± 0.01c | 4.11 ± 0.01b |
| TFC (mg QE/g) | 3.97 ± 0.01a | 0.41 ± 0.01c | 0.73 ± 0.01b |
| Tannins (mg CE/g) | 2.88 ± 0.01a | 0.86 ± 0.01b | 2.18 ± 0.03a |
| DPPH (mg AAE/g) | 1.19 ± 0.01a | 0.28 ± 0.01c | 0.75 ± 0.01b |
| FRAP (mg AAE/g) | 0.70 ± 0.01b | 0.19 ± 0.01c | 1.53 ± 0.01a |
| ABTS (mg AAE/g) | 7.05 ± 0.01a | 3.85 ± 0.01c | 6.16 ± 0.01b |
The data are shown as mean ± standard deviation (n = 3)
GAE gallic acid equivalents, QE quercetin equivalents, CE catechin equivalents, AAE ascorbic acid equivalents
a,b,cThe means in a row with significant difference (p < 0.05) using a one-way analysis of variance (ANOVA) and Tukey’s test
The TFC values in the black species were varied significantly from 0.41 ± 0.01 mg QE/g to 3.97 ± 0.01 mg QE/g. Among three black spices, the most abundant flavonoid compounds were found in black pepper (3.97 ± 0.01 mg QE/g), followed by black cardamom (0.73 ± 0.01 mg QE/g) and black cumin (0.41 ± 0.01 mg QE/g). The TFC of our black cumin sample was almost 2 times highrer than an Indian raw black cumin seeds, could be due to varietal difference or solvent extraction ratio (Liang et al. 2018). However, the TFC of black cumin was also compared with cumin seeds from South Korea (2.06 mg QE/g) (Assefa et al. 2018). Moreover, the TFC of South Korean cardamom pods (0.71 mg QE/g) was also similar to that of our black cardamom (Assefa et al. 2018).
Regarding total tannins in our selected three black spices, black pepper (2.88 ± 0.01 mg CE/g) had a higher level of tannins followed by black cardamom (2.18 ± 0.03 mg CE/g) and black cumin (0.86 ± 0.01 mg CE/g). The tannins in our black spices were lower than previously reported in one of the Indian cumin seeds (80.23 mg CE/g) (Bettaieb Rebey et al. 2012). It was highly possible that different extraction solvents contributed to the different extractability due to polarity differences of solvents, growing and agronomical conditions (Pitchaon et al. 2007).
Antioxidant activities (DPPH, FRAP and ABTS)
Antioxidant potential of three black spices was determined by DPPH, FRAP and ABTS assays, and the antioxidant activity was expressed as mg equivalents of ascorbic acid (AAE) per gram of sample.
The DPPH values of three spices varied from 0.28 to 1.19 mg AAE/g, with statistically significant difference (p < 0.05). The highest DPPH value was recorded in black pepper (1.19 mg AAE/g), followed by black cardamom (0.75 mg AAE/g) and black cumin (0.28 mg AAE/g). Previously, the DPPH values of 20 different pepper spices grown in Vietnam, India and Indonesia had been reported, ranging from 6.79 to 15.81 mg AAE/g, which was much higher than our black spices (Soňa et al. 2017). However, black cardamom in this study showed similar DPPH activity to that of South Korea cardamom (0.83 mg AAE/g) (Assefa et al. 2018).
FRAP assay was also conducted to provide comprehensive information on the antioxidant capacity of three black spices, since antioxidants with different mechanisms contributed to the antioxidant properties of spices (Nikolic et al. 2019). The FRAP assay is based on the reducing reaction of TPTZ complex to TPTZ complex, and it estimates the total concentrate of redox-active compounds, excepted thiol antioxidants (Konczak et al. 2010). The FRAP activity in three black species varied significantly from 0.19 to 1.53 mg AAE/g; the highest FRAP capacity was found in black cardamom (1.53 ± 0.01 mg AAE/g). The FRAP values of three black spices were within the range of Serbian’s black spices (0.14–2.40 mg AAE/g) (Nikolic et al. 2019).
Regarding ABTS, three black spices showed stronger antioxidant capacities measured by ABTS as compared to DPPH and FRAP assays. The ABTS antioxidant power was measured by dye’s decolorization ability (Breksa et al. 2010). Black pepper (7.05 ± 0.01 mg AAE/g) had significantly higher antioxidant properties than black cumin (3.85 ± 0.01 mg AAE/g). The ABTS+ radical scavenging activity of our three black spices was comparatively higher than that of Korean black pepper (3.34 mg AAE/g), cumin (3.199 mg AAE/g) and cardamom (1.09 mg AAE/g), the discrepancy could be explained by different sample preparation method (Assefa et al. 2018).
LC-ESI-QTOF/MS characterization of the phenolic compounds
An untargeted qualitative characterization of phenolic compounds in black pepper, black cumin and black cardamom was employed by LC-ESI-QTOF/MS in both negative and positive ionization modes (Figure 1S & 2S, Supplementary Material). The LC-ESI-QTOF/MS identified compounds with more than 80 library identification score were selected firstly, among them, compounds with a mass error less than ± 10 ppm were further selected for characterization and m/z verification (Table 1S–3S, Supplementary Material).
A total of 138 compounds were detected and tentatively characterized in black pepper, black cumin and black cardamom (Table 2). Eight polyphenol classes were tentatively identified in three black spices samples, while stilbenes were only found in black cumin and non-phenolic metabolites only presented in black pepper and black cardamom. Flavonoids and phenolic acids were the key phenolic compounds among all samples. In flavonoids, flavonol was the predominant subclass in black cardamom, while isoflavonoids and anthocyanins were the major subclasses for black cumin and black pepper respectively. For phenolic acids, hydroxycinnamic acids were main phenolic acids in all samples. Phenolic acids and flavonoids were reported as a main sources of antioxidant activities in spices (Konczak et al. 2010). According to our knowledge and systematic literature search, we identified 52 new compounds that were not previously identified in these three black species although they were found in different medicinal plants, fruits and vegetables, mentioned in Table 2.
Table 2.
Qualitative characterization of phenolic compounds in black pepper, black cumin and black cardamom by LC-ESI-QTOF/MS in positive and negative ionization modes
| Peak no. | Proposed compound | Molecular formula | RT (min) | Ionization mode | Molecular weight | Theoretical (m/z) | Observed (m/z) | Mass error (ppm) | Sample name |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||||
| Hydroxycinnamic acids | |||||||||
| 1 | Cinnamic acid | C9H8O2 | 9.169 | [M + H]+ | 148.0524 | 149.0597 | 149.0590 | − 4.70 | BCM2 |
| 2 | 3-Sinapoylquinic acid | C18H22O10 | 9.655 | [M − H]− | 398.1213 | 397.1140 | 397.1144 | 0.50 | BP2 |
| 3 | Caffeic acid 3-O-glucuronide | C15H16O10 | 11.306 | [M − H]− | 356.0743 | 355.0670 | 355.0698 | 7.89 | BCM2 |
| 4 | 1,2-Disinapoylgentiobiose | C34H42O19 | 13.655 | [M + H]+ | 754.2320 | 755.2393 | 755.2400 | 0.93 | BP2 |
| 5 | Ferulic acid 4-O-glucoside | C16H20O9 | 18.048 | [M − H]− | 356.1107 | 355.1034 | 355.1062 | 7.89 | BCM2 |
| 6 | Isoferulic acid | C10H10O4 | 18.197 | [M + H]+ | 194.0579 | 195.0652 | 195.0645 | − 3.59 | BCM2 |
| 7 | Caffeic acid 3-sulfate | C9H8O7S | 19.522 | [M − H]− | 259.9991 | 258.9918 | 258.9938 | 7.72 | BCM2 |
| 8 | Chlorogenic acid | C16H18O9 | 19.884 | [M + H]+ | 354.0951 | 355.1024 | 355.1010 | − 3.94 | *BP2, BCM2 |
| 9 | 3-O-Methylrosmarinic acid | C19H18O8 | 20.069 | [M + H]+ | 374.1002 | 375.1075 | 375.1070 | − 1.33 | BCM1 |
| 10 | Feruloyl tartaric acid | C14H14O9 | 22.145 | [M − H]− | 326.0638 | 325.0565 | 325.0583 | 5.54 | BCD1 |
| 11 | p-Coumaroyl glycolic acid | C11H10O5 | 25.038 | [M + H]+ | 222.0528 | 223.0601 | 223.0596 | − 2.24 | BCM2 |
| 12 | p-Coumaroyl malic acid | C13H12O7 | 25.375 | [M − H]− | 280.0583 | 279.0510 | 279.0511 | 0.36 | BP1 |
| 13 | 3,4-O-Dimethylgallic acid | C9H10O5 | 25.375 | [M − H]− | 198.0528 | 197.0455 | 197.0474 | 9.64 | BP1 |
| 14 | Avenanthramide 2f | C17H15NO6 | 33.409 | [M − H]− | 329.0899 | 328.0826 | 328.0816 | − 3.05 | BP2 |
| 15 | Caffeic acid | C9H8O4 | 19.146 | *[M − H]−/[M + H]+ | 180.0423 | 179.0350 | 179.0349 | − 0.56 | BP2, *BCD2 |
| 16 | m-Coumaric acid | C9H8O3 | 34.353 | [M − H]− | 164.0473 | 163.0400 | 163.0416 | 9.81 | BCD1 |
| 17 | p-Coumaroyl tyrosine | C18H17NO5 | 35.074 | [M + H]+ | 327.1107 | 328.1180 | 328.1170 | − 3.05 | BP1 |
| 18 | Sinapic acid | C11H12O5 | 35.988 | [M − H]− | 224.0685 | 223.0612 | 223.0621 | 4.03 | BCM2 |
| 19 | p-Coumaroyl tartaric acid | C13H12O8 | 49.461 | [M − H]− | 296.0532 | 295.0459 | 295.0483 | 8.13 | *BP1, BCM1 |
| 20 | p-Coumaric acid ethyl ester | C11H12O3 | 81.116 | [M − H]− | 192.0786 | 191.0713 | 191.0715 | 1.05 | BCD1 |
| 21 | Rosmarinic acid | C18H16O8 | 81.838 | [M + H]+ | 360.0845 | 361.0918 | 361.0920 | 0.55 | BCD2 |
| Hydroxybenzoic acids | |||||||||
| 22 | Vanillicacid 4-sulfate | C8H8O7S | 8.921 | [M − H]− | 247.9991 | 246.9918 | 246.9937 | 7.69 | BCM1 |
| 23 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 9.092 | [M − H]− | 316.0794 | 315.0721 | 315.0724 | 0.95 | BP1 |
| 24 | Protocatechuic acid | C7H6O4 | 12.289 | [M − H]− | 154.0266 | 153.0193 | 153.0205 | 7.84 | *BP2, BCM2 |
| 25 | 4-O-Methylgallic acid | C8H8O5 | 14.359 | *[M − H]−/[M + H]+ | 184.0372 | 183.0299 | 183.0301 | 1.09 | *BP2, BCM2 |
| 26 | Hippuricacid | C9H9NO3 | 14.569 | [M + H]+ | 179.0582 | 180.0655 | 180.0643 | − 6.66 | BCM2 |
| 27 | p-Hydroxybenzoic acid | C7H6O3 | 19.903 | [M − H]− | 138.0317 | 137.0244 | 137.0257 | 9.49 | BP2, *BCM2 |
| 28 | Paeoniflorin | C23H28O11 | 66.567 | [M − H]− | 480.1632 | 479.1559 | 479.1570 | 2.30 | BCM2 |
| Hydroxyphenylacetic acids | |||||||||
| 29 | 3,4-Dihydroxyphenylacetic acid | C8H8O4 | 23.735 | *[M − H]−/[M + H]+ | 168.0423 | 167.0350 | 167.0360 | 5.99 | *BP2, BCM2, BCD2 |
| 30 | 5-(3’,4’-dihydroxyphenyl)-valeric acid | C11H14O4 | 26.799 | [M − H]− | 210.0892 | 209.0819 | 209.0837 | 8.61 | BCD1 |
| 31 | 2-Hydroxy-2-phenylacetic acid | C8H8O3 | 31.794 | [M + H]+ | 152.0473 | 153.0546 | 153.0541 | − 3.27 | *BP1, BCD1 |
| 32 | Phenacetylglycine | C10H11NO3 | 32.249 | [M − H]− | 193.0739 | 192.0666 | 192.0675 | 4.69 | BCD2 |
| Hydroxyphenylpropanoic acids | |||||||||
| 33 | Dihydroferuloylglycine | C12H15NO5 | 26.826 | [M + H]+ | 253.0950 | 254.1023 | 254.1012 | − 4.33 | BCD1 |
| 34 | 3-Methoxyacetophenone | C9H10O2 | 77.515 | [M + H]+ | 150.0681 | 151.0754 | 151.0754 | 0.00 | BCD1 |
| 35 | 3-Hydroxyphenylpropionic acid | C9H10O3 | 79.587 | [M + H]+ | 166.0630 | 167.0703 | 167.0693 | − 5.99 | *BCM2, BCD2 |
| Hydroxyphenylpentanoic acids | |||||||||
| 36 | 3-Hydroxyphenylvaleric acid | C11H14O3 | 28.199 | [M + H]+ | 194.0943 | 195.1016 | 195.1017 | 0.51 | *BP1, BCD1 |
| Flavonoids | |||||||||
| Flavonols | |||||||||
| 37 | Kaempferol 7-O-glucoside | C21H19O11 | 8.246 | [M − H]− | 447.0927 | 446.0854 | 446.0852 | − 0.45 | BCD2 |
| 38 | Myricetin | C15H10O8 | 19.405 | [M + H]+ | 318.0376 | 319.0449 | 319.0456 | 2.19 | BCD2 |
| 39 | Kaempferol 3-O-glucosyl-rhamnosyl-galactoside | C33H40O20 | 26.244 | [M + H]+ | 756.2113 | 757.2186 | 757.2178 | − 1.06 | *BP2, BCM2, BCD2 |
| 40 | Patuletin 3-O-glucosyl-(1- > 6)-[apiosyl(1- > 2)]-glucoside | C33H40O22 | 29.974 | [M + H]+ | 788.2011 | 789.2084 | 789.2046 | − 4.81 | *BCM2, BCD2 |
| 41 | Kaempferol 3,7-O-diglucoside | C27H30O16 | 32.641 | [M − H]−/*[M + H]+ | 610.1534 | 611.1607 | 611.1581 | − 4.25 | *BCM1, BCD1 |
| 42 | Kaempferol 3,7,4’-O-triglucoside | C33H40O21 | 32.658 | [M − H]−/*[M + H]+ | 772.2062 | 773.2135 | 773.2114 | − 2.72 | *BCM1, BCD1 |
| 43 | Myricetin 3-O-rhamnoside | C21H20O12 | 39.662 | [M + H]+ | 464.0955 | 465.1028 | 465.1036 | 1.72 | *BP2, BCM1, BCD1 |
| 44 | Patuletin 3-O-(2’’-feruloylglucosyl)(1- > 6)-[apiosyl(1- > 2)]-glucoside | C43H48O25 | 32.691 | [M − H]− | 964.2485 | 963.2412 | 963.2410 | − 0.21 | BCM1 |
| 45 | Kaempferol 3-O-(2’’-rhamnosyl-galactoside) 7-O-rhamnoside | C33H40O19 | 41.733 | [M + H]+ | 740.2164 | 741.2237 | 741.2247 | 1.35 | *BP1, BCD1 |
| 46 | Isorhamnetin 3-O-glucoside7-O-rhamnoside | C28H32O16 | 52.144 | [M − H]− | 624.1690 | 623.1617 | 623.1620 | 0.48 | BP1 |
| Anthocyanins | |||||||||
| 47 | Malvidin 3,5-O-diglucoside | C29H35O17 | 6.750 | [M + H]+ | 655.1874 | 656.1947 | 656.1945 | − 0.30 | BCM1 |
| 48 | Delphinidin 3-O-glucosyl-glucoside | C27H31O17 | 15.088 | [M − H]− | 627.1561 | 626.1488 | 626.1497 | 1.44 | *BP1, BCM1 |
| 49 | Cyanidin 3,5-O-diglucoside | C27H31O16 | 23.238 | [M − H]− | 611.1612 | 610.1539 | 610.1531 | − 1.31 | *BP2, BCD2 |
| 50 | Peonidin 3-O-sambubioside-5-O-glucoside | C33H41O20 | 26.088 | [M − H]− | 757.2191 | 756.2118 | 756.2130 | 1.59 | *BP1, BCM1 |
| 51 | Cyanidin 3-O-rutinoside | C27H31O15 | 26.800 | [M − H]− | 595.1663 | 594.1590 | 594.1587 | − 0.50 | *BP2, BCD2 |
| 52 | Delphinidin 3-O-glucoside | C21H21O12 | 29.516 | [M − H]− | 465.1033 | 464.0960 | 464.0955 | − 1.08 | BCD1 |
| 53 | Cyanidin 3-O-diglucoside-5-O-glucoside | C33H41O21 | 32.575 | [M − H]− | 773.2140 | 772.2067 | 772.2091 | 3.11 | BCM1 |
| 54 | Pelargonidin 3-O-sambubioside | C26---H29O14 | 43.647 | [M − H]− | 565.1557 | 564.1484 | 564.1485 | 0.18 | BP1 |
| 55 | Cyanidin 3-O-(2-O-(6-O-(E)-caffeoyl-Dglucoside)-D-glucoside)-5-O-D-glucoside | C43H49O24 | 43.84 | [M − H]− | 949.2614 | 948.2541 | 948.2547 | 0.63 | BCM1 |
| 56 | Peonidin 3-O-rutinoside | C28H33O15 | 46.512 | *[M − H]−/[M + H]+ | 609.1819 | 608.1746 | 608.1730 | − 2.63 | *BP2, BCM2, BCD2 |
| 57 | Pelargonidin 3-O-rutinoside | C27H31O14 | 53.900 | [M − H]− | 579.1714 | 578.1641 | 578.1635 | − 1.04 | BP2 |
| Isoflavonoids | |||||||||
| 58 | 3’,4’,7-Trihydroxyisoflavan | C15H14O4 | 6.618 | [M − H]− | 258.0892 | 257.0819 | 257.0808 | − 4.28 | BCM1 |
| 59 | Sativanone | C17H16O5 | 13.705 | [M + H]+ | 300.0998 | 301.1071 | 301.1077 | 1.99 | BP2 |
| 60 | 3’-O-Methylequol | C16H16O4 | 16.855 | [M + H]+ | 272.1049 | 273.1122 | 273.1138 | 5.86 | BCM1 |
| 61 | 2’-Hydroxyformononetin | C16H12O5 | 18.710 | [M + H]+ | 284.0685 | 285.0758 | 285.0740 | − 6.31 | BCM1 |
| 62 | 3’,4’,7-Trihydroxyisoflavanone | C15H12O5 | 21.673 | [M + H]+ | 272.0685 | 273.0758 | 273.0750 | − 2.93 | *BP2, BCM2 |
| 63 | 6’’-O-Acetyldaidzin | C23H22O10 | 25.055 | [M − H]− | 458.1213 | 457.1140 | 457.1126 | − 3.06 | BCM1 |
| 64 | 3’,4’,5,7-Tetrahydroxyisoflavanone | C15H12O6 | 26.645 | [M + H]+ | 288.0634 | 289.0707 | 289.0712 | 1.73 | BCM1 |
| 65 | 6’’-O-Acetylgenistin | C23H22O11 | 26.990 | [M + H]+ | 474.1162 | 475.1235 | 475.1213 | − 4.63 | BP1 |
| 66 | Irilone | C16H10O6 | 29.923 | [M + H]+ | 298.0477 | 299.0550 | 299.0530 | − 6.69 | BCD2 |
| 67 | Quercetin | C15H10O7 | 67.231 | [M + H]+ | 302.0427 | 303.0500 | 303.0507 | 2.31 | BP2, *BCM1, BCD1 |
| 68 | 3’-Hydroxymelanettin | C16H12O6 | 46.671 | [M + H]+ | 300.0634 | 301.0707 | 301.0694 | − 4.32 | BCD2 |
| 69 | 3’-Hydroxydaidzein | C15H10O5 | 52.235 | [M + H]+ | 270.0528 | 271.0601 | 271.0592 | − 3.32 | BP2 |
| 70 | 4’-Methoxy-2’,3,7-trihydroxyisoflavanone | C16H14O6 | 66.291 | [M − H]− | 302.0790 | 301.0717 | 301.0742 | 8.30 | BCD1 |
| 71 | Kaempferol | C15H10O6 | 79.891 | *[M − H]−/[M + H]+ | 286.0477 | 285.0404 | 285.0411 | 2.46 | *BP2, BCM2, BCD2 |
| Flavones | |||||||||
| 72 | Cirsilineol | C18H16O7 | 18.694 | [M − H]− | 344.0896 | 343.0823 | 343.0839 | 4.66 | BCM2 |
| 73 | Luteolin 7-O-glucuronide | C21H18O12 | 22.145 | [M − H]− | 462.0798 | 461.0725 | 461.0734 | 1.95 | BCD1 |
| 74 | Gardenin B | C19H18O7 | 25.088 | [M + H]+ | 358.1053 | 359.1126 | 359.1120 | − 1.67 | BCM2 |
| 75 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 26.990 | [M − H]−/*[M + H]+ | 594.1585 | 595.1658 | 595.1633 | − 4.20 | *BP2, BCM2, BCD2 |
| 76 | Diosmin | C28H32O15 | 46.512 | *[M − H]− | 608.1741 | 607.1668 | 607.1693 | 4.12 | *BP2, BCM2, BCD2 |
| 77 | Apigenin 7-O-apiosyl-glucoside | C26H28O14 | 31.613 | [M + H]+ | 564.1479 | 565.1552 | 565.1557 | 0.88 | BCD2 |
| 78 | Kaempferol-3-glucoside | C21H20O11 | 45.874 | *[M + H]+ | 448.1006 | 449.1079 | 449.1076 | − 0.67 | BP2, *BCM1, BCD2 |
| 79 | Isorhoifolin | C27H30O14 | 35.306 | [M + H]+ | 578.1636 | 579.1709 | 579.1688 | − 3.63 | *BP2, BCD2 |
| 80 | Apigenin 6-C-glucoside | C21H20O10 | 35.289 | [M + H]+ | 432.1056 | 433.1129 | 433.1129 | 0.00 | *BP2, BCD2 |
| 81 | Chrysoeriol 7-O-glucoside | C22H22O11 | 46.604 | [M + H]+ | 462.1162 | 463.1235 | 463.1235 | 0.00 | BCD1 |
| Flavanones | |||||||||
| 82 | 6-Geranylnaringenin | C25H28O5 | 7.584 | [M − H]− | 408.1937 | 407.1864 | 407.1882 | 4.42 | *BP1, BCD1 |
| 83 | Naringin | C27H32O14 | 41.270 | [M + H]+ | 580.1792 | 581.1865 | 581.1842 | − 3.96 | BCD2 |
| 84 | Hesperidin | C28H34O15 | 45.477 | [M + H]+ | 610.1898 | 611.1971 | 611.1967 | − 0.65 | *BP2, BCD2 |
| Flavanols | |||||||||
| 85 | 3’-O-Methyl-(-)-epicatechin7-O-glucuronide | C22H24O12 | 42.246 | [M + H]+ | 480.1268 | 481.1341 | 481.1323 | − 3.74 | BP1 |
| 86 | 3’-O-Methylcatechin | C16H16O6 | 52.916 | [M + H]+ | 304.0947 | 305.1020 | 305.1001 | − 6.23 | BCD1 |
| Dihydrochalcones | |||||||||
| 87 | 3-Hydroxyphloretin2’-O-xylosyl-glucoside | C26H32O15 | 30.113 | [M − H]− | 584.1741 | 583.1668 | 583.1700 | 5.49 | BP1 |
| 88 | Phloretin 2’-O-xylosyl-glucoside | C26H32O14 | 43.061 | [M + H]+ | 568.1792 | 569.1865 | 569.1865 | 0.00 | BCM1 |
| Dihydroflavonols | |||||||||
| 89 | Dihydroquercetin 3-O-rhamnoside | C21H22O11 | 20.268 | [M − H]− | 450.1162 | 449.1089 | 449.1115 | 5.79 | BCM2 |
| Lignans | |||||||||
| Lignans | |||||||||
| 90 | Schisandrin C | C22H24O6 | 15.845 | [M − H]− | 384.1573 | 383.1500 | 383.1483 | − 4.44 | BCM2 |
| 91 | 7-Hydroxysecoisolariciresinol | C22H30O5 | 29.262 | [M + H]+ | 374.2093 | 375.2166 | 375.2198 | 8.53 | BCM1 |
| 92 | Sesaminol 2-O-triglucoside | C36H46O22 | 34.365 | [M − H]− | 830.2481 | 829.2408 | 829.2393 | − 1.81 | BCM1 |
| 93 | 7-Oxomatairesinol | C20H20O7 | 39.648 | [M + H]+ | 372.1209 | 373.1282 | 373.1283 | 0.27 | BCM2 |
| 94 | 1-Acetoxypinoresinol | C22H24O8 | 50.065 | [M + H]+ | 416.1471 | 417.1544 | 417.1542 | − 0.48 | BP2 |
| 95 | Lariciresinol-sesquilignan | C30H36O10 | 52.536 | [M − H]− | 556.2308 | 555.2235 | 555.2265 | 5.40 | BCM2 |
| 96 | Episesamin | C20H18O6 | 62.591 | [M − H]− | 354.1103 | 353.1030 | 353.1055 | 7.08 | BCM2 |
| 97 | Arctigenin | C21H24O6 | 74.697 | [M + H]+ | 372.1573 | 373.1646 | 373.1640 | − 1.61 | BP2 |
| 98 | Schisandrin | C24H32O7 | 76.735 | [M + H]+ | 432.2148 | 433.2221 | 433.2219 | − 0.46 | BP2 |
| 99 | Schisanhenol | C23H30O6 | 76.901 | [M + H]+ | 402.2042 | 403.2115 | 403.2111 | − 0.99 | BP2 |
| 100 | Cyclolariciresinol | C20H24O6 | 77.398 | [M − H]−/*[M + H]+ | 360.1573 | 361.1646 | 361.1645 | − 0.28 | *BP2, BCM2 |
| 101 | Conidendrin | C20H20O6 | 77.515 | [M + H]+ | 356.1260 | 357.1333 | 357.1321 | − 3.36 | BCD2 |
| 102 | Matairesinol | C20H22O6 | 80.819 | [M − H]− | 358.1416 | 357.1343 | 357.1336 | − 1.96 | BP2 |
| 103 | Dimethylmatairesinol | C22H26O6 | 81.274 | [M + H]+ | 386.1729 | 387.1802 | 387.1787 | − 3.87 | BP1 |
| 104 | Schisandrin B | C23H28O6 | 81.357 | [M + H]+ | 400.1886 | 401.1959 | 401.1948 | − 2.74 | BP2 |
| Other polyphenols | |||||||||
| Tyrosols | |||||||||
| 105 | Hydroxytyrosol 4-O-glucoside | C14H20O8 | 9.671 | [M − H]− | 316.1158 | 315.1085 | 315.1113 | 8.89 | *BP2, BCM2 |
| 106 | Hydroxytyrosol | C8H10O3 | 9.832 | [M − H]− | 154.0630 | 153.0557 | 153.0547 | − 6.53 | BCM2 |
| 107 | Oleosidedimethylester | C18H26O11 | 14.470 | [M − H]− | 418.1475 | 417.1402 | 417.1421 | 4.55 | BCM1 |
| 108 | Oleuropein | C25H32O13 | 21.880 | [M − H]− | 540.1843 | 539.1770 | 539.1798 | 5.19 | BCD2 |
| 109 | 3,4-DHPEA-AC | C10H12O4 | 23.255 | *[M − H]−/[M + H]+ | 196.0736 | 195.0663 | 195.0672 | 4.61 | *BP2, BCM2, BCD2 |
| 110 | Ligstroside-aglycone | C19H22O7 | 30.609 | [M − H]− | 362.1366 | 361.1293 | 361.1312 | 5.26 | BCD2 |
| 111 | p-HPEA-AC | C10H12O3 | 81.340 | [M − H]−/*[M + H]+ | 180.0786 | 181.0859 | 181.0852 | − 3.87 | *BP2, BCD2 |
| Hydroxycoumarins | |||||||||
| 112 | Scopoletin | C10H8O4 | 11.836 | [M − H]−/*[M + H]+ | 192.0423 | 193.0496 | 193.0487 | − 4.66 | *BCM2, BCD2 |
| 113 | Mellein | C10H10O3 | 19.014 | *[M − H]−/[M + H]+ | 178.0630 | 177.0557 | 177.0566 | 5.08 | *BP2, BCD2 |
| 114 | Coumarin | C9H6O2 | 26.660 | [M + H]+ | 146.0368 | 147.0441 | 147.0432 | − 6.12 | BCD2 |
| 115 | 4-Hydroxycoumarin | C9H6O3 | 49.073 | [M + H]+ | 162.0317 | 163.0390 | 163.0379 | − 6.75 | BCD2 |
| 116 | Esculetin | C9H6O4 | 78.510 | [M + H]+ | 178.0266 | 179.0339 | 179.0335 | − 2.23 | BCM2 |
| Hydroxybenzaldehydes | |||||||||
| 117 | p-Anisaldehyde | C8H8O2 | 24.514 | *[M − H]− | 136.0524 | 135.0451 | 135.0448 | − 2.22 | *BP2, BCM2, BCD2 |
| Hydroxybenzoketones | |||||||||
| 118 | 3-Hydroxy-3-(3-hydroxyphenyl) propionicacid | C9H10O4 | 33.766 | [M + H]+ | 182.0579 | 183.0652 | 183.0645 | − 3.82 | BCD1 |
| 119 | 2,3-Dihydroxy-1-guaiacylpropanone | C10H12O5 | 50.529 | [M + H]+ | 212.0685 | 213.0758 | 213.0751 | − 3.29 | *BP1, BCD1 |
| Hydroxyphenylpropenes | |||||||||
| 120 | Acetyleugenol | C12H14O3 | 62.774 | [M − H]− | 206.0943 | 205.0870 | 205.0884 | 6.83 | BCM2 |
| Alkylphenols | |||||||||
| 121 | 3-Methylcatechol | C7H8O2 | 10.566 | *[M − H]−/[M + H]+ | 124.0524 | 123.0451 | 123.0455 | 3.25 | *BP2, BCM2, BCD2 |
| 122 | 4-Vinylphenol | C8H8O | 34.353 | [M − H]− | 120.0575 | 119.0502 | 119.0512 | 8.40 | BCD1 |
| Alkylmethoxyphenols | |||||||||
| 123 | 4-Ethylguaiacol | C9H12O2 | 19.125 | [M − H]− | 152.0837 | 151.0764 | 151.0774 | 6.62 | *BCM2, BCD2 |
| Naphtoquinones | |||||||||
| 124 | 1,4-Naphtoquinone | C10H6O2 | 26.380 | [M + H]+ | 158.0368 | 159.0441 | 159.0444 | 1.89 | BCM2 |
| 125 | Juglone | C10H6O3 | 82.367 | [M + H]+ | 174.0317 | 175.0390 | 175.0396 | 3.43 | *BP2, BCM2 |
| Phenolic terpenes | |||||||||
| 126 | Rosmanol | C20H26O5 | 34.563 | [M + H]+ | 346.1780 | 347.1853 | 347.1834 | − 5.47 | BCM2 |
| 127 | Thymol | C10H14O | 69.678 | [M + H]+ | 150.1045 | 151.1118 | 151.1113 | − 3.31 | BP2 |
| Curcuminoids | |||||||||
| 128 | Demethoxycurcumin | C20H18O5 | 47.666 | [M + H]+ | 338.1154 | 339.1227 | 339.1227 | 0.00 | BCM2 |
| Other polyphenols | |||||||||
| 129 | Pyrogallol | C6H6O3 | 8.788 | [M + H]+ | 126.0317 | 127.0390 | 127.0389 | − 0.79 | *BCM2, BCD2 |
| 130 | 3,4-Dihydroxyphenylglycol | C8H10O4 | 9.003 | [M + H]+ | 170.0579 | 171.0652 | 171.0651 | − 0.58 | *BCM2, BCD2 |
| 131 | Catechol | C6H6O2 | 17.551 | [M + H]+ | 110.0368 | 111.0441 | 111.0435 | − 5.40 | BCM2 |
| 132 | Arbutin | C12H16O7 | 21.772 | [M + H]+ | 272.0896 | 273.0969 | 273.0951 | − 6.59 | BP2 |
| 133 | Isopropyl 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate | C12H16O5 | 29.582 | [M − H]− | 240.0998 | 239.0925 | 239.0919 | − 2.51 | BCD1 |
| 134 | Salvianolic acid C | C26H20O10 | 43.971 | [M + H]+ | 492.1056 | 493.1129 | 493.1104 | − 5.07 | BCD2 |
| Non-phenolic metabolites | |||||||||
| 135 | Vanilloylglycine | C10H11NO5 | 6.541 | [M − H]− | 225.0637 | 224.0564 | 224.0563 | − 0.45 | BP2 |
| 136 | 1,3,5-Trimethoxybenzene | C9H12O3 | 57.471 | [M + H]+ | 168.0786 | 169.0859 | 169.0844 | − 8.87 | BCD2 |
| Stilbenes | |||||||||
| Stilbenes | |||||||||
| 137 | 3’-Hydroxy-3,4,5,4’-tetramethoxystilbene | C17H18O5 | 27.192 | [M − H]− | 302.1154 | 301.1081 | 301.1100 | 6.31 | BCM2 |
| 138 | Resveratrol | C14H12O3 | 17.435 | [M + H]+ | 228.0786 | 229.0859 | 229.0869 | 4.37 | BCM2 |
*Example sample used for the LC-ESI-QTOF/MS parameters gathering for each phenolic compound in the selected mode
BP black pepper, BCM black cumin, BCD Black cardamom
1Compounds identified first time in a particular black specie sample but already been reported in other plant materials including fruits, vegetables and medicinal plants
2Compounds already been identified in a particular black specie sample
Phenolic acids
Phenolic acids were detected and characterized in all three black spices. In the present work, we tentatively characterized 5 subclasses, among these phenolic acids, two subclasses were all detected in three black spices (hydroxycinnamic acids and hydroxyphenylacetic acids), hydroxybenzoic acids and hydroxyphenylpentanoic acids were tentatively identified in both black pepper, while hydroxyphenylpropanoic acids were tentatively characterized in black cardamom and black cumin. Kanti Bhooshan and Syed Ibrahim (2009) reported that hydroxycinnamic acids are more common than hydroxybenzoic acids in most of the plant food. In this study, we tentatively characterized 21 different hydroxycinnamic acids and 8 hydroxybenzoic acids in three black spices.
Hydroxycinnamic acids
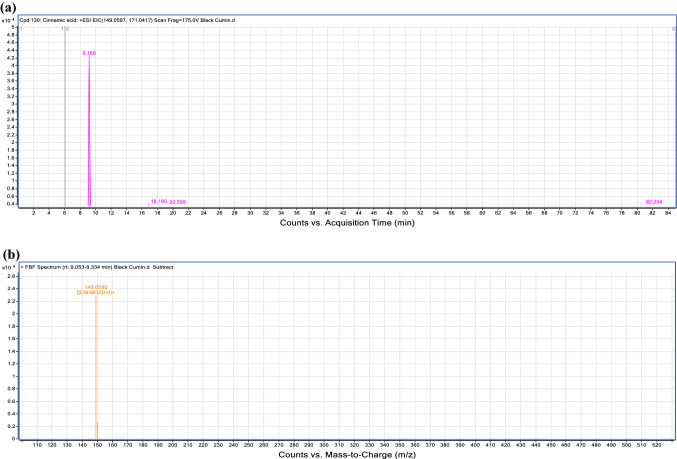
Hydroxycinnamic acids were the most abundant compounds in three spices samples. Compound (1) with [M + H]+ at m/z 149.0590 was tentatively identified as cinnamic acid. Cinnamic acid have also been identified in black cumin (Singh et al. 2004). Figure 1 showed the extracted ion chromatogram and the mass spectrum of cinnamic acid. Two compounds were both detected in black pepper and black cumin in ESI− and ESI+ modes. In black pepper and black cumin, compound (8) with [M + H]+ at m/z 355.1010 was tentatively characterized as chlorogenic acid while compound (19) in black pepper and black cumin with [M − H]− at m/z 295.0483 was tentatively identified as p-coumaroyl tartaric acid. However, these two compounds were not detected in black cardamom. Compound (15) with the molecular formula C9H8O4 and having the precursor ion at m/z 181.0492 in both positive and negative mode, were tentatively characterized as caffeic acid in both black pepper and black cardamom, in keeping with a previous report on pepper (Fenglin et al. 2018).
Fig. 1.
Extracted ion chromatogram and their mass spectrum. a A chromatograph of cinnamic acid (Compound 1, Table 2), Retention time (RT = 9.169 min) in the positive mode of ionization (ESI+/[M + H]+) tentatively identified only in black cumin; b mass spectra of cinnamic acid showing an observed m/z 149.0590
Two caffeic acid derivatives (Compound 3 and 7) were detected in the ESI− mode in black cumin with product ions at m/z 355.0698 and 258.9938 respectively. Caffeic acid had been identified in Tasmannia pepper berries in the study of Konczak et al. (2010). Compound (5) with [M − H]− at m/z 355.1062 was tentatively identified as ferulic acid 4-O-glucoside. Ferulic acid have also been identified in bitter cumin by Ani et al. (2006). In black cardamom (Compound 16) with the precursor ion at m/z 163.0416 in the ESI− mode was tentatively identified as m-coumaric acid. Coumaric acid was previously identified in Tasmannia pepper leaves (Konczak et al. 2010). Sruthi and Zachariah (2016) also identified hydroxycinnamic acids (including caffeic acid, and 4-coumaric acid) in Indian black pepper, which was consistent with our results.
Hydroxybenzoic acids
Hydroxybenzoic acids were detected in black pepper and black cumin, while not detected in black cardamom. A total of three hydroxybenzoic acids have been detected both in black pepper and black cumin, including 3-hihydroxybenzoic acid (Compound 24), 4-O-methylgallicacid (Compound 25) and 2-hydroxybenzoic acid (Compound 27). The compound (26) in black cumin with [M + H]+ at m/z 180.0643 was tentatively characterized as hippuric acid. In black cumin (Compound 22 and 28) with precursor ions at m/z 246.9937/479.1570 in ESI− mode were tentatively identified as vanillicacid 4-sulfate and paeoniflorin. Hydroxybenzoic acids were also identified in cumin by Mnif and Aifa (2015). Sruthi and Zachariah (2016) have previously identified hydroxybenzoic acids in black pepper collected from Kerala of India by LC–MS research.
Flavonoids
In the present work, we tentatively characterized eight different flavonoids derivatives from three spices. Among which, four subclasses (anthocyanins, flavonols, isoflavonoids and flavones) were tentatively identified in all samples in both positive and negative modes, while flavanones and flavanols were detected only in black pepper and black cardamom. Dihydrochalcones was tentatively identified in black pepper and black cumin while dihydroflavonols was only tentatively characterized in black cumin sample.
Flavonols
Flavonol was the predominant subclass in three black spices. We tentatively characterized 10 different flavonols in all three spices. Compound (39), with the molecular formula C33H40O20, having the precursor ion [M + H]+ at m/z 757.2178, was tentatively characterized as kaempferol 3-O-glucosyl-rhamnosyl-galactoside in all three black spices. And compound (43), with the precursor ion [M + H]+ at m/z 465.1036, was tentatively characterized as and myricetin 3-O-rhamnoside in all three black spices. Myricetin was also found in black cumin seedcake (Deepak and Lele 2017). Kaempferol was also identified in bitter cumin in the research lead by Ani et al. (2006). Three compounds were tentatively identified in both black cumin and black cardamom in positive and negative modes, being patuletin 3-O-glucosyl-(1– > 6)-[apiosyl (1– > 2)]-glucoside (Compound 40) with [M + H]+ ions at m/z 789.2046, kaempferol 3,7-O-diglucoside (Compound 41) with [M + H]+/[M − H]− ion at m/z 611.1581, and kaempferol 3,7,4’-O-triglucoside (Compound 42), with both positive and negative ions at m/z 773.2114. Kaempferol 3-O-(2’’-rhamnosyl-galactoside) 7-O-rhamnoside (Compound 45) was tentatively identified both in black pepper and black cardamom.
Anthocyanins
In the present work, we tentatively characterized 11 different anthocyanins, among which, seven anthocyanins were tentatively identified in black pepper, six were tentatively characterized in black cumin, and four were detected in black cardamom. Compound (56) with [M − H]−/[M + H]+ at m/z 608.1730 had been assigned as peonidin 3-O-rutinoside in black pepper, black cumin and black cardamom. Two compounds were tentatively identified in both black pepper and black cumin in negative ionization modes. Compound (50) in black pepper and black cumin with [M − H]− at m/z 756.2130 was tentatively characterized as peonidin 3-O-sambubioside-5-O-glucoside while compound (48) in black pepper and black cumin with [M − H]− at m/z 626.1497 was tentatively identified as delphinidin 3-O-glucosyl-glucoside. However, these two compounds were not detected in black cardamom. The other two compounds were both detected in black pepper and black cardamom in ESI− modes. In black pepper and black cardamom, compound (49) with [M − H]− at m/z 610.1531 was tentatively characterized as cyanidin 3,5-O-diglucoside while compound (51) with [M − H]− at m/z 594.1587 was tentatively identified as cyanidin 3-O-rutinoside. However, these two compounds were not detected in black cumin. Cyanidin 3-rutinoside and cyanidin 3-glucoside were reported as phenolic composition in Tasmannia pepper berry (Konczak et al. 2010). Fenglin et al. (2018) previously identified cyanidin and cyanidin derivatives in piper nigrum Linnaeus.
Isoflavonoids
A total of 14 isoflavonoids derivatives were detected and tentatively characterized in black spices. Two isoflavonoids were tentatively identified in three black spices, including 5,6,7,3′,4′-Pentahydroxyisoflavone (Compound 67) and 3′-Hydroxygenistein (Compound 71). In black cumin and black pepper, compound (62) with [M + H]+ at m/z 273.0750 was tentatively identified as 3′,4′,7-trihydroxyisoflavanone, which was not detected in black cumin. Black cardamom (Compound 66 and 70) with precursor ions [M + H]+ and [M − H]− at m/z 299.0530 and 301.0742 respectively, had been assigned as and irilone and 4′-methoxy-2′,3,7-trihydroxyisoflavanone.
Flavones
In this work, it was found that flavones derivatives were one of the most abundant compounds in three black spices. Thus, 10 compounds have been tentatively characterized in this subclass. Compound (75), with the molecular formula C27H30O15 and having the precursor ion [M − H]−/[M + H]+ at m/z 595.1633 was tentatively characterized as apigenin 6,8-di-C-glucoside in three black spices. Apigenin was also previously characterized in Indian black pepper by Sruthi and Zachariah (2016). The identification of apigenin derivatives in black pepper was consistent with the work of Fenglin et al. (2018) about Piper nigrum Linnaeus. Compound (76) with [M − H]− at m/z 607.1693 was tentatively identified as diosmin in all three black spices, and compound (78) in black pepper, black cumin and black cardamom with [M + H]+ at m/z 449.1060 was tentatively identified as 6-Hydroxyluteolin 7-O-rhamnoside. Compound (79) in black pepper and black cardamom with [M + H]+ at m/z 579.1688 was tentatively characterized as isorhoifolin.
Lignans and tyrosols
A total of 15 lignans derivatives have been detected in three black spices. Compound (100) with [M + H]+ at m/z 361.1645 was tentatively identified as cyclolariciresinol in both black pepper and black cumin. Compounds (104) in black pepper and compound (90) in black cumin with different modes (at m/z 401.1948 and 383.1483, respectively) were tentatively identified as schisandrin derivatives. Black cardamom (Compound 101) with precursor ion at 357.1321 in the ESI+ was tentatively characterized as conidendrin, which was the only lignans detected in black cardamom. In black pepper (Compound 94) with [M − H]− at m/z 417.1542 was assigned as 1-acetoxypinoresinol.
In the present work, we tentatively characterized seven different tyrosols, among which, three tyrosols were tentatively identified in black pepper, four were tentatively characterized in black cumin, and four were detected in black cardamom. Compound (109) in black pepper and black cumin with [M − H]−/[M + H]+ at m/z 195.0672 was tentatively characterized as 3,4-DHPEA-AC, who was also detected in black cumin. In black pepper and black cardamom, compound (111) with [M − H]−/[M + H]+ at m/z 181.0852 was tentatively identified as p-HPEA-AC, which was not identified in black cumin. One compound was tentatively characterized both in black pepper and black cumin, being hydroxytyrosol 4-O-glucoside (Compound 105), while not detected in black cardamom. In black cardamom (Compound 108) with [M − H]− at m/z 539.1798 was assigned to be oleuropein.
Non-phenolic metabolites and stilbenes
Both black cardamom and black pepper contained non-phenolic metabolites, who was not identified in black cumin. Black pepper (Compound 135) and black cardamom (Compound 136) with [M − H]− and [M + H]+ ions at m/z 224.0563 and 169.0844 were tentatively identified as vanilloylglycine and 1,3,5-trimethoxybenzene respectively. Stilbenes was only detected in black cumin. In black cumin (Compound 137 and 138) with different ion modes at m/z 301.1100 and 229.0869 were detected as 3′-hydroxy-3,4,5,4′-tetramethoxystilbene and resveratrol respectively.
The screening and characterization of polyphenolic compounds showed that some of the polyphenols presented in these black spices have strong antioxidant potential. Hydroxycinnamic acid derivatives, hydroxybenzoic acids and their derivatives, protocatechuic acid, chlorogenic acid, catechin, hydroxytyrosol, matairesinol, quercetin and kaempferol derivatives are regarded as potential compounds showing considerable free radical scavenging capacity (Ma et al. 2019; Peng et al. 2019; Tang et al. 2020). The presence of these antioxidant compounds indicates that black spices can be good sources of polyphenols and antioxidant potential. In short, black spices are a good source of polyphenols and could be utilized in food, feed, and pharmaceutical industries.
Quantitative analysis of polyphenol in three spices by HPLC
The HPLC technique is widely used to separate and quantify the phenolic compounds. Eight polyphenols were targeted to quantify through HPLC–PDA including 4 phenolic acids (protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid), 4 flavonoids (kaempferol-3-glucoside, quercetin, kaempferol and diosmin) based on the LC-ESI-QTOF/MS characterization and previously reported antioxidant activities (Supplementary Material, 3S1 & 3S2).
Protocatechuic acid was detected in all three black spices, and the highest content was found in black pepper (3.98 ± 0.07 mg/g), followed by black cumin (1.39 ± 0.01 mg/g) and black cardamom (0.36 ± 0.01 mg/g) (Table 3). The amount of the detected protocatechuic acid in black cumin was significantly higher than that in black cumin (0.13 mg/g) reported by Ani et al. (2006). p-hydroxybenzoic acid was detected to be the most predominant phenolic acid in black pepper and black cumin, with 38.18 ± 0.01 and 22.86 ± 0.01 mg/g respectively but not detected in black cardamom. The concentration of p-hydroxybenzoic acid in our black cumin was higher than Iranain black cumin sample (0.188 ± 0.21 mg/100 g) (Mariod et al. 2009). The only phenolic acid that did not quantify in black cumin was caffeic acid. The content of caffeic acid in black pepper and black cardamom were 2.15 ± 0.01 mg/g and 0.36 ± 0.01 mg/g respectively, who was reported as the main sources of antioxidant activities in Indian black pepper (Sruthi and Zachariah 2016).
Table 3.
Phenolic compounds (mg/g) in black pepper, black cumin and black cardamom
| No. | Compound name | RT (min) | Standard equation | Concentration (mg/g DW) | Polyphenol class | ||
|---|---|---|---|---|---|---|---|
| Black pepper | Black cumin | Black cardamom | |||||
| 1 | Kaempferol-3-glucoside | 47.111 | 22405x–33,766 | 0.06 ± 0.01b | 0.39 ± 0.01a | 0.04 ± 0.01b | Flavonoids |
| 2 | Diosmin | 49.249 | 1368x–18,098 | 4.42 ± 0.02b | 0.21 ± 0.01c | 23.94 ± 0.09a | Flavonoids |
| 3 | Quercetin | 70.098 | 2585.7x–29,267 | 0.24 ± 0.03c | 1.83 ± 0.02a | 0.69 ± 0.02b | Flavonoids |
| 4 | Kaempferol | 80.347 | 4425.8x–110,841 | 0.35 ± 0.03b | 9.81 ± 0.07a | 0.40 ± 0.02b | Flavonoids |
| 5 | Protocatechuic acid | 12.569 | 1824x–16,182 | 3.98 ± 0.07a | 1.39 ± 0.01b | 0.36- ± 0.01c | Phenolic acids |
| 6 | p-Hydroxybenzoic acid | 20.240 | 1387.5x + 5575.1 | 38.18 ± 0.01a | 22.86 ± 0.01b | – | Phenolic acids |
| 7 | Chlorogenic acid | 20.579 | 3043.6x + 4706.3 | 0.04 ± 0.01a | 0.02 ± 0.01a | – | Phenolic acids |
| 8 | Caffeic acid | 25.001 | 5622.4x + 23,944 | 2.15 ± 0.01a | – | 0.36 ± 0.01b | Phenolic acids |
Samples were analyzed as described in the method section and reported as means ± standard deviation (n = 3). The superscript letters were results of statistical analysis
In this study, four flavonoids were quantified in three black spices. Diosmin was quantified to be the predominant component in black cardamom, with 23.94 ± 0.09 mg/g, which was almost 5 times higher than that of black pepper (4.42 ± 0.02 mg/g). The highest content of kaempferol was quantified in black cumin (9.81 ± 0.07 mg/g), followed by black cardamom (0.40 ± 0.02 mg/g) and black pepper (0.35 ± 0.03 mg/g). The kaempferol content of our black cumin sample was higher than the Indian bitter cumin (94.7 g/g) (Ani et al. 2006).
The present study showed differences in the levels of phenolic compounds in the evaluated black spices. In short, all black spices are a good source of polyphenols and could be utilized in food, feed and pharmaceutical industries.
Conclusion
The LC-ESI-QTOF/MS analysis was successfully applied to identify the polyphenolic compounds from three different black species (black cumin, black pepper and black cardamom), they have distinct phenolic composition, mostly flavonoids and phenolic acids. A total of 138 compounds were tentatively identified from there black spices. Anthocyanins, flavonols, isoflavonoids, hydroxycinnamic acids, hydroxybenzoic acids, lignans and tyrosols were tentatively identified in black spices. In the HPLC analysis, p-hydroxybenzoic acid (phenolic acid) and diosmin (flavonoid) was the most abundant polyphenols in black spices. For the antioxidant activity, black pepper had the highest DPPH and ABTS values, whereas black cardamom had the highest FRAP activity. Our results indicated that antioxidant capacity was significantly correlated with polyphenolic composition of black spices. This study will provide valuable information for future exploitation of phenolic compounds as well as supporting the widespread use of black pepper, black cumin and black cardamom in food, nutrition and pharmaceutical industries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Dr. Nicholas Williamson, Dr. Shuai Nie and Dr. Michael Leeming from the Mass Spectrometry and Proteomics Facility, Bio21 Institute, VIC, Australia for providing access and support for the use of HPLC and LC-ESI-QTOF/MS and data analysis. We would also like to thank for Chunhe Gu, Rana Dildar Khan, Chao Ma, Danying Peng, Jiafei Tang, Biming Zhong, Danwei Yang, Yasir Iqbal and Akhtar Ali from the School of Agriculture and Food, Faculty of Veterinary and Agricultural Sciences, the University of Melbourne for their incredible support.
Author Contributions
Conceptualization, methodology, validation and investigation, Hafiz Suleria, Yuying Feng and Frank Dunshea; resources, Hafiz Suleria and Frank Dunshea.; writing—original draft preparation, Yuying Feng and Hafiz Suleria; writing—review and editing, Hafiz Suleria and Frank Dunshea.; supervision, Hafiz Suleria and Frank Dunshea.; funding acquisition, Hafiz Suleria and Frank Dunshea.
Funding
This research was funded by the University of Melbourne (Grant No. UoM-18/21) under the “McKenzie Fellowship Scheme” and the “Faculty Research Initiative Funds” funded by the Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Australia.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuying Feng, Email: yuyingf1@student.unimelb.edu.au.
Frank R. Dunshea, Email: fdunshea@unimelb.edu.au
Hafiz A. R. Suleria, Email: hafiz.suleria@unimelb.edu.au
References
- Ani V, Varadaraj M, Naidu K. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.) Eur Food Res Technol. 2006;224(1):109. doi: 10.1007/s00217-006-0295-z. [DOI] [Google Scholar]
- Assefa AD, Young-Soo K, Kumar Saini R. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J Food Meas Charact. 2018;12(3):1548–1555. doi: 10.1007/s11694-018-9770-z. [DOI] [Google Scholar]
- Bettaieb Rebey I, Bourgou S, Ben Slimen Debez I, Jabri Karoui I, Hamrouni Sellami I, Msaada K, Limam F, Marzouk B. Effects of extraction solvents and provenances on phenolic contents and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Food Bioprocess Technol. 2012;5(7):2827–2836. doi: 10.1007/s11947-011-0625-4. [DOI] [Google Scholar]
- Breksa AP, Takeoka GR, Hidalgo MB, Vilches A, Vasse J, Ramming DW. Antioxidant activity and phenolic content of 16 raisin grape (Vitis vinifera L.) cultivars and selections. Food Chemi. 2010;121(3):740–745. doi: 10.1016/j.foodchem.2010.01.029. [DOI] [Google Scholar]
- Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I, Berhe N, Willett WC, Phillips KM, Jacobs DR, Jr, Blomhoff R. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak K, Lele SS. Extraction, characterization and bioactive properties of Nigella sativa seedcake. J Food Sci Technol. 2017;54(12):3936–3947. doi: 10.1007/s13197-017-2853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenglin G, Guiping W, Yiming F, Hongying Z. Nontargeted metabolomics for phenolic and polyhydroxy compounds profile of pepper (Piper nigrum L.) products based on LC-MS/MS analysis. Molecules. 2018;8:1985. doi: 10.3390/molecules23081985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Howell K, Dunshea FR, Suleria HAR. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants. 2019;8(9):405. doi: 10.3390/antiox8090405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19(6):347–361. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandi S, Charles AL. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: estimation methods for EC50 using advanced statistical programs. Food Chem. 2019;275:41–49. doi: 10.1016/j.foodchem.2018.09.040. [DOI] [PubMed] [Google Scholar]
- Kanti Bhooshan P, Syed Ibrahim R. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;5:270. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak I, Zabaras D, Dunstan M, Aguas P. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010;122(1):260–266. doi: 10.1016/j.foodchem.2010.03.004. [DOI] [Google Scholar]
- Kumar Kandikattu H, Rachitha P, Jayashree GV, Krupashree K, Sukhith M, Abdul M, Narayanappa A, Farhath K. Anti-inflammatory and anti-oxidant effects of cardamom (Elettaria repens (Sonn.) Baill) and its phytochemical analysis by 4D GCXGC TOF-MS. Biomed Pharmacother. 2017;91:191–201. doi: 10.1016/j.biopha.2017.04.049. [DOI] [PubMed] [Google Scholar]
- Li A-N, Li S, Zhang Y-J, Xu X-R, Chen Y-M, Li H-B. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–6047. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LC, Yang TA, Easa AM, Zzaman W. Effect of conventional and superheated steam roasting on the total phenolic content, total flavonoid content and DPPH radical scavenging activities of black cumin seeds. Pertan J Trop Agric Sci. 2018;41(2):663–676. [Google Scholar]
- Ma C, Dunshea FR, Suleria HAR. LC-ESI-QTOF/MS characterization of phenolic compounds in palm fruits (jelly and fishtail palm) and their potential antioxidant activities. Antioxidants. 2019;8(10):483. doi: 10.3390/antiox8100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariod AA, Ibrahim RM, Ismail M, Ismail N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009;116(1):306–312. doi: 10.1016/j.foodchem.2009.02.051. [DOI] [Google Scholar]
- Mesfin H, Won Hee K. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation. 2019;5(1):29. doi: 10.3390/fermentation5010029. [DOI] [Google Scholar]
- Misharina TA. Antiradical properties of essential oils and extracts from coriander, cardamom, white, red, and black peppers. Appl Biochem Microbiol. 2016;52(1):79–86. doi: 10.1134/S0003683816010087. [DOI] [Google Scholar]
- Mnif S, Aifa S. Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem Biodivers. 2015;5:733. doi: 10.1002/cbdv.201400305. [DOI] [PubMed] [Google Scholar]
- Nazzaro F, Fratianni F, Coppola R, De Feo V. Essential oils and antifungal activity. Pharmaceuticals. 2017;10(4):86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic JS, Mitic VD, Stankov Jovanovic VP, Dimitrijevic MV, Stojanovic GS. Chemometric characterization of twenty three culinary herbs and spices according to antioxidant activity. J Food Meas Charact. 2019;13(3):2167–2176. doi: 10.1007/s11694-019-00137-0. [DOI] [Google Scholar]
- Ouyang H, Hou K, Peng W, Liu Z, Deng H. Antioxidant and xanthine oxidase inhibitory activities of total polyphenols from onion. Saudi J Biol Sci. 2018;25:1509–1513. doi: 10.1016/j.sjbs.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Zahid HF, Ajlouni S, Dunshea FR, Suleria HAR. LC-ESI-QTOF/MS profiling of Australian Mango Peel by-product polyphenols and their potential antioxidant activities. Processes. 2019;7(10):764. doi: 10.3390/pr7100764. [DOI] [Google Scholar]
- Pitchaon M, Maitree S, Rungnaphar P. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100(4):1409–1418. doi: 10.1016/j.foodchem.2005.11.032. [DOI] [Google Scholar]
- Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional indian medicinal plants. Indian J Pharm Sci. 2011;73(2):146–151. doi: 10.4103/0250-474X.91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf. 2018;17(3):512–531. doi: 10.1111/1541-4337.12330. [DOI] [PubMed] [Google Scholar]
- Singh UP, Singh DP, Maurya S, Maheshwari R, Singh M, Dubey RS, Singh RB. Investigation on the phenolics of some spices having pharmacotherapeutic properties. J Herb Pharmacother. 2004;4(4):27–42. doi: 10.1080/J157v04n04_03. [DOI] [PubMed] [Google Scholar]
- Soňa Š, Jiří M, Jana O, Tűnde J, Pavla D. Polyphenols content and antioxidant activity of paprika and pepper spices. Potravinarstvo. 2017;1:52. [Google Scholar]
- Spinola V, Pinto J, Castilho PC. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015;173:14–30. doi: 10.1016/j.foodchem.2014.09.163. [DOI] [PubMed] [Google Scholar]
- Sruthi D, Zachariah TJ. Phenolic profiling of piper species by liquid chromatography-mass spectrometry. J Spices Aromat Crops. 2016;25(2):123–132. [Google Scholar]
- Suhaj M. Spice antioxidants isolation and their antiradical activity: a review. J Food Compos Anal. 2006;19(6–7):531–537. doi: 10.1016/j.jfca.2004.11.005. [DOI] [Google Scholar]
- Tang J, Dunshea FR, Suleria HAR. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods. 2020;9(1):7. doi: 10.3390/foods9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodora C, Péter N, Constana G, János S, Maria-Magdalena M. Molecular mechanisms and bioavailability of polyphenols in prostate cancer. Int J Mol Sci. 2019;5:1062. doi: 10.3390/ijms20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan-Yuan Z, Fang Z, Kiran T, Ao-Te C, Hao W, Jian-Guo Z, Zhao-Jun W (2018) Effect of natural polyphenol on the oxidative stability of pecan oil. In: Food and chemical toxicology 119 (3rd international symposium on phytochemicals in medicine and food (3-ISPMF)), pp 489–495
- Yunfeng P, Tian D, Wenjun W, Yanju X, Xingqian Y, Mei L, Donghong L. Effect of harvest, drying and storage on the bitterness, moisture, sugars, free amino acids and phenolic compounds of jujube fruit (Zizyphus jujuba cv. Junzao) J Sci Food Agric. 2018;98(2):628–634. doi: 10.1002/jsfa.8507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.