Abstract
This study evaluated the interference of sub-lethal acid (SLA) stress and high isostatic pressure (HIP) stress on the survival of Lactobacillus rhamnosus GG (LGG) in mixed jussara and mango juice with the pH adjusted to 3.0 and 3.5, during 90 days of storage at 6 °C. The SLA stress at pH 4.0 previously applied to the LGG cells had no effect on the survival of this bacterium in mixed jussara and mango juices. On the other hand, the application of 200 MPa/5 min/25 °C treatment to LGG cells was shown to be efficient in the cross protection of this bacterium in an acid medium. Pressures above 400 MPa/5 min/25 °C caused a reduction in the viability in an acid medium and lower pressures (< 100 MPa) showed similar results compared to control treatment in the LGG survival. No changes in pH, acidity and soluble solids were observed in mixed juices. In addition, these products showed elevated levels of anthocyanins, phenolic compounds and antioxidant capacity. This highlights the capacity of the HIP process to promote the cross protection of LGG in an acid medium.
Keywords: Tropical fruits, Functional food, Probiotic, Sub-lethal stress
Introduction
Consumers are more conscious of the need to adopt a healthy, balanced diet by consuming functional, whole foods derived from fruits and vegetables. However, a conflict exists between the need to consume these foods and the accelerated rhythm of life of the populations. Thus, the industry has invested in new technologies that guarantee little change in the structure of the foods and little or no addition of chemical additives maintaining the appearance and freshness of the original foods (Al-Ghamdi et al. 2020; Rios-Corripio et al. 2020).
Whole fruit juices are rich in nutrients, vitamins and minerals and generally have a lower energy value than sweetened beverages such as soft drinks and nectars (De Ancos et al. 2020). A variety of fruits and vegetables can be used in their composition, including mixed juices, so as to unite the benefits of each ingredient and improve the palatability and sensory characteristics of the product (Malik et al. 2019). Jussara is a promising fruit for use in such juices, due principally to its elevated anthocyanin content (858–1008 μM Trolox/g) and antioxidant capacity (1552–1588 mg/100 g) (Campos et al. 2019). The mango (Mangifera indica) is a tropical fruit widely consumed and appreciated throughout the world due to its sweet taste and succulence (Moreira et al. 2017). Therefore, the mixed juices production has been widely explored in order to unite the benefits of various fruits, giving a product with improved nutritional value and good acceptance.
Many studies have been carried out to evaluate the viability of probiotic microorganisms in vegetable matrixes (Martins et al. 2015, 2016; Miranda et al. 2019; Perricone et al. 2014), and determined their survival during storage and under simulated gastrointestinal tract conditions. These products, in addition to being considered functional (Tripathi and Giri 2014), can also be consumed by people who cannot consume dairy products (Martins et al. 2013, 2015). Lactobacillus rhamnosus GG is an example of probiotic that has been used in the human diet, due to its resistance to digestive processes (Shah 2007) and its adaptability in different foods (Saxelin and Kajander 2009).
The biggest challenge to the manufacture and insertion of probiotic vegetable products on the market is maintenance of the survival of the probiotic cultures during storage, due to the naturally acid conditions of fruit juices, which can cause the death of the microorganism and its inability to resist the gastrointestinal tract conditions (Tripathi and Giri 2014; Perricone et al. 2015). In parallel, results obtained in studies carried out with microorganisms submitted to sub-lethal stresses, have shown modifications in their genic expression (Perricone et al. 2014; Reale et al. 2015), improving their survival during storage and giving them greater resistance to the conditions of the digestive process. However, there is a lack of information on the effect of sub-lethal baric and acid stresses on the resistance of L. rhamnosus GG in fruits. Thus, the objective of this study was to induce baric and sub-lethal acid stresses in L. rhamnosus GG and evaluate its resistance during the shelf life of a mixed jussara and mango juice.
Materials and methods
Preparation of the mixed jussara and mango juice
Three batches of frozen jussara (Euterpe edulis Martius) and mango (Mangifera indica L.) pulps (10 kg) were obtained from a producer in the city of Rio Pomba, MG, Brazil and packaged in polyethylene containers each with a capacity of 500 g. The jussara and mango pulps were frozen at − 18 °C. Posteriorly, the jussara and mango pulps were thawed at 4 °C, and 70 g of jussara pulp, 30 g of mango pulp and 7 g of sucrose were weighed and mixed together to make the mixed juice. Based on this composition, according to Brazilian legislation, this product can be classified as ready-to-drink sweetened tropical juice (Brazil 2018). The pH value was then adjusted to 3.0 and 3.5 using pro analysis (P.A.) citric acid (Proregi, Minas Gerais, Brazil).
After acidification, the mixed juices were filled into 100 mL sterile flasks and pasteurized in a water bath at 82 °C for 1 min. A flask containing the juice and a thermometer was used to check the temperature (Moreira et al. 2017). After pasteurization the flasks were cooled to room temperature in an ice bath at 2 °C and the product was stored at 6 °C.
Application of sub-lethal baric stress to L. rhamnosus GG cells and evaluation of their survival in the mixed jussara and mango juice
A loopful of the L. rhamnosus GG was taken from a capsule (Culturelle® ATTCC 53103, Connecticut, USA) and activated in de Man Rogosa Sharpe broth (MRS, Merck, Darmstadt, Germany) at 37 °C for 18 h. The broth was subsequently removed by centrifuging (Thermo Fisher Scientific, Sorvall™ Stratos™ Centrifuge Series, Germany) at 8500 ×g/5 °C for 15 min. The cell pellet obtained was washed twice consecutively with a phosphate buffer solution at pH 7.0 in order to avoid stressing the cells, re-suspended in 100 mL of the same solution obtaining a L. rhamnosus GG count of ~ 9 CFU.mL−1 of solution. Subsequently, aliquots (10 mL) were packaged in low density polyethylene bags and vacuum sealed.
The samples were subsequently submitted to a high isostatic pressure (HIP) process at pressures of 100, 200, 300, 400 and 500 MPa for 5 min at 25 °C. A control treatment (non-processed sample—0.1 MPa) was also prepared for comparative purposes. This process was carried out in a QFP 2L-700 equipment (Avure Technologies Inc, USA) with a 2L capacity, which allowed for a maximum operational pressure of up to 690 MPa, with the temperature controlled between 10 and 90 °C.
After HIP processing, the viability of the L. rhamnosus GG in the inoculum was determined in order to verify the number of surviving cells. The counts were obtained by deep plating in MRS agar (Merck, Darmstadt, Germany), incubating at 37 °C for 72 h (Richter and Vedamuthu 2001).
Subsequently, 1 mL of HIP processed sample was added to 9 mL of pasteurized juice with the pH adjusted to 3.0 and 3.5 to evaluate survival of the bacteria during storage at 6 °C. The juices were fractionated into microtubes containing 1 mL aliquots and the L. rhamnosus GG count determined (Richter and Vedamuthu 2001) after 0, 7, 14, 21, 28, 45 and 90 days of storage at 6 °C. After counting, the percent survival was calculated, considering the initial count (time of 0 h) as 100% and the survival after the other storage times calculated accordingly.
Elaboration of the mixed jussara and mango juice with added L. rhamnosus GG submitted or otherwise to sub-lethal acid stress
A loopful of L. rhamnosus GG was taken from a capsule (Culturelle®) and activated in MRS broth at 37 °C for 18 h. Subsequently, aliquots of 0.2; 0.3 or 0.4 mL of the culture were transferred to tubes containing 10 mL MRS broth and incubated under the same conditions until reaching a count of approximately 107 CFU.mL−1, equivalent to an absorbance of between 0.2 and 0.3 at 600 nm in a spectrophotometer (BEL® Photonics, SP 2000UV, São Paulo, Brazil).
The tube containing the culture which reached this absorbance value was diluted 100 times in four tubes each containing 45 mL of MRS broth at pH 5.7 ± 0.2, two not being acidified to pH 4.0 (control treatment) and two acidified to pH 4.0 (sub-lethal acid stress treatment). These tubes were incubated for 1 h at 37 °C with the objective of inducing the production of the alternative sigma factor (σA—sub-unit of the RNA polymerase enzyme responsible for recognizing regions promoting stress response genes) by the L. rhamnosus GG in response to the medium at pH 4.0.
The MRS broth was then removed from the tubes by centrifuging at 8500 x g/5 °C/15 min. (Thermo Fisher Scientific, Sorvall™ Stratos™ Centrifuge Series, Germany) and the cells re-suspended in 45 mL of juice with the pH adjusted to 3.0 and 3.5 to obtain four treatments: C3) control juice at pH 3.0 in which the L. rhamnosus GG was not submitted to prior sub-lethal stress at pH 4.0 for 1 h at 37 °C; E3) juice at pH 3.0 in which the L. rhamnosus GG was submitted to prior sub-lethal stress at pH 4.0 for 1 h at 37 °C; C3.5) control juice at pH 3.5 in which the L. rhamnosus GG was not submitted to prior sub-lethal stress at pH 4.0 for 1 h at 37 °C; E3.5) juice at pH 3.5 in which the L. rhamnosus GG was submitted to prior sub-lethal stress at pH 4.0 for 1 h at 37 °C.
The products obtained were transferred to sterile tubes and stored at 6 °C. The L. rhamnosus GG count and percent survival were determined as described in item 2.2 after 0, 7, 14, 21, 28, 45 and 90 days of storage.
Comparison of the percent survival of L. rhamnosus GG submitted to sub-lethal baric and acid stresses
After verifying the percent survival of L. rhamnosus GG processed by HIP and added to juices at pH 3.0 and 3.5, the process condition that guaranteed the greatest survival after 90 days of storage was selected and compared with the values for the percent survival after the application of acid stress at pH 4.0, to verify which type of stress applied to the cells guaranteed greater survival of the L. rhamnosus GG.
Characterization of the mixed jussara and mango juice
The color, pH, acidity, soluble solids, anthocyanins, phenolic compounds and antioxidant capacity were determined after obtaining the initial sample (T0) and after 14 (T14) and 28 (T28) days of storage of the products at 6.0 °C ± 0.5 °C, and the microbiological characteristics determined after zero (T0) and 28 (T28) days of storage.
The physicochemical and microbiological characterizations were only carried out on the juices in which the L. rhamnosus GG was submitted or otherwise to sub-lethal acid stress as described in item 2.3.
Color determination
The objective surface color of the juices was evaluated using a Konica Minolta CR10 (Tecnal, BR) colorimeter. The color was determined by a direct reading of the reflectance of the coordinates L*, a* and b* using the CIELAB scale adopted as the standard by the International Commission on Illumination.
Physicochemical analyses
The pH value, titratable acidity (g citric acid per 100 mL of product) and soluble solids content of the juices were determined according to the Association of Official Agricultural Chemists—AOAC (2016).
The phenolic compound contents of the samples were determined using the Folin–Ciocalteau method (Singleton et al. 1999). The results were expressed in mg of gallic acid equivalents (GAE) per 100 g of sample (mg GAE.100 g−1).
The anthocyanin contents of the juice samples were determined according to Lees and Francis (1972). The results were expressed in mg anthocyanin per 100 g of product.
Aliquots of the juices were tested for their antioxidant capacity according to the ABTS [2,2′-azinobis (3-ethylbenzthiazoline sulfonic acid-6)] radical capture method described by Re et al. (1999). The results were expressed in uM Trolox per g of product.
Evaluation of the microbiological quality of the juices
The contaminating microbiota of the juices was evaluated by standard plate counts for aerobic mesophylls, filamentous fungi and yeasts, total coliforms and E. coli, and by the evaluation for the presence of Salmonella sp.
The standard plate count for filamentous fungi and yeasts was carried out by surface plating of the decimal dilutions in Dichloran Rose Bengal Chloramphenicol agar (DRBC, Biolog, Belo Horizonte, MG, Brazil) with incubation at 25 °C for 5 days before counting (Beuchat and Cousin 2001).
The standard plate counts for aerobic mesophylls, total coliforms and Escherichia coli were carried out using Petrifilms® specific for each group of microorganisms, following the manufacturer’s instructions. Subsequently, the count of each microbial group was determined in log CFU.mL−1 of juice.
The determination of the presence or absence of Salmonella sp. was carried out using 25 mL of product homogenized with 225 mL of lactose broth (MicroMed/Isofar, Duque de Caxias, Rio de Janeiro, Brazil) according to the methodology of Andrews et al. (2001).
Statistical analyses
The analysis of the percent survival of L. rhamnosus GG submitted to baric stress was evaluated using an entirely random triple factorial design (2 × 5 × 7) with two juices (pH 3.0 and 3.5), five pressure levels (0,1, 100, 200, 300 and 400 MPa) and 7 analysis times (0, 7, 14, 21, 28, 45 and 90 days).
The analysis of the percent survival of L. rhamnosus GG submitted to sub-lethal acid stress was evaluated using an entirely random double factorial design (4 × 7) with 4 juices (C3, E3, C3.5 and E3.5) and 7 analysis times (0, 7, 14, 21, 28, 45 and 90 days).
The physicochemical and functional characteristics of the juices were evaluated using an entirely random double factorial design (4 × 3), with four treatments (C3, E3, C3.5 and E3.5) and three storage times (0, 14 and 28 days). The microbiological characteristics were evaluated using an entirely random double factorial design (4 × 2), with four treatments (C3, E3, C3.5 and E3.5) and two storage times (0 and 28 days). This storage time (up to 28 days) for physicochemical and microbiological characterization of the juices was chosen based on an average shelf life for a probiotic mixed juice considering microbiological standards, as well as a time commonly used for commercialization and consumption of pasteurized juice (Moreira et al. 2017).
In order to compare the survival of L. rhamnosus GG in the juices at pH 3.0 and 3.5 when the bacterium was submitted to sub-lethal stress by high pressure or with acid at pH 4.0, an entirely random double factorial design (4 × 7) was used, with four treatments (S3—juice at pH 3.0 with the addition of L. rhamnosus GG submitted to sub-lethal baric stress at 200 MPa/25 °C/5 min; S3.5—juice at pH 3.5 with the addition of L. rhamnosus GG submitted to sub-lethal baric stress at 200 MPa/25 °C/5 min; E3—juice at pH 3.0 with the addition of L. rhamnosus GG submitted to sub-lethal stress at pH 4.0; and E3.5—juice at pH 3.5 with the addition of L. rhamnosus GG submitted to sub-lethal stress at pH 4.0) and 7 analysis times (0, 7, 14, 21, 28, 45 and 90 days).
The results obtained were evaluated by an analysis of variance and Tukey’s test to compare the means at a 5% level of significance. All experiments were carried out with three repetitions. All statistical procedures were carried out considering a 5% level of probability and using the statistical software STATISTICA 7.0 (StatiSoft, Inc., Tulsa, Okla., USA).
Results and discussion
Influence of sub-lethal baric stress on the survival of L. rhamnosus GG in mixed jussara plus mango juices
After the HIP processes and prior to inoculation into the juices, L. rhamnosus GG counts greater than 9.0 log CFU.mL−1 were found for the pressures of 0.1 MPa (control treatment) and 100 MPa/5 min/25 °C and counts between 8.5 and 9.0 log CFU.mL−1 for the processes of 200, 300 and 400 MPa for 5 min at 25 °C. For processing at 500 MPa/5 min/25 °C the bacterial count was only 3.0 log CFU.mL−1, representing a reduction of 6 logarithmic cycles, and after inoculation into the juices with the pH adjusted to 3.0 and 3.5, it was shown that the effect of the acid pH value together with the application of the more drastic process (500 MPa/5 min/25 °C) was lethal to the L. rhamnosus GG cells, and there was no growth of the bacteria in any of the samples, so this treatment was discarded.
Pressure levels of up to 400 MPa/5 min/25 °C were not sufficient to reduce the initial L. rhamnosus GG population by even 1 logarithmic cycle, which demonstrates the elevated resistance of this strain to the HIP process. Thus more studies are suggested to evaluate the potential of the application of this technology in the mixed jussara and mango juice processing as a conservation method by inactivating undesirable microbiota without reducing the count of the probiotic bacterium inoculated into the product.
According to FDA recommendations, a minimum pressure of 580 MPa should be applied at room temperature for 3 min to guarantee pasteurization of low-acid foods and commercial sterility in acid foods (pH < 4.0) and their stability during storage (FDA 2010; Moreira et al. 2017). This processing condition assures inactivation of Escherichia coli O157:H7, Listeria spp., Salmonella spp. and Staphylococcus sp. However, such treatment also inactivated the L. rhamnosus GG. The combined effect of the low pH value of the product and of the high pressure treatment, even when at levels inferior to 580 MPa could guarantee the inactivation of pathogenic microorganisms, as reported by Buzrul et al. (2008), who reported that a treatment of 300 MPa/5 min/20 °C was efficient in eliminating E. coli and Listeria innocua from pineapple and kiwi juices.
While evaluating the interaction between the pressure levels applied, the pH values of the juices and the storage times, the process condition of 200 MPa/5 min/25 °C was shown to be the best induced baric stress treatment, since after 90 days of storage in the juice adjusted to pH 3.0, the L. rhamnosus GG maintained a survival rate of 86.9% and did not differ (p < 0.05) from its survival rate at zero time (100%). However, the processes of 0.1 MPa and 100 MPa/5 min/25 °C applied to this probiotic bacterium reduced its survival (p < 0.05) to approximately 78% after 90 days of storage, and the processes of 300 MPa and 400 MPa for 5 min at 25 °C led to the death of the bacterium when inoculated into juice adjusted to pH 3.0 (Fig. 1a).
Fig. 1.
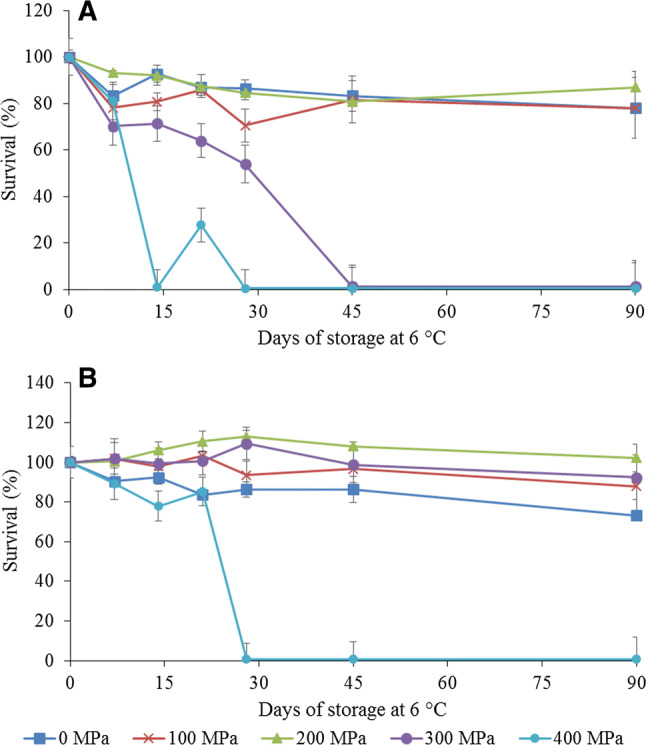
Survival of L. rhamnosus GG submitted to baric stress and inoculated into juices with the pH value adjusted to 3.0 (a) and 3.5 (b)
In the mixed juice at pH 3.5, no difference in the survival of the L. rhamnosus GG after application of 200 MPa/5 min/25 °C was noted during the 90 days of evaluation, whereas the survival of the bacterium not submitted to stress (0.1 MPa) was reduced by 26.84% during the same period, but did not differ (p < 0.05) from the process using 100 MPa/5 min/25 °C, which showed 87.9% of survival (Fig. 1b). For the process applying 400 MPa/5 min/25 °C, the bacterium did not survive after 28 days of storage of the juice (Fig. 1b). Finally after 90 days of storage, the process which applied 300 MPa/5 min/25 °C maintained 92.41% survival of the bacterium in the juice, not differing (p < 0.05) from the initial survival rate. However, for the same HIP application, the bacterium did not survive in the juice at pH 3.0 (p < 0.05), demonstrating that the low pH value of the juice together with the elevated pressure represents a lethal factor for the cells (Figs. 1a, b). In other studies, it was found that other technologies such as high pressure homogenization reduced the resistance of L. acidophilus while increased it for L. paracasei (Tabanelli et al. 2013). This could be explained by the mechanisms of stress response to the initial conditions (i.e. pressure, low pH). In addition, the combination of multiple stresses, as well as the greater severity of stress conditions may lead to a decrease of the maximum growth rate and an extended length of the lag phase (Yáñez et al. 2008).
Thus, the process condition of 200 MPa/5 min/25 °C was efficient in causing sub-lethal stress in L. rhamnosus GG cells, which resulted in an increase in the production of stress metabolites. These substances may help the cells to withstand external deleterious effects of the environment (Ananta and Knorr 2003). However, the condition of 400 MPa/5 min/25 °C together with the elevated acid condition of the juice functioned as a barrier for the survival of the probiotic, that is, it impeded maintenance of its survival during the 90 days of storage. Scheyhing et al. (2004) evaluated the cross protection of various stress factors and observed that the stress induced by a pressure of 80 MPa only affected its sensitivity to heat and had no effect on the resistance of Lactobacillus sanfransiscensis to pH values between 3.0 and 6.0, confirming that low pressure values had no effect on cross protection at low pH values, as also found in the present study. In another study, Ananta and Knorr (2003) found that L. rhamnosus LGG pre-processed at 100 MPa/37 °C for 10 min showed higher survivability to thermal stress. On the other hand, pressure above 200 MPa resulted in microbial inactivation (Ananta and Knorr 2003). This confirms that for each microorganism in a given matrix there is a pressure limit to be applied and that in general low and medium pressures can offer cross protection to stressful conditions in the environment (Mota et al. 2018). Other technologies may also offer cross protection, in this case, Burns et al. (2008) noted an increase in the viability of lactic acid bacteria in buttermilk after high pressure homogenization and this may be attributed to the increased availability of low molecular weight peptides and free fatty acids.
Influence of sub-lethal acid stress on the survival of L. rhamnosis GG in mixed jussara and mango juice
There was no difference between the treatments in terms of the survival rate of the L. rhamnosis GG cells up to 28 days of storage. However, after 45 days the L. rhamnosis GG cells not submitted to prior sub-lethal stress at pH 4.0 and added to the mixed juice at pH 3.0, showed a reduction in survival resulting in 70% of the initial viability, differing from the other treatments (Fig. 2). However, after 90 days of storage there was again no difference between the treatments, since the mean counts of L. rhamnosis GG had reduced in all treatments (Fig. 2).
Fig. 2.
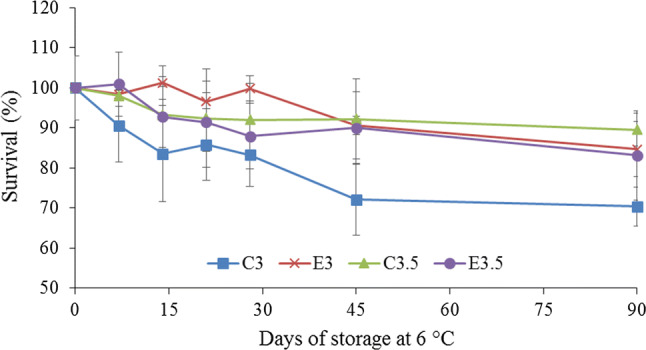
Survival of L. rhamnosus GG in mixed jussara and mango juice submitted or not to sub-lethal acid stress at pH 4.0. C3 and C3.5: L. rhamnosus not subjected to acid stress and added in juice with pH 3.0 and 3.5, respectively. E3 and E3.5: L. rhamnosus submitted to acidic stress (pH 4.0) and added in juice with pH 3.0 and 3.5, respectively
The behavior of different L. rhamnosis strains when exposed to different stress situations has also been evaluated by Reale et al. (2015) and Perricone et al. (2015). Reale et al. (2015) showed that the strain of L. rhamnosis CTC1676 showed good growth capacity after 24 h of incubation at pH 3.5, whereas the strain DIAL40 showed the opposite behavior. In general the low viability of probiotic cultures in fruit juices can be overcome by adaptation and the induction of resistance by exposing the probiotics to sub-lethal stress, which will induce a type of resistance in response to the subsequent stress (Perricone et al. 2015).
Although an increase in the survival of L. rhamnosis GG previously submitted to sub-lethal acid stress was not shown in this study, it was verified that the control microorganism added to the mixed juice at pH 3.0 showed the lowest viability during storage when compared to the other samples evaluated (Fig. 2). In addition, the results obtained showed the great capacity of this bacterium to survive for up to 90 days in the acid conditions of the mixed jussara and mango juice, since the smallest percent survival was 70% in relation to zero time (Fig. 2) and the count was above 5.07 log CFU.mL−1 in all treatments for up to 90 days of storage. The International Dairy Federation suggests that probiotic products must contain at least 106 viable probiotic bacteria per gram or milliliter of product at the time of consumption to exert beneficial effect on the host’s health (Moreira et al. 2017). Thus, the daily consumption of 100 mL of the mixed jussara and mango juice would be sufficient to ingest 107 of viable L rhamnosis GG cells, and hence reap the health benefits conferred by this microorganism.
Comparison of the percent survival of L. rhamnosis GG submitted to sub-lethal baric and acid stress
On comparing the sub-lethal stress under the process condition of 200 MPa/5 min/25 °C with the sub-lethal acid stress at pH 4.0, a significant (p < 0.05) difference was found between the treatment, time and interaction for the two factors. After 14 days of storage of the juices, there was no difference in survival of the L. rhamnosus GG cells, but after 21 days of evaluation, the bacterium showed a greater survival rate after being submitted to baric stress and added to the mixed juice with the pH value adjusted to 3.5, in relation to the bacterium submitted to prior acid stress at pH 4.0 and added to juices at pH values of 3.0 and 3.5, the same situation also occurring after 45 days of analysis (Fig. 3).
Fig. 3.
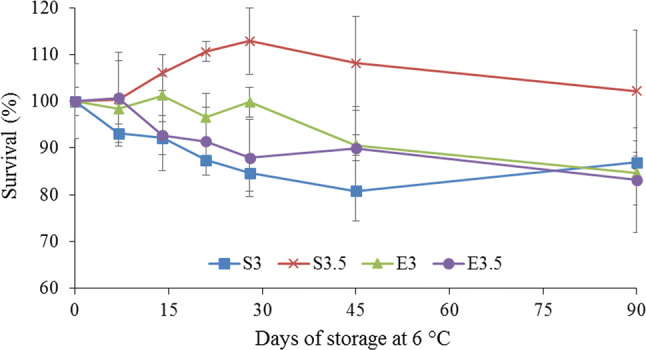
Survival of L. rhamnosus GG submitted to sub-lethal acid (pH 4.0) or baric (200 MPa/5 min/25 °C) stress added to juices with the pH adjusted to 3.0 and 3.5. S3: juice at pH 3.0 with the addition of L. rhamnosus GG submitted to sub-lethal baric stress at 200 MPa/25 °C/5 min; S3.5: juice at pH 3.5 with the addition of L. rhamnosus GG submitted to sub-lethal baric stress at 200 MPa/25 °C/5 min; E3: juice at pH 3.0 with the addition of L. rhamnosus GG submitted to sub-lethal stress at pH 4.0; E3.5: juice at pH 3.5 with the addition of L. rhamnosus GG submitted to sub-lethal stress at pH 4.0
After 90 days of treatment, the L. rhamnosus GG cells submitted to sub-lethal baric stress at 200 MPa/5 min/25 °C presented a greater survival rate in juices at pH 3.5 than cells of the bacterium submitted to sub-lethal acid stress, but there was no difference in survival rate for the cells of this bacterium submitted to the same high pressure treatment and added to juice at pH 3.0 (Fig. 3).
Quality of the mixed jussara and mango juices
With respect to the color of the mixed juices adjusted to different pH values, no differences were detected between the samples for the parameters of L* and a* (p > 0.05) (Table 1). However, during storage the values of the coordinate b* increased (Table 1), indicating an intensification of the yellow tonality of the mixed juices, probably due to separation of the fat from the product.
Table 1.
Analysis of mixed jussara and mango juice for color, pH, titratable acidity and soluble solids
| Juices | Time (days) | L* | a* | b* | pH | Acidity (g citric acid/100 mL) | Soluble solids (°Brix) |
|---|---|---|---|---|---|---|---|
| Control pH 3.0 | 0 | 23.82 ± 0.88 | 3.15 ± 0.64 abcde | 0.27 ± 1.11 bc | 3.35 ± 0.09 cde | 1.13 ± 0.07 abc | 16.67 ± 1.27 a |
| 14 | 26.37 ± 0.72 | 3.87 ± 0.58 ab | 0.63 ± 0.12 abc | 3.27 ± 0.13 de | 1.18 ± 0.39 abc | 13.77 ± 2.23 a | |
| 28 | 24.37 ± 1.70 | 2.13 ± 0.80 cde | 1.70 ± 0.36 a | 3.16 ± 0.09 e | 1.40 ± 0.31 ab | 12.43 ± 2.98 a | |
| Stress pH 3.0 | 0 | 24.27 ± 0.71 | 2.57 ± 0.47 bcde | 0.37 ± 0.50 bc | 3.37 ± 0.12 bcde | 0.79 ± 0.21 bc | 16.60 ± 2.47 a |
| 14 | 25.97 ± 0.98 | 3.42 ± 0.23 abcd | 1.03 ± 0.12 abc | 3.24 ± 0.15 de | 0.76 ± 0.20 c | 12.77 ± 2.46 a | |
| 28 | 24.87 ± 0.95 | 2.16 ± 0.84 cde | 1.97 ± 0.81 a | 3.26 ± 0.09 de | 1.14 ± 0.15 abc | 12.87 ± 3.67 a | |
| Control pH 3.5 | 0 | 23.87 ± 0.60 | 3.23 ± 0.55 abc | 0.17 ± 0.75 c | 3.68 ± 0.13 a | 0.73 ± 0.12 c | 12.23 ± 2.68 a |
| 14 | 25.50 ± 0.53 | 3.73 ± 0.45 abc | 0.60 ± 0.20 ab | 3.67 ± 0.17 ab | 0.88 ± 0.13 bc | 10.80 ± 2.70 a | |
| 28 | 24.77 ± 0.49 | 1.96 ± 0.31 de | 2.23 ± 0.50 a | 3.5 ± 0.04 abcd | 1.15 ± 0.14 abc | 14.70 ± 2.61 a | |
| Stress pH 3.5 | 0 | 24.37 ± 1.16 | 2.00 ± 0.56 de | 0.20 ± 0.38 c | 3.62 ± 0.05 abc | 1.20 ± 0.09 abc | 13.37 ± 1.78 a |
| 14 | 25.37 ± 0.75 | 4.26 ± 0.32 a | 1.20 ± 0.36 abc | 3.68 ± 0.07 a | 1.31 ± 0.10 abc | 11.53 ± 3.28 a | |
| 28 | 24.63 ± 1.26 | 1.73 ± 0.64 e | 1.70 ± 0.38 a | 3.49 ± 0.03 abcd | 1.70 ± 0.19 a | 10.87 ± 1.50 a |
Means followed by the same letter in the same column do not differ from each other according to Tukey’s test at 5% of probability (n = 9)
Although there was slight change in absolute terms for the parameters of pH, acidity and soluble solids, statistically the pH value and titratable acidity remained stable (p > 0.05) during 28 days of storage of the product at 6 °C, and, as expected, there was no significant difference (p > 0.05) in the values for soluble solids between the treatments and times evaluated (Table 1). Such results may be justified due to the low metabolism of bacteria at low temperatures.
The bioactive compounds (anthocyanins, antioxidant capacity and phenolic compounds) did not differ (p > 0.05) between treatment and time, demonstrating the acid stress previously applied to the L. rhamnosus GG cells did not alter the bioactive characteristics of the juices, which maintained their functional characteristics during the 28 days of storage (Table 2).
Table 2.
Analysis of mixed jussara and mango juice for the total anthocyanin contents, antioxidant capacities and phenolic compound contents
| Juices | Time (days) | Anthocyanin content (mg/100 g) | Antioxidant capacity (µM trolox/g) | Phenolic compound content (mg EAG/100 g) |
|---|---|---|---|---|
| Control pH 3.0 | 0 | 472.50 ± 61.13 a | 176.51 ± 45.91 a | 2703.65 ± 279.00 a |
| 14 | 496.96 ± 32.78 a | 192.92 ± 42.81 a | 2888.72 ± 202.09 a | |
| 28 | 448.06 ± 38.49 a | 167.74 ± 40.55 a | 3185.97 ± 193.90 a | |
| Stress pH 3.0 | 0 | 568.22 ± 133.06 a | 152.59 ± 66.12 a | 3036.57 ± 221.24 a |
| 14 | 474.20 ± 24.89 a | 172.54 ± 98.73 a | 2724.68 ± 53.75 a | |
| 28 | 483.03 ± 44.30 a | 146.34 ± 25.49 a | 2794.00 ± 431.15 a | |
| Control pH 3.5 | 0 | 535.30 ± 65.40 a | 202.03 ± 54.09 a | 2898.15 ± 542.10 a |
| 14 | 468.77 ± 47.94 a | 254.09 ± 57.45 a | 2752.95 ± 229.14 a | |
| 28 | 466.39 ± 14.15 a | 185.08 ± 40.15 a | 2649.67 ± 140.87 a | |
| Stress pH 3.5 | 0 | 528.80 ± 24.13 a | 224.02 ± 61.54 a | 2704.40 ± 272.99 a |
| 14 | 513.23 ± 45.11 a | 180.80 ± 90.23 a | 2783.78 ± 494.51 a | |
| 28 | 513.23 ± 45.11 a | 167.10 ± 25.85 a | 2498.62 ± 62.35 a |
Means followed by the same letter in the same column do not differ from each other according to Tukey’s test at 5% of probability (n = 9)
Although anthocyanins normally undergo degradation during storage, the stability of the anthocyanins in the present study could be justified by the low pH value of the juices, since anthocyanins are stable at pH values between 1.0 and 4.0, being degraded at pH values above 7.0 (Castañeda-Ovando et al. 2009).
The products obtained showed low microbial counts of aerobic mesophylls, filamentous fungi and yeasts and total coliforms and there was no difference (p > 0.05) between the samples and times evaluated (Table 3). In addition, although there was no statistical difference, the control sample at pH 3.0 showed 1 log CFU.mL−1 less compared to the control sample at pH 3.5 for aerobic mesophyll after 28 days. This is probably due to the greater lethal effect of the lower pH (pH 3.0) preventing the growth of this microbial group over time. Thus, the results obtained demonstrated the adoption of good practices during processing and that the heat treatment adopted was satisfactory to promote the reduction of microbial counts of the products to levels that were safe for human consumption.
Table 3.
Microbial quality of mixed jussara and mango juice subjected to acid stress at different pH
| Juice | Time (days) | Aerobic mesophiles | Filamentous fungi and yeasts | Coliforms at 30 °C | E. coli | Salmonella sp. |
|---|---|---|---|---|---|---|
| Control pH 3.0 | 0 | 1.23 ± 2.13 | 0.57 ± 0.98 | < 1.00 | < 1.00 | Absent |
| 28 | 1.19 ± 2.07 | 0.52 ± 0.54 | 0.87 ± 1.50 | < 1.00 | Absent | |
| Stress pH 3.0 | 0 | 1.02 ± 1.76 | 0.43 ± 0.75 | < 1.0 | < 1.00 | Absent |
| 28 | < 1.00 | 0.55 ± 0.59 | 0.83 ± 1.43 | < 1.00 | Absent | |
| Control pH 3.5 | 0 | < 1.00 | 0.43 ± 0.75 | < 1.00 | < 1.00 | Absent |
| 28 | 2.12 ± 1.84 | 0.85 ± 0.22 | < 1.00 | < 1.00 | Absent | |
| Stress pH 3.5 | 0 | < 1.00 | 0.40 ± 0.75 | < 1.00 | < 1.00 | Absent |
| 28 | 1.16 ± 2.01 | 0.77 ± 0.40 | 1.06 ± 0.92 | < 1.00 | Absent |
Mean counts ± SD (log CFU.mL−1) for aerobic mesophilic microorganisms, filamentous fungi and yeasts, coliforms at 30 °C, E. coli and Salmonella sp. in the mixed jussara and mango juices (n = 9)
Conclusion
The sub-lethal acid stress at pH 4.0 previously applied to the L. rhamnosus GG cells had no effect on the survival of this bacterium in jussara and mango juices wth the pH value adjusted to 3.0 and 3.5.
The application of 200 MPa/5 min/25 °C to L. rhamnosus GG cells was shown to be efficient in the cross protection of this bacterium in an acid medium. Pressures above 400 MPa/5 min/25 °C caused a reduction in the viability in an acid medium and lower pressures showed similar results compared to control treatment on the maintenance of LGG survival during the 90 days of storage of the mixed juice. Thus the treatment of 200 MPa/25 °C/5 min was more efficient than acid stress to promote the survival the bacterium in mixed juice.
Continuation of the studies with the use of high pressure as a stress factor is required in order to elucidate how probiotic strains behave after being submitted to HIP in vegetable matrixes. Evaluations of the resistance of L. rhamnosus GG cells to simulated in vivo conditions of the gastrointestinal tract after the application of sub-lethal stresses, are also required.
The mixed jussara and mango juices processed at 82 °C for 1 min and stored at 6 °C for 28 days were safe for commercialization and consumption. In addition they presented elevated levels of anthocyanins, phenolic compounds and antioxidant capacity, which did not differ between the treatments and did not degrade in 28 days of refrigerated storage.
Acknowledgements
The authors are grateful to the National Council for Scientific and Technological Development (CNPq-Brazil—Grant No. 467824/2014-2), the Foundation for Research of the State of Minas Gerais (FAPEMIG—Grant No. MPR-00005-13), the PET Agricultural Sciences Group and the Federal Institute of Southeast Minas Gerais, for their financial support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Ghamdi A, Sonar CR, Patel J, Albahr Z, Sablani SS. High pressure-assisted thermal sterilization of low-acid fruit and vegetable purees: microbial safety, nutrient, quality, and packaging evaluation. Food Control. 2020;114:107233. doi: 10.1016/j.foodcont.2020.107233. [DOI] [Google Scholar]
- Ananta E, Knorr D. Pressure-induced thermotolerance of Lactobacillus rhamnosus GG. Food Res Int. 2003;36:991–997. doi: 10.1016/j.foodres.2003.07.001. [DOI] [Google Scholar]
- Andrews WH, Flower RS, Silliker J, Bailey JS. Salmonella. In: Downes FP, Ito K, editors. Compendium of methods for microbilological examination of foods. 4. Washington DC: APHA; 2001. pp. 357–380. [Google Scholar]
- AOAC (2016) Official methods of analysis of the Association of Official Analytical Chemists. 20th edn. Washington, D.C
- Beuchat IR, Cousin MA. Years and molds. In: Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods. 4. Washington DC: APHA; 2001. pp. 209–215. [Google Scholar]
- Brazil (2018) Ministry of Agriculture, Cattle and Supplying. Normative Instruction No. 49, September 26, 2018. Regulates the identity and quality standards of fruit juice and pulp (Brasília, DF), Federal Official Gazette
- Burns P, Vinderola G, Molinari F, Reinheimer J. Suitability of whey and buttermilk for the growth and frozen storage of probiotic lactobacilli. Int J Dairy Technol. 2008;61:2. doi: 10.1111/j.1471-0307.2008.00393.x. [DOI] [Google Scholar]
- Buzrul S, Alpas H, Largeteau A, Demazeau G. Inactivation of Escherichia coli and Listeria innocua in kiwifruit and pineapple juices by high hydrostatic pressure. Int J Food Microbiol. 2008;124:275–278. doi: 10.1016/j.ijfoodmicro.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Campos RCAB, Martins EMF, Pires BA, Peluzio MCG, Campos ANR, Ramos AM, Leite Junior BRC, Martins ADO, Silva RR, Martins ML. In vitro and in vivo resistance of Lactobacillus rhamnosus GG carried by a mixed pineapple (Ananas comosus L. Merril) and jussara (Euterpe edulis Martius) juice to the gastrointestinal tract. Food Res Int. 2019;116:1247–1257. doi: 10.1016/j.foodres.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Castañeda-Ovando A, Pacheco-Hernandez ML, Paez-Hernandez ME, Rodriguez JÁ, Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- De Ancos B, Rodrigo MJ, Sánchez-Moreno C, Cano MP, Zacarías L. Effect of high-pressure processing applied as pretreatment on carotenoids, flavonoids and vitamin C in juice of the sweet oranges ‘Navel’ and the red-fleshed ‘Cara Cara’. Food Res Int. 2020;132:109105. doi: 10.1016/j.foodres.2020.109105. [DOI] [PubMed] [Google Scholar]
- FDA (2010) Kinetics of microbial inactivation for alternative food processing technologies—high pressure processing. U.S Food and Drug Administration. http://www.fda.gov/Food/FoodScienceResearch/SafePracticesforFoodProcesses/ucm101456.htm. Accessed August 2018
- Lees DH, Francis FG. Standardization of pigment analysis in cranberries. HortScience. 1972;7:83–84. [Google Scholar]
- Malik M, Bora J, Sharma V. Growth studies of potentially probiotic lactic acid bacteria (Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus casei) in carrot and beetroot juice substrates. J Food Process Pres. 2019;43:e14214. doi: 10.1111/jfpp.14214. [DOI] [Google Scholar]
- Martins EMF, Ramos AM, Vanzela ESL, Stringheta PC, Pinto CLOP, Martins JM. Products of a vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int. 2013;51:764–770. doi: 10.1016/j.foodres.2013.01.047. [DOI] [Google Scholar]
- Martins EMF, Ramos AM, Martins ML, Oliveira PM, Stringheta PC. Minimally processed fruit salad enriched with Lactobacillus acidophilus: viability of anti-browning compounds in the preservation of color. Afr J Biotechnol. 2015;14:2022–2027. doi: 10.5897/AJB2015.14444. [DOI] [Google Scholar]
- Martins EMF, Ramos AM, Martins ML, Leite Júnior BRC. Fruit salad as a new vehicle for probiotic bacteria. Food Sci Technol. 2016;36:540–548. doi: 10.1590/1678-457X.03316. [DOI] [Google Scholar]
- Miranda RF, De Paula MM, Da Costa GM, Barão CE, et al. Orange juice added with L. casei: is there an impact of the probiotic addition methodology on the quality parameters? LWT—Food Sci Technol. 2019;106:186–193. doi: 10.1016/j.lwt.2019.02.047. [DOI] [Google Scholar]
- Moreira RM, Martins ML, Leite Junior BRC, Martins EMF, Ramos AM, Cristianini M, Campos ANR, Stringheta PC, Silva VRO, Canuto JW, Oliveira DC, Pereira DCS. Development of a juçara and Ubá mango juice mixture with added Lactobacillus rhamnosus GG processed by high pressure. LWT—Food Sci Technol. 2017;77:259–268. doi: 10.1016/j.lwt.2016.11.049. [DOI] [Google Scholar]
- Mota MJ, Lopes RP, Sousa S, Gomes AM, Delgadillo I, Saraiva JA. Lactobacillus reuteri growth and fermentation under high pressure towards the production of 1,3-propanediol. Food Res Int. 2018;113:424–432. doi: 10.1016/j.foodres.2018.07.034. [DOI] [PubMed] [Google Scholar]
- Perricone M, Corbo MR, Sinigaglia M, Speranza B, Bevilacqua A. Viability of Lactobacillus reuteri in fruit juices. J Funct Foods. 2014;10:421–426. doi: 10.1016/j.jff.2014.07.020. [DOI] [Google Scholar]
- Perricone M, Bevilacqua A, Altieri C, Sinigaglia M, Corbo MR. Challenges for the production of probiotic fruit juices. Beverages. 2015;1:95–103. doi: 10.3390/beverages1020095. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reale A, Renzo TD, Rossi F, Zotta T, Iacumin L, Preziuso M, Parente E, Sorrentino E, Coppola R. Tolerance of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus strains to stress factors encountered in food processing and in the gastro-intestinal tract. LWT—Food Sci Technol. 2015;60:721–728. doi: 10.1016/j.lwt.2014.10.022. [DOI] [Google Scholar]
- Richter RL, Vedamuthu ER. Milk and milk products. In: Downes FP, Ito K, editors. Compendium of Methods for the Microbiological Examination of Foods. 4. Washington DC: APHA; 2001. pp. 483–505. [Google Scholar]
- Rios-Corripio G, Welti-Chanes J, Rodríguez-Martínez V, Guerrero-Beltrán JA. Influence of high hydrostatic pressure processing on physicochemical characteristics of a fermented pomegranate (Punica granatum L.) beverage. Innov Food Sci Emerg Technol. 2020;59:102249. doi: 10.1016/j.ifset.2019.102249. [DOI] [Google Scholar]
- Saxelin M, Kajander K. Commercially available human probiotic microorganisms. In: Salminen S, editor. Handbook of probiotics and prebiotics. 2. New York: Wiley; 2009. pp. 469–475. [Google Scholar]
- Scheyhing CH, Hörmann S, Ehrmann MA, Vogel RF. Barotolerance is inducible by preincubation under hydrostatic pressure, cold-, osmotic-and acid-stress conditions in Lactobacillus sanfranciscensis DSM 20451T. Lett Appl Microbiol. 2004;39:284–289. doi: 10.1111/j.1472-765X.2004.01578.x. [DOI] [PubMed] [Google Scholar]
- Shah NP. Functional cultures and health benefits. Int Dairy J. 2007;17:1262–1277. doi: 10.1016/j.idairyj.2007.01.014. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Method Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Tabanelli G, Patrignani F, Vinderola G, Reinheimer JA, Gardini F, Lanciotti R. Effect of sub-lethal high pressure homogenization treatments on the in vitro functional and biological properties of lactic acid bacteria. LWT—Food Sci Technol. 2013;53:580–586. doi: 10.1016/j.lwt.2013.03.013. [DOI] [Google Scholar]
- Tripathi MK, Giri SK. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. 2014;9:225–241. doi: 10.1016/j.jff.2014.04.030. [DOI] [Google Scholar]
- Yáñez R, Marques S, Gírio FM, Roseiro JC. The effect of acid stress on lactate production and growth kinetics in Lactobacillus rhamnosus cultures. Process Biochem. 2008;43:356–361. doi: 10.1016/j.procbio.2007.12.014. [DOI] [Google Scholar]


