Abstract
As the coronavirus disease 2019 (COVID-19) pandemic strains the healthcare system, radiology residents across the United States have become a vital part of the redeployed workforce. Through a series of four cases of COVID-19 patients encountered on the wards, we highlight the insight and unique set of skills redeployed radiology residents possess that are essential to patient care during this crisis. By increasing visibility through active participation on the clinical team, we demonstrate the fundamental role radiology has in the greater field of medicine.
Keywords: COVID-19, Radiology, Redeployment, Patient care
Highlights
-
•
Radiology residents are an asset to the redeployed workforce and have meaningful contribution to patient care.
-
•
Dynamic imaging rounds became critical in clinical decision-making and expediting appropriate management.
-
•
Redeployment increases the visibility of radiology and highlights the essential role of the radiologist.
1. Introduction
The rapidly growing coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) created an enormous burden on the healthcare system. With millions of confirmed cases worldwide, the spread of COVID-19 and the severity of its symptoms have challenged many hospital systems facing inadequate space and supply of resources [1,2]. In addition to the shortage of personal protective equipment (PPE), there was a crucial ongoing need for staff since healthcare workers at the frontline are most at risk [[3], [4], [5]]. Many hospitals in New York City, the epicenter of the U.S. pandemic, required redeployment of healthcare providers including trainees from various specialties into roles on the frontlines directly managing COVID-19 patients.
Radiology residents were in a unique position having both general internship experience and radiology-specific knowledge. In addition, radiology departments deferred elective imaging during the crisis in order to conserve PPE and protect patients and staff, resulting in fewer cases to read and fewer image-guided procedures to perform [4,6]. For these reasons, radiology residents became a readily available cohort for redeployment. However, it was only during our first-hand experience on the medical wards when we realized that radiology residents were an asset to the redeployed workforce and had meaningful contributions to patient care. Through our newfound role on the medical team, we directly impacted clinical decision making by: 1) dynamic consultation on imaging during medical rounds, 2) prompt communication with the radiology department, including interventional radiology (IR), as imaging-related questions arose, 3) retrospective review of imaging to explain uncertain clinical findings, and 4) guidance on ordering appropriate imaging given a specific clinical scenario.
2. Case series
We report four cases of imaging related to COVID-19 morbidity and mortality that we encountered during our redeployment experience, illustrating the pivotal role that redeployed radiology residents have in patient care and management.
2.1. Case 1
A 38-year-old man with a history of hypertension presented with five days of subjective fevers, cough and pleuritic chest pain. In addition, he noted right calf pain for about two weeks. His initial chest radiograph demonstrated patchy peripheral opacities. A subsequent chest computed tomography (CT) scan revealed bilateral peripheral and predominantly posterior ground-glass opacities and consolidation, which can be seen with COVID-19 (Fig. 1A, B) [7]. It also revealed extensive filling defects within the distal left main pulmonary artery and all five lobar pulmonary arteries (Fig. 1C). Given the two findings, the possibility of pulmonary infarcts was mentioned in the impression.
Fig. 1.

A. Axial CT scan (lung windows) of Patient 1. Bilateral peripheral ground-glass opacities are predominantly posterior (black arrows), a pattern of findings commonly seen in COVID-19 infection.
B. Coronal image of the same patient. Note the dense wedge-shaped peripheral consolidation (black arrow). This morphology is highly suggestive of pulmonary infarction especially in the setting of extensive pulmonary emboli.
C. Axial contrast enhanced CT scan of the same patient. There is a filling defect within the distal left main pulmonary artery (black arrow). There were additional extensive filling defects involving all five lobar pulmonary arteries.
As radiology residents deployed to the medicine unit, we actively reviewed the patient's imaging with the team on rounds, specifically drawing attention to an area of dense peripheral wedge-shaped consolidation, which is atypical for COVID-19 [8,9]. On subsequent questioning at bedside, the patient reported episodes of hemoptysis which is a rare symptom and found in less than 1% of COVID-19 patients as reported in one study [10]. Active discussion of the imaging findings along with new clinical information led the team to strongly suspect pulmonary infarction. His anticoagulation was changed from enoxaparin to unfractionated heparin, which is readily reversible, as we became acutely aware of his potential for rapid decompensation.
Due to time constraints associated with a high volume of sick patients, the details of the initial report were overlooked. However, as integrated members of the team, we reemphasized the possibility of a pulmonary infarct during rounds which directly led to focused questioning at the bedside and subsequent changes in clinical management.
2.2. Case 2
A 77-year-old man with no past medical history presented with hypoxia and was found to be positive for SARS-CoV-2 on nasopharyngeal RT-PCR swab. He was also noted to have new atrial fibrillation with rapid ventricular rate on physical exam. His chest CT revealed an acute saddle pulmonary embolism with extension into the segmental and subsegmental branches of all lobes (Fig. 2A). There were findings of right heart strain as evidenced by flattening of the interventricular septum and relative enlargement of the right ventricle compared to the left ventricle (Fig. 2B) [11]. The patient developed persistent hypotension and tachycardia, thus meeting criteria for a massive pulmonary embolism [12,13].
Fig. 2.

A. Axial contrast enhanced CT scan of Patient 2. There are filling defects within the right and left main pulmonary arteries consistent with an acute saddle pulmonary embolus (black arrow).
B. Axial contrast enhanced CT scan of the same patient. Patient had flattening of the interventricular septum (black arrow) and relative enlargement of the right ventricle as compared to the left ventricle, indicating evidence of right heart strain secondary to an acute saddle pulmonary embolus.
Having had clinical experience in IR, we immediately recognized the need for an IR consultation to evaluate for possible intervention. We then promptly discussed the case with our colleagues and organized an interdisciplinary discussion between the primary medical team, IR and the critical care team. Hours later, the patient underwent a catheter-directed thrombolysis with IR with immediate clinical improvement, as evidenced by an intraprocedural decrease in pulmonary arterial pressures, increased systemic blood pressures and a normalized heart rate [14,15]. This case highlighted our unique position and ability to expedite communication between teams to better facilitate patient care in a time-sensitive manner.
2.3. Case 3
A 79-year-old man with a history of insulin-dependent diabetes was brought to the hospital by his family due to three days of confusion and a new “puffy left eye”. On physical exam, the patient had crepitus around his left eye. A chest radiograph demonstrated bilateral parenchymal opacities which can be seen with COVID-19, as well as extensive subcutaneous emphysema, pneumomediastinum, and trace apical pneumothoraces (Fig. 3 ). A follow up chest CT showed extensive pneumomediastinum and pneumopericardium thought to be in the setting of tracheobronchial trauma secondary to coughing (Fig. 4A). The medical team had questions about the etiology of the left eye crepitus. By carefully reviewing a same-day head CT, we revealed the extension of the thoracic subcutaneous emphysema cranially to the prevertebral soft tissues, skull base, and left preseptal orbital soft tissues (Fig. 4B). Our initiative helped the team better understand the extent of his disease and further explain his atypical physical exam findings.
Fig. 3.

Portable radiograph of Patient 3. Bilateral peripheral airspace opacities are suggestive of COVID-19 infection. There was suspicion for pneumomediastinum given a continuous diaphragm sign (white arrow) and associated subcutaneous emphysema (black arrow).
Fig. 4.

A. Axial CT scan (lung windows) of the same patient. The findings on chest radiograph were confirmed on the CT. There was evidence of spontaneous pneumothorax (red arrow), pneumopericardium (black arrow) and subcutaneous emphysema (white arrow) associated with parenchymal findings of COVID-19.
B. Axial CT scan of the same patient. The thoracic subcutaneous emphysema was found to extend to the skull base and left periorbital septal soft tissues (white arrow), explaining the patient's development of left eye crepitus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Case 4
A 53-year-old female healthcare worker presented with one week of dyspnea, fevers, and myalgias and was diagnosed with COVID-19. A lower extremity doppler that was positive for acute deep venous thrombosis (DVT) in the right peroneal vein. She was started on therapeutic enoxaparin. A day after the initiation of anticoagulation, the patient developed abdominal pain and thigh numbness. A non-contrast CT of the abdomen and pelvis revealed a large right retroperitoneal hematoma extending into and expanding the right psoas muscle as well as a small left iliopsoas intramuscular hematoma (Fig. 5 ).
Fig. 5.
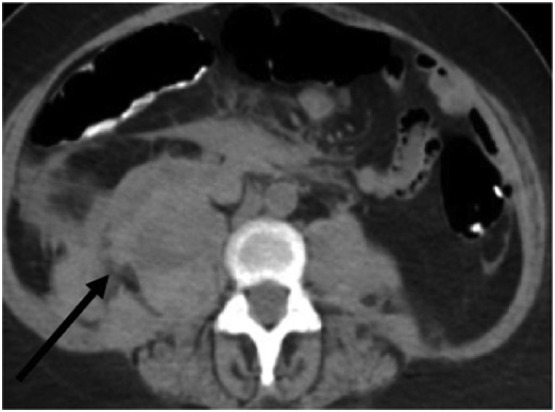
Axial non-contrast abdominal CT of Patient 4. Mixed attenuation within the retroperitoneal space represents a large right retroperitoneal hematoma extending into and expanding the right psoas muscle (black arrow).
Because the patient had an acute drop in hemoglobin and worsening abdominal pain, we recognized the need for possible intervention and immediately ordered CT angiography (CTA) as the next best diagnostic study. When the CTA demonstrated a focus of active extravasation, we consulted IR for an abdominopelvic angiography for possible embolization. As radiology residents, we knew the appropriate imaging to order when the patient was decompensating, promptly placed the electronic order ourselves, and communicated with our IR colleagues, expediting patient care.
3. Discussion
As the need for healthcare providers increased during the COVID-19 pandemic, radiology residents became a crucial part of the redeployed workforce and were able to make significant contributions to patient care. The face-to-face interaction with clinicians, real-time review of imaging findings, and facilitation of communication with the radiology department proved to be invaluable during our redeployment experience. Traditionally, radiology consultation with film rounds was considered standard clinical practice, and more recently, resident-driven clinical imaging rounds have been shown to have a positive impact on training, education, and patient outcomes [[16], [17], [18]]. During redeployment, imaging rounds became critical in clinical decision-making, expediting appropriate management, potentially avoiding unnecessary tests, and decreasing overall healthcare costs. The redeployment experience increased the visibility of radiology and gave us an opportunity to showcase our expertise, highlighting the fundamental role of the radiologist within the field of medicine.
As integrated members of the medical team, we gained a deeper understanding of the internal medicine workflow and the barriers clinicians face when trying to place orders, communicate with the department, or review imaging with a radiologist. This was particularly evident during a time of crisis when many providers were redeployed into roles that were unfamiliar to them and where normal workflow was disrupted. We addressed many of the team's radiology-specific concerns immediately as they arose, easing the administrative burden and allowing more focus on patients directly. In turn, we learned how to efficiently find clinical information through our electronic medical record system and how to communicate more effectively with our hospital's HIPAA approved mobile software.
In addition, we relied heavily on our colleagues for their clinical knowledge and developed a greater appreciation for the COVID-19 disease process and its associated characteristics. This clinical knowledge is essential in the interpretation of COVID-19-related imaging as we resume our role as radiology residents, enhancing our reports to more accurately answer the clinical question. For example, understanding the hypercoagulable state associated with the disease [19] will allow us to appropriately calibrate the pre-test probability for thrombotic events, including pulmonary emboli, deep venous thromboses, mesenteric and peripheral arterial thromboses, and acute infarctions. This, in turn, will aid in selecting appropriate protocols, tailoring image interpretation, and optimizing consultation and communication of results with the primary team.
On a personal level, we established relationships with many residents and attendings from various specialties which will undoubtedly affect our reporting and communication in the future. Working together under these unique circumstances boosted morale and helped strengthen the relationship between the radiology and internal medicine departments. This added benefit would not have been possible without being directly part of the medical team. Importantly, the toll that redeployment takes on mental health varies tremendously across healthcare workers even within the same hospital system [3,20,21]. However, interdependence within our team fostered a strong sense of purpose and camaraderie amongst the house staff, which may be beneficial when coping with the stresses of being on the frontlines of a pandemic.
Ultimately, redeployment to a medical team gave us a newfound perspective on the importance of radiology within the greater healthcare system. It reminded us first-hand how imaging can play a pivotal role in clinical decision-making, especially during this time of crisis. Direct communication between the radiologist and the clinical team through resident-driven clinical imaging rounds is not only beneficial for patients but can improve our diagnostic capabilities as well. In turn, radiology residents have the power to increase radiology visibility and reinforce the essential value of the radiologist.
Declaration of competing interest
Authors have nothing to disclose.
References
- 1.World Health Organization . 2020. Coronavirus disease (COVID-19) situation report - 109. [Google Scholar]
- 2.Khullar D., Bond A.M., Schpero W.L. COVID-19 and the financial health of US hospitals. JAMA. 2020 doi: 10.1001/jama.2020.6269. [DOI] [PubMed] [Google Scholar]
- 3.Adams J.G., Walls R.M. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020;323:1439–1440. doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- 4.Alvin M.D., George E., Deng F., Warhadpande S., Lee S.I. The impact of COVID-19 on radiology trainees. Radiology. 2020:201222. doi: 10.1148/radiol.2020201222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Strategies to mitigate healthcare personnel staffing shortages. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/mitigating-staff-shortages.html
- 6.Cavallo J.J., Forman H.P. The economic impact of the COVID-19 pandemic on radiology practices. Radiology. 2020 doi: 10.1148/radiol.2020201495. 201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 9.He Hongying W., Stein Marjorie B., Zalta Benjamin B., Haramati Linda B. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21:1–7. doi: 10.1097/01.rti.0000187433.06762.fb. [DOI] [PubMed] [Google Scholar]
- 10.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 1708;2020:382. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlan A., Aghayev A., Chang C.-C.H., Patil A., Jeon K.N., Park B. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265:283–293. doi: 10.1148/radiol.12110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soloff L.A., Rodman T. Acute pulmonary embolism: II. Clinical. American Heart Journal. 1967;74:829–847. doi: 10.1016/0002-8703(67)90102-0. [DOI] [PubMed] [Google Scholar]
- 13.Sista A.K., Kuo W.T., Schiebler M., Madoff D.C. Stratification, imaging, and management of acute massive and submassive pulmonary embolism. Radiology. 2017;284:5–24. doi: 10.1148/radiol.2017151978. [DOI] [PubMed] [Google Scholar]
- 14.Kuo M.D.W.T. Endovascular therapy for acute pulmonary embolism. J Vasc Interv Radiol. 2012;23:167–179.e4. doi: 10.1016/j.jvir.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Kuo W.T., Banerjee A., Kim P.S., DeMarco F.J., Levy J.R., Facchini F.R. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT) Chest. 2015;148:667–673. doi: 10.1378/chest.15-0119. [DOI] [PubMed] [Google Scholar]
- 16.Porter M.E. What is value in health care? N Engl J Med. 2010;363:2477. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 17.Cizman Z., Hammer M., Mollard B., Morgan R., Ballenger Z., Runner G.J. A resident perspective on adding value as radiologists. Acad Radiol. 2016;23:517–520. doi: 10.1016/j.acra.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Salama G.R., Sullivan C., Holzwanger D., Giambrone A.E., Min R.J., Hentel K.D. Improving care and education through a radiology resident-driven clinical consultation service. Acad Radiol. 2017;24:1175–1181. doi: 10.1016/j.acra.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg N., Docherty M., Gnanapragasam S., Wessely S. Managing mental health challenges faced by healthcare workers during covid-19 pandemic. BMJ. 2020;368 doi: 10.1136/bmj.m1211. [DOI] [PubMed] [Google Scholar]
- 21.Lima C.K.T., Carvalho PM de M., Lima I de A.A.S., Nunes JVA de O., Saraiva J.S., de Souza R.I. The emotional impact of coronavirus 2019-nCoV (new coronavirus disease) Psychiatry Res. 2020:287. doi: 10.1016/j.psychres.2020.112915. [DOI] [PMC free article] [PubMed] [Google Scholar]


