Abstract
Background and aims
Given the limited information describing the connection between metabolic syndrome (MetS) and Coronavirus Disease 2019 (COVID-19), we aimed to assess the impact of MetS on morbidity and mortality among COVID-19 patients.
Methods
This retrospective cohort study was performed from 1st April to May 3, 2020 on 157 ICU-admitted COVID-19 patients in Shahid Modarres Hospital in Tehran, Iran. Patients’ clinical, laboratory and radiological findings, and subsequent complications, were collected and compared between MetS and non-MetS groups.
Results
74 of all cases had MetS. Among the MetS components, waist circumference (p-value = 0.006 for men; p-value<0.0001 for women), Triglycerides (p-value = 0.002), and Fasting Blood Sugar (p-value = 0.007) were significantly higher in MetS group; with no statistical difference found in HDL levels (p-value = 0.21 for men; p-value = 0.13 for women), systolic blood pressure(p-value = 0.07), and diastolic blood pressure (p-value = 0.18) between two groups. Length of ICU admission (p-value = 0.009), the need for invasive mechanical ventilation (p-value = 0.0001), respiratory failure (p-value = 0.0008), and pressure ulcers (p-value = 0.02) were observed significantly more in MetS group. The Odds Ratio (OR) of mortality with 0(OR = 0.3660), 1(OR = 0.5155), 2(OR = 0.5397), 3(OR = 1.9511), 4(OR = 5.7018), and 5(OR = 8.3740) MetS components showed an increased mortality risk as the components’ count increased. The patient with BMI>40 (OR = 6.9368) had more odds of fatality comparing to those with BMI>35 (OR = 4.0690) and BMI>30 (OR = 2.5287). Furthermore, the waist circumference (OR = 8.31; p-value<0.0001) and fasting blood sugar (OR = 2.4588; p-value = 0.0245) were obtained by multivariate logistic regression as independent prognostic factors for mortality.
Conclusion
The findings suggest a strong relationship between having MetS and increased risk of severe complications and mortality among COVID-19 ICU-admitted patients.
Keywords: COVID-19, Metabolic syndrome, Diabetes
Highlights
-
•
There is limited information about the connection between metabolic syndrome (MetS) and the outcome of COVID-19 patients.
-
•
The hospitalized COVID-19 cases with MetS undergoing intensive cares are more likely to have severe clinical course.
-
•
The need for prioritizing screening, detection, and aggressive therapy in MetS patients must be considered.
-
•
MetS is associated with increased risk of severe complications and mortality among COVID-19 ICU-admitted patients.
Abbreviations
- MetS
Metabolic Syndrome
- COVID-19
Coronavirus Disease 2019
- SARS-CoV-2
Severe Acute Respiratory Coronavirus-2 Syndrome
- WHO
World Health Organization
- CDC
Centers for Disease Control and Prevention
- BMI
Body Mass Index
- ICU
Intensive care units
- ARDS
Acute respiratory distress syndrome
- AKI
Acute kidney injury
- ALI
Acute liver injury
- ACI
Acute cardiac injury
- NCEP
National Cholesterol Education Program
- ATP
Adult Treatment Panel
- IIV3
Trivalent Inactivated Influenza Vaccine
- ACE2
Angiotensin Converting Enzyme 2
1. Introduction
The pandemic of novel Coronavirus Disease 2019 (COVID-19) has raised a global health concern [1] and the rapidity of its spread and transmission along with its variable mortality among different countries has remained as an enigma [2]. We are indeed still learning, what exactly put someone at high risk of severe outcomes and death due to COVID-19. But what we do know, is that cases at risk for an adverse prognosis of Severe Acute Respiratory Coronavirus-2 syndrome (SARS-CoV-2) were described to have pre-existing conditions like cancer, chronic respiratory disease, diabetes, cardiovascular disease or hypertension, as well as being elderly [3], [4], [5], [6], [7]. Metabolic syndrome (MetS) as a common metabolic disorder in general population [8] is defined by World Health Organization (WHO) as a pathologic state determined by abdominal obesity, insulin resistance, hyperlipidemia, and hypertension(8). Even though it might not be a disease per se, it is a term that highlights traits that may have an increased risk of comorbidities [9]. Accordingly, as reported by the Centers for Disease Control and Prevention (CDC), it is estimated that individuals with MetS following diabetes mellitus type 2 might have up to ten times greater risk of death due to COVID-19 [10]. Hyperglycemia as one of the defining factors of MetS, was obtained as an independently important predictive factor of mortality and morbidity in cases with COVID-19, and its control was considered as key in COVID-19 patients [11]. Moreover, individuals with higher Body Mass Index (BMI), which is also an indicator of MetS, may be more susceptible to develop a severe course of COVID-19; to the extent that, for 4103 COVID-19 patients in New York City, the United States, BMI>40 kg/m2 was revealed to be the second independent predictor of hospitalization after old age [12]. Obesity in itself is likely to be a prominent risk factor of increased mortality and morbidity among COVID-19 cases [13]. Given the widely recognized fact that the existence of the comorbidities is notably related to the seriousness of clinical course and complications of COVID-19 infection, exploring the connection between MetS and the severity of COVID-19 has been of major clinical significance and well-conducted studies to elucidate these associations are urgently needed. So, this study is aimed at providing an overview of MetS and its impact on clinical course, complications, prognosis, and mortalities in patients presenting with COVID-19.
2. Methods
Study design: The current retrospective cohort research was carried out from 1st April to May 3, 2020 on 157 SARS-CoV2 infected patients they had been admitted to the intensive care units (ICU) of Shahid Modarres Hospital, in Tehran, Iran. Shahid Modarres Hospital is a tertiary referral center having 279 beds in which determined as one of the major referral hospitals during the outbreak of COVID-19. All the patients enrolled in the study were admitted based on WHO confirmation guidelines [14], [15]; and the patient’s nasopharyngeal swab sample was confirmed by RT- PCR test for COVID-19 diagnosis during their hospitalization. The written informed consent was obtained from all the cases or their families to utilize their medical history for research purposes.
Participants and Procedures: Clinical charts and nursing records of laboratory-confirmed COVID-19 patients were reviewed. Demographic and Anthropometric data, as well as clinical characteristics and laboratory findings, were gathered with designed data collection forms. Three types of research separately reviewed and triple-checked the obtained data. Missing data were gathered by the direct contact of medical researchers or family medicine physicians with a family member of the patients. We included the COVID-19 ICU admitted patients with a final status of being either discharged or expired. Among 237 eligible patients, 63 cases were excluded due to insufficient anthropometric and laboratory data. Also, 17 patients who had malignancies and were under chemotherapy/radiotherapy have been further excluded from the research because of the particular condition of such patients [16]. So overall, 157 cases have been included in the analysis, of whom 74 patients had the MetS and 83 patients were placed in the none-MetS group as a comparison.
Definitions: Even though there are various definitions for MetS, we used the clinic-oriented nature of the National Cholesterol Education Program (NCEP)-Adult Treatment Panel (ATP)-III criteria [17] because of the widespread use of these criteria in adults and availability of required data from our patient’s medical records. Therefore, MetS diagnosis was accomplished considering the presence of three or more of the further-mentioned measures; a fasting blood glucose surpassing 110 mg/dL, an arterial blood pressure exceeding 130/85 mmHg, a waist circumference of over 102 cm for male, and 88 cm for female individuals, serum triglyceride level of more than 150 mg/dl, serum HDL concentration<40 mg/dl for male, and <50 mg/dl for female individuals. Also, the treatment therapy for each factor of diabetes, hypertension, and dyslipidemia was considered as one criterion. Of note, the levels of waist circumference and HDL which are defined differently for men and women(16), were considered to be reported and analyzed separately for each gender. The disease onset date was considered as the day when the onset of symptoms was acclaimed. Acute kidney injury (AKI) was defined based on the levels of serum creatinine(17). Acute cardiac injury (ACI) was identified based on the serum troponin-I in the state of being above the 99th percentile upper reference limit [18]. Acute liver injury (ALI) was defined as the presence of coagulopathy and hepatic encephalopathy [19]. Acute respiratory distress syndrome (ARDS) was defined based on the Berlin definition [20]. Sepsis and septic shock were considered under the Third International Consensus Definitions for Sepsis and Septic Shock [21].
Statistical analysis: The primary analysis was a comparison of epidemiologic and clinical characteristics between MetS group and non-MetS group. The complications that occurred during ICU admission, were compared between the two groups. Descriptive data of continuous variables and categorical variables were demonstrated as median (IQR) and number [%], respectively. Chi-square test, Fisher’s exact test, and Mann-Whitney U test have been taken into use for the comparison of outcomes between the two groups, as necessary. Multivariable logistic-regression analysis has been performed to assess the MetS components’ effects considering wrist circumference, increased blood glucose, elevated blood pressure, low HDL, and high levels of triglyceride on the likelihood of COVID-19 mortality in the ICU. Also, the relation between the number of MetS components as well as BMI categories (BMI> 30, BMI> 35, and BMI> 40), based on the critical role of BMI on the occurrence of MetS [22], on the mortality of ICU admitted patients were ascertained. The odds ratios and confidence intervals of 95% have been reckoned. A two-sided α of less than 0·05 has been deemed as statistically significant. Furthermore, the median clinical course of significant signs and also complications of two groups were designed in figures as a comparison. The statistical examinations were conducted utilizing the IBM SPSS statistics (version: 22.0).
3. Results
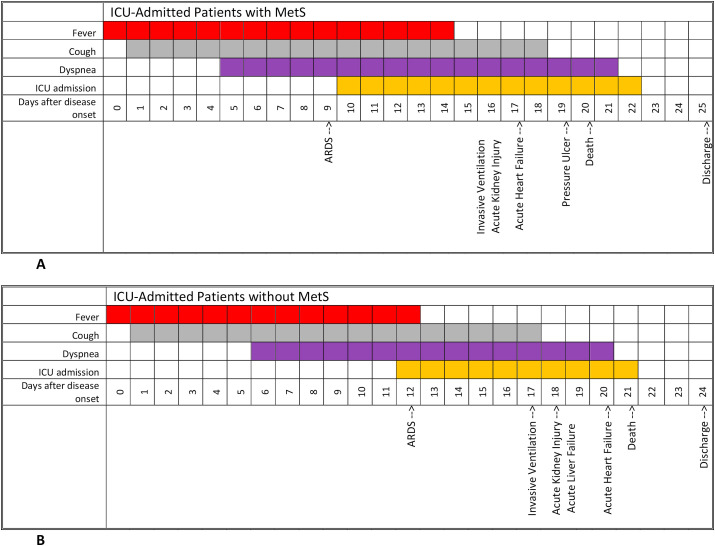
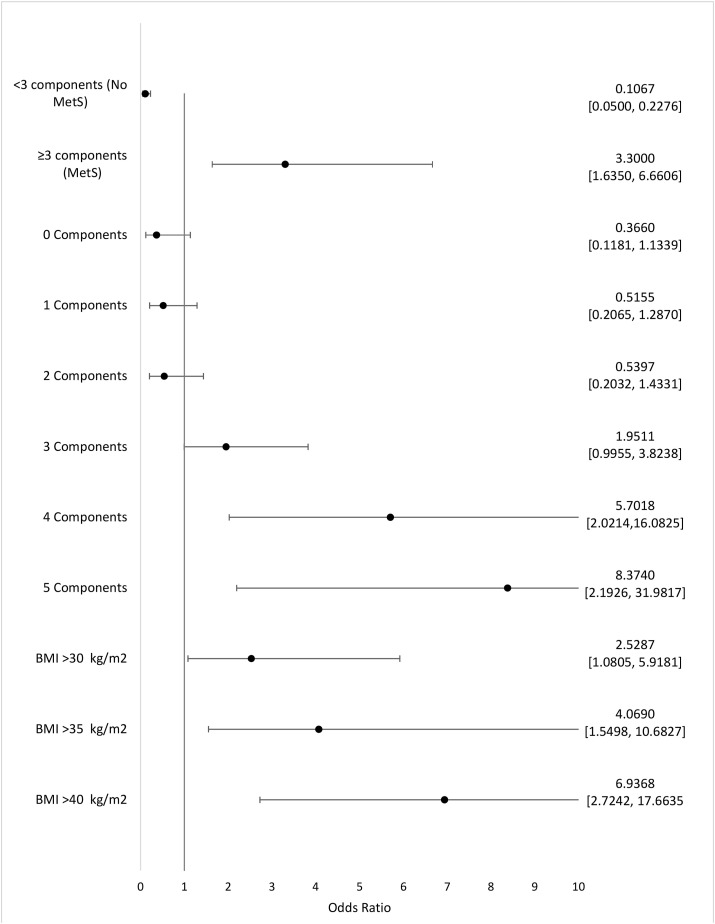
Overall, 157 cases have been included in this study; 74 (47.1%) patients had MetS and 83 (52.9%) cases were placed in Non-MetS group as a comparison. 66 (89.2%) of MetS group and 72 (86.74%) of non-MetS group were men which shows a prepotency of male gender among ICU admitted patients in both groups. The difference between age of MetS patients [66(63–70) years] and non-MetS patients [69 (64–72) years], was not significant (P-value = 0.09). Pack-year history of smoking in non-MetS group was significantly more than MetS group (p-value = 0.03). A marked High BMI, (P = 0.02) and large Waist circumference were obtained in patients with MetS as shown in Table .1 . Among the MetS components, waist circumference (men: p-value = 0.006; women: p-value <0.0001), triglyceride (p-value = 0.002), and fasting blood glucose (p-value = 0.007) had significantly higher amount in MetS group. Moreover, individuals with MetS were strongly known to be at increased risk of presenting Ischemic heart diseases (p-value = 0.03), Diabetes (p-value = 0.008), and Dyslipidemia (p-value = 0.04) but this difference was not significant in Chronic Respiratory diseases, Chronic kidney diseases and Hypertensive disorders. As shown in Table .1, the clinical manifestation of Respiratory Rate (p-value = 0.008), and Blood Oxygenic Saturation< 75% (p-value = 0.04) in MetS group showed significant differences in comparison to non-MetS group; while no static difference was observed in other presentations and signs. As the laboratory findings are summarized in Table .1, in the MetS group, the initial white blood cell counts (p-value = 0.01), D-dimer (p-value = 0.02), Lactate dehydrogenase (p-value = 0.006), and Hemoglobin A1C (p-value = 0.005), were significantly increased in comparison with non-MetS group. Also, lymphocyte count was obtained significantly lower in MetS group (p-value <0.0001). In radiologic characteristics, patients with MetS presented a marked Bilateral infiltration compared to non-MetS group (p-value = 0.02). Fig. 1 illustrates a visual presentation of how the courses of some important features compare between MetS patients and non-MetS patients. Starting with fever, as it is shown in the Chart, the median period for which the patients had been febrile, were longer among those with MetS (14 days vs. 12 days). Also, while the median length of coughing was just slightly greater in MetS patients (18 days vs. 17 days), the median number of days for which cases were admitted in ICU, was notably greater among individuals with MetS (13 days vs. 10 days). Moreover, as it can be interpreted from Fig. 1, dyspnea and the ICU admission had in fact, started respectively 1 and 2 days earlier in the MetS cohort, in comparison to non-MetS cohort. Besides, almost all of the concerning complications had taken place sooner in MetS patients; as an instance ARDS and acute heart failure had both occurred 3 days sooner among those with MetS (day 9 vs. day 12, and day 17 vs. day 20, respectively). The onset of AKI and ALI as another two complications were both reported to be 2 days earlier in MetS patients (day 16 vs. day 18). In addition to that, Invasive ventilation and death had both happened 1 day later among non-MetS patients (day 17 vs. day 16, and day 21 vs. day 20, respectively). While expectedly, the discharge had taken place 1 day sooner for non-MetS patients (day 24 vs. day 25). Table .2 attempts to demonstrate the comparison of various clinical features and complications of the two groups. The period of stay within ICU had a median of 8 days for patients without MetS, and 10 days for patients with MetS, marking a significant difference (p-value = 0.009). Also, the median length of total stay within hospital among patients with MetS has been 18 days which was significantly greater than the 11-day stay recorded for those who didn’t have MetS (p-value = 0.02). Additionally, the need for invasive mechanical ventilation was observed remarkably more in patients with MetS; mechanical ventilation was utilized in 77.0% of MetS patients, whereas 46.9% of patients without MetS needed it, underlining a p-value of 0.0001. Another important index with a significant difference between the two groups was SOFA score upon admission, which the median score has been 4 for MetS patients and 3 for non-MetS patients (p-value = 0.01). Several complications were observed in patients, two of which highlighted a significant difference; respiratory failure and pressure ulcer. The former was seen in 78.4% of MetS patients and 53.0% of non-MetS patients (p-value = 0.0008) and the latter was seen in 13.5% and 3.6% of MetS and non-MetS patients respectively. Other mentionable complications that were noted more frequently among MetS patients are as follows; pulmonary embolism (p-value = 0.22), septic shock (p-value = 0.88), AKI (p-value = 0.10), ALI (p-value = 0.17), ACI (p-value = 0.12), myocardial infarction (p-value = 0.32), and coagulopathy (p-value = 0.087). But as stated before, the recorded differences for these complications were indeed not significant. As it is indicated in Table .3 , regarding the mortality of ICU-admitted SARS-CoV-2 cases, the Odds ratio for those with MetS is 3.3, which represents the odds of mortality in SARS-CoV-2 cases with MetS, compared to that in patients without MetS (p-value = 0.0009). Accordingly, the Odds ratio for patients with no components of MetS was 0.3660, whereas the Odds ratio of mortality in patients with 1, 2, 3, 4 and 5 of MetS defining components were 0.5155, 0.5397, 1.9511, 5.7018, and 8.3740 respectively. Table .3 also shows how a BMI of over 30 is significantly effective in increasing the Odds ratio of mortality. In fact, as the BMI increases the Odds ratio rises as well; when the BMI is over 30 the Odds ratio is 2.5287 with a significant p-value of 0.0325, when the BMI is over 35 the Odds ratio is 4.0690 with a significant p-value of 0.0044, and when the BMI rises above 40 the Odds Ratio is 6.9368. (P-value<0.0001). This is also illustrated in Fig. 2 , where having 3 or more components of MetS has resulted in an odds ratio of more than 1, while having 2, 1 or no components has put the Odds ratio mark before x = 1 line. In fact, the statistical analyses revealed, that as the number of MetS defining factors goes up, the Odds ratio of mortality increases. Although, it is crucial to mention that these results are statistically significant only for having 4 and 5 MetS components, with a p-value of 0.0010 and 0.0002, respectively. Another important statistical finding was that the Odds Ratio of mortality in patients without MetS is 0.1067, which considering how it’s way less than 1, means that not having MetS decreases the odds of mortality as an outcome (p-value<0.0001). In a quick glance over Fig. 2, it’s also comprehensible that the Odds Ratio of mortality in patients with a BMI of over 30 is noticeably elevated. It’s quite clear how the mere rise in BMI (from 30 to 40) can raise the Odds ratio exponentially for patients, posing a significantly effective risk factor for poor prognosis. Table .4 shows the extent to which different defining components of MetS affect the odds of mortality. The most effectual MetS components are increased waist circumference and increased blood glucose which resulted in an Odds ratio of 8.3171 and 2.4588, respectively marking a significant difference with p-values of <0.0001 and 0.0245, respectively. The other three defining factors of MetS, including Increased blood pressure, Impaired levels of Triglyceride, and Impaired levels of HDL, created Odds ratios only slightly more than 1; being respectively 1.6716, 1.2045, and 1.08032. (P-value = 0.1381, 0.6010, and 0.0906 respectively).
Table 1.
Comparison of MetS components, demographics, and clinical information upon arrival to hospital (a Median (IQR);b n [%]).
| With MetS (n = 74) | Without MetS (n = 83) | p-value | |
|---|---|---|---|
| Primary information | |||
| Age (years)a | 66 (63–70) | 69 (64–72) | 0.09 |
| Gender (male)b | 66[89.2%] | 72[86.74%] | 0.63 |
| Smoker (pack/year)b | 9 [[5], [6], [8], [9], [10], [11], [12], [13], [14], [16], [23]] | 10.5 [[8], [9], [10], [11], [12], [13], [23]] | 0.03 |
| Body mass index (kg/m2)a | |||
| Male | 26.1(23–28.6) | 24.9(22.4–25.9) | 0.02 |
| Female | 30(28.6–32.2) | 27.2(25.3–30.1) | <0.0001 |
| MetS components | |||
| Waist circumference (cm) a | |||
| Male | 94(87–97) | 87 (82–93) | 0.006 |
| Female | 95 (84–100) | 89 (74–95) | <0.0001 |
| Systolic blood pressure (mmHg)a | 136(123–145) | 134(121–143) | 0.07 |
| Diastolic blood pressure (mmHg)a | 82(72–87) | 80(69–86) | 0.18 |
| HDL (mg/dL) a | |||
| Male | 34(32–34) | 39(36–42) | 0.21 |
| Female | 31(30–33) | 38(36–39) | 0.13 |
| Triglyceride (mg/dL)a | 171(151–186) | 138(116–141) | 0.002 |
| >200 b | 13 [17.6%] | 7 [8.4%] | 0.09 |
| Fasting blood glucose (mg/dL)a | 104 (91–124) | 101(84–108) | 0.007 |
| >127 b | 15[20.3%] | 9[10.8%] | 0.12 |
| Underlying diseaseb | |||
| Hypertensive disorders | 48 [64.9%] | 49 [59.0%] | 0.45 |
| Ischemic heart disease | 38 [51.3%] | 29 [34.9%] | 0.03 |
| Diabetes | 45 [60.8%] | 33 [39.7%] | 0.008 |
| Dyslipidemia | 30 [40.5%] | 21 [25.3%] | 0.04 |
| Chronic Respiratory diseases | 18 [24.3%] | 24 [28.9%] | 0.46 |
| Chronic kidney diseases | 10 [13.5%] | 8 [9.6%] | 0.44 |
| Sing and symptoms | |||
| Temperaturea | 37.3 (37.1–38) | 37 (36.9–37.4) | 0.48 |
| T > 39.5 °C b | 14 [18.9%] | 11 [13.2%] | 0.38 |
| Heart ratea | 94(84–103) | 96(83–100) | 0.23 |
| Respiratory ratea | 26(22–28) | 23(19–27) | 0.008 |
| Blood oxygenic saturationa | 87(83–91) | 90(86–93) | 0.0003 |
| <75 b | 10 [13.5%] | 3[3.6%] | 0.04 |
| Shortness of breathb | 70 [94.6%] | 81 [97.5%] | 0.42 |
| Coughingb | 42 [56.7%] | 38 [45.8%] | 0.20 |
| Muscle acheb | 50 [67.6%] | 63 [75.9%] | 0.28 |
| Diarrheab | 11 [14.9%] | 9 [10.8%] | 0.48 |
| Nausea or Vomitingb | 17 [23.0%] | 25 [30.1%] | 0.36 |
| Anosmia/hyposmiab | 12 [%16.2] | 11 [13.2%] | 0.65 |
| Ageusia/dysgeusiab | 9 [12.2%] | 13 [15.7%] | 0.64 |
| Laboratory findings | |||
| White blood cell count × 109 per littera | 8.9 (5.9_13.6) | 8.6(5.4–12.3) | 0.01 |
| >11b | 32 [43.2%] | 19 [22.9%] | 0.01 |
| Lymphocyte × 109 per littera | 0.7 (0.5–1,0) | 1.1(0.8–1,1) | <0.0001 |
| <0.8 b | 53 [71.6%] | 38 [45.8%] | 0.001 |
| D-dimer mcg/Mla | 4.5(1.6–15.1) | 4.3(1.5–14.9) | 0.02 |
| >2 b | 31 [41.9%] | 29 [39.2%] | .041 |
| Lactate dehydrogenase, U/La | 393(312–523) | 306(239–408) | 0.006 |
| >280 b | 69 [93.2%] | 63 [75.9%] | 0.003 |
| Total cholesterola | 206(193–217) | 188(176–196) | 0.09 |
| >240 b | 11[14.9%] | 4 [4.8%] | 0.06 |
| Hemoglobin A1Ca | 7(6–7.7) | 5.1(3.8–6.1) | 0.005 |
| >6.4 b | 17[23.0%] | 8[9.6%] | 0.02 |
| Radiological findingsb | |||
| Consolidation | 53 [71.6%] | 48 [57.8%] | 0.07 |
| Bilateral infiltration | 43 [58.1%] | 33 [39.7%] | 0.02 |
| Ground glass opacity | 46 [62.2%] | 40 [48.2%] | 0.10 |
Fig. 1.
The comparison of median range of major symptoms, along with clinical course and complications’ occurrences between A: ICU-Admitted Patients with MetS, & B: ICU-Admitted Patients without MetS.
Table 2.
Comparison of different clinical features and complications (a Median (IQR);b n [%]).
| MetS patients (n = 74) | Non-MetS patients (n = 83) | p-value | |
|---|---|---|---|
| ICU length of staya | 10 [[6], [8], [9], [10], [11], [12], [13], [14], [23]] | 8 [[5], [6], [8], [9], [10], [11], [12], [13], [23]] | 0.009 |
| Hospital length of staya | 18 [[9], [10], [11], [12], [13], [14], [16], [17], [24]] | 11 [[9], [10], [11], [12], [13], [14], [16]] | 0.02 |
| Invasive mechanical ventilationb | 57 [77.0%] | 39 [46.9%] | 0.0001 |
| SOFA score (upon admission to ICU)a | 4 [[2], [3], [4], [5], [6], [8], [23]] | 3 [[2], [3], [4], [5], [6], [8]] | 0.01 |
| Complicationsb | |||
| Acute Respiratory Distress Syndrome (ARDS) | 60 [81.1%] | 72 [86.7%] | 0.33 |
| Respiratory failure | 58 [78.4%] | 44 [53.0%] | 0.0008 |
| Pulmonary embolism | 2 [2.7%] | 0 [0%] | 0.22 |
| Secondary infection | 13 [17.6%] | 15 [18.1%] | 0.93 |
| Septic shock | 3 [4.1%] | 3 [3.6%] | 0.88 |
| Acute kidney failure | 39 [52.7%] | 33 [39.7%] | 0.10 |
| Acute liver failure | 41 [55.4%] | 37 [44.6%] | 0.17 |
| Acute cardiac injury | 11 [14.9%] | 6 [7.2%] | 0.12 |
| Myocardial infarction | 4 [5.4%] | 2 [2.4%] | 0.32 |
| Coagulopathy | 23 [31.1%] | 16 [19.3%] | 0.087 |
| Hyperproteinemia | 7 [9.5%] | 13 [15.7%] | 0.24 |
| Acidosis | 45 [60.8%] | 51 [61.4%] | 0.93 |
| Pressure Ulcer | 10[13.5%] | 3 [3.6%] | 0.02 |
Table 3.
Odds Ratios of different risk factors for COVID-19 mortality in ICU-admitted patients, including the state of having MetS, not having Mets, and having different number of MetS components, along with BMI categories.
| Odds ratio (95% Confidence level) | p-value | ||
| Absence of MetS (<3 components) | 0.1067 | 0.0500–0.2276 | <0.0001 |
| Presence of MetS (≥3 components) | 3.3000 | 1.6350–6.6606 | 0.0009 |
| Number of components for MetS | |||
| 0 | 0.3660 | 0.1181–1.1339 | 0.0815 |
| 1 | 0.5155 | 0.2065–1.2870 | 0.1557 |
| 2 | 0.5397 | 0.2032–1.4331 | 0.2158 |
| 3 | 1.9511 | 0.9955–3.8238 | 0.0515 |
| 4 | 5.7018 | 2.0214–16.0825 | 0.0010 |
| 5 | 8.3740 | 2.1926–31.9817 | 0.0002 |
| Body Mass Index (BMI) | |||
| >30 kg/m2 | 2.5287 | 1.0805–5.9181 | 0.0325 |
| >35 kg/m2 | 4.0690 | 1.5498–10.6827 | 0.0044 |
| >40 kg/m2 | 6.9368 | 2.7242–17.6635 | <0.0001 |
Fig. 2.
Relation of having MetS, not having MetS, the existence of 0–5 components of MetS, and different BMI categories, to mortality.
Table 4.
Multivariate analysis of COVID-19 mortality in the intensive care unit according to metabolic risk-factors.
| Odds ratio (95% Confidence level) | p-value | ||
|---|---|---|---|
| Increased waist circumference | 8.3171 | 3.7331–18.5301 | <0.0001 |
| Increased blood glucose | 2.4588 | 1.1228–5.3846 | 0.0245 |
| Increased blood pressure | 1.6716 | 0.876–3.2965 | 0.1381 |
| Impaired levels of HDL | 1.08032 | 0.9110–3.5692 | 0.0906 |
| Impaired levels of Triglyceride | 1.2045 | 0.5998–2.4187 | 0.6010 |
4. Discussion
MetS, as a maladaptation to a modern world, has been considered as one of the most common reasons for morbidity and mortality in the communities, and it is emerging to become a principal health issue. The global prevalence of MetS has been increased from 1.1% in 1980 to 3.85% in 2015(8). However, it needs to be notified that the increasing numbers of MetS individuals show an uneven distribution due to the societal lifestyle and variations based on age, gender, and ethnicity among different communities. It seems that the risen prevalence of MetS in older COVID-19 patients in Iran and Italy, in comparison to China, may be the cause of differences in mortality between the countries. Moreover, the increasing prevalence of MetS in many affected countries by COVID-19, and also the previous experience with the role of MetS on 2009 H1N1 Influenza patient mortality should increase the sensitivity of physicians, caring for COVID-19 patients with MetS. We have gained multiple pieces of information about influenza in patients suffering from MetS, which were expected to be in line with the novel coronavirus outbreak. Several reports around the world has identified obesity and MetS as prognostic factors of hospitalization, ICU admission and the need for invasive ventilation. During the 2009 H1N1 pandemic, obesity (BMI>30) was known to be an independently substantial risk factor for severe complications among cases infected by influenza [25]. Of instance, In a study of 1088 hospitalized/expired H1N1 Influenza patients between April and August 2009 in California, United States, the prevalence of BMI >30 and BMI >40 was 58% and 67% respectively, and 66% of those patients with BMI >30 had underlying diseases related to MetS considering diabetes and hypertensive disorders [26]. Additionally, in a cross-sectional study of B. Bijani et al. on patients afflicted with 2009 influenza H1N1, MetS was revealed to be considered as an independent prognostic factor for blood oxygenic saturation of less than 90% (OR = 18.66; 95% CI, 1.60 to 217.47; p = 0.019) [27]. Of equal emphasis, a study has shown two times greater risk of influenza-like syndromes in individuals with BMI >30 who were vaccinated with Trivalent Inactivated Influenza Vaccine (IIV3) in comparison to normal BMI population, despite receiving influenza vaccine [28]. Based on what we obtained from this study, the respiratory failure (p-value = 0.0008) and invasive ventilation requirement (p-value = 0.0001) were the complications observed significantly higher in Mets group. Similar to what we revealed, a recent retrospective study by A. Simonnet et al. which evaluated the relationship between BMI and invasive ventilation requirement among 124 COVID-19 ICU admitted patients [29], it has been revealed the proportion of invasive ventilation raised as the BMI level increased (p-value<0.01). Etiologically, elevated BMI levels are shown to be related with decreased compliance of pulmonary system, functional capacity, and expiratory reserve volume which worsen the condition of the patient in respiratory infections. Increased peripheral obesity, cause the respiratory function to become more compromised in supine position due to reduced diaphragmatic excursion, making the ventilation more difficult [30]. Pathologically, patients with obesity and MetS have increased inflammatory mediators considering interleukin 6 (IL-6) which may have effects on the pulmonary parenchyma and bronchus [31,32]. The abnormal increase in the secretion of cytokines and adipokines including interferons and TNF-alpha points to a chronic inflammation in peripheral obesity, and may therefore cause an impaired immune response [33]. Also, obesity and MetS is revealed to impair the immune responses to infection based on the damages of macrophages effective response and related immune pathways [34]. We also found that imbalanced blood glucose independently increases the rate of mortality in the COVID-19 patients. Similarly, the evidence suggests that hyperglycemia, especially during the hospitalization, aggravate the prognosis of COVID-19 patients in both diabetic/MetS patients and non-diabetic ones [35,36]. There are two main causes why increased levels of glycaemia, notably in acute states, is associated with adverse prognosis during COVID-19 infection. The first one is that an elevated blood glucose is associated with a significant increase of inflammatory mediators [37]; Understanding the role of cytokine storming and progressed states of COVID-19 infection [38] will emphasize how important it is to be alert to patient’s blood glucose and subsequent induced cytokine release. The second reason is the virus attachment to angiotensin converting enzyme 2 (ACE2) [39]. The glycosylation of the ACE2, which could be increased in the state of hyperglycemia, is required for the binding of SARS-CoV-2 to the cellular receptors [40]. Therefore, elevated glycosylation of ACE2 in different organs which is induced by uncontrolled blood glucose may suggest the higher cellular invasion of SARS-CoV2 and subsequently increased severity of COVID-19 infection and its complications. Of note, the impact of COVID-19 also will not be limited to the ICU. The obese individuals who are self-isolating and minimizing contact with society to be protected against the virus, are already stigmatized and suffering from different levels of depression and psychological problems. More than every other time, the clinicians need to pay attention to psychological aspects of obese patients in this pandemic. As conclusion, based on what we obtained in our study, and the discussion about the experience of 2009 H1N1 Influenza pandemic, it is important to exercise more caution about the screening and treatment of patients with obesity, especially those with higher number of comorbidity history regarding MetS. The hospitalized COVID-19 patients with MetS who should undergo intensive cares are specifically susceptible to have more severe clinical course, respiratory complications, and mortality. These findings underline the need for prioritizing screening, detection, and aggressive therapy in such patients.
Declaration of competing interest
Authors declare no conflict of interests.
References
- 1.World Health O. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 2020 [
- 2.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(3):211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malekpour Alamdari, Afaghi, Rahimi, Esmaeili Tarki, Tavana, Zali Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran. The Tohoku Journal of Experimental Medicine. 2020;252:73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 8.Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 9.Samson S.L., Garber A.J. Metabolic syndrome. Endocrinol Metab Clin N Am. 2014;43(1):1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Covid C., Covid C., Covid C., Chow N., Fleming-Dutra K., Gierke R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR (Morb Mortal Wkly Rep) 2020;69(13):382. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020:1–2. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020:1–2. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan D.H., Ravussin E., Heymsfield S. COVID 19 and the patient with obesity–the editors speak out. Obesity. 2020 doi: 10.1002/oby.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization W.H. World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. [Google Scholar]
- 15.safari, keyvani, alamdari Abdominal Surgery in Patients with COVID-19: Detection of SARS-CoV-2 in Abdominal and Adipose Tissues. Annals of surgery. 2020;272 doi: 10.1097/sla.0000000000004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Quteimat O.M., Amer A.M. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020 doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsy R.J. The National Cholesterol education Program adult treatment Panel III guidelines. J Manag Care Pharm. 2003;9(1 Suppl):2–5. doi: 10.18553/jmcp.2003.9.s1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canbay A., Tacke F., Hadem J., Trautwein C., Gerken G., Manns M.P. Acute liver failure: a life-threatening disease. Dtsch Arztebl Int. 2011;108(42):714–720. doi: 10.3238/arztebl.2011.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azoulay E., Mokart D., Kouatchet A., Demoule A., Lemiale V. Acute respiratory failure in immunocompromised adults. Lancet Respir Med. 2019;7(2):173–186. doi: 10.1016/S2213-2600(18)30345-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnlov J., Sundstrom J., Ingelsson E., Lind L. Impact of BMI and the metabolic syndrome on the risk of diabetes in middle-aged men. Diabetes Care. 2011;34(1):61–65. doi: 10.2337/dc10-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saklayen M.G. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 25.Louie J.K., Acosta M., Winter K., Jean C., Gavali S., Schechter R. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. Jama. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 26.Venkata C., Sampathkumar P., Afessa B. Hospitalized patients with 2009 H1N1 influenza infection: the Mayo Clinic experience. Mayo Clin Proc. 2010;85(9):798–805. doi: 10.4065/mcp.2010.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bijani B., Pahlevan A.A., Qasemi-Barqi R., Jahanihashemi H. Metabolic syndrome as an independent risk factor of hypoxaemia in influenza A (H1N1) 2009 pandemic. Infez Med. 2016;24(2):123–130. [PubMed] [Google Scholar]
- 28.Neidich S.D., Green W.D., Rebeles J., Karlsson E.A., Schultz-Cherry S., Noah T.L. Increased risk of influenza among vaccinated adults who are obese. Int J Obes. 2017;41(9):1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones R.L., Nzekwu M.M. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Zheng J., Zhang L., Liu Y., Chen G.P., Zhang H.P. Systemic inflammation mediates the detrimental effects of obesity on asthma control. Allergy Asthma Proc. 2017 doi: 10.2500/aap.2018.39.4096. [DOI] [PubMed] [Google Scholar]
- 32.Peters M.C., McGrath K.W., Hawkins G.A., Hastie A.T., Levy B.D., Israel E. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huttunen R., Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37(3):333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 34.Morris A.E., Stapleton R.D., Rubenfeld G.D., Hudson L.D., Caldwell E., Steinberg K.P. The association between body mass index and clinical outcomes in acute lung injury. Chest. 2007;131(2):342–348. doi: 10.1378/chest.06-1709. [DOI] [PubMed] [Google Scholar]
- 35.Ceriello A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract. 2020;163:108186. doi: 10.1016/j.diabres.2020.108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma W.X., Ran X.W. [The management of blood glucose should be emphasized in the treatment of COVID-19] Sichuan Da Xue Xue Bao Yi Xue Ban. 2020;51(2):146–150. doi: 10.12182/20200360606. [DOI] [PubMed] [Google Scholar]
- 37.Akbari M., Hassan-Zadeh V. Hyperglycemia affects the expression of inflammatory genes in peripheral blood mononuclear cells of patients with type 2 diabetes. Immunol Invest. 2018;47(7):654–665. doi: 10.1080/08820139.2018.1480031. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Zhang X.R., Ju Z.Y., He W.F. [Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies] Zhonghua Shaoshang Zazhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. 0. [DOI] [PubMed] [Google Scholar]
- 39.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceriello A., Zarich S.W., Testa R. Lowering glucose to prevent adverse cardiovascular outcomes in a critical care setting. J Am Coll Cardiol. 2009;53(5 Suppl):S9–S13. doi: 10.1016/j.jacc.2008.09.054. [DOI] [PubMed] [Google Scholar]