Abstract
Drought is one of the major abiotic stresses that affects crop yield worldwide. An eco-friendly tool that can broadly improve plants' tolerance to water stress is bioionocula comprising plant growth-promoting rhizobacteria (PGPR). In this study, the effect of two PGPR Cupriavidus necator 1C2 (B1) and Pseudomonas fluorescens S3X (B2), singly and/or co-inoculated at two inocula sizes (S1 - 3 × 103 cells g−1 dry weight (dw) soil and S2 - 3 × 106 cells g−1 dw soil), on growth, nutrient uptake, and use efficiency was assessed in maize (Zea mays L.) plants grown at three levels of irrigation (80% of water holding capacity (WHC) – well-watered, 60% of WHC - moderate water deficit stress, and 40% of WHC - severe water deficit stress) in a greenhouse experiment. The impact of water deficit and bioinoculants on soil microbial activity (fluorescein diacetate hydrolysis) was also evaluated.
Moderate and severe water deficit negatively affected soil microbial activity, as well as, maize growth, by reducing plants' shoot biomass and increasing root/shoot ratio at 60 and 40% of WHC. Bioinoculants mitigated the negative effects on shoot biomass, especially when PGPR were co-inoculated, increasing up to 89% the aerial biomass of plants exposed to moderate water deficit. Bioinoculation also increased nitrogen (N) and phosphorous (P) use efficiency, which may have led to higher maize growth under water deficit conditions. The size of the inocula applied had marginal influence on biometric and nutrient parameters, although the higher concentration of the mixture of PGPR was the most effective in improving shoot biomass under moderate water deficit.
This study shows that rhizobacterial strains are able to increase nutrient use efficiency and to alleviate water stress effects in crops with high water demands and have potential applications to keep up with productivity in water stress scenarios.
Keywords: Agricultural sciences, Biotechnology, Microbiology, Bioinoculants, Drought, Inocula size, Water deficit, Water scarcity, Agricultural soil science, Agricultural water management, Crop biomass, Microbial biotechnology, Nutrient availability
Agricultural sciences; Biotechnology; Microbiology; Bioinoculants; Drought; Inocula size; Water deficit; Water scarcity; Agricultural soil science; Agricultural water management; Crop biomass; Microbial biotechnology; Nutrient availability
1. Introduction
The limitation of water availability in some areas of the world along with the increase of human population, the expansion of agricultural, energy, and industrial sectors have already intensified dramatically the demand for water in the last decades (FAO, 2011; Gosling and Arnell, 2016).
Drought is a major limiting factor for crop production as it causes plant growth disturbances and crop failure (Mahajan and Tuteja, 2005), leading to huge economic losses and to the decline of food availability across the world (FAO, 2002; United Nations, 2006). Water deficit may cause morphological, biochemical, and physiological injuries on plants affecting various important cellular processes (Farooq et al., 2009). Among the most deleterious effects are damages on the photosynthetic apparatus (Chaves et al., 2009; Lawlor and Cornic, 2002) and oxidative injuries on proteins, membrane lipids and other cellular components (Farooq et al., 2009; Reddy et al., 2004; Zlatev and Lidon, 2012). In addition, water deficit can reduce the size of crops' organs, delay/hinder flowering and fertilization, and decline grain yield and quality (Farooq et al., 2009; Prasad et al., 2008). These negative effects are often associated with decreases in the microbial activity of the soil (Rousk et al., 2013) and in the amount of macro and micronutrients available to plants (Hu et al., 2007).
Maize (Zea mays L.) is one of the most produced food crops in the world (http://faostat3.fao.org), contributing to the survival of billions of people (Awika, 2012). However, maize yield suffered a reduction up to 40% on a global scale due to drought, according to a meta-analysis performed by Daryanto et al. (2016), based on published data between 1980 and 2015. Finding mitigation strategies to tackle the impact of water shortage on maize production is a priority. Indeed, the development of new stress-tolerant varieties through conventional breeding and/or by plant genetic engineering (Ashraf, 2010; Atkinson and Urwin, 2012; Bakhsh and Hussain, 2015), as well as the application of inorganic and organic chemicals, including osmoprotectants and plant hormones (Travaglia et al., 2010) has been used to improve maize tolerance to drought. However, some drawbacks have been associated to these approaches, since they are time-consuming, cost and labour-intensive, and there is the risk of unwanted transfer of transgenic genes to the environment (Atkinson and Urwin, 2012).
The application of beneficial microorganisms as bioinoculants appears as an environmental friendly biotechnological tool for sustainable agricultural practices (Dimpka et al., 2009; Dodd and Pérez-Alfocea, 2012). The inoculation of plants with plant growth-promoting rhizobacteria (PGPR) has been shown as an effective tool to alleviate maize stress caused by several environmental factors (Moreira et al., 2016a,b; Pereira and Castro (2014a,b) including drought (Naseem and Bano, 2014; Sandhya et al., 2010, 2011; Shirinbayan et al., 2019). According to Kaushal and Wani (2016), PGPR application attenuates the negative effects of drought on plants through a process called rhizobacterial-induced drought endurance and resilience, which involves a plethora of physiological and biochemical processes in plants, including changes in the levels of phytohormones, activation of the antioxidant defense system, and the accumulation of several compatible organic solutes like sugars, amino acids and polyamines. However, the efficiency of PGPR inoculation is highly dependent on the interactions between the indigenous populations in the rhizosphere and the introduced strains (van Veen et al., 1997). As such, the size of the inocula applied should be taken into account as the number of microbial cells introduced in soil can determine the success of bioinoculants (Moreira et al., 2016b; Pillay and Nowak, 1997). To date only a few authors reported the effect of inoculum size on plant growth, and to the best of our knowledge this issue was never addressed before in plants grown under water stress conditions.
Therefore, the aim of this study was to evaluate the effect of PGPR inoculation at two inocula sizes, either singly or co-inoculated, on maize grown under different water regimes (80, 60, and 40% of water holding capacity - WHC) and on soil microbial activity. We hypothesize that the inoculation of rhizobacteria may improve nitrogen (N) and phosphorous (P) use efficiency by maize plants grown under water deficit conditions, mitigating the negative effects of water shortage on its growth.
2. Material and methods
2.1. Bacterial inoculants
The rhizobacterial strains Cupriavidus necator sp. 1C2 (B1) and Pseudomonas fluorescens S3X (B2) were previously isolated from an environmental degraded area (Pires et al., 2017; Pereira et al., 2015a). These bacterial strains were selected based on their plant growth promoting traits (Pereira et al., 2015b), comprising very good siderophore and indole acetic acid (IAA) production, and 1-aminocyclopropane-1-carboxylic acid (ACC)-deaminase activity, and on their proven ability to enhance maize growth under stressful conditions, such as metal pollution (Moreira et al., 2016b, 2019).
Both bacterial strains were tested for their osmotic tolerance in trypticase soy broth (TSB) medium amended with different concentrations of polyethylene glycol (PEG) 6000 to obtain different osmotic potentials (0; -0.10; -0.20; -0.30; -0.70 MPa) (Michel and Kaufmann, 1973). Three replicates were prepared for each strain and osmotic potential. After 72 h of incubation at 28 °C and 120 rpm in an orbital shaker, bacterial growth was recorded by measuring the optical density (OD) at 600 nm using a Unicam Helios spectrophotometer (Waltham, USA). The percentage of growth inhibition was calculated for both strains. Both isolates were also screened for their ability to produce IAA under -0.30 and -0.70 MPa. This assay was performed according to the method described by Gordon and Weber (1951).
2.2. Greenhouse pot experimental design
The greenhouse experiment consisted in a factorial design of seven bacterial treatments and three irrigation regimes: well-watered, moderate, and severe water deficit, corresponding to 80, 60, and 40% of WHC, with four replicates. Bacterial treatments were applied in two different sizes - S1 and S2 - corresponding to 3 × 103 cells g−1 dry weight (dw) soil and 3 × 106 cells g−1 dw soil, respectively, as presented in Table 1.
Table 1.
Bacterial treatments applied to maize plants grown at different irrigation regimes (80, 60, and 40% of WHC).
| Treatments | Inocula size (cells g−1 dw) | |
|---|---|---|
| Control | non-inoculated soil – no bacteria | - |
| B1 S1 | soil inoculated with C. necator 1C2 | 3 × 103 |
| B2 S1 | soil inoculated with P. fluorescens S3X | 3 × 103 |
| B1 S2 | soil inoculated with C. necator 1C2 | 3 × 106 |
| B2 S2 | soil inoculated with P. fluorescens S3X | 3 × 106 |
| MIX S1 | soil inoculated with a mixture of C. necator 1C2 + P. fluorescens S3X | 3 × 103 |
| MIX S2 | soil inoculated with a mixture of C. necator 1C2 + P. fluorescens S3X | 3 × 106 |
Zea mays seeds var. DKC3014 (a variety not known to be resistant to drought following information on the technical sheet from the supplier) provided by Dekalb, France were surface sterilized with 0.5% (v/v) NaOCl for 10 min and rinsed several times with deionized sterile water. Sterilized seeds were germinated in water-agar plates and seven maize seedlings were sown in pots (13 × 15 cm) containing 1 kg of sieved soil. After one week, plants were thinned to 5 per pot. Soil was randomly collected from a depth of 0–20 cm in an agricultural area of northern Portugal (41°16′36.10″N; 8°42′50.57″W). Soil properties were as follows: pH, 6.06 ± 0.01 (potentiometric); organic matter content, 2.15 ± 0.01 (%; Walkley-Black); electrical conductivity, 116 ± 2 (μS cm−1; conductimetric); total N, 0.12 ± 0.02 (%; conductimetric); extractable P, 13.9 ± 0.4 (mg kg−1; Mehlich 3); extractable K, 128.2 ± 1.3 (mg kg−1; Mehlich 3).
For the inoculation of seedlings, bacterial strains were grown in TSB medium overnight at 30 °C and 120 rpm in an orbital shaker. Cells in the exponential phase were harvested by centrifugation at 9000 rpm for 10 min, washed twice with sterile saline solution (0.85% NaCl) and resuspended in sterile saline solution to get an inoculum density of ca. 108 CFU ml−1. The two sizes of inocula (S1 and S2) were sprayed into the soil surface one week after the seedlings were transferred to pots.
Three water regimes, 80, 60, and 40% of WHC were applied eight weeks after sowing. Pots were placed in a controlled growth room (12 h photoperiod, 450 μmol m−2 s−1 photosynthetically active radiation, 18–21 °C temperature range, 50–60 % relative humidity range) at Centro de Biotecnologia e Química Fina, Universidade Católica Portuguesa, Porto, Portugal.
2.3. Plant analysis
A Soil Plant Analysis Development (SPAD) Chlorophyll Meter (SPAD 502 Plus, Spectrum Technologies, Inc.) was used to estimate the chlorophyll content (Ling et al., 2011) of leaves before harvest (13 weeks). Readings were taken in each plant of each pot at three locations of the third expanded leaf. Plants were then harvested, separated in roots and shoots and washed with deionized water. Shoot elongation was determined and both plant tissues were oven dried at 65 °C for 2 weeks, after which root and shoot dry weight was assessed. The root/shoot ratio was calculated as dry mass of roots divided by dry mass of shoots.
Dry shoot and roots samples were ground and digested in a PerkinElmer MicroWave using a H2SO4:H2O2 mixture (1:1). Digested samples were used to determine the total N and P in the roots and shoots as described by Wallinga et al. (1989). Briefly, for total N determination two reagents were added to the digests: reagent 1 - a mixture of 50 mM disodium hydrogen phosphate buffer (pH = 12.3) and 4% bleach; reagent 2 - a mixture of 1 M sodium salicylate solution, 1 mM sodium nitroprusside and 3 mM ethylenediamine tetraacetic acid (EDTA). For total P colorimetric determination, two reagents were added to the digests: reagent 1 - 30 mM ascorbic acid solution and reagent 2 - a mixture of 6 mM antimonyl tartarate, 5 mM ammonium molybdate and 0.7 M sulphuric acid solutions. N and P concentrations were determined using an Unicam Helios spectrophotometer (Waltham, USA) at 660 and 880 nm, respectively. The total N and P content were used to calculate the physiological nutrient use efficiency for N (NUE) and P (PUE) according to the formula (Nguyen et al., 2014):
| Nutrient use efficiency = Total dry biomass / Total nutrient absorbed |
where Total nutrient absorbed = Nutrient concentration x Total dry biomass.
2.4. Fluorescein diacetate (FDA) hydrolysis
In order to estimate the overall microbial activity in soil samples FDA test was performed (Adam and Duncan, 2001). Briefly, 5 ml of 60 mM sodium phosphate buffer (pH 7.6) and 25 μl of 4.8 mM fluorescein diacetate were added to 0.5 g of fresh soil. Samples were incubated for 20 min at 25 °C and 150 rpm. The reaction was stopped by adding 5 ml of acetone. Samples were centrifuged at 8000 rpm for 10 min at room temperature and the amount of hydrolyzed FDA was determined at 490 nm. A calibration curve was prepared using a fluorescein standard solution (0–100 mg l−1).
2.5. Statistical analysis
Statistical analyses were performed using the statistical software package SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Two-way ANOVA was performed to assess the significant differences of the effect of bacterial treatments and water regimes on each tested parameter. One-way ANOVA with Duncan post hoc analysis, was also performed to assess the effects of bacterial treatments on the different plant parameters for each water regime.
3. Results
3.1. Shoot elongation, dry biomass, and chlorophyll content
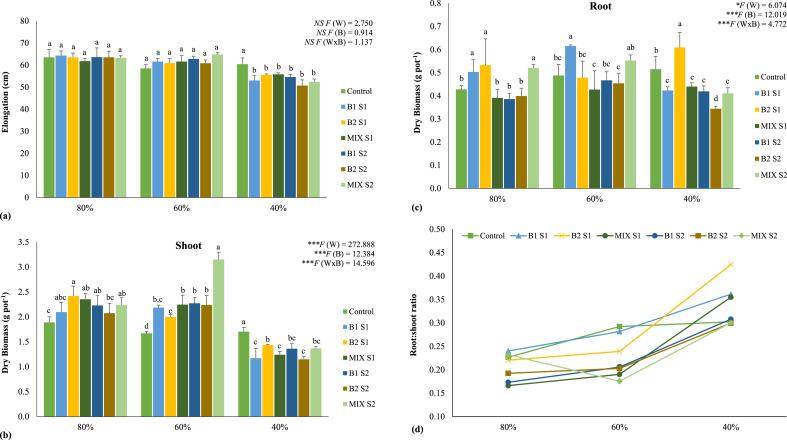
The effect of water regimes and bacterial inoculation on maize growth is shown in Figure 1. At 80% of WHC, shoot elongation ranged from 61.8 to 64.3 cm, while at 60% and 40%, shoot length varied between 58.5 and 64.8 cm and between 50.7 and 60.4 cm, respectively (Figure 1a).
Figure 1.
Shoot elongation (a), shoot (b) and root (c) dry biomass, and root/shoot ratio (d) of maize plants grown under different water regimes (80, 60, and 40% of WHC) and inoculated with different bacterial treatments: control (non-inoculated soil – no bacteria), B1 S1 (soil inoculated with C. necator 1C2 – S1), B2 S1 (soil inoculated with P. fluorescens S3X – S1), MIX S1 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S1; B1 S2 (soil inoculated with C. necator 1C2 – S2), B2 S2 (soil inoculated with P. fluorescens S3X – S2), MIX S2 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S2). Values are means ± to standard deviation (n = 4). A two-way ANOVA was performed to determine the influence of bacterial treatments and of water regimes on shoot elongation and on shoot and root dry biomass. (W - water regimes; B - bacterial treatments; W x B - water regimes x bacterial treatments) and as NS – Non-significant at the level P > 0.05; ∗ significant at the level P < 0.05; ∗∗ significant at the level P < 0.01; ∗∗∗ significant at the level P < 0.001, respectively. A one-way ANOVA was performed to determine the influence of bacterial treatments on shoot elongation and on root and shoot dry biomass for each water regime. Means for the same water regime showing different letters are significantly different from each other (P < 0.05) according to Duncan test. For shoot elongation, the F values of one-way ANOVA are NS F = 0.450, NS F = 0.655, and ∗∗∗F = 6.039 for 80, 60,and 40% of WHC, respectively. For shoot dry biomass, the F values of one-way ANOVA are ∗F = 2.991, ∗∗∗F = 37.282, and ∗∗∗F = 11.186, respectively for 80, 60, and 40% of WHC. For root dry biomass, the F values of one-way ANOVA are ∗F = 4.674, ∗F = 4.885, and ∗∗∗F = 17.361 for 80, 60, and 40% of WHC, respectively.
Water regimes and bacterial treatments did not affect (P > 0.05) shoot elongation at 80 and 60% of WHC. However, at 40% of WHC the inoculation of bacterial strains significantly (P < 0.05) decreased shoot elongation.
Despite the minor effects of water regimes and bioinoculation on shoot length, the biomass of plants was significantly influenced by both factors (Figure 1b). Shoot biomass varied between 1.89 and 2.42 g dw at 80%, 1.670 and 3.15 g dw at 60%, and 1.15 and 1.70 g dw at 40% of WHC. The increase of water deficit in soil had low influence on the aboveground biomass of non-inoculated plants (control). In general, bioinoculation enhanced shoot biomass at 80% and 60% of WHC. At 80% of WHC, four bacterial treatments (B2 S1, MIX S1, B1 S2, and MIX S2) promoted shoot biomass, with increases of 28 and 26% in B2 S1 and MIX S1 inoculated plants, respectively. Nonetheless, at 40% of WHC, the six bacterial treatments decreased significantly (P < 0.05) this parameter, as for shoot elongation. All bacterial treatments had a beneficial effect on plants irrespective of the amount of inocula (S1 and S2) or bacterial strains applied. Despite the general marginal effect of inocula size on shoots biomass, an increase of 89% at 60% of WHC was observed when the highest amount of bacterial mixture (MIX S2) was applied.
Water regimes and bacterial treatments also significantly (P < 0.05) influenced root biomass (Figure 1c), which ranged from 0.39 to 0.53 g dw at 80%, 0.43–0.62 g dw at 60%, and 0.34–0.61 g dw at 40% of WHC. Overall, roots' biomass was increased by the decline of moisture in soils, both in non-inoculated and inoculated plants. At 80% of WHC, the inoculation of bacterial strains C. necator 1C2 (B1) and P. fluorescens S3X (B2) at the lowest inocula size (S1) enhanced roots' biomass by 18 and 25%, respectively. The same was observed in plants grown at 60 and 40% of WHC when single inoculated with strains B1 (26%) and B2 (18%), respectively.
Differences in root and shoot growth of maize plants among irrigation regimes and bacterial treatments resulted in variations in root/shoot ratios (Figure 1d), which were generally higher at 40% of WHC. At this water regime, bacterial treatments B1 S1, B2 S1 and MIX S1 greatly increased root/shoot ratio of maize when compared to non-inoculated plants.
SPAD measurements of leaves of plants grown under different water regimes at the end of the experiment are presented in Table 2. Water regimes and bacterial inoculation did not significantly (P > 0.05) affect this parameter.
Table 2.
Chlorophyll content of leaves of plants grown under different water regimes (80, 60, and 40% of WHC) and inoculated with different bacterial treatments: control (non-inoculated soil – no bacteria), B1 S1 (soil inoculated with C. necator 1C2 – S1), B2 S1 (soil inoculated with P. fluorescens S3XS1), MIX S1 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S1; B1 S2 (soil inoculated with C. necator 1C2 – S2), B2 S2 (soil inoculated with P. fluorescens S3X – S2), MIX S2 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S2) at the end of experiment.
| Water regimes |
|||
|---|---|---|---|
| 80% | 60% | 40% | |
| Control | 29.9 ± 0.3a | 30.4 ± 0.7a | 29.9 ± 0.9a |
| B1 S1 | 30.0 ± 1.6a | 31.3 ± 0.5a | 29.4 ± 0.4a |
| B2 S1 | 30.1 ± 0.9a | 31.0 ± 0.4a | 28.0 ± 1.3a |
| MIX S1 | 30.1 ± 1.1a | 30.5 ± 1.2a | 29.6 ± 1.7a |
| B1 S2 | 29.8 ± 1.0a | 32.0 ± 0.7a | 27.7 ± 1.2a |
| B2 S2 | 29.5 ± 0.5a | 30.7 ± 0.3a | 28.0 ± 0.4a |
| MIX S2 | 29.8 ± 1.2a | 30.8 ± 0.6a | 30.2 ± 0.2a |
| NS F (W) = 0.418 | |||
| NS F (B) = 1.050 | |||
| NS F (WxB) = 1.028 | |||
Values are means ± standard deviation (n = 5). A two-way ANOVA was performed to determine the influence of bacterial treatments and of water regimes on chlorophyll content. The results are shown with the test statistic for each case (W: water regimes; B: bacterial treatments; W x B: water regimes x bacterial treatments) and as NS: Non significant at the level P > 0.05; ∗ significant at the level P < 0.05; ∗∗ significant at the level P < 0.01; ∗∗∗ significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of bacterial treatments on chlorophyll content for each water regime at the end of the experiment. Means for the same water regime showing different letters are significantly different from each other (P < 0.05) according to Duncan test. The F values are NS F = 0.378, NS F = 0.335, and NS F = 1.963 for 80, 60, and 40% of WHC, respectively.
3.2. N and P accumulation in plant tissues
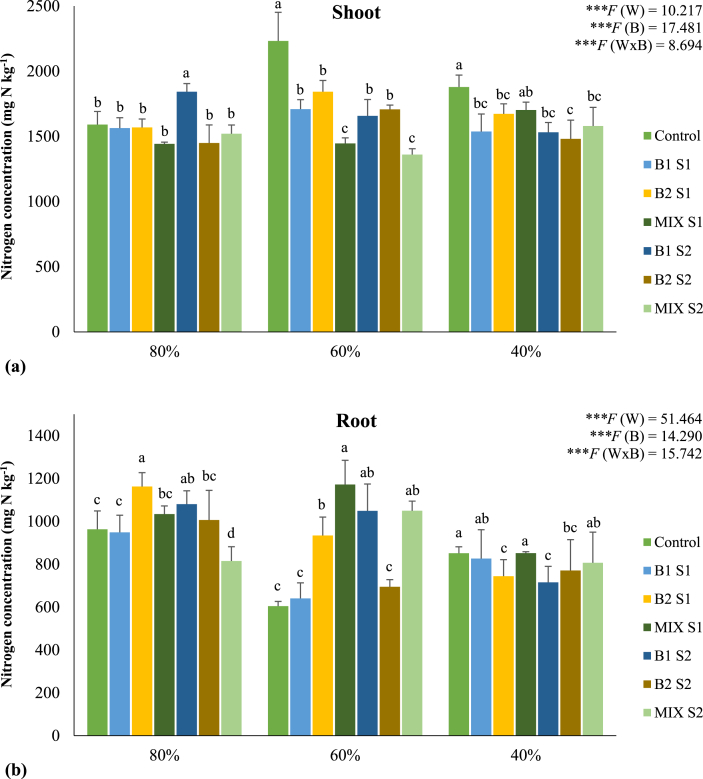
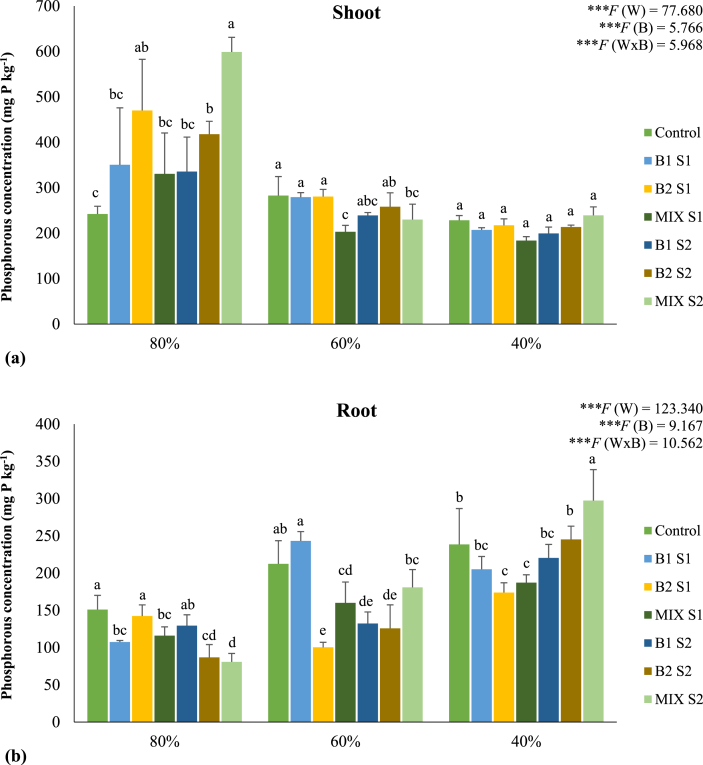
Water regimes and bacterial treatments significantly (P < 0.001) affected N accumulation in plant tissues. The concentration of N was higher in shoots than in roots for all bacterial treatments and water regimes applied (Figure 2). Bioinoculants tended to decrease N accumulation in shoots of plants grown at moderate and severe water deficit, while at 80% of WHC no effect was observed, with the exception of treatment B1 S2 where an increase of 16% was registered. It was observed a marked reduction of N concentration in the roots of non-inoculated plants subjected to moderate and severe water deficit. At 60% of WHC, bacterial inoculation clearly attenuated this decline. As observed for N, the accumulation of P was higher in shoots than in roots of maize (Figure 3). The accumulation of P was higher in the shoots of plants grown under well-watered conditions, being observed a slight decrease under moderate and severe water deficit. The positive effect of bioinoculants on P accumulation in roots was only observed at 80% of WHC, with the treatment MIX S2 showing the best performance. On the other hand, P concentration in roots tended to be higher in plants under moderate and severe water deficit, regardless of bacterial inoculation. At 40% of WHC, there was a significant increase in the levels of P in the roots of the plants inoculated with the highest load of the bacterial mixture (MIX S2). The effect of water regimes and bacterial treatments on NUE and PUE are presented in Table 3. In general, bacterial inoculation enhanced the efficiency of use of both nutrients. Under well-watered conditions, bioinoculation decreased or maintained NUE and PUE values, while at moderate and severe water deficit both indices were increased by bacterial inoculation. The highest increases were observed in plants grown at 60% of WHC and inoculated with a mixture of bacterial strains (MIX S1 and MIX S2), enhancing NUE by 32 and 41% and PUE by 35 and 21%, respectively, when compared to non-inoculated plants.
Figure 2.
Nitrogen concentration (mg kg−1) in shoots (a) and roots (b) of maize plants grown under different water regimes (80, 60, and 40% of WHC) and inoculated with different bacterial treatments: control (non-inoculated soil – no bacteria), B1 S1 (soil inoculated with C. necator 1C2 – S1), B2 S1 (soil inoculated with P. fluorescens S3X – S1), MIX S1 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S1; B1 S2 (soil inoculated with C. necator 1C2 – S2), B2 S2 (soil inoculated with P. fluorescens S3X – S2), MIX S2 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S2). Values are means ± standard deviation (n = 4). A two-way ANOVA was performed to determine the influence of bacterial treatments and of the water regimes on nitrogen concentration in roots and shoots (W: water regimes; B: bacterial treatments; W x B: water regimes x bacterial treatments) and as NS: Non significant at the level P > 0.05; ∗ significant at the level P < 0.05; ∗∗ significant at the level P < 0.01; ∗∗∗ significant at the level P < 0.001, respectively. A one-way ANOVA was performed to determine the influence of bacterial treatments on nitrogen concentration in roots and shoots for each water regime. Means for the same water regime showing different letters are significantly different from each other (P < 0.05) according to Duncan test. For shoots, the F values are ∗∗∗F = 7.843, ∗∗∗F = 20.828, and ∗∗F = 4.751 for 80, 60, and 40% of WHC, respectively. For roots, the F values are ∗∗∗F = 9.241, ∗∗∗F = 19.407, and ∗∗∗F = 7.028 for 80, 60, and 40% of WHC, respectively.
Figure 3.
Phosphorous concentration (mg kg−1) in shoots (a) and roots (b) of maize plants grown under different water regimes (80, 60, and 40% of WHC) and inoculated with different bacterial treatments: control (non-inoculated soil – no bacteria), B1 S1 (soil inoculated with C. necator 1C2 – S1), B2 S1 (soil inoculated with P. fluorescens S3X – S1), MIX S1 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S1; B1 S2 (soil inoculated with C. necator 1C2 – S2), B2 S2 (soil inoculated with P. fluorescens S3X – S2), MIX S2 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S2). Values are means ± standard deviation (n = 4). A two-way ANOVA was performed to determine the influence of bacterial treatments and of the water regimes on nitrogen concentration in roots and shoots (W: water regimes; B: bacterial treatments; W x B: water regimes x bacterial treatments) and as NS: Non significant at the level P > 0.05; ∗ significant at the level P < 0.05; ∗∗ significant at the level P < 0.01; ∗∗∗ significant at the level P < 0.001, respectively. A one-way ANOVA was performed to determine the influence of bacterial treatments on nitrogen concentration in roots and shoots for each water regime. Means for the water regime showing different letters are significantly different from each other (P < 0.05) according to Duncan test. For shoots, the F values are ∗∗∗F = 6.358, ∗∗F = 4.436, and NS F = 1.062 for 80, 60, and 40% of WHC, respectively. For roots, the F values are ∗∗∗F = 11.026, ∗∗∗F = 14.351, and ∗∗∗F = 6.825 for 80, 60, and 40% of WHC, respectively.
Table 3.
Nitrogen use efficiency (NUE) and phosphorus use efficiency (PUE) in maize plants grown under different water regimes (80, 60, and 40% of WHC) and inoculated with different bacterial treatments: control (non-inoculated soil – no bacteria), B1 S1 (soil inoculated with C. necator 1C2 – S1), B2 S1 (soil inoculated with P. fluorescens S3X – S1), MIX S1 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S1; B1 S2 (soil inoculated with C. necator 1C2 – S2), B2 V2 (soil inoculated with P. fluorescens S3X – S2), MIX S2 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S2).
| Nitrogen Use Efficiency (NUE) |
Phosphorous Use Efficiency (PUE) |
||||||
|---|---|---|---|---|---|---|---|
| 80% | 60% | 40% | 80% | 60% | 40% | ||
| Control | 682.0 ± 38.6a | 541.1 ± 57.5d | 610.2 ± 35.2c | Control | 4467.7 ± 309.8a | 3785.3 ± 451.6c | 4354.5 ± 418.1c |
| B1 S1 | 693.7 ± 31.3a | 679.3 ± 27.2b | 746.1 ± 61.0ab | B1 S1 | 3623.4 ± 1007.1ab | 3690.4 ± 127.1c | 4852.6 ± 168.2b |
| B2 S1 | 668.9 ± 26.7a | 601.0 ± 11.7c | 717.5 ± 22.6ab | B2 S1 | 2600.2 ± 737.5bc | 4078.2 ± 223.0bc | 4900.2 ± 266.8b |
| MIX S1 | 722.7 ± 4.9a | 713.6 ± 21.5ab | 676.4 ± 18.0bc | MIX S1 | 3529.7 ± 803.8ab | 5119.8 ± 298.6a | 5419.5 ± 153.3a |
| B1 S2 | 578.6 ± 14.6b | 644.9 ± 36.5bc | 747.4 ± 26.7ab | B1 S2 | 3424.2 ± 698.6ab | 4525.4 ± 100.3ab | 4916.0 ± 356.3b |
| B2 S2 | 731.3 ± 59.8a | 651.2 ± 8.1bc | 765.0 ± 59.4a | B2 S2 | 2760.1 ± 227.7bc | 4282.9 ± 409.1bc | 4527.8 ± 25.2bc |
| MIX S2 | 722.3 ± 32.5a | 761.9 ± 24.5a | 718.1 ± 55.4ab | MIX S2 | 2001.6 ± 114.9c | 4564.2 ± 562.3ab | 3980.7 ± 295.2c |
| ∗∗∗F = 7.211 | ∗∗∗F = 16.552 | ∗∗∗F = 4.203 | ∗F = 4.6826 | ∗∗∗F = 6.086 | ∗∗∗F = 9.660 | ||
| ∗∗∗F (W) = 11.752 | ∗∗∗F (W) = 6.387 | ||||||
| ∗∗∗F (B) = 11.957 | ∗∗∗F (B) = 63.948 | ||||||
| ∗∗∗F (WxB) = 5.865 | ∗∗∗F (WxB) = 5.240 | ||||||
Values are means ± standard deviation (n = 4). A two-way ANOVA was performed to determine the influence of bacterial treatments and of the water regimes on NUE and PUE. (W: water regimes; B: bacterial treatments; W x B: water regimes x bacterial treatments) and as NS: Non significant at the level P > 0.05; ∗ significant at the level P < 0.05; ∗∗ significant at the level P < 0.01; ∗∗∗ significant at the level P < 0.001, respectively. One-way ANOVA was performed to determine the influence of bacterial treatments on NUE and PUE for each water regime. Means for the same concentration showing different letters are significantly different from each other (P < 0.05) according to Duncan test.
3.3. FDA activity in soils
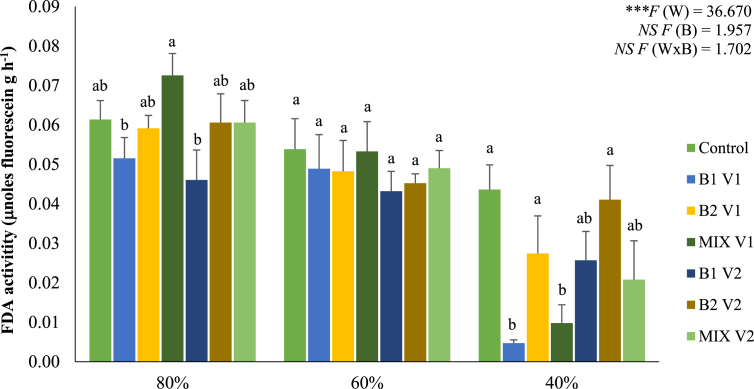
Water regimes negatively affected (P < 0.01) FDA activity in soils (Figure 4). In fact, the hydrolysis of FDA was higher in the rhizosphere of plants growing at 80% of WHC, being significantly reduced at 60 and 40%. Bacterial inoculation did not significantly (P > 0.05) influence this parameter.
Figure 4.
FDA activity in rhizospheric soil of maize plants grown under different water regimes (80, 60, and 40% of WHC) and inoculated with different bacterial treatments: control (non-inoculated soil – no bacteria), B1 S1 (soil inoculated with C. necator 1C2 – S1), B2 S1 (soil inoculated with P. fluorescens S3X–S1), MIX S1 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S1; B1 S2 (soil inoculated with C. necator 1C2 – S2), B2 S2 (soil inoculated with P. fluorescens S3X – S2), MIX S2 (soil inoculated with a mixture of C. necator 1C2 and P. fluorescens S3X – S2). Values are means ± standard error (n = 5 to 6). A two-way ANOVA was performed to determine the influence of bacterial treatments and of water regimes on FDA activity in soil. (W: water regimes; B: bacterial treatments; W x B: water regimes x bacterial treatments) and as NS: Non significant at the level P > 0.05; ∗ significant at the level P < 0.05; ∗∗ significant at the level P < 0.01; ∗∗∗ significant at the level P < 0.001, respectively. A one-way ANOVA was performed to determine the influence of bacterial treatments on FDA activity for each water regime. Means for the same water regime showing different letters are significantly different from each other (P < 0.05) according to Duncan test. The F values are NS F = 1.978, NS F = 0.309, and ∗F = 2.940 for 80, 60, and 40% of WHC, respectively.
4. Discussion
Maize is one of the most important cereals in the world. However, it is a crop with high water demand, which is a hurdle under the current scenario of climate change where water availability is reducing. This study showed that bioinoculants, especially the mixture of PGPR (P. fluorescens S3X + C. necator 1C2) were able to mitigate the effects of moderate water deficit (60% of WHC) in maize growth, regardless the inocula size applied.
Drought is undoubtedly one of the major abiotic stresses to crop growth and productivity (Fahad et al., 2017). Several authors have reported the adverse impacts of water scarcity on maize growth and development, including a significative reduction of shoot elongation, biomass, leaf area, chlorophyll, and nutrient content (Sandhya et al., 2010; Shirinbayan et al., 2019). In this work, water limitation in soil had a reduced impact on the aerial biomass of plants grown at 60 and 40% of WHC. However, higher reductions in shoot biomass have been reported in maize plants grown under similar deficient irrigation regimes. For instance, Fan et al. (2015) showed a decrease of above 40% in aerial biomass of the maize plants grown at 45% of WHC if compared to plants grown at 80%. Coherently with these results, Shirinbayan et al. (2019) showed that plants grown at 40% of WHC produced less 40% of shoot biomass than well-watered plants. Drought hinders the synthesis of photosynthetic pigments often leading to a reduction in the plant's photosynthetic rates (Ashraf and Harris, 2013). Nonetheless, in this work, according to SPAD measurements water deficiency in soil did not induce significant changes in chlorophyll content of maize leaves. Indeed, at the end of the experiment, no symptoms of chlorosis were observed, which may explain the low impact of severe water deficit on shoot biomass.
Bacterial soil inoculation increased shoot biomass of well-irrigated plants and was even more effective on plants exposed to moderate (60% of WHC) water deficit conditions. Similarly, a meta-analysis including 52 papers on the inoculation of PGPR under drought conditions showed that bacterial treatments are generally more effective under water scarcity than under good irrigation conditions (Rubin et al., 2017). Fan et al. (2015) also showed that bioinoculation slightly increased shoot dry biomass of maize plants grown under moderate and severe water deficits. The effectiveness of PGPR in mitigating deleterious effects of drought in crop growth and productivity has been reported in the literature (Naveed et al., 2014; Sandhya et al., 2010, 2011; Shirinbayan et al., 2019). These beneficial effects are often the result of the production of phytohormones such as IAA, gibberellic acid and cytokinins, and exopolysaccharides (EPS) by bacterial strains (Naseem and Bano, 2014; Niu et al., 2018; Sandhya et al., 2011). PGPR may induce significative changes in the architecture of root system through the production of phytohormones (e.g. IAA), which enhance lateral root branching and formation of root hairs (Vacheron et al., 2013). These changes will increase nutrient uptake by the root systems that foster plant growth. In fact, strains C. necator 1C2 and P. fluorescens S3X were able to withstand low osmotic potentials (-30 and -70 MPa), while producing high levels of IAA (data not shown), which may explain the beneficial effects observed in plants grown under moderate water deficit. Moreover, the bacterial strain S3X may also have contributed to increase plant growth by the production of EPS, since pseudomonads are often reported as efficient EPS producing-bacteria (Niu et al., 2018; Sandhya et al., 2009). Indeed, Khan et al. (2017) reported that the EPS produced by PGPR enhanced the water holding capacity in the rhizosphere of wheat plants, contributing to increase their root growth and biomass.
Contrary to what was observed in the aboveground tissues, non-inoculated plants grown at 60 and 40% of WHC showed higher root biomass than well-watered ones, which was accompanied by an increase in root/shoot ratio. Several authors have also reported a greater impact of water stress on aerial growth than on root growth (Franco et al., 2011; Sharp et al., 2004), leading to an increase of root/shoot ratio under low soil water content. This increase of roots is usually attributed to a greater nutrient allocation in belowground tissues (Kozlowski and Pallardy, 2002), rapid osmotic adjustment of roots, and enhanced loosening ability of root cell walls (Sharp et al., 2004). Some bacterial treatments can also enhance root biomass of plants exposed to moderate water deficit, although to a lesser extent compared to shoots. In accordance, Rubin et al. (2017) reported that under soil water limitation, PGPR are generally more effective on the improvement of shoots than roots biomass.
In this work, the beneficial effects of bioinoculants were not consistently observed across the water regimes applied, since inoculated plants under severe water deficit (40% of WHC) presented lower root and shoots' biomass than non-inoculated ones, suggesting that the effect of bacterial inoculation was dependent on the intensity of water stress. Ulrich et al. (2019) also reported that microbial communities may induce different plant responses to drought, including positive effects under good irrigation conditions and moderate water deficit and negative impacts during severe drought. In addition, the effectiveness of bioinoculants on the enhancement of plant growth under stressful conditions may also be compromised by the low survival in soil and by the competition with the indigenous community (Delshadi et al., 2017). Indeed, the effects on plants may not only be the result of synergistic/antagonistic interactions between inoculated strains and native microbiota but may also be related to the induction or repression of indigenous microbial populations (Trabelsi and Mhamdi, 2013).
The selection of the adequate strains to be inoculated in soil is an issue of utmost importance to increase the success of bioinoculation under environmental stressful conditions. In the present work, the inoculated PGPR are strains that have been shown to be efficient under very inhospitable conditions, including high levels of metals in soils (Moreira et al., 2016b, 2019). It is possible that the selected PGPR were not efficient at the most extreme conditions (40% of WHC) because they could not survive. Thus, the application of bacterial strains isolated from drought-affected areas could have enhanced the effectiveness of bioinoculation under severe water deficit. According to Marulanda et al. (2009) indigenous bacterial strains are better adapted to stress conditions, which will contribute to increase their survival in soil, allowing them to express their plant growth promoting activities throughout time.
Our results also showed that native soil microbial populations were negatively affected by severe water deficit (40% of WHC), as a remarkable reduction of FDA hydrolysis at this water regime was observed. Similar results were obtained by Aseri and Tarafdar (2006) that showed a decline of FDA activity in arid soils. Moreover, bacterial inoculation further decreased FDA hydrolysis, suggesting a repression of indigenous microbiota by bioinoculants.
The number of inoculated cells is also a crucial point to increase the success of bioinoculation, however, to date only a few authors reported the effect of inocula size on plant growth (Moreira et al., 2016b). In the present study, in general, the application of both inocula sizes (3 × 103 cells g−1 dw soil and 3 × 106 cells g−1 dw soil) was not discriminatory for plant growth and nutritional parameters so that we could clearly state that the amount of inoculum had influence on plant performance under different irrigation regimes. Nonetheless, the highest increment on shoot biomass occurred in plants inoculated with a mixture of both bacterial strains at the highest inocula size applied (MIX S2). On the contrary, Moreira et al. (2016b) showed that doubling the inoculum size caused a reduction in shoot's biomass, however that study was performed with contaminated soil and that outcome was related to the increased accumulation of Cd in plant tissues when a higher number of bacterial cells was applied. It is also important to highlight that regardless the size of inoculum, combined inoculation of PGPR seems to have more effect on maize development than single inoculation, by increasing nutrient use efficiency under water stress conditions. Similarly, Kumar et al. (2016) also reported that the synergistic application of two bacterial strains boosted plant growth under water stress, compared to single strain application. Co-inoculation of several bacterial strains can improve their adaptability to the environmental stressful conditions and increase their colonization in the rhizosphere, allowing to compete with the native communities.
In this work, it was observed a higher accumulation of N in shoots than in roots, which may be explained by the need for N for the photosynthesis in leaves (Yang et al., 2014). In addition, under water deficit stress both non-inoculated and inoculated plants tended to increase the accumulation of N in shoots. In fact, according to several authors plants often increase the concentration of N in leaves to maintain growth under dry conditions (Cunningham et al., 1999; Wright et al., 2003). This was particularly evident in non-inoculated plants, where the concentration of N in shoots was increased by 40% and 18% at 60 and 40% of WHC, respectively, which may explain the low reduction observed in shoot growth at these water regimes. A different trend was observed for roots, since the accumulation of N decreased with the reduction of water moisture in soils, which was offset by the application of different bacterial treatments under moderate water deficit.
As noted for N, the concentration of P was higher in shoots than in roots of maize plants. The highest accumulation of P in shoots was recorded in plants growing at 80% of WHC and a slight reduction of this nutrient was observed with the raise of water deficiency in soils, while, in roots, the accumulation of P increased. This might be related to plants' ability to balance the distribution of the nutrients among its compartments, as a strategy to respond to the changes occurring in the environment (Yang et al., 2014).
Overall, under moderate and severe water deficit, inoculation promoted the efficiency of use of both nutrients. MIX S1 and MIX S2 were the most efficient treatments in improving NUE and PUE, especially at 60% of WHC. Zeffa et al. (2019) reported the increase of NUE in maize plants inoculated with Azospirillum sp., suggesting that plants with a higher NUE can reduce the damages caused by N limitation. Adesemoye et al. (2008) also reported that microbial inoculants not only promoted maize growth but also N and P uptake efficiency, reducing potential losses of these nutrients to the environment. Nonetheless, despite PGPR's ability to enhance nutrient use efficiency by plants has been widely studied, little data are available for plants under drought stress.
5. Conclusion
This study clearly showed that the inoculation of maize plants with the bacterial strains P. fluorescens S3X and C. necator 1C2 had important effects on tolerance of maize to moderate water stress. Bioinoculants significantly promoted shoot biomass and P and N use efficiency by maize plants. These results suggest that these PGPR can be used to reduce the effects of drought stress, helping to maintain maize productivity with a less water supply.
Declarations
Author contribution statement
Sofia IA Pereira: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Daniela Abreu: Performed the experiments; Analyzed and interpreted the data.
Helena Moreira: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Alberto Vega: Performed the experiments.
Paula ML Castro: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the project - Biological tools for adding and defending value in key agro-food chains (bio– n2–value), nº NORTE-01- 0145-FEDER-000030, funded by Fundo Europeu de Desenvolvimento Regional (FEDER), under Programa Operacional Regional do Norte-Norte2020. The authors would like to thank the scientific collaboration of CBQF under the Fundação Ciência e Tecnologia (FCT) project UIDB/50016/2020. Daniela Abreu would like to thank ADP Fertilizantes for the financial support
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adam G., Duncan H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001;33:943–951. [Google Scholar]
- Adesemoye A., Torbert H., Kloepper J. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 2008;54:876–886. doi: 10.1139/w08-081. [DOI] [PubMed] [Google Scholar]
- Aseri G.K., Tarafdar J.C. Fluorescein diacetate: a potential biological indicator for arid soils. Arid Land Res. Manag. 2006;20(2):87–99. [Google Scholar]
- Ashraf M. Inducing drought tolerance in plants: recent advances. Biotechnol. Adv. 2010;28:169–183. doi: 10.1016/j.biotechadv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Ashraf M., Harris P. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51(2):163–190. [Google Scholar]
- Atkinson N.J., Urwin P.E. The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 2012;63(10):3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- Awika J.M. Major cereal grains production and use around the world. In: Awika J.M., Piironen V., Bean S., editors. Advances in Cereal Science: Implications to Food Processing and Health Promotion. ACS Symposium Series. American Chemical Society; Washington DC: 2012. pp. 1–13. [Google Scholar]
- Bakhsh A., Hussain T. Engineering crop plants against abiotic stress: current achievements and prospects. Emir. J. Food Agric. 2015;27:24–39. [Google Scholar]
- Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham S.A., Summerhayes B., Westoby M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 1999;69:569–588. [Google Scholar]
- Daryanto S., Wang L., Jacinthe P.-A. Global synthesis of drought effects on maize and wheat production. PloS One. 2016;11(5) doi: 10.1371/journal.pone.0156362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delshadi S., Ebrahimi M., Shirmohammadi E. Influence of plant-growth-promoting bacteria on germination, growth and nutrients’ uptake of Onobrychis sativa L. under drought stress. J. Plant Interact. 2017;12(1):200–208. [Google Scholar]
- Dimpka C., Weinand T., Asch F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- Dodd I., Pérez-Alfocea F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012:3415–3428. doi: 10.1093/jxb/ers033. [DOI] [PubMed] [Google Scholar]
- Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saud S., Ihsan M.Z., Alharby H., Wu C., Wang D., Huang J. Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Hu H., Huang G., Huang F., Li Y., Palta J. Soil inoculation with Burkholderia sp. LD-11 has positive effect on water-use efficiency in inbred lines of maize. Plant Soil. 2015;390:337–349. [Google Scholar]
- FAO . World Food Day; Rome, Italy: 2002. Crops and Drops Making the Best Use of Water for Agriculture. [Google Scholar]
- FAO . Food and Agriculture Organisation of the United Nations; Rome, Italy: 2011. Climate Change, Water and Food Security. [Google Scholar]
- Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: mechanisms and management. Agron. Sustain. Dev. 2009;29:185–212. [Google Scholar]
- Franco J.A., Bañón S., Vicente M.J., Miralles J., Martínez-Sánchez J.J. Root development in horticultural plants grown under abiotic stress conditions – a review. J. Hortic. Sci. Biotechnol. 2011;86(6):543–556. [Google Scholar]
- Gordon S.A., Weber R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling S.N., Arnell N.W. A global assessment of the impact of climate change on water scarcity. Climatic Change. 2016;134:371–385. [Google Scholar]
- Hu Y., Burucs Z., Schmidhalter U. Short-term effect of drought and salinity on growth and mineral elements in wheat seedlings. J. Plant Nutr. 2007;29:2227–2243. [Google Scholar]
- Kaushal M., Wani S.P. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016;66:35–42. [Google Scholar]
- Khan N., Bano A., Babar M.D.A. The root growth of wheat plants, the water conservation and fertility status of sandy soils influenced by plant growth promoting rhizobacteria. Symbiosis. 2017;72:195–205. [Google Scholar]
- Kozlowski T.T., Pallardy S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002;68(2):270–334. [Google Scholar]
- Kumar M., Mishra S., Dixit V., Agarwal L., Singh P. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.) Plant Signal. Behav. 2016;11(1) doi: 10.1080/15592324.2015.1071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.W., Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Ling Q., Huang W., Jarvis P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011;107:209–214. doi: 10.1007/s11120-010-9606-0. [DOI] [PubMed] [Google Scholar]
- Mahajan S., Tuteja N. Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Marulanda A., Barea J.M., Azcón R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009;28:115–124. [Google Scholar]
- Michel B.E., Kaufmann M.R. The osmotic potential of Polyethylene Glycol 6000. Plant Physiol. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira H., Pereira S.I.A., Marques A., Rangel A., Castro P. Mine land valorization through energy maize production enhanced by the application of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2016;23:6940–6950. doi: 10.1007/s11356-015-5914-4. [DOI] [PubMed] [Google Scholar]
- Moreira H., Pereira S.I.A., Marques A., Rangel A., Castro P. Selection of metal resistant Plant Growth-Promoting Rhizobacteria for the growth and metal accumulation of energy maize in a mine soil – effect of the inoculum size. Geoderma. 2016;278:1–11. [Google Scholar]
- Moreira H., Pereira S.I.A., Marques A.P.G.C., Rangel A.O.S.S., Castro P.M.L. Effects of soil sterilization and metal spiking in plant growth promoting rhizobacteria selection for phytotechnology purposes. Geoderma. 2019;334:72–81. [Google Scholar]
- Naseem H., Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014;9:689–701. [Google Scholar]
- Naveed M., Mitter B., Reichenauer T., Wieczorek K., Sessitsch A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014;97:30–39. [Google Scholar]
- Nguyen H., Pham C., Bertin P. The effect of nitrogen concentration on nitrogen use efficiency and related parameters in cultivated rices (Oryza sativa L. subsp. indica and japonica and O. glaberrima Steud) in hydroponics. Euphytica. 2014;198(1):137–151. [Google Scholar]
- Niu X., Song L., Xiao Y., Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S.I.A., Castro P.M.L. Diversity and characterization of culturable bacterial endophytes from Zea mays and their potential as plant growth-promoting agents in metal-degraded soils. Environ. Sci. Pollut. Res. 2014;21:14110–14123. doi: 10.1007/s11356-014-3309-6. [DOI] [PubMed] [Google Scholar]
- Pereira S.I.A., Castro P.M.L. Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol. Eng. 2014;73:526–535. [Google Scholar]
- Pereira S.I.A., Pires C., Henriques I., Correia A., Magan N., Castro P.M.L. Assessment of rhizospheric culturable bacteria of Phragmites australis and Juncus effusus from polluted sites. J. Basic Microbiol. 2015;55:1–12. doi: 10.1002/jobm.201500010. [DOI] [PubMed] [Google Scholar]
- Pereira S.I.A., Barbosa L.V., Castro P.M.L. Rhizobacteria isolated from a metal polluted area enhance plant growth in zinc and cadmium contaminated soil. Int. J. Environ. Sci. Technol. 2015;12:2127–2142. [Google Scholar]
- Pires C., Franco A., Pereira S.I.A., Henriques I., Correia A., Magan N., Castro P.M.L. Metal(loid) contaminated soils as a source of heterotrophic culturable aerobic bacteria for remediation applications. Geomicrobiol. J. 2017;34:760–768. [Google Scholar]
- Pillay V.K., Nowak J. Inoculum density, temperature, and genotype effects on in vitro growth promotion and epiphytic and endophytic colonization of tomato (Lycopersicon esculentum L.) seedlings inoculated with a pseudomonad bacterium. Can. J. Microbiol. 1997;43(4):354–361. [Google Scholar]
- Prasad P.V.V., Staggenborg S.A., Ristic Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In: Ahuja L.R., Reddy V.R., Saseendran S.A., Yu Q., editors. Response of Crops to Limited Water: Understanding and Modelling Water Stress Effects on Plant Growth Processes. ASA, CSSA, SSSA; Madison, USA: 2008. pp. 301–355. [Google Scholar]
- Reddy A.R., Chaitanya K.V., Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Rubin R.L., van Groenigen K.J., Hungate B.A. Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis. Plant Soil. 2017;416:309–323. [Google Scholar]
- Rousk J., Smith A.R., Jones D.L. Investigating the long-term legacy of drought and warming on the soil microbial community across five European shrubland ecosystems. Global Change Biol. 2013;19:3872–3884. doi: 10.1111/gcb.12338. [DOI] [PubMed] [Google Scholar]
- Sandhya V., Ali S.Z., Grover M., Reddy G., Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils. 2009;46(1):17–26. [Google Scholar]
- Sandhya V., Ali S.Z., Grover M., Reddy G., Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010;62:21–30. [Google Scholar]
- Sandhya V., Ali S.Z., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011;6:1–14. [Google Scholar]
- Sharp R.E., Poroyko V., Hejlek L.G., Spollen W.G., Springer G.K., Bohnert H.J., Nguyen H.T. Root growth maintenance during water deficits: physiology to functional genomics. J. Exp. Bot. 2004;55(407):2344–2351. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- Shirinbayan S., Khosravib H., Malakoutia M.J. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl. Soil Ecol. 2019;133:138–145. [Google Scholar]
- Trabelsi D., Mhamdi R. Microbial inoculants and their impact on soil microbial communities: a review. BioMed Res. Int. 2013;863240:1–11. doi: 10.1155/2013/863240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglia C., Reinoso H., Cohen A., Luna C., Tommasino E., Castillo C., Bottini R. Exogenous ABA increases yield in field-grown wheat with moderate water restrictionn. J. Plant Growth Regul. 2010;29:366–374. [Google Scholar]
- Ulrich D.E.M., Sevanto S., Ryan M., Albright M.B.N., Johansen R.B., Dunbar J.M. Plant-microbe interactions before drought influence plant physiological responses to subsequent severe drought. Sci. Rep. 2019;9:249. doi: 10.1038/s41598-018-36971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . 2006. Coping with Water Scarcity. A Strategic Issue and Priority for System-wide Action.www.unwater.org [Google Scholar]
- Vacheron J., Desbrosses G., Bouffaud M.-L., Touraine B., Moënne-Loccoz Y., Muller D., Legendre L., Wisniewski-Dyé F., Prigent-Combaret C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen J.A., van Overbeek L.S., van Elsas J.D. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 1997;61:121–135. doi: 10.1128/mmbr.61.2.121-135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinga I., Vark W., Houba V., Lee J. Springer Netherlands; Netherlands: 1989. Plant Analysis Procedures; pp. 47–76. [Google Scholar]
- Wright I.J., Reich P.B., Westoby M. Least-cost input mixtures of water and nitrogen for photosynthesis. Am. Nat. 2003;161:98–111. doi: 10.1086/344920. [DOI] [PubMed] [Google Scholar]
- Yang X., Tang Z., Ji C., Liu H., Ma W., Mohhamot A., Shi Z., Sun W., Wang T., Wang X., Wu X., Yu S., Yue M., Zheng C. Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across northern China. Sci. Rep. 2014;4:5448. doi: 10.1038/srep05448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeffa D.M., Perini L.J., Silva M.B., de Sousa N.V., Scapim C.A., Oliveira A.L.M., Júnior T.A., Gonçalves L.S.A. Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PloS One. 2019;14(4) doi: 10.1371/journal.pone.0215332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatev Z., Lidon F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 2012;24:57–72. [Google Scholar]