Abstract
Coronaviruses are pleomorphic, enveloped, or spherical viruses, which have a size ranging from 80 to 120 nm. These viruses act on receptors that cause the triggering of fusion. Coronaviruses were first described after cultivation from patients with common colds by Tyrell and Bynoe in 1966. There are various subtypes of coronavirus, 7 out of these can cause infection in human beings. The Alpha subtype is responsible for mild infection showing symptoms or infection without any prevailing symptoms. On the other hand, the beta subtype is responsible for very serious diseases leading to fatality. The lineage of this novel SARS-CoV-2 falls under the beta lineage of the beta coronavirus which has been observed to have a relation to the MERS and SARS coronavirus. In the Huanan market selling seafood, the transition of this novel virus in humans from animals has occurred. It has the potential to be the cause of widespread fatality amongst the people of the globe. On August 16, 2020, the World Health Organisation had reported 2,1294,845 cases which are confirmed to date out of which 413,372 deaths have occurred. Currently, no targeted antiviral vaccines or drugs to fight against COVID-19 infection have been approved for use in humans. This pandemic is fast emerging and drug repurposing is the only ray of hope which can ensure quick availability. Vaccine development is progressing each day with various platforms such as DNA, Live Attenuated Virus, Non-Replicating Viral Vector, Protein Subunit, and RNA, being utilized for the development. COVID-19 attacks the immune system of the host & this can result in a cytokine storm. As a result, various herbal agents both acting as antivirals and immunomodulatory can also be used. Convalescent Plasma Therapy and Mesenchymal Stem Cell therapy are also being explored as a plausible therapeutic. There remains a considerable unmet need for therapeutics to be addressed. The development and availability of accessible and efficient therapy are essential for the treatment of patients. This review discusses the epidemiology, pathogenesis, the tale of origin, and transmission of COVID-19 or Sars-Cov2 virus and gives evidence of potential therapeutic agents that can be explored to cast away this pandemic.
Keywords: Coronavirus, COVID-19, SARS-COV2, Drug repurposing, Vaccines, Convalescent plasma therapy and mesenchymal stem cell therapy
Highlights
-
•
World Health Organisation (WHO) declared COVID-19 or SARS-Cov2 as a global public health emergency on 30th January 2020.
-
•
COVID-19 is the third known zoonotic coronavirus disease after SARS and the Middle East respiratory syndrome (MERS).
-
•
At present drug repurposing is the only ray of hope which can ensure quick availability.
-
•
Vaccine development along with Convalescent Plasma Therapy and Mesenchymal Stem Cell therapy are also being focussed upon.
-
•
Various herbal agents can also be used as a prophylaxis or as an adjunct therapy for cytokine storm.
1. Introduction
Coronaviruses are a part of a huge family of single-stranded, positive-sense enveloped RNA viruses that can infect human beings as well as various animal hosts (Mousavizadeh and Ghasemi, 2020). They are currently classified as one of the two genera in the family Coronaviridae (Masters, 2006). The coronavirus genetic composition encodes for 4 structural proteins which include, the spike, nucleocapsid, envelope, and membrane protein; some coronaviruses also have a hemagglutinin esterase protein. The most dominantly present surface protein is the Spike Glycoprotein which is accountable for attaching the virus and consequently, fusing with the membrane (Cong and Ren, 2014). The consequent series of coronavirus disease cases had come up in Wuhan situated in China began in December of 2019. A huge population was infected by the virus through the animal market situated in the city of Wuhan wherein the sale of wet animals was very common. Consequently, It has been postulated that COVID-19 may have a zoonotic origin (Rothan and Byrareddy, 2020). Inevitably, the World Health Organisation had designated Coronavirus Disease 2019 as a public health emergency that affects the population across all countries on January 30, 2020. On the other hand, the International Committee on Taxonomy of Viruses which constitutes a coronavirus committee named this disease as a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It includes the cluster of beta coronaviruses. It is the third zoonotic disease associated with coronavirus. Earlier, Severe Acute Respiratory syndrome and the Middle East respiratory syndrome have also gravely affected the globe (Sun et al., 2020). The symptoms of the infection are only identifiable after an incubation period of 5.2 days. On the other hand, the period after which symptoms can be identified by the time of death ranges from about 6 to 41 days and the median duration of the same is 14 days (Rothan and Byrareddy, 2020). With it's growing spread this pandemic has observed various scientists and researchers around the globe are persistently exploring drugs which would prove to be the ray of hope in the fight against COVID-19. At present, no specifically verified antiviral therapeutics against COVID-19 (Dong et al., 2020). The pandemic is fast emerging and drug repurposing is the only ray of hope which can ensure quick availability. At present, there are no therapies be it a vaccine or a drug for COVID-19. However, there are several established drugs such as Chloroquine, Hydroxychloroquine, Ivermectin, and Niclosamide, Azithromycin, Remdesvir, and Lopinavir, etc. All these drugs have shown in vitro potency and also in silico lead for the fight in combating SARS CoV 2 (Fisher and Heymann, 2020). Vaccine development is progressing each day with various platforms like DNA, Live Attenuated Virus, Non-Replicating Viral Vector, Protein Subunit, and RNA being utilized for the development. COVID-19 attacks the immune system of the host resulting in a cytokine storm. As a result, various herbal agents both acting as antivirals and immunomodulators can be used as a prophylactic agent or as an adjunct therapy along with some anti-rheumatic drugs which can also be repurposed for mitigating the cytokine storm. Various studies have suggested that some abietane-type diterpenoid and lignoid compounds possess a strong activity against SARS-CoV effects (Wen et al., 2007). Many phytoconstituents possess an effective antiviral activity. In the absence of potent chemical therapy, alternative herbal therapy can be explored. Convalescent Plasma Therapy and Mesenchymal Stem Cell therapy are also being explored as a plausible therapeutic. Several studies have shown that when treated with convalescent plasma therapy, the patients stayed in the hospital for a lesser duration and the mortality rate was also lowered (Chen et al., 2020). Mesenchymal stem cell therapy can prevent the release of cytokines and enhance endogenous repair (Golchin et al., 2020). The safety, toxicity and efficacy of the candidate therapeutics for the treatment of COVID-19 has to be evaluated in preclinical and clinical trials. With this background, in this review we will divulge into understanding the pathogenesis and structure of Covid-19 as well as take an insight into the potential therapeutic agents which could target this virus.
2. Coronavirus
Coronaviruses (CoVs) belong to the order Nidovirales, family Coronaviridae, and subfamily Coronavirinae. These viruses contain a single-stranded RNA genome. The size of their genetic material ranges from 26 to 32 kb (Schoeman and Fielding, 2019). They can be classified into 4 genera which comprise alpha, beta, gamma, and delta. Out of these, the beta genera has further 4 lineages named as A, B, C, and D. These viruses are the cause of various enteric as well as respiratory disorders in mammals as well as birds. In 2002–2004, the outbreak of severe acute respiratory syndrome took place and in 2012 the Middle East respiratory syndrome had posed a major threat to mankind. However, the other four coronaviruses are accountable for 30% of mild infections of the respiratory tracts which are most commonly observed in patients who have a weaker immune system and in populations including children and elders (Tortorici and Veesler, 2019).
3. Covid-19 pandemic, a threat to the human race
In December, the newly identified β-coronavirus was observed as the cause of various of pneumonia cases in the population in the city of Wuhan situated in China. Initially, the virus was named as the 2019-novel coronavirus by the WHO which was later renamed by the Coronavirus Study Group from the International Committee on Taxonomy of Viruses as SARS-COV-2 (Guo et al., 2020).
3.1. Origin and transmission
The early infected patient had been traced to the Huanan wholesale market selling seafood in the city of Wuhan situated in China. The Chinese Center for Disease Control and Prevention performed laboratory evaluation to rule out common respiratory pathogens like SARS-CoV, adenovirus, influenza, avian influenza, MERS-CoV. Consequently, COVID-19 was identified as the CDC pathogen causing the outbreak. COVID-19 has the potential to cause critical illness in the geriatric population and can be transmitted easily among other populations (She et al., 2020). The origin of this novel coronavirus associated infection has been established as bats. After elucidating the full-length genome sequences, Zhou and colleagues noted that COVID-19 is 96% similarity at the whole-genome level to a bat coronavirus. After performing a phylogeny derived analysis on the entire viral genetic makeup, Wu and colleagues observed that this novel virus has a possible relation to the SARS group which had been already obtained as samples through bats from China (Cheng and Shan, 2020).
3.2. Epidemiology
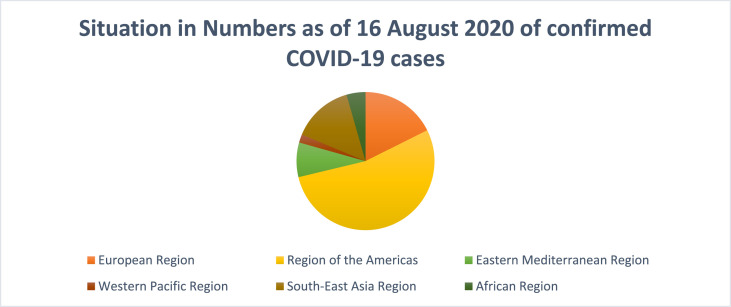
The reports of the initially infected patients were obtained in December 2019. During the period through December 18 to December 29, one of the 5 aforementioned patients died due to critical distress in the respiratory passage. 41 admitted patients were diagnosed with laboratory-confirmed COVID-19 infection by January 2, 2020. Less than half of the infected individuals already were suffering from disorders like diabetes and hypertension. (Rothan and Byrareddy, 2020) has the potential to be the cause of widespread fatality amongst the people of the globe. On August 16, 2020, the World Health Organisation had reported 2, 1294,845 cases which are confirmed to date out of which 413,372 deaths have occurred. Fig. 1 depicts the situation in numbers as of June 12, 2020 of confirmed COVID-19. As of August 16, 2020, the number of confirmed cases per million are 61 in China, 433 in Japan, 7332 in Spain, 4192 in Italy, 2667 in Germany, 4660 in The United Kingdom, 3096 in France, 1877 in India, 15,887 in the United States of America and 3223 in Canada. Out of these Italy, the United States, Spain, Germany, UK, France, and Canada have reported community transmission while China, Japan, and India report clusters of cases (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200504-covid-19-sitrep-105.pdf?sfvrsn=4cdda8af_2, 2020).
Fig. 1.
Situation in Numbers as of August 16, 2020 of confirmed COVID-19 cases.
3.3. Symptoms and diagnosis
The most frequently seen symptoms are fever, dry cough, myalgia, or fatigue. On the other hand, sputum production, headache, hemoptysis, shortness of breath, and diarrhea are less frequently observed (C. Huang et al., 2020). Some other symptoms experienced by patients are chills, shivers, sore throat, loss of taste, and smell. Respiratory viruses have the potential to interact with each other and affect susceptibility to acute respiratory diseases (X. Huang et al., 2020).
Reverse transcriptase-polymerase chain reaction remains the reference standard for the diagnosis. Characteristic chest CT imaging features include multifocal bilateral ground-glass opaqueness along with patchy consolidations which are prominently present in the peripheral sub-pleural distribution and preferred posterior part or lower lobe predilection. Wuhan exposure, travel history, or a close contact history are suggestive of COVID-19 infection (Zu et al., 2020). Serological antibody testing is a valuable assay that produces the result in a short period (Ahn et al., 2020). Recently, WHO has listed two emergency use diagnostic tests for the COVID-19 pandemic which would facilitate quality-assured accurate tests. The genesis Real-Time PCR Coronavirus (COVID-19) test has been designed by Primerdesign in the United Kingdom which is suited for laboratories with modest sample testing scope as it is an open system assay. Another test namely the cobas® SARS-CoV-2 has been developed for utilization on the cobas® 6800/8800 Systems developed by Roche in the United States of America and is suited for larger laboratories because it is a closed system assay (https://www.who.int/news-room/detail/07-04-2020-who-lists-two-covid-19-tests-for-emergency-use, 2020). Various platforms are being explored in laboratories. These include paper-based systems, electrochemical sensors, aptamer-based biosensors, and surface-enhanced Raman scattering based systems (Udugama et al., 2020). Novel CRISPR–Cas12-based detection can differentiate SARS-CoV-2 without any cross-reactivity for other relating coronavirus strains utilizing N gene gRNA and expecting cross-reactivity for E gene gRNA. These emerging technological platforms utilize portable microfluidic-based cartridges and lyophilized reagents to run their assay and could establish a method of testing outside of the laboratory (Broughton et al., 2020).
3.4. Structure of COVID-19
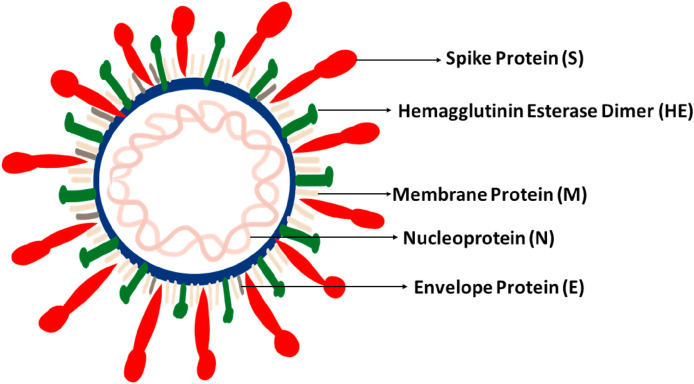
The spike protein (S) present on the surface of COVID-19 is responsible for the attachment of host cells followed by viral-host cell membrane fusion and internalization of the virus (Tufan et al., 2020). The envelope of the virus contains club-shaped glycoprotein projections (E). It also contains a hemagglutinin esterase protein (HE). Membrane glycoprotein (M) is the most common structural protein since it spans across the membrane bilayer three times. RNA is incorporated with a nucleoprotein (N) within a capsid comprising of the genetic material in the form of a matrix protein. The membrane glycoprotein is involved in the intracellular forming of the virus constituents without the need of spike protein (Mousavizadeh and Ghasemi, 2020) (Fig. 2 ).
Fig. 2.
Structure of COVID-19.
3.5. Pathogenesis
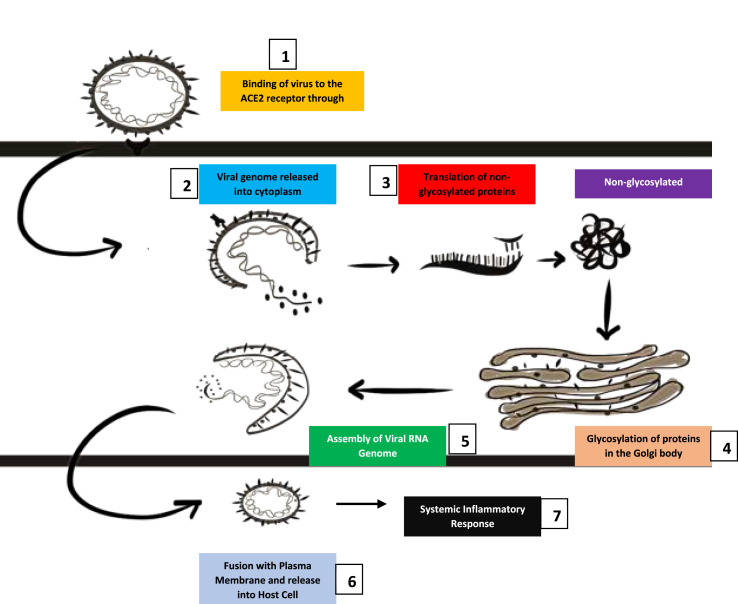
SARS-COV-2 virus possesses a heavily glycosylated spike protein. To gain entry into the endoplasmic reticulum and mediate attachment to the host receptor, the N-terminal signal sequence is utilized (Lu et al., 2020). S protein has been identified as a key determinant factor to facilitate virus entry into the cell of the host organism. The glycoprotein spike envelope of the virus binds to the angiotensin-converting enzyme 2 cellular receptors (Li et al., 2003). The fusion of the vesicles which contain the virus with the plasma membrane leads to the release of the virus inside the cell. The virus then proceeds to enter the cell and the viral RNA genome subsequently releases in the cytoplasm which is further translated into structural proteins and two polyproteins. After protein production, the viral genome begins replication. The formation of nucleocapsid is influenced by both genomic RNA and nucleocapsid protein after the newly produced glycoproteins for the envelope insert into the endoplasmic reticulum or the Golgi bodies. (De Wit et al., 2016). The virus-specific cytotoxic T lymphocytes recognize the antigenic peptides released which are presented by the major histocompatibility complex. The tissue injury leads to heightened proinflammatory cytokine production and recruitment which results in a cytokine storm also known as secondary hemophagocytic lymphohistiocytosis or macrophage activation syndrome, thus leading to further tissue damage (Tufan et al., 2020). The acute phase response is associated with a severe decrease of CD4+ T and CD8+ T cells. The inflammatory storm leads to the release of abundant pro-inflammatory cytokines and chemokines. The virus can induce the production of vesicles that possess a double membrane and lack pattern recognition receptors which ensures that they are not detected by the host dsRNA. Coronavirus also can influence the antigen presentation (Li et al., 2020). Fig. 3 depicts the pathogenesis of COVID-19.
Fig. 3.
Pathogenesis of COVID-19.
1. The virus binds to the angiotensin-converting enzyme 2 receptors through the spike protein. 2. Consequently, the viral genetic material releases into the cytoplasm. 3. Translation produces non-glycosylated proteins. 4. The non-glycosylated proteins are transferred to the Golgi bodies and translated. 5. The viral genome is assembled. 6. Fusion with plasma membrane followed by release into the host cell. 7. A systemic inflammatory response prevails.
4. Potential therapeutic agents
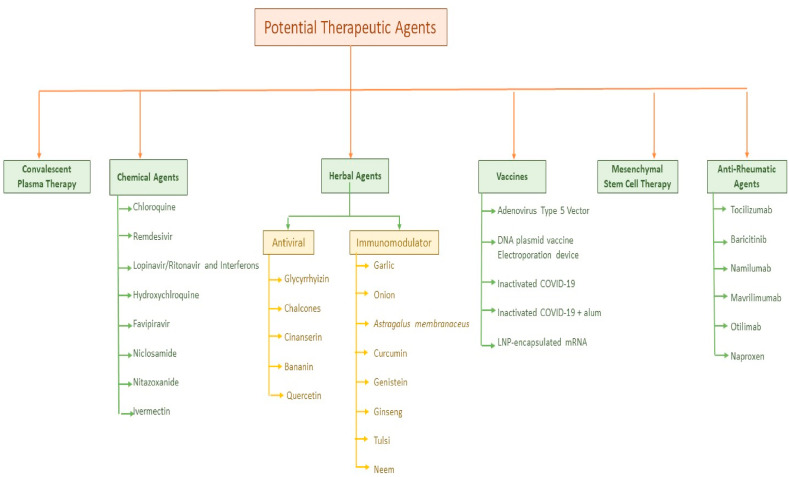
The pandemic of COVID-19 is progressing each day. Currently, there are no approved vaccines against COVID-19. Drug repurposing offers a ray of hope to provide treatment to the patients. Many repurposed drugs are showing promising outcomes in trials and further investigations are ongoing to accurately evaluate their efficacy. Additionally, antiviral and immune-boosting herbal therapies have the potential to curb the heightened immune response (cytokine storm) associated with the disease and can be utilized as prophylaxis or as an adjunct therapy. Consequently, Convalescent Plasma Therapy and Mesenchymal Stem- Cell Therapy is also being explored as a viable therapeutic apart from the ongoing research to develop vaccines for SARS-COV-2. Fig. 4 depicts the schematic representation of the potential therapeutics.
Fig. 4.
Potential therapeutic agents for COVID-19 PANDEMIC.
4.1. Chemical agents
4.1.1. Chloroquine
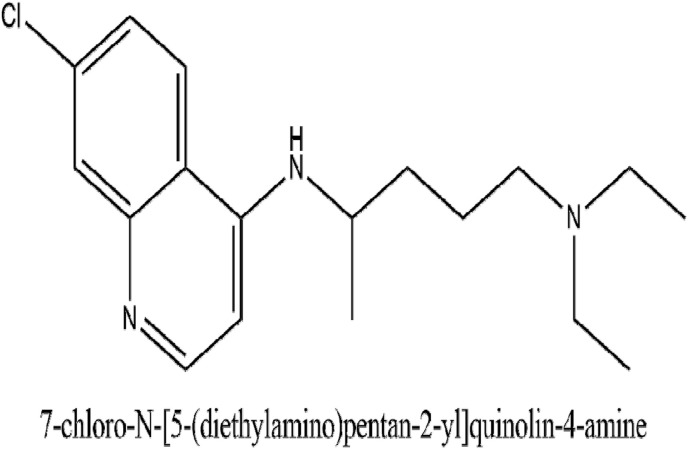
Chloroquine who's IUPAC name is 4-N-(7-chloroquinolin-4-yl)-1-N,1-N-diethylpentane-1,4-diamine has been used conventionally for prophylaxis and treatment of malaria. Its dose for malaria prophylaxis is 500 mg orally 2 weeks before, during, and up to 8 weeks after exposure to an endemic area. It is taken as a weekly dose. When used for the treatment of extraintestinal amoebas, its dose is 21 mg/kg for 3 weeks (Goel and Gerriets, 2020). Its mechanism of action as evaluated by various studies reveals that it can increase the endosomal pH needed for the fusion of the virus and the cell. It also has the potential in interfering with the glycosylation of receptors inside the cell of SARS coronavirus. Due to the emergence of this severe pandemic, there is a pertinent clinical demand, and chloroquine phosphate can be used for treating pneumonia associated with COVID-19 (Gao et al., 2020). The in-vitro evaluation of chloroquine for treating SARS-CoV was seen in China. A culture test on Vero E6 cells using 50% and 90% concentrations was performed and the antiviral activity observed on the addition of drugs was 1.13 and 6.90 μM respectively. The subsequent in vivo record obtained from a clinical study done in China shows that Chloroquine can decrease the duration of hospital stay and improving the progress of COVID-19 pneumonia. As a result of which, administering 500 mg of chloroquine two times a day in patients in patients with varying forms of COVID-19 pneumonia was advised. (Colson et al., 2020). Another time-of-addition assay demonstrates that chloroquine acted both at the entry as well as at the post-entry stages of the infection by COVID-19 in VERO E6 cells. In Vivo, Chloroquine also possesses an immune-modulating action that holds the potential of synergizing the antiviral effect. Since chloroquine has a wider distribution in the whole body which includes the lungs, it shows promising effects against the COVID-19 infection (Wang et al., 2020). Fig. 5 illustrates the IUPAC name and structure of chloroquine. Table 1 outlines some of the ongoing clinical trials for the evaluation of Chloroquine. Currently, 83 trials are going on in different countries to study the activity of chloroquine in COVID-19.
Fig. 5.
The IUPAC name and structure of Chloroquine.
Table 1.
Various Ongoing clinical trials for evaluation of Chloroquine (https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04328493 | Treatment with Chloroquine in patients in Vietnam | 2 | the loading dose of 1200 mg, 300 mg base orally once daily for 9 days | Vietnam |
| NCT04344951 | An Open-Label, Non-Randomized Clinical Trial utilizing Chloroquine Phosphate Against COVID-19 | 2 | 500 mg two times a day for seven days. | Greece |
| NCT04333628 | The utilization of Chloroquine for Mild Symptomatic and Asymptomatic COVID-19 | 2,3 | Low dose- 125 mg every day for 7 days High dose- 500 mg two times a day for 7 days |
Israel |
| NCT04349371 | Determination of the clinical efficacy of Chloroquine in health care workers | 2 | 250 mg once daily for 1 week then 2 tabs of 250 mg for once for 7 days for 3 months | United States |
| NCT04351724 | Aimed at the evaluation of the efficacy of various anti-viral treatments | 2,3 | 250 mg | Austria |
4.1.2. Remdesivir
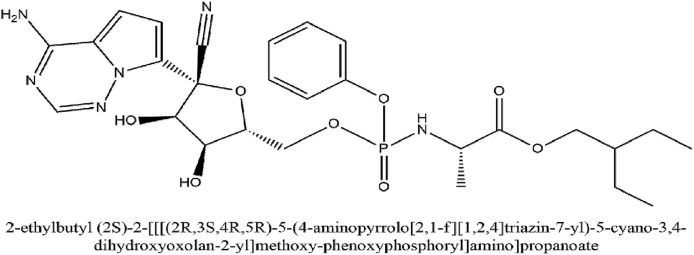
Remdesivir (2-ethyl butyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo [2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate) has a development code of GS-5734. It is a broad-spectrum antiviral drug which was originally innovated against Ebola virus infection by Gilead Sciences in 2017. It is a monophosphoramidate prodrug adenosine analog who's metabolization into its active form leads to the formation of GS-441524. The active form inhibits viral RNA polymerase (RdRP) as well as is successful in evading proofreading by exonuclease of the virus which causes a reduction in viral RNA load. The antiviral action for remdesivir is the cessation of the chain of the viral RNA of the virus (Al-Tawfiq et al., 2020). Fig. 6 illustrates the IUPAC name and structure of Remdesivir. Table 2 outlines some of the clinical studies for the evaluation of Remdesivir.
Fig. 6.
The IUPAC name and structure of Remdesivir.
Table 2.
Ongoing clinical trials for evaluation of Remdesivir (https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04280705 | Adaptive COVID-19 Treatment Clinical Trial | 3 | 200 mg i.v. on the first day, 100 mg once a day up to 10 days | United States |
| NCT04321616 | Evaluating the Efficacy of various Anti-viral Drugs against COVID 19 | 2,3 | 100 mg i.v. daily up to 10 days; loading dose 200 mg at inclusion will be given. | Norway |
| NCT04315948 | Trial of Treatments against COVID-19 in Adult Hospitalized patients | 3 | 100 mg i.v. loading dose on the first day, 100 mg once-daily I.v. maintenance dose up to 10 days | France |
| NCT04292899 | Clinical Trial Study for Evaluation of the Safety and Activity of Remdesivir in Patients With Severe Coronavirus Disease | 3 |
PartA: (Patients that are not mechanically ventilated) 200 mg given on the first day followed by 100 mg on Days 2–5 PartA: (Patients Not Mechanically Ventilated) 200 mg on the first day and then 100 mg on Days 2–10. PartB: (Extension) 200 mg on the first day then 100 mg on Days 2–10. Part B: (Patients provided with Mechanical Ventilation) 200 mg on the first day followed 100 mg on Days 2–10 |
United States |
| NCT04292730 | Clinical Trial for Evaluation of the Safety and Activity of Remdesivir in Patients | 3 |
PartA: (Patients not mechanically ventilated) 200 mg on the first day followed by 100 mg on Days 2–5 PartA: (Patients Not Mechanically Ventilated) 200 mg on the first day followed by 100 mg on Days 2–10. PartB: (Extension) 200 mg on the first day followed by 100 mg on Days 2–10. Part B: (Patients provided with Mechanical Ventilation) 200 mg on the first day followed by 100 mg on Days 2–10 |
United States |
Its activity has been evaluated in vitro like primary human airway epithelial cell cultures with submicromolar IC50 values, bat coronaviruses, bat coronaviruses, and human coronavirus in primary human lung cells wherein it was observed that Remdesivir causes inhibition of both SARS and MERS coronavirus replication. In a study done in mice, the administration of GS-5734 was seen to reduce lung viral load and improve clinical symptoms of infection and respiratory function (Sheahan et al., 2017).
In primary human lung epithelial cell culture, remdesivir has shown potent activity against circulating human, MERS, SARS, and related zoonotic bat coronaviruses. It was also observed that remdesivir improved infection outcomes and at the same time, a reduction in viral loads of SARS coronavirus infected mice was seen. It is the only therapeutic alternative that efficiently reduces pulmonary pathology. The emerging evidence enclosed depicts the potential of Remdesivir to treat CoV infections (Sheahan et al., 2020). US FDA had authorized the use of Remdesivir in coronavirus treatment on May 2, 2020 (https://www.aninews.in/news/world/us/us-fda-authorises-emergency-use-of-remdesivir-in-coronavirus-treatment20200502042752).
4.1.3. Lopinavir/Ritonavir and Interferons
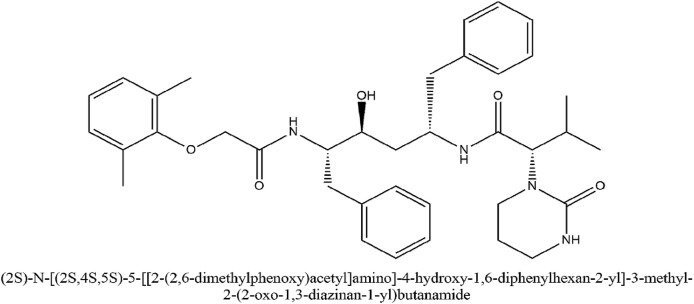
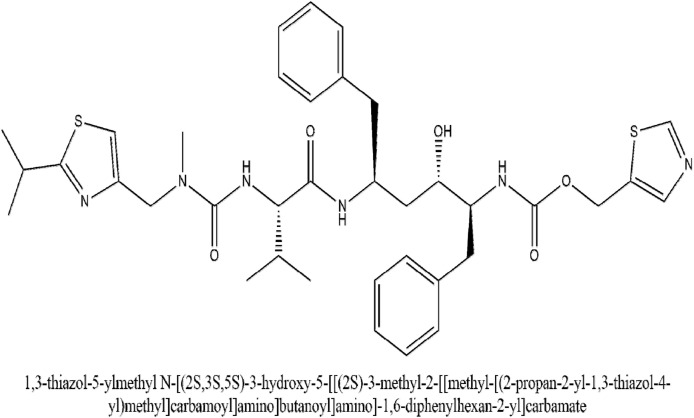
Proteinase is a pertinent enzyme in coronavirus polyprotein processing. Lopinavir is a proteinase inhibiting drug that has a structural relation to ritonavir. The IUPAC name of lopinavir (2S)-N-[(2S,4S,5S)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide. It was approved by the USFDA as a co-formulation with ritonavir having the brand name Kaletra. Ritonavir increased lopinavir drug exposure by inhibition of cytochrome P450 isoenzyme 3A4 (Mangum and Graham, 2001). The peak serum concentration of 9.6 μg/ml and trough serum concentration of 5.5 μg/ml inhibit SARS-CoV. Additionally, the post-entry step in the MERS coronavirus replication cycle is also blocked. Ritonavir can inhibit the CYP3A4 associated metabolizing of lopinavir and hence it increases the serum concentration of lopinavir (Yao et al., 2020). The IUPAC name of ritonavir is 1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[[(2S)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate. A high-throughput assay for antiviral activity associated with the cytopathic effect due to the MERS coronavirus disease in Vero and Huh7 cells showed lopinavir's potential to inhibit CoV replication (De Wilde et al., 2014). When lopinavir was given in combination with ritonavir, the plasma half-life of lopinavir was increased through the inhibition of cytochrome P450. An open-label clinical trial study (NCT04276688) done on patients with SARS with a control group was done, in which patients were administered ribavirin. Additionally, when 400 mg and 100 mg of lopinavir and ritonavir respectively was added to the therapy, a significant reduction in the risk of adverse clinical outcomes and viral load was seen among patients having COVID-19 infection (Chu et al., 2004).
The very first patient of COVID-19 infection imported from the city of Wuhan to Korea was given 400 mg and 100 mg of lopinavir and ritonavir respectively on day 4 of the disease. Gradually, her dyspnea had started improving which reduced oxygen need, and the lesion in the lung begun to vanish in radiography of the chest (Kim et al., 2020). Another 54-year-old patient who returned from Wuhan to Korea was given the same combination of lopinavir and ritonavir on the tenth day of the infection. The subsequent day onwards viral load had begun to reduce and very few titers of the virus were observed (Lim et al., 2020). A clinical study involving a total of 199 adult population diagnosed with COVID-19 infection was conducted, in which 99 infected individuals were given the combination of lopinavir and ritonavir and 100 patients were given standard care. The patients received this combination of lopinavir-ritonavir in the doses of 400 mg and 100 mg, respectively two times a day for two weeks days randomly in a 1:1 ratio. The primary endpoint was the time from randomization to either discharge from the hospital or improvement of two points on a seven-category ordinal scale. The mortality rate was 19.2% at 28 days in the group that received lopinavir and ritonavir and 25.0% in the standard care group (Cao et al., 2020).
A study was done on marmosets with the severe disease which resembled MERS in humans. Treatment with lopinavir and ritonavir-treated and interferon-β1b combination therapy was given which had better outcomes than the untreated animals. The mean clinical scores decreased by 50.9%–95.0% and the weight loss also decreased. Radiological findings showed minimal pulmonary infiltrates. Bronchointerstitial pneumonia was observed to be very moderate and reduction in mean viral load was observed in necropsied lung (Chan et al., 2015). A study was done on 10 patients which presented with symptoms of COVID-19 infection in which the patients were given combined therapy of lopinavir 400 mg once in 12 h and interferon α2b, 5 million Units two times a day or solely drug Lopinavir. Some patients showed an improving opacity of computed tomography images. However, adverse digestive effects and hypokalemia were a cause of concern by clinicians (Liu et al., 2020). The above data indicate its potential as an anti-viral therapeutic in the experimental paradigm of SARS-COV2 infection. Fig. 7, Fig. 8 illustrate the IUPAC name and structure of Lopinavir and Ritonavir respectively. Table 3 outlines some of the clinical studies for the evaluation of Lopinavir/Ritonavir and Interferons.
Fig. 7.
IUPAC name and structure of Lopinavir.
Fig. 8.
The IUPAC name and structure of Ritonavir.
Table 3.
Clinical Studies for evaluation of Lopinavir/Ritonavir and Interferons (https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04276688 | Utilization of Lopinavir/Ritonavir, Ribavirin and IFN-beta Combination against nCoV Treatment | 2 |
Lopinavir/ritonavir: 400 mg and 100 mg respectively administered twice daily for 14 days Ribavirin: 400 mg twice administered daily for 14 days Interferon Beta-1B: 0.25 mg subcutaneous injection administered alternatively for 3 days |
Hong Kong |
| NCT04251871 | Treatment and Prevention of COVID-19 infection with Traditional Chinese Medicines | Not Applicable |
alfa interferon: aerosol inhalation lopinavir and ritonavir:400 mg of lopinavir and 100 mg of ritonavir bid for 2 weeks. |
China |
| NCT04315948 | Clinical Trial of various Treatments for Hospitalized adult patients against COVID-19 | 3 |
Lopinavir/ritonavir: 200 mg Lopinavir and 50 mg Ritonavir IFN-ß-1a: one dose 44 μg/0.5 ml |
France |
4.1.4. Hydroxychloroquine
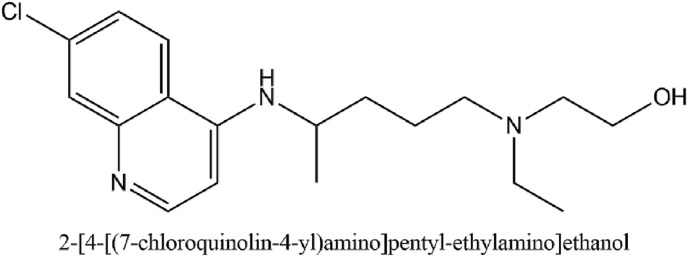
Hydroxychloroquine has immunosuppressive, anti-autophagy, and antimalarial activity (Lim et al., 2009). It's IUPAC name is 2-[4-[(7-chloroquinolin-4-yl)amino]pentyl-ethylamino]ethanol. When administered for malaria prophylaxis in adults, hydroxychloroquine is started with 400 mg once a week from 2 weeks before until 8 weeks after travel to an endemic area. For rheumatic diseases, the most common dose is 400 mg per day (Hickley et al., 2011). A study conducted on Vero cells showed an EC50 of 34 ± 5 μM against SARS-CoV. Another study on feline coronavirus on the Crandell–Reese feline kidney cells showed an EC50 of 28 ± 27 μM (Colson et al., 2020).
A clinical study was conducted in March on French COVID-19 patients in which patients received 600 mg hydroxychloroquine. The endpoint was considered as the presence of the virus. Consequently, a significant reduction in the viral carriage was observed compared to controls. Azithromycin proved to make the activity of hydroxychloroquine more efficient in virus elimination (Gautret et al., 2020) Various clinical studies are currently ongoing for evaluation of the activity of hydroxychloroquine. A Phase 2 multi-center open-labeled randomized clinical trial is being conducted in Korea on 150 participants which aim at investigating whether hydroxychloroquine reduces the viral load from the respiratory specimen in patients. The study tests if hydroxychloroquine is successful in preventing symptomatic COVID-19 disease and if it can prevent disease progression in persons. A Phase 3 study is being conducted on 3000 participants who were given 200 and 800 mg tablet orally once followed by a 600 mg tablet in 6–8 h then a 600 mg tablet one time a day for the following 4 days (Singh et al., 2020). Hydroxychloroquine is a remarkable drug that has been used for decades because of its anti-inflammatory and antiviral properties. They are cheap and would be easily available for use against COVID-19. However, they have a narrow therapeutic index. Cardiac toxicity may occur as a result of prolongation of QT interval and sodium channel inhibition which may cause ventricular arrhythmia and blockade in conduction (Mégarbane, 2020). Considering the potential benefit and risk balance of hydroxychloroquine, it could prove to be a valid treatment option in the absence of any approved drugs. Fig. 9 illustrates the IUPAC name and structure of hydroxychloroquine. Table 4 outlines the clinical studies for the evaluation of Hydroxychloroquine.
Fig. 9.
The IUPAC name and structure of Hydroxychloroquine.
Table 4.
Clinical studies for evaluation of Hydroxychloroquine (https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04345692 | A Randomized Controlled Clinical Study utilizing Hydroxychloroquine for Treating Hospitalized Patients | 3 | 400 mg two times a day 1, then 200 mg two times a day for days 2–5 | United States |
| NCT04340544 | Utilization of Hydroxychloroquine for the Treatment of Mild COVID-19 Disease | 3 | 600 mg daily for 7 days | Germany |
| NCT04349228 | Assessing the Efficacy and Safety of Hydroxychloroquine as a Prophylactic therapeutic for Health Professionals against COVID19 | 3 | 200 mg/day | Tunisia |
| NCT04328272 | Effectiveness of Hydroxychloroquine in Covid-19 Patients | 3 |
Day1: 3 tablets (200 mg per tablet), after 6 h, 3 tablets (200 mg per tablet) Day2-7: (maintenance dose), 2 tablets twice a day. |
Pakistan |
| NCT04359953 | Efficacy of Hydroxychloroquine, Telmisartan, and Azithromycin for Elder Patients | 3 | 200 mg two times a day for 2 weeks | France |
| NCT04342221 | Hydroxychloroquine for COVID-19 | 3 |
Day 1:800 mg Day2 onwards: 600 mg (3 capsules) one time a day |
Germany |
| NCT04318015 | Hydroxychloroquine as a Chemoprophylactic therapeutic for Healthcare Personnel who have come in Contact With COVID-19 infected individuals | 3 | 200 mg per day for 60 days. | Mexico |
| NCT04332991 | Evaluation of Outcomes associated with COVID-19 when Treatment With Hydroxychloroquine Among Symptomatic patients was done | 3 | 400 mg two times a day, 200 mg two times a day for the following 4 days | United States |
4.1.5. Favipiravir
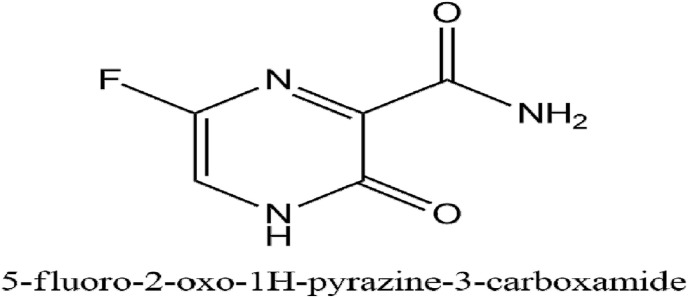
Favipiravir sold under the brand name Avigan can selectively and potently cause inhibition of the RNA-dependent RNA polymerase found in RNA viruses. It gets converted into an activated form, favipiravir-RTP (favipiravir ribofuranosyl-5′-triphosphate), RNA-dependent RNA polymerase recognizes it as its substrate, and inhibits the RNA polymerase activity (Furuta et al., 2017). The IUPAC name of Favipiravir is 5-fluoro-2-oxo-1H-pyrazine-3-carboxamide.
It can block the replication of many other RNA viruses, including arenaviruses, phleboviruses, sandfly fever, hantaviruses, flaviviruses, enteroviruses, an alphavirus, western equine encephalitis virus; a paramyxovirus, respiratory syncytial virus, and noroviruses (Furuta et al., 2017). A clinical study for evaluation of the safety and efficacy of favipiravir on 80 patients in the treating COVID-19 (ChiCTR2000029600) was done in Shenzhen in which 45 individuals were taken as control and 35 patients received favipiravir and showed reduced viral clearance time as well as an improvement in chest imaging (http://www.chictr.org.cn/showprojen.aspx?proj=49042, 2020). A multicenter randomized clinical study suggested that on treatment with favipiravir, the seventh-day recovery rate of recovery increased from 55.86% to 71.43% (Du and Chen, 2020). Fig. 10 illustrates the IUPAC name and structure of Favipiravir. Table 5 outlines clinical studies for the evaluation of Favipiravir.
Fig. 10.
The IUPAC name and structure of Favipiravir.
Table 5.
Clinical Study for evaluation of Favipiravir (https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04346628 | Administration Favipiravir orally comparing with the Standard Supportive Care given in Subjects against Mild COVID-19 | 2 | Day1: 1800 mg then 800 mg twice daily for the next 9 days | United States |
| NCT04349241 | Efficacy and Safety of Favipiravir in Management of COVID-19 | 3 | 1600 mg after every 12 h loading dose on the first day followed by 1200 mg maintenance dose on day 2–10 | Egypt |
| NCT04333589 | Utilization of Favipiravir for Patients whose Nucleic Acids Changed From Negative to Positive | Not Applicable |
Day 1: 1600 mg twice a day Day 2-7: 600 mg twice a day. |
China |
| NCT04358549 | Clinical Trial to Study of the Use of Favipiravir for Hospitalized patients | 2 |
Day 1: 1800 mg BID Days 2-14: 1000 mg BID For subjects with Child-Pugh: Days 2-14: 800 mg BID plus Standard care |
United States |
| NCT04336904 | Evaluation of The safety and performance Of Favipiravir against COVID-19 | 3 | Day 1: 1800 mg BID Days 2-14: 600 mg TID | Italy |
| NCT04359615 | Favipiravir in Hospitalized COVID-19 Patients (FIC) | 4 | Not specified | Tehran, Iran |
| NCT04310228 | Favipiravir and Tocilizumab combination | Not applicable | Favipiravir group On Day 1, 1600 mg, two times a day; from the 2nd to the 7th day, 600 mg, two times a day. Tocilizumab group 4 ~ 8 mg/kg |
China |
4.1.6. Niclosamide
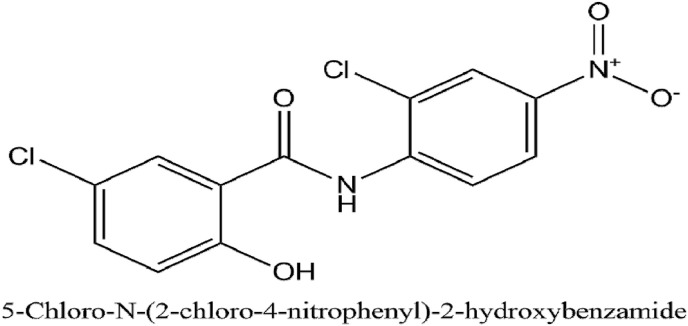
Niclosamide is an antihelminthic drug that has been used for 50 years for treating tapeworm infections. The anti-parasite activity is mediated by inhibition of mitochondrial oxidative phosphorylation and anaerobic ATP production (Pan et al., 2012). The maximum adult dose given is 2g. The IUPAC name of niclosamide is 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide.
A study was done with Vero E6 cells with the multiplicity of infection being 0.1. Niclosamide was successful in inhibiting the replication of SARS-CoV. The viral antigen production was destroyed at niclosamide concentration of 1.56 μM which was revealed by immunoblot analysis (Wu et al., 2004). Niclosamide unlike other nitro anilide based peptides does not get cleaved by SARS-CoV 3CL protease enzyme but shows a slight inhibitory action against the enzyme (Shie et al., 2005).
Another cell-based assay of cytopathogenic effect done in Vero E6 cells infected with the SARS virus showed a high level of inhibition mediated by niclosamide at concentrations between 3.3 and 20 μM. Additionally, a significant inhibiting effect was observed (Wen et al., 2007). Fig. 11 illustrates the IUPAC name and structure of Niclosamide. A Phase 3 clinical trial (NCT04345419) in 100 participants is currently ongoing to evaluate the use of Niclosamide against COVID-19 (https://clinicaltrials.gov/ct2/show/NCT04345419?term=niclosamide+covid&draw=2&rank=1, 2020). The results would potentiate and provide cardinal clues on the use of Niclosamide as a therapeutic for novel coronavirus infection.
Fig. 11.
The IUPAC name and structure of Niclosamide.
4.1.7. Nitazoxanide
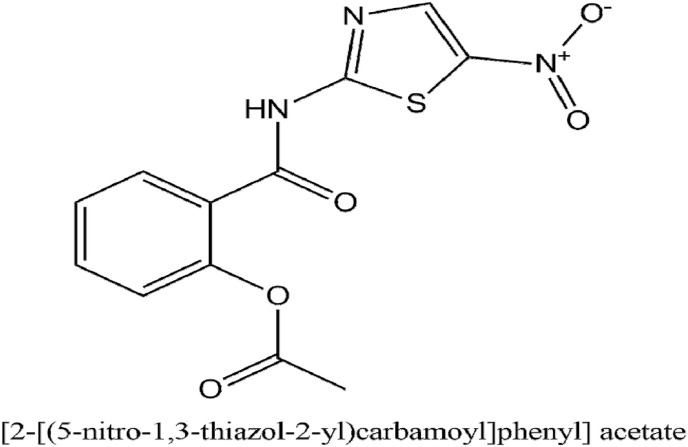
Nitazoxanide is an antiprotozoal and a broad-spectrum antiviral therapeutic which has been repurposed for treating influenza. It is widely sold in Latin America and India where it is taken for treatment of intestinal parasitic infections. It can block the maturing viral hemagglutinin at the post translating stage against influenza (Rossignol, 2014). The IUPAC name of nitazoxanide is [2-[(5-nitro-1,3-thiazol-2-yl)carbamoyl]phenyl] acetate.
Tizoxanide is the active metabolite of nitazoxanide. In various in vitro studies, It has been seen that tizoxanide is successful in inhibiting the replication of canine coronavirus S-378 grown in A72 cells with an IC50 of 1 μg/ml. Additionally, it has been revealed that it can inhibit bovine coronavirus strain L9, murine coronavirus, and human enteric coronavirus 4408 growing in mouse astrocytoma DBT and fibroblast 17Cl-1 cells with IC50s of about 0.3 μg/ml mediated by inhibiting viral N protein. Both tizaoxanide and nitazoxanide have an inhibiting action on MERS-CoV cultured in LLC-MK2 cells with IC50s of 0.92 nitazoxanide and 0.83 μg/ml tizoxanide respectively (Rossignol, 2016). Fig. 12 illustrates the IUPAC name and structure of Nitazoxanide. As of April 26, 2020, there are 3 clinical trials (NCT04341493, NCT04361318, NCT04360356) ongoing to evaluate the plausible usage of Nitazoxanide against COVID-19 which would provide key information on its efficacious utilization.
Fig. 12.
The IUPAC name and structure of Nitazoxanide.
4.1.8. Ivermectin
Ivermectin is an anti-parasitic drug with a broad spectrum of activity. It is highly efficacious and has a wide margin of safety. Since 1987, it has been used in veterinary medicine and humans. It was developed by Merck & Co. (Canga et al., 2008).
A study utilized SARS-CoV-2 infected Vero/hSLAM cells in which 5 μM ivermectin was added. Just after one day, a 93% reduced viral RNA supernatant was observed on comparing with the vehicle DMSO. Also, a 99.8% reduced cell-associated viral RNA depicting destruction virions was seen. Just after 2 day period, the effect was seen to increase to approximately 5000 times in reducing viral RNA. The effect is likely due to the inhibition of IMPα/β1 associated nuclear import of proteins of the virus (Caly et al., 2020). Most of the RNA viruses depend on IMPα/β1 for the progress of infection and the inhibitory action of ivermectin could be prospective for its action against COVID-19 (Choudhary et al., 2020). Fig. 13 illustrates the IUPAC name and structure of Ivermectin. Table 6 outlines the clinical studies for the evaluation of ivermectin.
Fig. 13.
The IUPAC name and structure of Ivermectin.
Table 6.
Clinical Studies for evaluation of Ivermectin (https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04351347 | Evaluating the efficacy of Ivermectin and Nitazoxanide | 2,3 | Not Specified | Egypt |
| NCT04360356 | Evaluating the efficacy of Ivermectin and Nitazoxanide Combination therapy | 2,3 | Ivermectin 200 mcg/kg plus Nitazoxanide 500 mg two times a day for 6 days | Not yet recruiting |
| NCT04343092 | Hydroxychloroquine and Azithromycin with an adjunct of Ivermectin | 1 | Ivermectin 0.2 mg/kg (single dose at once = 2 tablets of 6mg/weekly) | Iraq |
| NCT04374279 | Clinical Study for Promoting Recovery With Ivermectin or Endocrine Therapy | 2 | Drug: Bicalutamide 150 mg Once a day for 7 days Ivermectin 600 μg/kg once a day for 3 days |
United States |
| NCT04373824 | Ivermectin and Standard of Care Treatment evaluation | Not applicable | Ivermectin 200 to 400mcg/kg first day and the second day was given with standard care | Max Super Speciality Hospital, New Delhi, India |
4.1.9. Anti-rheumatic drugs against COVID-19
A cytokine storm syndrome associated with an uncontrolled release of inflammatory cytokines is seen in COVID-19. Along with this, immunosuppression leads to suggest secondary hemophagocytic lymphohistiocytosis (Misra et al., 2020). An anti-IL-6 monoclonal antibody, tocilizumab can be used for treating rheumatoid arthritis. Many trials are ongoing to check its efficacy against COVID-19 (Perricone et al., 2020). Glucocorticoids are among the most common agent in rheumatological clinical practice. They cause inhibition of the migration and chemokine production which can impair viral clearance. Their use against COVID-19 in-patients is doubtful (Strehl et al., 2019). The viral spike protein induces a tumor necrosis factor-α-converting enzyme-depending shed of the domain of the Angiotensin-converting enzyme-2 receptor. The use of TNF inhibitors can prove to be effective in reducing SARS-CoV2 infection. Baricitinib is a selectively acting inhibitor of Janus kinases 1 and 2. It dampens the inflammatory response that causes severe interstitial pneumonia by inhibiting IL-6 and interferon-gamma. Due to its anti-inflammatory response, it could be used as an agent against COVID-19 (Favalli et al., 2020). Granulocyte-monocyte colony-stimulating factor mediates the proliferation and maturation of granulocytes, monocytes, and macrophages. Many Granulocyte-monocyte colony-stimulating factor receptor inhibitor agents namely namilumab, mavrilimumab, and otilimab are currently under investigation for use against COVID-19. Nonsteroidal anti-inflammatory drugs are prescribed for relieving pain, fever, and inflammation. While it has been established that they may cause masking of symptoms when given in-case of COVID-19, some drugs are being tested for their plausible therapeutic use. Naproxen is an inhibitor of both COX-2 and Influenza A virus nucleoprotein. COX-2 is an important mediator of inflammation and its inhibition may contribute to the antiviral effect of naproxen (Perricone et al., 2020). Due to the converging pathologies of rheumatoid disorders and COVID-19, various plausible agents have been proposed, however detailed investigative results are awaited to confirm their use. Table 7 gives an insight into the various clinical trials ongoing concerning these agents.
Table 7.
Ongoing clinical trials for evaluation of anti-rheumatic drugs ((https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04356937 | Evaluating the Efficacy of Tocilizumab | 3 | 8 mg | United States |
| NCT04345445 | Clinical trial Study for Evaluating if Tocilizumab and corticosteroids are efficacious for coronavirus infection | 3 | 8 mg | Malaysia |
| NCT04317092 | Evaluating the Efficacy of Tocilizumab | 2 | 8 mg | Italy |
| NCT04358614 | Baricitinib Therapy in COVID-19 | 2,3 | 4 mg | Italy |
| NCT04340232 | Studying the Safety and Efficacy of Baricitinib | 2,3 | 2 mg | United States |
| NCT04346147 | Studying the Safety and Efficacy of Baricitinib | 2 | 4 mg | Spain |
| NCT04325633 | Evaluation of the Efficacy when Naproxen is added in treating of Critically Ill Patients | 3 | 250 mg twice daily | Not Specified |
4.2. Convalescent plasma therapy
Passive immunization has been utilized for preventing and treating human infectious disorders since the 20th century when specific antibodies were acquired from the serum of stimulated animals. Convalescent blood products are obtained by collecting whole blood or plasma from a patient who has survived infection and developed consequent humoral immunity against the virus. This acts as a source of various antibodies whose transfusion can neutralize the pathogen which eventually leads to its eradication from the blood circulation (Marano et al., 2016).
Alternatively, non-neutralizing antibodies can get bound to the virus thus, contributing to prophylaxis as well as recovery. On the other hand, administering a passive antibody can be another option to provide quick immunity. A pilot study was done in ten patients with a severe form of COVID-19 infection in which they were transfused with convalescent plasma with neutralizing antibodies. All the patients showed an improvement in fever, cough, chest pain, and shortness of breath within three days of transfusion. A radiology improvement in pulmonary lesions was also seen (Bloch et al., 2020). Several studies also show a shorter duration in the hospital and reduced mortality rate in patients given convalescent plasma than those not given the same treatment (Chen et al., 2020). Another study was done to determine the benefits of convalescent plasma transfusion in patients who were gravely at the infectious disease department located in the Shenzhen Third People's Hospital in China. It was conducted in 5 patients who received antiviral therapy along with methylprednisolone. After plasma transfusion, it was observed that the body temperature normalized and the viral load had reduced which became negative within 12 days after the transfusion (Shen et al., 2020). Table 8 outlines some of the clinical studies for the evaluation of Convalescent plasma therapy.
Table 8.
Clinical studies for evaluation of Convalescent Plasma Therapy (https://clinicaltrials.gov/ct2/results).
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04345679 | Anti COVID-19 Convalescent Plasma Therapy | Early Phase 1 | ~200 ml over 4 h | Not yet recruiting |
| NCT04346446 | Evaluating the Efficacy of Convalescent Plasma Therapy | 2 | Not Specified | India |
| NCT04345523 | Evaluation of Convalescent Plasma Therapy against the Standard care for the Treatment | 2 | fresh plasma from donor immunized against COVID-19 | Spain |
| NCT04356534 | Convalescent Plasma Therapy Clinical Trial in COVID -19 Patients | Not Applicable | 400 ml given as 200 ml over 2 h in 2 consecutive days | Bahrain |
| NCT04342182 | Utilizing Convalescent Plasma as Therapy for Covid-19 | 2,3 | 300 ml | Netherlands |
| NCT04359810 | Convalescent Plasma utilization for in Critically Ill Patients | 2 | 1 unit; ~200–250 ml | United States |
| NCT04358783 | The use of Convalescent Plasma comparing with Best available Therapeutic for the Treatment | 2 | single 200 ml dose | Mexico |
| NCT04343261 | Convalescent Plasma utilization in the Treatment | 2 | 2 Units | United States |
| NCT04343755 | Utilization of Convalescent Plasma in Hospitalized Subjects | 2 | Not Specified | United States |
| NCT04340050 | COVID-19 Convalescent Plasma | Early Phase 1 | ~300 ml over 4 h | United States |
| NCT04345289 | Evaluating Efficacy and Safety of Novel Treatment- Convalescent Plasma for Adults | 3 | Single Infusion 2 × 300 ml | Denmark |
| NCT04345991 | Evaluation of the Efficacy of Convalescent Plasma | 2 | Two units of 200–220 ml each transfused i.v. | France |
| NCT04333355 | Safety in Convalescent Plasma Transfusion to COVID-19 | 1 | Not Specified | Mexico |
| NCT04347681 | Evaluating the Potential Efficacy of Convalescent Plasma | 2 | 10–15 ml/kg body weight of the recipient | Saudi Arabia |
| NCT04346446 | Evaluating the Efficacy of Convalescent Plasma Therapy for Severely Sick Patients | 2 | 200–600 ml of convalescent plasma will be transfused to patients. | New Delhi, India |
4.3. Mesenchymal stem cell therapy
In patients suffering from COVID-19, a large number of inflammatory mediators leading to a cytokine storm has been observed. Mesenchymal stem cell therapy can prevent the release of cytokines and enhance endogenous repair. Mesenchymal stem cells can be obtained by isolating the same from peripheral blood, adipose tissue, bone marrow, placenta, umbilical cord, Wharton jelly, amniotic fluid, and cord blood (Golchin et al., 2020). In the lungs, Mesenchymal stem cells release various soluble mediators like antimicrobial peptides, angiogenic growth factors, anti-inflammatory cytokines, and angiogenic growth factors (Khoury et al., 2020). Many clinical trials (NCT04302519, NCT04276987, NCT04313322, NCT04333368, NCT04355728) are being conducted to evaluate its activity as an effective therapy against COVID-19. Table 9 presents an overview of the ongoing clinical trials for mesenchymal stem cells.
Table 9.
Ongoing clinical trials for evaluation of Mesenchymal Stem Cell Therapy.
| Clinical Trial Number | Study Description | Phase | Dose | Location |
|---|---|---|---|---|
| NCT04348435 | Clinical Trial for Determination of the Safety and Efficacy of Allogeneic Mesenchymal Stem Cell Therapy | 2 | 200 million cells in each dose | United States |
| NCT04349631 | Determining the Safety and Efficacy of Autologous Mesenchymal Stem Cell Therapy | 2 | Five I.V. infusion | United States |
| NCT04341610 | Evaluating the use of Allogeneic adipose-derived mesenchymal stromal cell Therapy | 1,2 | 100 million cells diluted in 100 ml saline | Denmark |
| NCT04313322 | Wharton's Jelly-Mesenchymal Stem Cells therapy for COVID-19 patients | 1 | WJ-MSCs suspended in 25 ml of a Saline solution which contains 0.5% human Serum Albumin | Jordan |
| NCT04288102 | Evaluating the use of Mesenchymal Stem Cells | 2 | 4.0*10E7 cells per time | China |
| NCT04346368 | Evaluating utilization of Bone Marrow-Derived Mesenchymal Stem Cell Treatment | 1,2 | 1*10E6/kg body weight | China |
| NCT04339660 | Evaluation of the use of Human Mesenchymal Stem Cells | 1,2 | 1*10E6 UC-MSCs/kg body weight after suspending in 100 ml saline | China |
| NCT04333368 | Evaluation of Umbilical cord-derived Mesenchymal Stromal Cells | 1,2 | 1 Million/kg | France |
| NCT04361942 | Allogeneic Mesenchymal Stromal Cells evaluation for use against COVID-19 infection | 2 | 1 million MSV cells/kg in 100 ml of saline | Spain |
4.4. Vaccines
Vaccines prove to be the most effective strategy against infectious disease because they are cost-effective as compared to treatment. They can cause a reduction in mortality as well as morbidity without any effects that may prevail for long (Ahn et al., 2020). Scientists across the globe are working with different approaches such as whole virus, recombinant protein subunit, antibody, and nucleic acid vaccine. Whole virus vaccines utilize the attenuated form of the coronavirus. Such vaccines can prove to be effective to provide immunity but can pose a risk in some people as they can develop symptoms as a result of vaccination. This risk can be eliminated by developing vaccines based on the recombinant protein subunit vaccine which targets the spike protein of the virus. Antibody-based vaccines are another approach that is based on antibodies obtained from SARS-COV or SARS-COV2 infection. Nucleic acid vaccine having an injection of DNA or RNA into the host cell is another promising approach. Injected nucleic acid will undergo translation post-injection into the cell resulting in the production of proteins. This can help the immune system to fight the infection. Bacillus Calmette-Guerin is a live attenuated strain obtained from an isolate of Mycobacterium bovis. It is used as the first line of evidence due to its protection against various viral infections which has been evaluated from various in vitro and in vivo studies in mice. On the other hand, clinical evidence for its action is limited (Moorlag et al., 2019). Currently, 9 Phase 3 clinical trials are being pursued to evaluate the efficacy of BCG as a candidate vaccine against COVID-19 in various countries including Australia, the United States, Egypt, and the Netherlands (“Search, 2020, ClinicalTrials, 2020a). According to the draft of COVID-19 vaccines as of August 20, 2020, there are currently 8 prospective vaccines being evaluated in Phase 3 amongst the overall 30 being tested clinically and 139 preclinically (“Draft landscape of COVID-19 candidate vaccines,” 2020). A phase I clinical trial (ChiCTR2000030906) for recombinant novel coronavirus vaccine with adenoviral vector in healthy adults is being conducted in Wuhan, China. The study is divided into 3 groups each receiving either a low dose (5E10vp), middle dose (1E11vp), and high dose (1E11vp) with sample sizes of 36 each. (http://www.chictr.org.cn/showprojen.aspx?proj=51154, 2018). Another phase II clinical study (ChiCTR2000031781) for Adenovirus Vector in healthy adults is being conducted in Wuhan, China. The study is divided into 3 groups each receiving either middle dose (1E11vp), low dose (5E10vp) or placebo with sample sizes of 250,125 and 125 respectively (http://www.chictr.org.cn/showprojen.aspx?proj=52006, 2018). The Non-replicating Adenovirus Type 5 vector in both the aforementioned trials has been made by CanSino Biological Inc. and the Beijing Institute of Biotechnology. It was a candidate for the Ebola vaccine and has been repurposed for its use against COVID-19. A phase 1 clinical study (NCT04336410) is being conducted in the United States for evaluating the mmunological profile, safety and tolerability of INO-4800. The study has been divided into 2 experimental groups one of which receives one 1 mg injection while the other experimental group receives two 1 mg injections. This DNA vaccine Electroporation device has been developed by Inovio Pharmaceuticals. It was a candidate for various viruses like Lassa, Nipah, HIV, Filovirus, Zika, and Hepatitis B (Inovio Pharmaceuticals, 2019). Another Phase 1 (NCT04283461) clinical study is being conducted in the United States in both males and non-pregnant females with good well being. This clinical study assesses the safety, reactogenicity, and immunogenicity of mRNA-1273 which has been developed by ModernaTX, Inc. It is a nanoparticle mRNA based vaccine. The study has been divided into 2 experimental groups one of which receives 25 mcg of mRNA-1273 and the other receives 100 mcg of mRNA-1273, both of these are administered on Days 1 and 29 respectively. (https://clinicaltrials.gov/ct2/show/NCT04283461?term = vaccine&cond = covid-19&draw = 2, 2020). Recently, a Phase three clinical trial (NCT0447047) has started studies on mRNA-1273. Bharat Biotech in collaboration with Indian Council of Medical Research has developed a vaccine in India namely, COVAXIN which is currently being pursued to evaluate its activity in a Phase1/2 trial (NCT04471519). It is a whole cell virion inactivated based vaccine aimed at intramuscular delivery with 2 dosing at day 0 and 14 (“Draft landscape of COVID-19 candidate vaccines,” 2020). A phase I/II clinical study is being conducted for inactivated viral vaccine in china. It was developed by Beijing Institute of Biological Products and Wuhan Institute of Biological Products (http://www.chictr.org.cn/showprojen.aspx?proj=52227, 2020). Lastly, A Phase 1 clinical study is being conducted to evaluate inactivated novel coronavirus and alum as a plausible vaccine which was developed by Sinovac. It has been earlier used for SARS. (https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf, 2020). Additionally, it is also being evaluated in 2 Phase 3 clinical trials (NCT04456595 and 669/UN6. KEP/EC/2020). AstraZeneca and the University of Oxford have collaborated in the development of a plausible vaccine ChAdOx1 nCoV-19 which was made by the Jenner Institute and Oxford Vaccine Group at the University of Oxford. The vaccine uses a viral weakened version of adenovirus which contains the spike protein of COVID-19. The surface spike protein is generated in the body which provides the immune response to attack the virus (https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-oxford-university-announce-landmark-agreement-for-covid-19-vaccine.html, 2020). An ongoing phase I/II multicentre clinical study (NCT04324606) which is randomized and single-blind is being conducted to determine efficacy, safety and immunogenicity of ChAdOx1 nCoV-19 in healthy adults in United Kingdom. It involves 1112 participants and is being conducted by Oxford University (https://clinicaltrials.gov/ct2/show/NCT04324606). It's efficacy is also being evaluated in 2 Phase three clinical trials (ISRCTN89951424, NCT04516746). An inactivated vaccine being developed by Wuhan Institute of Biological products is also undergoing Phase 3 clinical trial (chiCTR2000034780). It is aimed to be administered intramuscularly on days 0, 14 or 0,21 comprising a total of 2 doses. In a collaborative effort by BioNTech, Fosun Pharma and Pfizer, a RNA based vaccine 3 LNP-mRNAs is being evaluated in Phase 3 clinical trial (NCT04368728). It is aimed to be given intramuscularly on days 0 and 28 (“Draft landscape of COVID-19 candidate vaccines,” 2020).
4.5. Herbal therapy
4.5.1. Antiviral herbal therapy
A study done on glycyrrhizin which is a major active constituent of licorice root used seldom as a herb in Traditional Chinese Medicine has shown its potential to inhibit the replicating isolates of the SARS virus (Yang et al., 2020). The activity of various herbs has been evaluated for activity against COVID-19. Glycyrrhizin causes inhibition of SARS-associated coronavirus penetration, adsorption, and replication (Fiore et al., 2008). Chalcones containing perhydroxyl group from Angelica keiskei exhibit potent inhibitory activity against SARS-CoV proteases (Park et al., 2016). Cinanserin is a serotonin antagonist obtained from the Houttuynia cordata of family Saururaceae. Being another example of an agent that causes inhibition of SARS-CoV replication, it may inhibit the protease enzyme as well. Bananins are a type of compounds which have a trioxa-adamantane moiety bounded with a pyridoxal moiety. Bananin was also found to inhibit SARS-CoV replication in fetal rhesus kidney 4 cells with over 300 μM of 50% cytotoxicity concentration (De Clercq, 2006). Quercetin is an aglycone found in onions that possess a virucidal activity against a large variety of viruses. In a study done on quercetin flavonoid obtained from Houttuynia cordata, a successful inhibition of murine coronavirus was seen (Chiow et al., 2016).
4.5.2. Immune boosting
COVID-19 attacks the immune system of the patient which implicates a possibility of the use of immunomodulatory herbs for prophylaxis and prevention of coronavirus infection. The immune system is an incredibly intricate network that contains specialized cells that helps in preventing infections (Sultan et al., 2014). It has been established that garlic and onion extracts contain many organosulfur compounds some of which possess phagocytic activity. They can suppress neutrophil infiltration and damage in rat intestines decreases nitric oxide synthesis and reduces levels of interleukins formed by macrophages (Schepetkin et al., 2019). Astragalus membranaceus is a popular traditional Chinese herb. Astragalosides IV can cause inhibition of nitric oxide and pro-inflammatory cytokine release (Qi et al., 2017). Curcumin causes negative regulation of proinflammatory, cytokines, monocyte chemoattractant protein by down-regulating Janus kinase and signal transducers and activators of the transcription signaling pathway. (Kocaadam and Şanlier, 2017). Genistein, a soy isoflavone, has anti-inflammatory action against macrophages by inhibiting NF-κB (Lyons and Roche, 2018). Ginseng has been traditionally used as an herbal supplement in various Asian countries. The action influences various inflammatory cytokines (Nguyen and Nguyen, 2019). Tulsi is one of the most commonly utilized medicinal plants in traditional medicine. The beneficial properties are attributed to its active constituents namely eugenol, carvacrol, ursolic acid β-caryophyllene, and rosmarinic acid. Various studies show its influence on both cellular and humoral immunity (Qi et al., 2017). Azadirachta indica having a common name neem possesses various medicinal properties. It can decrease lipid peroxidation and provide anti-inflammatory action (Subapriya and Nagini, 2005). The use of the immune-modulating herbal drug can offer a synergistic approach with chemical therapeutics to tackle the COVID-19 pandemic. Hence, herbal anti-viral agents and immunomodulatory agents can be utilized as a prophylactic or as an adjunct therapy.
5. Future perspectives & conclusion
As we ponder into the upcoming future, controlling the Covid-19 pandemic will take several months until we successfully develop a novel treatment option or a vaccine. Hence, the best possible route is to take adequate measures to prevent the spread of this deadly virus by adapting to the “new normal”. Taking into account social distancing and improving personal hygiene should be the foremost practice that should be adopted by the public. These would be effective in controlling the spread of the virus and reducing peak incidence. The healthcare system of various countries should increase the number of tests conducted, contact tracing, quarantine of infected individuals, and precautionary self-isolation of individuals who were in contact. These measures would be critical in ensuring a reduction in the number of new cases.
At the same time, it is also important that as we uplift the lockdown and the life returns to its normalcy, the public should follow the social-distancing guidelines and SOPs maintained by every state or province which would help in containing the spread of the virus. Rapid-antibody testing must be implemented on a large scale to identify asymptomatic cases.
The organizations should make it imperative for its staff to follow WHO guidelines on using alcohol-based hand-sanitizers and regularly do handwashing with soap and water. We must avoid touching of eyes, nose, and mouth, and practice respiratory hygiene by wearing face masks in public (Kratzel et al., 2020).
Disinfection of surfaces and sanitization drives are extremely important as coronavirus can survive on surfaces for a long time. Scientific pieces of evidence have indicated that it can survive on plastic, steel, cardboard, and copper for 72 h, 48 h, 24 h, and 4 h respectively. Hence, surface disinfection of homes, public places, and hospitals using 70% IPA, or 0.1% sodium hypochlorite solution or 0.5% hydrogen peroxide should be done on regular basis (Kampf et al., 2020; Van Doremalen et al., 2020). Health care workers are advised to use personal protective equipment (PPE) along with N95 masks and to also properly dispose of the equipment. Any individual suffering from fever, cough, and difficulty in breathing is advised to seek medical attention. Social distancing (minimum 6 feet) is recommended both at individual and community levels (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public, 2020).
Community transmission can be prevented by reducing the spread of infection by early detection, isolation, and contact tracing of positive cases of Covid-19 and quarantining those who were in contact. All the public places such as places of mass gathering, like schools, libraries, places of worship, malls, and cinemas should be closed as of now and all social events must be suspended. The public places can start re-opening in the non-containment zones by following all the regulations of social-distancing and regular thermal screening should be introduced at public places such as airports, railway stations, and bus stations, as well as hospitals, malls, banks, and courts. The unlock strategies for public places and social events should be such that states must introduce strategies to contain the spread of the virus by following the SOPs laid down by the government by ensuring a minimum number of people at the gathering and to follow the norms of social distancing, sanitization and thermal screening (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public, 2020).
Results are awaited for the multiple ongoing clinical trials to develop novel treatment options as well as a vaccine to treat the respiratory syndrome. Moreover, it will take several months before a vaccine is developed, approved, and reaches to the market through the clinical trials that have been expedited (Zhou et al., 2020). Due to the absence of any approved drugs, investigational drug repurposing has acted as the ray of hope in the scarcity of any purposed therapeutic. Alternatively, convalescent plasma therapy and mesenchymal stem cell therapy are tools that are emerging as effective in many patients. The most vulnerable groups are the healthcare providers, people involved in essential services, children especially less than 5 years old, and elderly people who need to be taken care of most during this pandemic. We have to adapt to staggered shifts to mitigate the transmission of Covid-19 in the future. The use of online platforms can be utilized for months. The use of telemedicine needs to be implemented (Mattiuzzi and Lippi, 2020).
COVID-19 attacks the immune system of the patient which implicates a possibility of the use of immunomodulatory herbs for prophylaxis and prevention of coronavirus infection. The immune system is an incredibly intricate network that contains specialized cells that helps in preventing infections (Sultan et al., 2014). The use of the immune-modulating herbal drug can offer a synergistic approach with chemical therapeutics to tackle the COVID-19 pandemic. Hence, herbal anti-viral agents and immunomodulatory agents can be utilized as a prophylactic or as an adjunct therapy. The focus should be laid on boosting the immune system by taking immune-boosting supplements such as curcumin, ashvaghandha, Vitamin C, zinc, tulsi, neem, etc. which also have been discussed earlier as prophylaxis (Elsayed and Khan, 2020). The activity, safety, and efficacy of these agents are being evaluated in clinical trials around the globe.
This Covid-19 outbreak has indeed taught the world the importance of containment measures, contact tracing, thermal screening, antibody-testing for infections as well as sanitation drives and maintenance of personal hygiene to combat any outbreak if no treatment or vaccine is available. These preventive measures can help in minimizing the economic burden of disease and help us in understanding the mechanism of a disease so that we can respond promptly. These strategies would also help in preventing and combating future pandemics based on our present experience. The race to developing a cure and treatment has united the globe and there are substantial grounds to develop an effective therapeutic in the coming future to combat this widespread pandemic of COVID-19 (Table 10 ).
Table 10.
A brief overview of the plausible therapeutics against COVID-19.
| S.No | Treatment | Mechanism | Reference |
|---|---|---|---|
| 1. | Chloroquine and hydroxychloroquine | 4 increase endosomal pH 5 interfering with the glycosylation of receptors |
Gao et al. (2020) |
| 2. | Remdesivir |
|
Al-Tawfiq et al. (2020) |
| 3. | Favipiravir |
|
Furuta et al. (2017) |
| 4. | Lopinavir and Rotinavir |
|
Chu et al. (2004) |
| 5. | Niclosamide |
|
Wu et al. (2004) |
| 6. | Nitazoxanide |
|
Rossignol (2016) |
| 7. | Ivermectin |
|
Caly et al. (2020) |
| 8. | Convalescent Plasma Therapy |
|
Shen et al. (2020) |
| 9. | Mesechymal Stem Cell Therapy |
|
Golchin et al. (2020) |
| 10 | Glycyrrhizin |
|
Fiore et al. (2008) |
| 11 | Cinanserin |
|
De Clercq (2006) |
| 12 | Chalcones containing perhydroxyl group from Angelica keiskei |
|
Park et al. (2016) |
| 13 | Garlic and Onion |
|
Schepetkin et al. (2019) |
| 14. | Astragalus membranaceus |
|
Qi et al. (2017) |
| 15 | Curcumin |
|
Kocaadam and Şanlier (2017) |
| 16 | Genistein |
|
Lyons and Roche (2018) |
| 17 | Ginseng |
|
Nguyen and Nguyen (2019) |
| 18 | Tulsi |
|
Qi et al. (2017) |
| 19 | Azadirachta indica |
|
Subapriya and Nagini (2005) |
| 20 | tocilizumab |
|
Perricone et al. (2020) |
| 21 | Baricitinib |
|
Favalli et al. (2020) |
| 22 | Namilumab, mavrilimumab, and otilimab |
|
Perricone et al. (2020) |
| 23 | Naproxen |
|
Perricone et al. (2020) |
CRediT authorship contribution statement
Ranjana Bhandari: Conceptualization, Literature Search, Writing - original draft, preparation, Writing - review & editing, Editing and Revision. Garima Khanna: Writing - original draft, Literature Search and writing. Anurag Kuhad: Supervision, Writing - review & editing, Reviewing and Editing.
Acknowledgements
Department of Technology, Government of India funded project to Dr. Anurag Kuhad File no. (EMR/2017/003589) has been duly acknowledged.
References
- Ahn D.-G., Shin H.-J., Kim M.-H., Lee S., Kim H.-S., Myoung J., Kim B.-T., Kim S.-J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Trav. Med. Infect. Dis. 2020:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AstraZeneca and Oxford University announce landmark agreement for COVID-19 vaccine. 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-oxford-university-announce-landmark-agreement-for-covid-19-vaccine.html [WWW Document]
- Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., van Buskirk C., Grossman B.J., Joyner M., Henderson J.P., Pekosz A., Lau B., Wesolowski A., Katz L., Shan H., Auwaerter P.G., Thomas D., Sullivan D.J., Paneth N., Gehrie E., Spitalnik S., Hod E., Pollack L., Nicholson W.T., Pirofski L.-A., Bailey J.A., Tobian A.A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.-Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38(7):870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canga A.G., Prieto A.M.S., Diez Liébana M.J., Martínez N.F., Sierra Vega M., García Vieitez J.J. The pharmacokinetics and interactions of ivermectin in humans - a mini-review. AAPS J. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang Jingli, Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li Huadong, Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li Hui, Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang Juan, Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P., Cai J.P., Chu H., Zhou J., Chen H., Qin C., Yuen K.Y. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERSCoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Clinical Trial Register (ChiCTR) - the world health organization international clinical trials registered organization registered platform. 2018. http://www.chictr.org.cn/showprojen.aspx?proj=51154 [WWW Document]
- Chinese Clinical Trial Register (ChiCTR) - the world health organization international clinical trials registered organization registered platform. 2018. http://www.chictr.org.cn/showprojen.aspx?proj=52006 [WWW Document]
- Chinese Clinical Trial Register (ChiCTR) - the world health organization international clinical trials registered organization registered platform. 2020. http://www.chictr.org.cn/showprojen.aspx?proj=49042 [WWW Document]
- Chinese Clinical Trial Register (ChiCTR) - the world health organization international clinical trials registered organization registered platform. 2020. http://www.chictr.org.cn/showprojen.aspx?proj=52227 [WWW Document]
- Chiow K.H., Phoon M.C., Putti T., Tan B.K.H., Chow V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016;9(1):1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary R., Sharma A.K., Choudhary R. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. New microbes new Infect. 2020:100684. doi: 10.1016/j.nmni.2020.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng C.C., Hung F.N., Wong M.M.L., Chan H., Chan S., Kao Y.T., Poon L.L.M., Wong L.P., Guan Y., Peiris J.S.M., Yuen Y., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev. Anti Infect. Ther. 2006;4(2):291–302. doi: 10.1586/14787210.4.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safety and immunogenicity study of 2019-nCoV vaccine ( mRNA-1273 ) for prophylaxis SARS CoV-2 infection. 2020. https://clinicaltrials.gov/ct2/show/NCT04283461?term=vaccine&cond=covid-19&draw=2 1-10.
- A real-life experience on treatment of patients with COVID 19 - full text view - ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04345419?term=niclosamide+covid&draw=2&rank=1 [WWW Document]
- Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Ren X. Coronavirus entry and release in polarized epithelial cells: a review. Rev. Med. Virol. 2014;24:308–315. doi: 10.1002/rmv.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., De Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draft Landscape of COVID-19 Candidate Vaccines. 2020. [WWW Document] [Google Scholar]
- Du Y.-X., Chen X.-P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020;108(2):242–247. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- Elsayed Y., Khan N.A. Immunity-boosting spices and the novel coronavirus. ACS Chem. Neurosci. 2020;11(12):1696–1698. doi: 10.1021/acschemneuro.0c00239. [DOI] [PubMed] [Google Scholar]
- Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun. Rev. 2020;19(5):102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D., Bielenberg J. Antiviral effects of Glycyrrhiza species. Phyther. Res. 2008;22(2):141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D., Heymann D. Q&A: the novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18:57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Goel P., Gerriets V. StatPearls Publishing; 2020. Chloroquine, StatPearls. 31855356. [PubMed] [Google Scholar]
- Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev. Reports. 2020:1–7. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickley N.M., Al-Maskari A., McKibbin M. Chloroquine and hydroxychloroquine toxicity. Arch. Ophthalmol. 2011;129(11):1506–1507. doi: 10.1001/archophthalmol.2011.321. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Wei F., Hu L., Wen L., Chen K. IRANIAN epidemiology and clinical characteristics of COVID-19. Acad. Med. Sci. I.R. Iran. 2020;23:268–271. doi: 10.34172/aim.2020.09. [DOI] [PubMed] [Google Scholar]
- Inovio Pharmaceuticals . 2019. Safety, Tolerability and Immunogenicity of INO-4500 in Healthy Volunteers - Full Text View - ClinicalTrials.Gov. [WWW Document] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R.M., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur. Respir. J. 2020;55(6):2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Choe P.G., Oh Y., Oh K.J., Kim J., Park S.J., Park J.H., Na H.K., Oh M.D. The first case of 2019 novel coronavirus pneumonia imported into korea from wuhan, China: implication for infection prevention and control measures. J. Kor. Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaadam B., Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017;57:2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- Kratzel A., Kratzel A., Todt D., V’kovski P., V’kovski P., Steiner S., Steiner S., Gultom M., Gultom M., Thao T.T.N., Thao T.T.N., Ebert N., Ebert N., Holwerda M., Holwerda M., Steinmann J., Steinmann J., Niemeyer D., Dijkman R., Dijkman R., Kampf G., Drosten C., Steinmann E., Thiel V., Thiel V., Pfaender S. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg. Infect. Dis. 2020;26:1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasllieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greeneugh T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(2):102–108. doi: 10.1016/J.JPHA.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.-S., Im J.-S., Cho J.-Y., Bae K.-S., Klein T.A., Yeom J.-S., Kim T.-S., Choi J.-S., Jang I.-J., Park J.-W. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob. Agents Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J. Kor. Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e79. [DOI] [PubMed] [Google Scholar]
- Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K., Yu W., Zhang J. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons C.L., Roche H.M. Nutritional modulation of AMPK-impact upon metabolic-inflammation. Int. J. Mol. Sci. 2018;19(10):3092. doi: 10.3390/ijms19103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangum E.M., Graham K.K. Lopinavir-ritonavir: a new protease inhibitor. Pharmacotherapy. 2001;21(11):1352–1363. doi: 10.1592/phco.21.17.1352.34419. [DOI] [PubMed] [Google Scholar]
- Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M., Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2)(152–7):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzi C., Lippi G. Which lessons shall we learn from the 2019 novel coronavirus outbreak? Ann. Transl. Med. 2020;8 doi: 10.21037/atm.2020.02.06. 48–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégarbane B. Chloroquine and hydroxychloroquine to treat COVID-19: between hope and caution. Clin. Toxicol. 2020:1–2. doi: 10.1080/15563650.2020.1748194. [DOI] [PubMed] [Google Scholar]
- Misra D.P., Agarwal V., Gasparyan A.Y., Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin. Rheumatol. 2020;1 doi: 10.1007/s10067-020-05073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019;12:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N.H., Nguyen C.T. Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology. 2019;27:871–883. doi: 10.1007/s10787-019-00630-4. [DOI] [PubMed] [Google Scholar]
- Pan J.X., Ding K., Wang C.Y. Niclosamide, an old antihelminthic agent, demonstrates antitumor activity by blocking multiple signaling pathways of cancer stem cells. Chin. J. Canc. 2012;43(2):549–561. doi: 10.5732/cjc.011.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y., Ko J.A., Kim D.W., Kim Y.M., Kwon H.J., Jeong H.J., Kim C.Y., Park K.H., Lee W.S., Ryu Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzym. Inhib. Med. Chem. 2016;31:23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- Perricone C., Triggianese P., Bartoloni E., Cafaro G., Bonifacio A.F., Bursi R., Perricone R., Gerli R. The anti-viral facet of anti-rheumatic drugs: lessons from COVID-19. J. Autoimmun. 2020;111:102468. doi: 10.1016/j.jaut.2020.102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Gao F., Hou L., Wan C. Anti-inflammatory and immunostimulatory activities of astragalosides. Am. J. Chin. Med. 2017;45:1157–1167. doi: 10.1142/S0192415X1750063X. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antivir. Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health. 2016;9:227. doi: 10.1016/J.JIPH.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Balasubramanian N., Quinn M.T. Neutrophil immunomodulatory activity of natural organosulfur compounds. Molecules. 2019;24 doi: 10.3390/molecules24091809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2020. Search of: bcg | COVID-19 | phase 3 - list results - ClinicalTrials.gov. [WWW Document] [Google Scholar]
- She J., Jiang J., Ye L., Hu L., Bai C., Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin. Transl. Med. 2020;9:19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., MacKman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Yang, Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Yan, Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA, J. Am. Med. Assoc. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie J.J., Fang J.M., Kuo C.J., Kuo T.H., Liang P.H., Huang H.J., Yang W. Bin, Lin C.H., Chen J.L., Wu Y.T., Wong C.H. Discovery of potent anilide inhibitors against the severe acute respiratory syndrome 3CL protease. J. Med. Chem. 2005;48:4469–4473. doi: 10.1021/jm050184y. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:241. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehl C., Ehlers L., Gaber T., Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front. Immunol. 2019;24(10):1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subapriya R., Nagini S. Medicinal properties of neem leaves: a review. Curr. Med. Chem. Anti Canc. Agents. 2005;5(2):149–156. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- Sultan M.T., Buttxs M.S., Qayyum M.M.N., Suleria H.A.R. Immunity: plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014;54:1298–1308. doi: 10.1080/10408398.2011.633249. [DOI] [PubMed] [Google Scholar]
- Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID‐19 based on current evidence. J. Med. Virol. jmv. 2020;25722 doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Veesler D. Advances in Virus Research. Academic Press Inc.; 2019. Structural insights into coronavirus entry; pp. 93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J., Chan W.C.W. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14,(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., Lee C.K., Chang S.T., Kuo C.J., Lee S.S., Hou C.C., Hsiao P.W., Chien S.C., Shyur L.F., Yang N.S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- WHO (No title) 2020. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf [WWW Document]
- (No title) 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200504-covid-19-sitrep-105.pdf?sfvrsn=4cdda8af_2 [WWW Document]
- Advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-publichttps://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public n.d., [WWW Document] accessed 8.25.20.
- WHO lists two COVID-19 tests for emergency use. 2020. https://www.who.int/news-room/detail/07-04-2020-who-lists-two-covid-19-tests-for-emergency-use [WWW Document]
- De Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., Van Nieuwkoop S., Bestebroer T.M., Van Den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.J., Jan J.T., Chen C.M., Hsieh H.P., Hwang D.R., Liu H.W., Liu C.Y., Huang H.W., Chen S.C., Hong C.F., Lin R.K., Chao Y.S., Hsu J.T.A. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004;48:2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020;92(6):556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Zhang X., Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front. Med. 2020;14(2):126–135. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Z.Y., Jiang M. Di, Xu P.P., Chen W., Ni Q.Q., Lu G.M., Zhang L.J. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]