Abstract
A series of trimethyltin(IV) carboxylates (1-7), having general formula [(CH3)3SnOOCR], where R = 3-CF3C6H4- (1), 3,4-(OCH2CH3)2 C6H3- (2), 3-FC6H4- (3), 4-ClC6H5- (4), 2,4-(OCH3)2C6H3- (5), 4-NO2C6H4- (6), 5-CH3C4H2N2- (7) have been synthesized and are characterized by using analytical techniques like, FT-IR, 1H, 13C and 119Sn NMR spectroscopy in addition to elemental analyses. To validate the structural motif, one of the complex (2) was also analyzed by single crystal XRD technique. The single crystal X-ray diffraction analysis revealed that the molecular species exist in one dimensional polymeric chain, wherein five coordinated tin is attached to two oxygens of the carboxyl and three carbon atoms of CH3 groups, whereas the bond angles for C-Sn-C (117.20–121.65°), C-Sn-O (87.87–98.60°) and O-Sn-O (173.75°) lie in the predictable range of distorted trigonal bipyramidal geometry. All the synthesized complexes were also subjected to preliminary screening for various biocidal applications such as antimicrobial, anti-diabetic, protein kinase inhibition and brine shrimp lethality test to check their efficacy. The compound 6 exhibits very good antibacterial activity having MIC value 6.25 μg/mL against K. pneumoniae, whereas antidiabetic and protein kinase inhibition data revealed that 1 and 2 may serve as good to moderately effective inhibitors, respectively.
Keywords: Organic chemistry, Trimethyltin(IV), Carboxylates, X-ray structure, Tau, Enzyme inhibition, Antimicrobial
Organic Chemistry, Trimethyltin(IV), Carboxylates, X-ray structure, Tau, Enzyme Inhibition, Antimicrobial.
1. Introduction
The organotin(IV) carboxylates have wide spectrum efficacy owing to its potential use in agricultural [1], industrial [2], synthetic [3] and biological [4] fields. They also find applications as heat stabilizer, PVC stabilizers and biocides [5, 6]. The biological effectiveness of these compounds may be further supported by structure to activity (SAR) correlation which is mainly dealt by their structural features. Organotin(IV) complexes have got particular interest due to their antibacterial [7], antifungal [8], antiviral [9] and antitumor activity [10], as several studies confirmed that organometallic compounds having O and N groups are specifically involved in antitumor activity. In general, antimicrobial activities of organotin complexes are related to the number and nature of R and to a lesser extent on L- (anionic) groups, the order of activity may be depicted in that way; R3SnL > R2SnL2 > RSnL3. The structural aspects of organotin(IV) carboxylates mainly reveal single organic moieties, most of which are 1D chain and 2D plane structures, however evidences regarding mixed ligand complexes are also obtained that may yield polymeric species [11, 12].
The biological efficacy of organotin(IV) complexes is mainly affected by the nature, number of donor ligands, coordination number and their chemical structures [13]. Eight bacterial and fungal pathogenic strains were selected to check the antimicrobial potential of the synthesized complexes. Selection of these strains was made on the basis of various ailments, they caused, in living organisms (humans and plants) i.e. S. aureus (Food poisoning, sinusitis, skin infection), B. subtilus (food pathogen), K. pneumonia (infect lungs, liver and bladder), E. coli (intestinal infections), A. niger (black mold), A.flavus (aflatoxin & carcinogen contamination), A. fumigatus (bronchopulmonary aspergilosis) and Mucor (Mucormycosis).
Diabetes mellitus is multifaceted and progressive metabolic disorder which is characterized by hyperglycemia i.e. blood containing high level of glucose sugar over a long period of time due to lack of insulin that may lead to micro and macro-vascular complications which are difficult to manage. The major hallmark in the cure of this disease is the management of blood glucose level. Where alpha amylase is a prominent enzyme found in human saliva and pancreatic juice that catalyzes large insoluble molecules of starch into small counterparts of absorbable in nature. An effective means of lowering the level of postprandial hyperglycemia is offered by alpha amylase inhibitors [14]. It has been observed that least attention has been paid to investigate the antidiabetic potential of organotin(IV) compounds, and a few of them have been reported in literature having effective role as alpha amylase inhibitors [15, 16]. Protein kinase inhibitors are the prominent class that are intensively encouraged as anticancer therapeutics. Protein kinase inhibition is a preliminary assay to speculate the antitumor activity of synthesized compounds. To date, nearly eighty small kinase inhibitors have been developed that are at some stage of clinical appraisal. Protein kinases are generally responsible for adding up a phosphate group to protein in a process called phosphorylation. Kinase phosphorylation is usually correlated with number of ailments such as cardiovascular disorder, cancer and various inflammatory diseases. Eventually protein kinases are accountable to block the aerial hyphae formation of Streptomyces species, and thus may be hypothesized to inhibit the proliferation of cancer cells. The organotin(IV) compounds are also being explored in this domain as a chemo preventive measure as few reports manifested [17, 18].
In continuation to our preceding work [19, 20, 21, 22, 23], we report herein the synthetic route, characterization and a few important biocidal activities of these organotin derivatives (1-7) to further unravel their structural and biological aspects. Keeping in view the medicinal importance of carboxylic acids, they are complexed with the triorganotin(IV) moiety for exploring their biological significance [24, 25]. The results obtained here are quite promising ones and the facts showed that they could furnish a key scaffold for enhanced antimicrobial activity, but structural alterations/modifications should be the prerequisites in determining their therapeutic potential for future drug discovery processes.
2. Experimental
2.1. Materials and methods
The commercially available chemicals; trimethyltin(IV) chloride, 3-(trifuoromethyl)benzoic acid, 3,4-diethoxybenzoic acid, 3-fluorobenzoic acid, 4-chlorobenzoic acid, 2,4-dimethoxybenzoic acid, 4-nitrobenzoic acid and 5-methylpyrazine-2-carboxylic acid were used as received. All the solvents used were of analytical grade and dried by reported procedures [26]. The electro thermal melting point apparatus (model MP-D Mitamura Riken Kogyo Japan) was used in order to determine melting points of the synthesized compounds. The IR spectra were recorded on KBr discs, using Bio-Rad Excalibur (model FTS 3000 MX). The multinuclear (1H, 13C, 119Sn) NMR spectrum were documented on Bruker Advance Digital 300 MHz FT-NMR spectrometer in deuterated CDCl3 as solvent relative to tetramethyl silane (TMS) as internal reference. The single crystal XRD analysis was performed on Kappa APEXII CCD (Bruker) diffractometer equipped with graphite monochromatic radiation [27]. The single crystal X-ray structure of (2) was completed at 296(2) K by mounting a crystal of suitable size on thin glass fiber, where structures was solved using ShelXT structure solution program using Direct Methods and structural refinement was done with 'SHELXL-2018/3′ refinement package using Least squares minimization [28]. The disordered C-atoms were treated as having similar thermal parameters. The H-atoms were positioned geometrically with C-H bond distance and refined as riding. The methyl group was allowed to rotate, but not to tip, to best fit the electron density. The constraint Uiso (H) = 1.2Ueq (C) or 1.5Ueq (methyl C) was applied.
2.2. Synthesis
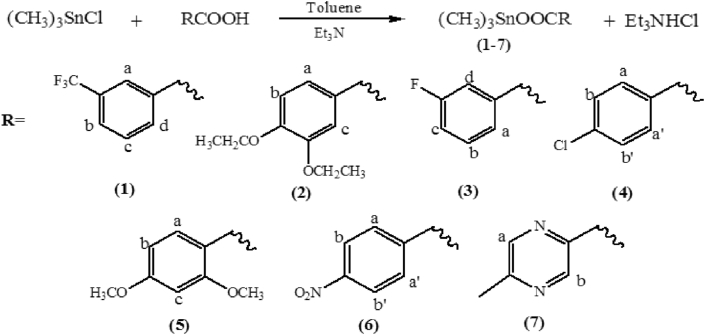
The target compounds were synthesized by using a reported procedure with slight modifications [22]. To get the respective product, the substituted benzoic acids were stirred in the presence of triethyl amine for one hour under argon at room temperature in dry toluene. The trimethyltin(IV) chloride was, then, added into it and stirred for another 3–4 h under reflux conditions. The corresponding triethylammonium chloride salt was filtered off as a byproduct and filtrate could evaporate at room temperature to obtain the crude products (Scheme 1). The raw compounds were recrystallized by dissolving them in a minimum quantity of chloroform and then added slight in excess of acetone to yield (1-7) as pure products. However suitable crystals for (2) could only be obtained for single crystal XRD analysis.
Scheme 1.
Synthetic procedure for (1-7).
2.2.1. Trimethylstannyl-3-(trifluoromethyl) benzoate (1)
Quantities used were; 3-(trifluoromethyl)benzoic acid (0.19 g, 1 mmol), triethylamine (0.14 mL, 1 mmol), trimethyltin chloride (0.19 g, 1 mmol), yield: 81%, crystalline solid, m.p: 128–130 °C, Anal. Calcd. for C11H13O2F3Sn (352.92 g/mol), C, 37.44, H, 3.71%, Found, C, 37.46, H, 3.69 %; FT-IR (KBr, ν, cm−1) C-Haromatic (2994), C-Haliphatic (2921), COOasym (1573), COOsym (1375), Δν (198), C-F (1279), Sn-C (547), Sn-O (452); 1H NMR (300 MHz, CDCl3, δ(ppm), J(Hz)) 8.34 (s, 1H, Ar-Ha), 8.26–8.23 (d, 1H, J = 7.8 Hz, Ar- Hd), 7.79–7.76 (d, 1H, J = 7.8 Hz, Ar-Hb), 7.58–7.53 (t, 1H, J = 7.8 Hz, Ar-Hc), 0.78–0.58 (s, 9H, -CH3, 2J [119/117Sn-1H = 58.2 Hz, 55.8 Hz]); 13C NMR (75 MHz, CDCl3, δ(ppm), J(Hz)) 170.3 (C=O), 133.5 (Ar-C6), 132.8 (C3-CF3), 128.7 (C-COO), 128.6 (Ar-C4), 128.5 (Ar-C2), 125.6 (-CF3), 0.41- -4.84 (1J [119/117Sn-13C = 393.7 and 376.5 Hz]), 119Sn NMR (DMSO, δ (ppm)) -138.84.
2.2.2. Trimethylstannyl-3,4-diethoxybenzoate (2)
Quantities used were 3,4-Diethoxybenzoic acid (0.21 g, 1 mmol), triethylamine (0.14 mL, 1 mmol), trimethyltin chloride (0.19 g, 1 mmol), yield: 85 %, crystalline solid, m.p: 132–134 °C, Anal. Calcd. for C14H22O4Sn (373.04 g/mol), C, 45.08, H, 5.94 %, Found, C, 45.07, H, 5.92 %; FT-IR (KBr, ν, cm−1) C-Haromatic (2978), C-Haliphatic (2925), COOasym (1547), COOsym (1372), Δν (175), Sn-C (551), Sn-O (469); 1H NMR (300 MHz, CDCl3, δ(ppm), J(Hz)) 7.68–7.65 (d, 1H, J = 8.4 Hz, Ar- Ha), 7.58 (s, 1H, Ar-Hc), 6.88–6.85 (d, 1H, J = 8.4 Hz, Ar- Hb), 4.18–4.11 (q, 2H, J = 6.9 Hz, -CH2), 1.50–1.44 (t, 3H, J = 6.9 Hz, CH3), 0.63–0.53 (s, 9H, -CH3, 2J [119/117Sn-1H = 58.2 Hz, 56.1 Hz]); 13C NMR (75 MHz, CDCl3, δ(ppm), J(Hz)) 171.7 (C=O), 152.2 (C3-OEt), 147.8 (C4-OEt), 123.9 (C-COO), 123.8 (Ar-C6), 114.3 (Ar-C2), 111.4 (Ar-C5), 64.4 (-CH2), 14.7 (-CH3), 0.43- -4.85 (1J [119/117Sn-13C = 378 and 396 Hz]), 119Sn NMR (DMSO, δ (ppm)) -138.96.
2.2.3. Trimethylstannyl-3-fluorobenzoate (3)
Quantities used were 3-fluorobenzoic acid (0.14 g, 1 mmol), triethylamine (0.14 mL, 1 mmol), trimethyltin chloride (0.19 g, 1 mmol), yield: 78 %, crystalline solid, m.p: 112–115 °C, Anal. Calcd. for C10H13O2FSn (302.92 g/mol), C, 39.65, H, 4.33 %, Found, C, 39.66, H, 4.30 %; FT-IR (KBr, ν, cm−1) C-Haromatic (2998), C-Haliphatic (2915), COOasym (1558), COOsym (1355), Δν (175), C-F (1255), Sn-C (551), Sn-O (471); 1H NMR (300 MHz, CDCl3, δ(ppm), J(Hz)) 7.87–7.84 (d, 1H, J = 7.2 Hz, Ar- Ha), 7.76–7.73 (d, 1H, J = 9.0 Hz, Ar-Hc), 7.28 (s, 1H, Ar-Hd), 7.24–7.18 (t, 1H, J = 8.1 Hz, Ar-Hb), 0.75–0.56 (s, 9H, -CH3, 2J [119/117Sn-1H = 58.8 Hz, 56.4 Hz]); 13C NMR (75MHz, CDCl3, δ(ppm), J(Hz)) 171.6 (C=O), 164.1 (C3-F), 129.7 (C-COO) 125.7 (Ar-C5), 119.2 (Ar-C6), 117.0 (Ar-C4), 0.59- -4.72 (1J [119/117Sn-13C = 375 and 398.5 Hz]).
2.2.4. Trimethylstannyl-4-chlorobenzoate (4)
Quantities used were 4-chlorobenzoic acid (0.15 g, 1 mmol), triethylamine (0.14 mL, 1 mmol), trimethyltin chloride (0.19 g, 1 mmol), yield: 85 %, crystalline solid, m.p: 129–131 °C, Anal. Calcd. for C10H13O2ClSn (319.37 g/mol), C, 37.61, H, 4.10 %, Found, C, 37.63, H, 4.15 %; FT-IR (KBr, ν, cm−1) C-Haromatic (2990), C-Haliphatic (2918), COOasym (1592), COOsym (1376), Δν (216), C-Cl (766), Sn-C (551), Sn-O (471); 1H NMR (300 MHz, CDCl3, δ(ppm), J(Hz)) 8.01–7.98 (d, 2H, J = 8.4 Hz, Ar- Ha, a’), 7.40–7.37 (d, 2H, J = 8.4 Hz, Ar-Hb, b), 7.28 (s, 1H, Ar-Hd), 0.75–0.56 (s, 9H, -CH3, 2J [119/117Sn-1H = 58.5 Hz, 56.1 Hz]); 13C NMR (75MHz, CDCl3, δ(ppm), J(Hz)) 171.2 (C=O), 138.4 (C4-Cl), 131.5 (Ar-C2, 6), 130.4 (C-COO), 128.3 (Ar-C3, 5), 0.6- -4.5 (1J [119/117Sn-13C = 393 and 376 Hz]).
2.2.5. Trimethylstannyl-2,4-dimethoxybenzoate (5)
Quantities used were 3,4-Dimethoxybenzoic acid (0.18 g, 1 mmol), triethylamine (0.14 mL, 1 mmol), trimethyltin chloride (0.19 g, 1 mmol), yield: 90 %, crystalline solid, m.p:168 = 170 °C, Anal. Calcd. for C12H18O4Sn (344.98 g/mol), C, 41.78, H, 5.26 %, Found, C, 41.80, H, 5.27 %; FT-IR (KBr, ν, cm−1) C-Haromatic (2998), C-Haliphatic (2934), COOasym (1610), COOsym (1403), Δν (207), Sn-C (549), Sn-O (440); 1H NMR (300 MHz, CDCl3, δ(ppm), J(Hz)) 8.02–7.99 (d, 1H, J = 9.0 Hz, Ar- Ha), 6.50–6.47 (d, 1H, J = 7.5 Hz, Ar- Hb), 6.51 (s, 1H, Ar-Hc), 3.91 (s, 6H, -OCH3), 0.72–0.52 (s, 9H, -CH3, 2J [119/117Sn-1H = 58.2 Hz, 55.8 Hz]); 13C NMR (75 MHz, CDCl3, δ(ppm), J(Hz)) 164.0 (C=O), 161.4 (C4-OMe), 135.1 (C2-OMe), 112.9 (Ar-C3), 104.3 (C-COO), 98.8 (Ar-C5), 55.4 (O-CH3), 0.60- -4.65 (1J [119/117Sn-13C = 387 and 377 Hz]), 119Sn NMR (DMSO, δ (ppm)) -127.46 ppm.
2.2.6. Trimethylstannyl-4-nitrobenzoate (6)
Quantities used were 4-nitrobenzoic acid (0.16 g, 1 mmol), triethylamine (0.14 mL, 1 mmol), trimethyltin chloride (0.19 g, 1 mmol), yield: 82 %, crystalline solid, m.p: 157–159 °C, Anal. Calcd. for C10H13NO4Sn (329.92 g/mol), C, 36.40, H, 3.97, N, 4.25 %, Found, C, 36.42, H, 4.23, N, 4.23 %; FT-IR (KBr, ν, cm−1) C-Haromatic (3110), C-Haliphatic (2915), COOasym (1518), COOsym (1345), Δν (173), Sn-C (550), Sn-O (440); 1H NMR (300 MHz, CDCl3, δ(ppm), J(Hz)) 8.27–8.18 (m, 4H, Ar- Ha, b, a’, b), 0.78–0.59 (s, 9H, -CH3, 2J [119/117Sn-1H = 58.5 Hz, 56.4 Hz]); 13C NMR (75MHz, CDCl3, δ(ppm), J(Hz)) 169.4 (C=O), 149.8 (C4-NO2), 138.6 (C-COO), 130.9 (Ar-C2, 6), 123.2 (Ar-C3, 5), 0.5- -4.7 (1J [119/117Sn-13C = 395 Hz and 378 Hz]).
2.2.7. Trimethylstannyl-5-methylpyrazine-2-carboxylate (7)
Quantities used were 5-methylpyrazine-2-carboxylic acid (0.13 g, 1 mmol), triethylamine (0.14 mL, 1 mmol), trimethyltin chloride (0.19 g, 1 mmol), yield: 85 %, amorphous solid, m.p: 132–134 °C, Anal. Calcd. for C9H14O2N2Sn (300.93 g/mol), C, 39.92, H, 4.69, N, 9.31 %, Found, C, 39.91, H, 4.71, N, 9.35 %; FT-IR (KBr, ν, cm−1) C-Haromatic (3066), C-Haliphatic (2927), COOasym (1616), COOsym (1380), Δν (236), Sn-C (547), Sn-O (468); 1H NMR (300 MHz, CDCl3, δ(ppm), J(Hz)) 9.28 (s, 1H, Ar-Ha), 8.56 (s, 1H, Ar-Hd), 2.67 (s, 3H, -CH3),` 0.91–0.56 (s, 9H, -CH3, 2J [119/117Sn-1H = 63 Hz]); 13C NMR (75 MHz, CDCl3, δ(ppm), J(Hz)) 162.0 (C=O), 157.1 (C5-CH3), 145.7 (Ar-C3), 143.1 (Ar-C6), 140.6 (C-COO), 21.8 (-CH3), 3.06- -2.77 (1J [119/117Sn-13C = 447 and 427 Hz]).
2.3. Biocidal applications
2.3.1. Antibacterial activity
To evaluate the antibacterial activity for 1-7, broth dilution technique was used [29], where bacterial cultures of K. pneumoniae (ATCC-1705), S. aureus (ATCC-6538), E. coli (ATCC-25922) and B. subtilis (ATCC-6633) with pre-adjusted turbidity were used in inoculum of nutrient broth to ascertain their minimum inhibitory concentration (MIC). Inoculum was prepared by suspending 8 g of nutrient broth in one liter distilled water, which was heated with constant agitation and boiled until complete dissolution and sterilized in autoclave at 121 °C. Dilute the above cultures with nutrient broth using 10-fold dilution (1:10 mL) i.e. 1 mL test culture in 9 ml nutrient broth. The bacterial cultures were adjusted for their turbidity to estimate the concentration in the media. The bacterial cultures were read at the wavelength of 600 nm and their absorbance was set between 0.08 to 0.1. If the absorbance was greater than 0.1, it was diluted with the growth medium to achieve desired concentration. The absorbance >0.082 or < than 0.1 suggested the cell density of 5∗108 CFU/mL. Each tested compound (5 μL from 10 mg/mL DMSO), standard drug (cefixime monohydrate and roxithromycin, 5 μL from 4 mg/mL in DMSO) and negative control (5 μL DMSO) were loaded in 96 well plate along with the addition of 195 μL of fresh bacterial inoculum in each well to have final test concentration of 50 μg/mL). All the 96 well plates were incubated for two hours at 37 °C and start taking reading exponentially with the help of micro plate reader at 630 nm. After the incubation of 24 h growth was monitored spectrophotometrically and compounds with clear wells were further taken into an account for determining MIC at two fold dilutions i.e. 50, 25, 12.5, 6.25 μg/mL. The MIC of synthesized complexes 1-7 were determine both visually as well as spectrophotometrically at 630 nm, the absorbance was measured both at 0 h and later after incubation of 24 h. The obtained results were subtracted and compared with positive and negative control.
2.3.2. Antifungal activity
Antifungal activity was explored using agar well disc diffusion method [30]. The spores of the fungal strains, namely Mucor species (FCBP-0300), A. flavus (FCBP-0064) A. fumigatus (FCBP-66), and A. niger (FCBP-0198) were suspended in Tween 20 solution (0.02%). The fungal cultures were adjusted for their turbidity to estimate the concentration in the media. The cultures were read at the wavelength of 600 nm and their absorbance was set between 0.08 to 0.1. If the absorbance was greater than 0.1, it was diluted with the growth medium to achieve desired concentration. The absorbance >0.082 or < than 0.1 suggested the cell density of 5∗108 CFU/mL. Then 100 μL of each strain was swabbed on each agar plates which was prepared by pouring Sabouraud dextrose agar on pre sterilized glass plates. The filter paper discs soaked with 5 μL of tested compound (10 mg/mL in DMSO), DMSO (as negative control) and clotrimazole (as positive standard, 4 mg/mL DMSO) were also placed on the dextrose agar plates. The average diameter (mm) of zone of inhibition were quantified and documented after an incubation period of 24–48 h at 28 °C. Active samples were tested for MIC at low concentration by two folds i-e 50, 25, 12.5, 6.25, 3.125 μg/mL. These plates were incubated for 24 h at 37 °C and the growth was examined visually. Here zone of inhibition values greater than or equal to 12 mm is considered active and minimum inhibitory concentration was decided on this basis.
2.3.3. Alpha amylase inhibition assay
Antidiabetic potential of trimethyltin derivatives (1-7) was quantified using alpha amylase inhibition activity utilizing standard protocol with trivial changes [31]. The mixture having 15 μL phosphate buffer with preadjusted at pH of 6.8, 25 μL of amylase enzyme (0.14 U/ml), 10 μL of synthesized compound 1-7 (1 mg/mL in DMSO) and 40 μL starch solution (2 mg/ml) was incubated at 50 °C for 30 min in 96 well plate, that was followed by the addition of 20 μL of HCl (1 M) in order to stop the reaction. About 90 μL of iodine solution (5 mM iodine and 5 mM potassium iodide in phosphate buffer solution) was inserted in each well. Blank was also made using DMSO and the phosphate buffer, whereas DMSO was used as negative control, that was prepared by adding DMSO in place of the trimethylstannyl(IV) carboxylates (1-7). Acarbose was used as positive control in this assay. The absorbance of reaction mixture was evaluated at 540 nm. The potential activity of the synthesized compounds were quantified as % age α-amylase inhibition.
2.3.4. Protein kinase inhibition assay
Protein kinase inhibition assay was performed by utilizing isolates of purified Streptomyces 85E bacterial strain. The culture was refreshed in tryptone soy broth for 24 h at 30 °C. The bacterial culture was swabbed on petri plates containing ISP4 media under sterilized condition. The sterile filter paper discs were loaded with 100 μg/mL of the synthesized compounds (in DMSO) and placed on the media seeded with Streptomyces 85E. Surfactin (5 μL of 4 mg/mL) and DMSO impregnated discs were used as positive and negative controls respectively. These plates were incubated at 30 °C for 72 h (time required for hyphae formation in Streptomyces 85E) and results were visually interpreted as clear and bald zones of inhibition (mm) [32].
2.3.5. Brine shrimp lethality assay (cytotoxicity)
Cytotoxicity assay was done according to the standard protocol with slight changes [30]. Firstly, the eggs of Artemia salina (Ocean 90, USA) were incubated in simulated sea water that was presaturated with oxygen (38 g/l supplemented with 6 mg/L dried yeast) for 24 h at 30 °C. A specifically designed tank having two separate compartments was used having perforated partition. In one compartment eggs was placed in sea water solution and other compartment was covered with foil and kept under constant illumination (the hatched nauplii travelled prototropically to the other compartment through the pores). The nauplii were transferred to a small beaker with Pasteur pipette, which was then used to harvest them separately. About 10 shrimp larvae were transferred to the 96 well plate from beaker having the test samples (1-7) in serial dilutions. The volume of each well was made up with sea water in such a way that concentration of DMSO remains <1%. The compounds were tested for brine shrimp lethality assay at two-fold concentrations that range 200–25 μg/mL, where, doxorubicin (in 4 mg/mL DMSO) served as positive. After 24 h, the degree of lethality for each compounds were quantified by considering the number of alive larvae. The median LD50 of the synthesized compounds with ≥50% mortality was considered using table curve 2D v 5.01 software.
3. Result and discussion
3.1. Chemistry
The salt metathesis technique was employed here in order to prepare the compounds (1-7) by stoichiometric reaction of trimethyltin chloride and the respective carboxylic acid in the presence of a base as detailed in Scheme 1. The synthesized compounds are stable species and were characterized by FT-IR, multi nuclear (1H, 13C, 119Sn) NMR spectroscopy and complete data are given in the experimental section.
3.2. FT-IR data
The metal to ligand binding mode and the presence of certain functionalities in compounds (1-7) could be investigated by vibrational spectroscopy. The IR absorption band for νOH which is present around 3200-3400 cm−1 in the ligand but its absence in the synthesized complexes indicate the deprotonation of carboxylic acid and its successful coordination with the trimethyltin group. Furthermore, lowering of symmetric and asymmetric stretching band of carboxylate moiety also indicates its successful binding with the metal center. The characteristic IR stretching frequencies for certain functionality, present in the compounds, are in ranges; 2978-3110, 2995-2915, 539-551 and 440-471 cm−1 for (C-Haromatic), (C-Haliphatic), (Sn-C) and (Sn-O) respectively.
The carbonyl stretching frequencies for (COOasy), and (COOsy), appear around 1518-1616 and 1345-1403 cm−1 respectively. The binding mode of the carboxylate moiety with the organotin moiety can easily be anticipated by taking the difference between asymmetric and symmetric stretching bands, [Δν = ν (COOasy) - ν(COOsy)] as earlier reports dictated [33]. It has been observed that upon binding with the organotin moiety, the value of ν(COOasy) decreases and corresponding ν(COOsy) increases, leading to a significant decrease in Δν. Here the values are greater or equal to 200 cm−1 indicating its monodentate mode of coordination for these compounds in solid phase [34].The FT-IR results showed closeness with the data obtained by single crystal XRD analysis as for 2. The Δν value can also be theoretically calculated by using XRD data with the help of following equation [35],
| Δν = 1818.1 δr + 16.47 (θcoo – 120) + 66.8 |
Where θ is the angle between O1-C1-O2 and δr is the difference between C1-O1 and C1-O2 bond lengths. The actual and theoretical values of Δν for 2 are 175 and 166 cm−1 respectively and are in close agreement with each other. The data further support the unidentate bridging mode of chelation for the carboxylate moiety present in the synthesized products.
3.3. NMR data
The chemical nature of (1-7) were further confirmed by multinuclear (1H,13C and 119Sn) NMR spectroscopy. The coupling constant and integration values explicitly determined the magnetically non-equivalent protons present in the trimethyltin(IV) carboxylates. The 1H NMR data revealed that deprotonation of -COOH functionality occur as the resonance peak at about 11 ppm is missing in the final products, which verify the link of carboxylate to the organotin moiety. The resonance peak for aliphatic -CH2 and -CH3 protons appear within range 4.18–4.11 and 1.50–1.44 ppm respectively, while resonances for aromatic protons appear at their usual region (8.32–6.51 ppm). The signal for Sn-CH3 protons appear in range 0.91–0.52 ppm with coupling constant [2J 119Sn-1H] values in range 63–56 Hz confirming the coordination number four with tetrahedral geometry (in solution) whereas single crystal XRD data for (2) manifest five coordinated tin in the solid state as the XRD. The θ values for all the synthesized compounds, as determined from the J -values, fall in range 114–111° (Table1) that justified the tetrahedral environment for the molecular species in solution phase. The θ value can be calculated by using following equation [36];
| θ = 0.0161 [2J]2–1.32 [2J] + 133.4 |
Table 1.
θ (°), [2J119Sn-1H] and [1J119Sn-13C] values for (1-7).
| Compounds | θ (°) | [2J119Sn-1H] (Hz) | [1J119Sn-13C] (Hz) |
|
|---|---|---|---|---|
| Actual 1J | Theoretical 1J | |||
| 1 | 111.11 | 58.2 | 394 | 391.5 |
| 2 | 111.11 | 58.2 | 396 | 391.6 |
| 3 | 111.44 | 58.8 | 398 | 395.5 |
| 4 | 111.27 | 58.5 | 393 | 393.5 |
| 5 | 111.11 | 58.2 | 387 | 391.6 |
| 6 | 111.27 | 58.5 | 395 | 393.5 |
| 7 | 114.14 | 63.0 | 427 | 426.1 |
The 13C NMR data for (1-7) clearly manifest the presence of magnetically non-equivalent carbons in their chemical structures. The resonance for -C=O was observed in range 171.7–162.0 ppm. The resonance peaks for -CH2 and -CH3 moiety appear at 64.4 and 14.7 ppm, respectively. The most characteristic peak for Sn-13C appears in range 0.77- -2.08 ppm with [1J 119Sn-13C] values of 387–427 Hz. The actual values, calculated from 13C NMR spectra for [1J 119Sn-13C], are in well agreement with the theoretically calculated values as obtained from Lockhart-Manders equation [37, 38], which lies in between four and five coordinated geometry as mentioned in Table 1.
| [1J119Sn-13C] = 11.4 θ–875 |
The 119Sn NMR spectroscopy was used for the determination of coordination number and geometry of synthesized trimethyltin(IV) carboxylates. The chemical shift values obtained for 1, 2 and 5 exist in range -90 to -190 ppm which refers to five coordinated tin in the complexes. The data provide a strong evidence that the geometry of the synthesized organotins may be referred as distorted trigonal bipyramidal in solution [39, 40].
3.4. X-ray crystallography
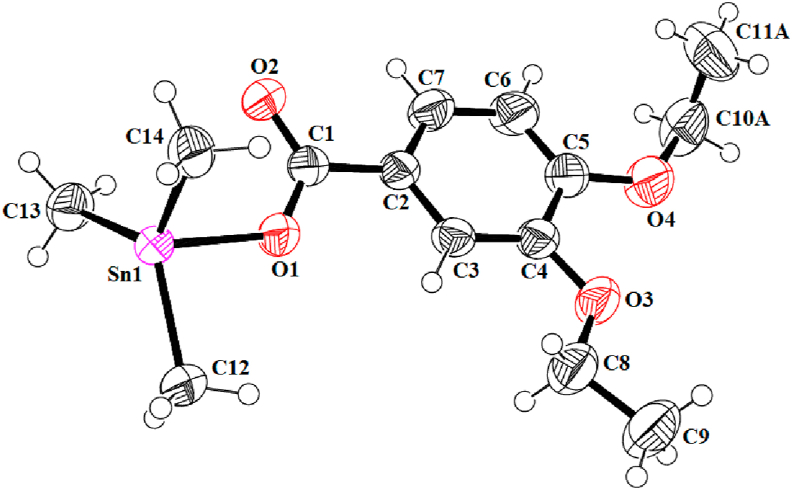
One of the synthesized complexes; (2) was analyzed by using single crystal XRD technique. The ORTEP diagram with displacement ellipsoids of heavy atoms drawn at 50 % probability level along with the partial packing diagram showing the polymeric chains with both parts of the disordered ethyl group are delineated as Figures 1 and 2, respectively. The crystallographic and structural refinement data are summarized in Table 2. The crystal system for 2 is monoclinic having space group P21/c. The ORTEP diagram clearly suggests that the complex exists in 1D-polymeric chain formation, where Sn is attached to three C atoms (Sn1-C12, Sn1-C13 and Sn1-C14) and two O atoms (Sn1-O1 and Sn1-O2i) having bond lengths 2.112(3), 2.109(3), 2.105(3), 2.146(19) and 2.471(19) A° respectively. The Sn1-O1bond length [2.146(19) A°], which is approximately equal to the sum of covalent radius of Sn and O (2.13 A°), which depicts a strong metal to ligand interaction between Sn1 and O1 moiety. The packing diagram also revealed that one of the ethoxy group at 4 position of the aromatic ring (in the ligand) is distorted whereas the second ethoxy group at 3 position is stable. The C-atoms of ethyl group in 4-ethoxy are disordered at two set of sites with occupancy ratio 0.508(9): 0.492(9). The disordered C-atoms were treated as having a similar anisotropic thermal ellipsoid. The bond lengths for 4-ethoxy group i.e. C10A-O4 and C10B-O4 are 1.471(13) and 1.410(12) respectively whereas for 3-ethoxy group bond length for C8-O3 is 1.404(4) depicting slight distortion in that moiety. The O-C and C-C distance in the disordered group was restrained to 1.42 and 1.50 Å, respectively in the refinement. The bond angles for O3-C8-C9, O4-C10A-C11A and O4-C10B-C11B are 108.9(3), 107.7(10) and 107.4(9) also demonstrate insignificant amount of distortion (around 1.5°) in 4-ethoxy group as compare to stable 3-ethoxy moiety [41, 42].
Figure 1.
ORTEP diagram of asymmetric unit for 2.
Figure 2.
The partial packing diagram for 2 showing the polymeric chains and the both parts of the disordered ethyl group.
Table 2.
Crystallographic data and refinement details∗ for (2).
| Identification Codes | 2 |
| CCDC # | 1968935 |
| Empirical formula | C14H22O4Sn |
| Formula weight | 373.00 |
| Temperature/K | 296 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 14.408(2) |
| b/Å | 9.7669(12) |
| c/Å | 12.6795(18) |
| α/° | 90 |
| β/° | 115.107(5) |
| γ/° | 90 |
| Volume/Å3 | 1615.6(4) |
| Z | 4 |
| μ/mm−1 | 1.59 |
| 2θ range for data collection | 2.605°–27.0° |
| Crystal size/mm3 | 0.42 × 0.38 × 0.20 |
| Radiation | MoKα (λ = 0.71073) |
| Rint | 0.037 |
| Goodness of Fit | 1.039 |
| Index ranges | -18 ≤ h ≤ 17, -7 ≤ k ≤ 12, -11 ≤ l ≤ 16 |
| R[F2 > 2σ(F2)], wR(F2) | 0.0262, 0.0702 |
| No. of reflections | 3505 |
| No. of parameters | 197 |
| No. of restraints | 64 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.48−0.46 |
∗Symmetry codes: (i) −x+1, y−1/2, −z+3/2; (ii) −x+1, y+1/2, −z+3/2.
The structural parameter, tau (τ), indicates geometry of the coordination center and here the data demonstrate the existence of 5-coordinated tin center with trigonal bipyramidal molecular shape for the synthesized compounds in solid phase. The tau is defined as τ = (β-α)/60; where β = first largest basal angle around Sn(IV) center, α = second largest angle around coordination sphere, and its value lies between 0-1 [ that is, the value for regular square pyramidal is around zero while a regular trigonal bipyramidal geometry may have the value around one] as the literature described [43]. The tau parameter for (2) was calculated and found to be 0.82, which is close to one which confirmed its molecular geometry as distorted trigonal bipyramidal. The bond angles for C-Sn-C (117.20–121.65°), C-Sn-O (87.87–98.60°) and O-Sn-O (173.75°) lie in the predictable range of five coordinated distorted trigonal bipyramidal geometry [12, 44].
3.5. Biocidal applications
3.5.1. Antimicrobial data
All the synthesized trimethyltin(IV) derivatives (1-7) were exposed to four bacterial (Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae and Escherichia coli) and four fungal strains (Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus and Mucor) for exploring their possible anti-microbial properties. The data, as MIC (μg/mL) values, for antibacterial and antifungal activities are reported in Table 3 The compounds 2, 4, 6 and 7 show significant MIC value of 12.5 μg/mL against S. aureus, K. pneumoniae, A.flavus, A. niger and A. fumigatus. The most exceptional inhibition of 6.25 μg/mL was observed for 6 against K. pneumoniae.
Table 3.
Antimicrobial activity data for (1-7).
|
Number of compounds |
MIC(μg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | K. pneumoniae | E. coli | A. flavus | A. niger | A. fumigatus | Mucor | |
| 1 | - | 25 | - | 50 | 12.5 | 12.5 | 12.5 | 50 |
| 2 | 50 | - | 12.5 | 50 | 12.5 | 12.5 | 12.5 | >50 |
| 3 | - | 25 | 25 | - | 25 | 50 | 12.5 | >50 |
| 4 | - | 12.5 | - | - | 12.5 | 12.5 | 12.5 | >50 |
| 5 | 50 | 25 | 12.5 | 50 | 12.5 | 50 | 50 | >50 |
| 6 | - | - | 6.25 | - | 25 | 25 | 25 | >50 |
| 7 | 50 | 25 | 12.5 | 50 | 12.5 | 12.5 | 12.5 | >50 |
| Cefixime∗ | 1.11 | 0.334 | 0.334 | 1.11 | - | - | - | - |
| Roxithromycin∗ | 0.334 | 0.334 | 0.334 | 0.334 | - | - | - | - |
| Clotrimazole∗ | - | - | - | - | 10 | 5 | 5 | 2.5 |
| DMSO | - | - | - | - | - | - | - | - |
∗ Standard drug.
- No activity.
The results of the present study can be explained in light of Tweedy's theory of chelation [45]. The enhanced activity of trimethylstannyl(IV) carboxylates is attributed to the chelation of tin metal ion. The hydrophobicity for the synthesized products has been enhanced by the presence of three donor methyl groups, which qualifies to the partial sharing of electropositive tin metal with the orbitals of carboxylate moiety and increase the π electron delocalization over the chelate ring. This successive enhancement of lipophilicity promotes the penetration of the complexes into the lipid bilayer and as a consequent, blocking the active sites present in the enzymes of the microorganism. The obtained results are substantial in comparison to the already reported ones [13, 46], which discovered that antibacterial and antifungal potential of these synthesized organotin become improved when substituted carboxylic acids were attached with trimethyltin moiety as exhibited by their MIC values.
3.5.2. Alpha amylase inhibition data
These trimethyltin(IV) derivatives (1-7) were preliminary bioassyed for their possible antidiabetic activity. The % age alpha amylase inhibition was documented at concentration of 100 μg/mL, using acarbose as positive. The data suggest significant antidiabetic potential for (1) with a value of 51.81 μg/mL, which is comparable to the reference drug (69.29 μg/mL) as mentioned in Table 4. The obtained results were compared with the reported ones, which demonstrated that trimethylstannyl-3-(trifluoromethyl)benzoate (1) may be explore further for its candidacy in future drug discovery and development processes [15, 16].
Table 4.
Antidiabetic, Protein kinase inhibition & Cytotoxicity data for (1-7) with LD50 values.
|
Number of compounds |
Antidiabetic assay |
Protein kinase inhibition assay |
Cytotoxicity (BSLA) |
|||||
|---|---|---|---|---|---|---|---|---|
| %age inhibition (100 μg/mL) | Zones (100 μg/disc) |
%age Mortality |
LD50 (μg/mL) | |||||
| Clear zone (mm) | Bald zone (mm) | 200 (μg/mL) | 100 (μg/mL) | 50 (μg/mL) | 25 (μg/mL) | |||
| 1 | 51.81 | 12 | - | 100 | 100 | 100 | 100 | 0.22 |
| 2 | 10.39 | 15 | 20 | 100 | 100 | 90 | 80 | 10.83 |
| 3 | 25.99 | 20 | - | 100 | 100 | 90 | 90 | 3.39 |
| 4 | 12.39 | 15 | - | 100 | 100 | 100 | 90 | 23.24 |
| 5 | 10.39 | 15 | - | 100 | 100 | 100 | 90 | 23.24 |
| 6 | 6.77 | - | 15 | 100 | 100 | 100 | 90 | 23.24 |
| 7 | 9.93 | 15 | 18 | 100 | 100 | 100 | 90 | 23.24 |
| Acarbose∗ | 69.29 | |||||||
| Surfactin∗ | - | 30 | ||||||
| Doxorubicin∗ | 90 | 80 | 60 | 30 | 5.93 | |||
| DMSO | - | - | - | - | - | - | - | - |
∗ Standard drug.
- No activity.
3.5.3. Protein kinase inhibition data
Protein kinase inhibition assay was performed for (1-7), using agar disc diffusion method [42], the data have been mentioned in Table 4, wherein DMSO and surfactin were used as negative and positive control, respectively. The absence of inhibition zone on tryptone soy broth surface confirms the non-toxic nature of DMSO. A significant activity was observed for 2 and 7 showing 20- and 18-mm bald zone of inhibition at 100 μg/mL concentration. The inhibition zone greater than or equal to 20 mm clear and bald zones has been considered as promising activity and cytotoxic nature of the compound, respectively. The assay specifically provides testimony about cytotoxic and anticancer nature of the trimethyltin(IV) derivatives (1-7). Protein kinases are involved in phosphorylation of numerous eukaryotic cell, thus playing a role in phosphorylation that may cause proliferation of the cancerous cells as well, whereas protein kinase inhibitors block these kinases and restrict the diseases. The data exhibit the significant activity for 2 that may serve as a lead compound after certain structural modifications in future studies [18].
3.5.4. Brine shrimp lethality data
The Brine shrimp lethality assay is a less expensive and robust method for the assessment of toxic nature and to acquire toxicity appraisal among the synthesized compounds (1-7) with reference to the reference drug. They were tested in two-fold serial dilution (200, 100, 50 and 25 μg/mL) to check the percentage mortality of the tested organism, Artemia salina (Table 4). The least LD50 values were obtained for 1 and 3 as 0.22 and 3.39 μg/mL respectively depicting their high level of toxicity, whereas compounds 4-7 are equally least toxic with LD50 value of 23.24 μg/mL. The obtained results suggest that trimethyltin derivatives are mildly toxic with the exception of 1 and 3 but can be further augmented in future drug discovery processes by tailoring various substituents in their structural motifs [18].
To develop a limited structure to activity relationship (SAR) various substituents on different positions of phenyl ring, present in the synthesized compounds (1-7), were taken into consideration. The compounds (1, 3, 4 and 6) contain -CF3, -F, -Cl and -NO2 functionality (having electron withdrawing effects) show MIC values of 25, 25, 12.5 μg/mL against S. aureus and 6.25 μg/mL against K. pneumoniae respectively. Similarly, the data for antifungal activity are also encouraging ones that may provide guidelines to the researchers working in this field. The preliminary screening data for alpha amylase inhibition demonstrate that 1 and 3 exhibit highest percentage inhibition of 51.81 and 25.99 μg/mL respectively because of the presence of -CF3 and -F groups in their molecules, justifying the obtained results at 100 μg/mL disc loading. The brine shrimp lethality assay data also exhibit that fluorinated stannyl carboxylates (1 & 3) have the least LD50 value of 0.22 and 3.39 μg/mL, that makes them most toxic among all the synthesized complexes. Thus one can infer from the presented data that a compromise has to be maintained between activity and toxicity of such compounds so as to make them amenable in future drug delivery system, and for that some adjustments/alterations, like physicochemical nature of the complexing agents, nature/length of alkyl chain of organotin moiety and hydrophobicity have to be taken into account.
4. Conclusions
Seven trimethyltin carboxylates (1-7) were successfully synthesized and characterized by FT-IR, multinuclear (1H, 13C and 119Sn) NMR spectroscopy and single crystal X-ray diffraction technique The distinguishing feature of the article is the understanding of some structural aspects of the compounds both in solid and solution phases by calculating/corelating their various spectroscopic and XRD parameters such as 2J (119Sn-1H) [58.2–63.0 ppm], 1J (119Sn-13C) [387-427 ppm], θ (111.11–114.14°) and tau τ (0.82) values. The synthesized complexes were also bio-assayed for their possible antibacterial, antifungal, antidiabetic (alpha amylase inhibition), protein kinase inhibition and cytotoxicity assay and some of them show promising results. An endeavor has been made to develop a limited structure to activity relationship among the synthesized compounds. The data show good to significant antibacterial and antifungal activity for some of the synthesized compounds with MIC ranging 50–6.25 μg/mL, whereas complex 1 exhibit excellent % alpha amylase inhibition of 51.81 μg/mL at a concentration of 100 μg/mL when compared with the respective reference drugs.
Declarations
Author contribution statement
Mehwish Mehmood: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Imtiaz-ud-Din: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ahmad Raheel: Analyzed and interpreted the data.
Ihsan-ul Haq, Muhammad N. Tahir: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to Quaid I Azam University Islamabad, Pakistan, for providing financial assistance and granting funds for the project.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
The CCDC for the reported compounds are 1968935. It can be free downloaded from CCDC data base. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
References
- 1.Shibata I., Baba A., Matsuda H. Regioselective ring cleavage of oxiranes catalyzed by organotin halide-triphenylphosphine complex. Tetrahedron Lett. 1986;27:3021–3024. [Google Scholar]
- 2.da Silva M.A., dos Santos A.S.S., dos Santos T.V., Meneghetti M.R., Meneghetti S.M.P. Organotin(IV) compounds with high catalytic activities and selectivities in the glycerolysis of triacylglycerides. Catal. Sci. Technol. 2017;7:5750–5757. [Google Scholar]
- 3.Abdellah M.A., Hadjikakou S.K., Hadjiliadis N., Kubicki M., Bakas T., Kourkoumelis N., Simos Y.V., Karkabounas S., Barsan M.M., Butler I.S., Synthesis Characterization, and biological studies of organotin(IV) derivatives with o-or p-hydroxybenzoic acids. Bioinorgan. Chem. Appl. 2009:2009. doi: 10.1155/2009/542979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu Baul T.S. Antimicrobial activity of organotin(IV) compounds: a review. Appl. Organomet. Chem. 2008;22:195–204. [Google Scholar]
- 5.Rabinovitch E.B., Dorsch J.L., Summers J.W., Quisenberry J.G. Role of organotin mercaptide stabilizer in heat and light stability of PVC. J. Vinyl Techn. 1986;8:73–78. [Google Scholar]
- 6.Arkış E., Balköse D. Thermal stabilisation of poly (vinyl chloride) by organotin compounds. Polym. Degrad. Stabil. 2005;88:46–51. [Google Scholar]
- 7.Sharma N., Kumar V., Kumari M., Pathania A., Chaudhry S. Synthesis, characterization, and antibacterial activity of triorganotin(IV) complexes of 2-methylphenol. J. Coord. Chem. 2010;63:3498–3515. [Google Scholar]
- 8.Rehman W., Baloch M.K., Muhammad B., Badshah A., Khan K.M. Characteristic spectral studies andin vitro antifungal activity of some Schiff bases and their organotin(IV) complexes. Chin. Sci. Bull. 2004;49:119–122. [Google Scholar]
- 9.Carraher C.E., Jr., Roner M.R. Organotin polymers as anticancer and antiviral agents. J. Organomet. Chem. 2014;751:67–82. [Google Scholar]
- 10.Hadjikakou S.K., Hadjiliadis N. Antiproliferative and anti-tumor activity of organotin compounds. Coord. Chem. Rev. 2009;253:235–249. [Google Scholar]
- 11.Ma C., Li J., Zhang R., Wang D. Syntheses and characterization of triorganotin complexes: X-ray crystallographic study of triorganotin pyridinedicarboxylates with trinuclear, 1D polymeric chain and 2D network structures. J. Organomet. Chem. 2006;691:1713–1721. [Google Scholar]
- 12.Ma C., Li J., Zhang R., Wang D. Syntheses and crystal structures of trimethyltin(IV) coordination polymers based on mixed ligands of 5-nitroisophthalate and 2, 2′(4, 4′)-bipy or phen. Inorganica chim. Acta. 2006;359:2407–2416. [Google Scholar]
- 13.Roy M., Devi S.S., Roy S., Singh C., Singh K.S. Synthesis, characterization, crystal structures and in vitro antimicrobial activities of triorganotin(IV) complexes of azo-dicarboxylates. Inorg. Chim. Acta. 2015;426:89–98. [Google Scholar]
- 14.Kazeem M., Adamson J., Ogunwande I. Modes of inhibition of. BioMed Res. Int. 2013 doi: 10.1155/2013/527570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy M., Roy S., Singh K.S., Kalita J., Singh S.S. Synthesis, characterisation and anti-diabetic activities of triorganotin(IV) azo-carboxylates derived from amino benzoic acids and resorcinol: crystal structure and topological study of a 48 membered macrocyclic-tetrameric trimethyltin (IV) complex. Inorg. Chim. Acta. 2016;439:164–172. [Google Scholar]
- 16.Roy M., Roy S., Singh K.S., Kalita J., Singh S.S. Synthesis, characterization and anti-diabetic assay of diorganotin(IV) azo-carboxylates: crystal structure and topological studies of azo-dicarboxylic acid ligand and its cyclic tetranuclear dimethyltin(IV) complex. New J. Chem. 2016;40:1471–1484. [Google Scholar]
- 17.Zhang J., Yang P.L., Gray N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Canc. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javed F., Ali S., Shahzadi S., Sharma S.K., Qanungo K., Tahir M.N., Shah N.A., Khan M.R., Khalid N. Synthesis, structural characterization, theoretical calculations and in vitro biological activities of organoti(IV) complexes with [O, O] donor ligand. J. Inorg. Organomet. Polym. Mater. 2016;26:48–61. [Google Scholar]
- 19.Imtiaz-ud-Din, Mazhar M., Ali S., Khan K.M. Lower homologues (methyl, ethyl) of diorganotin derivatives of germyl (substituted) propanoic acids: spectroscopic elucidations and biological studies. Nat. Prod. Res. 2007;21:749–758. doi: 10.1080/14786410601130299. [DOI] [PubMed] [Google Scholar]
- 20.Imtiaz-ud-Din, Mazhar M., Molloy K., Khan K.M. Synthesis, structural characterization of some germanium substituted chiral diethyltin derivatives. J. Organomet. Chem. 2006;691:1643–1648. [Google Scholar]
- 21.Raheel A., Imtiaz-ud-Din, Taj M.B., Ayub R., Tahir M.N., Raftery J., Al-Shakban M. Synthesis, characterization and DFT study of bioactive 2-[(2-Methylpropanoyl) amino] propanoic acid and its polymeric tributyltin(IV) derivative. ChemistrySelect. 2019;4:8638–8644. [Google Scholar]
- 22.Mehmood M., Imtiaz-ud-Din, Tahir M.N., Haq I.-u., Zahra S.S. Synthetic stratagem, characterization and biocidal applications of triorganotin(IV) complexes derived from hydrazide/hydrazone analogues. Inorg. Chim. Acta. 2019;486:387–394. [Google Scholar]
- 23.Mehmood M., Imtiaz-ud-Din, Abbas S., Azam S.S., Tahir M.N., Parvaiz N., Tameez-ud-Din Asim. Bioactive heteroleptic Bismuth(V) carboxylates: synthetic Stratagem, characterization and binding pattern validation. J. Organomet. Chem. 2020;921:1–11. 121357. [Google Scholar]
- 24.Shah F.A., Sabir S., Fatima K., Ali S., Qadri I., Rizzoli C. Organotin(IV) based anti-HCV drugs: synthesis, characterization and biochemical activity. Dalton Trans. 2015;44:10467–10478. doi: 10.1039/c5dt00862j. [DOI] [PubMed] [Google Scholar]
- 25.Ali S., Helliwell M., Shahzadi S. (3-Amino-4-chlorobenzoato) trimethyltin(IV) Acta Crystalloger E. 2006;62:m1778–m1779. [Google Scholar]
- 26.Pangborn A.B., Giardello M.A., Grubbs R.H., Rosen R.K., Timmers F.J. Safe and convenient procedure for solvent purification. Organometallics. 1996;15:1518–1520. [Google Scholar]
- 27.Bruker A. 2007. A. Bruker, Inc., 5465 East Cheryl Parkway, Madison, WI, 53711. [Google Scholar]
- 28.Sheldrick G., Kruger G., Goddard R. SHELXS-86, structure solution program. Acta Crystallogr. A: Found. Crystallogr. A. 1990;46:467–473. [Google Scholar]
- 29.Mannan H.A., Ahmed I., Hussain I., Jamil M., Miza B. Antibacterial activity and brine shrimp toxicity of Artemisia dubia extract. Pakistan J. Bot. 2012;44:1487–1490. [Google Scholar]
- 30.Ahmed M., Fatima H., Qasim M., Gul B. Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Compl. Alternative Med. 2017;17:386. doi: 10.1186/s12906-017-1894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zahra S.S., Ahmed M., Qasim M., Gul B., Zia M., Mirza B., Haq I.-u. Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Compl. Alternative Med. 2017;17:1–16. doi: 10.1186/s12906-017-1951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fatima H., Khan K., Zia M., Ur-Rehman T., Mirza B., Haq I.-u. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: an in vitro biological and phytochemical investigation. BMC Compl. Alternative Med. 2015;15:376. doi: 10.1186/s12906-015-0891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imtiaz-ud-Din, Molloy K.C., Mazhar M., Dastgir S., Ali S., Mahon M.F. Some tricyclohexyltin carboxylates containing germanium: synthesis, spectral and crystallographic characterization. Appl. Organomet. Chem. 2003;17:781–787. [Google Scholar]
- 34.Sirajuddin M., Ali S., Shah F.A., Ahmad M., Tahir M.N. Potential bioactive Vanillin–Schiff base di-and tri-organotin(IV) complexes of 4-((3, 5-dimethylphenylimino) methyl)-2-methoxyphenol: synthesis, characterization and biological screenings. J. Iran. Chem. Soc. 2014;11:297–313. [Google Scholar]
- 35.Nara M., Torii H., Tasumi M. Correlation between the vibrational frequencies of the carboxylate group and the types of its coordination to a metal ion: an ab initio molecular orbital study. Open J. Phys. Chem. 1996;100:19812–19817. [Google Scholar]
- 36.Lockhart T.P., Manders W.F. Structure determination by NMR spectroscopy. Dependence of | 2J (119Sn, 1H)| on the Me-Sn-Me angle in methyltin(IV) compounds. Inorg. Chem. 1986;25:892–895. [Google Scholar]
- 37.Nadvornik M., Holeček J., Handlíř K., Lyčka A. The 13C and 119Sn NMR spectra of some four-and five-coordinate tri-n-butyltin(IV) compounds. J. Organomet. Chem. 1984;275:43–51. [Google Scholar]
- 38.Holeček J., Nadvornik M., Handlíř K., Lyčka A. 13C and 119Sn NMR Study of some four-and five-coordinate triphenyltin (IV) compounds. J. Organomet. Chem. 1983;241:177–184. [Google Scholar]
- 39.Lyčka A., Holeček J., Nadvornik M., Handlíř K. 13C, 15N and 119Sn NMR spectral evidence for tin five-coordination in triorganotin(IV) oxinates. J. Organomet. Chem. 1985;280:323–329. [Google Scholar]
- 40.Willem R., Bouhdid A., Mahieu B., Ghys L., Biesemans M., Tiekink E.R., de Vos D., Gielen M. Synthesis, characterization and in vitro antitumour activity of triphenyl-and tri-n-butyltin benzoates, phenylacetates and cinnamates. J. Organomet. Chem. 1997;531:151–158. [Google Scholar]
- 41.Chandrasekhar V., Nagendran S., Baskar V. Organotin assemblies containing Sn-O bonds. Coord. Chem. Rev. 2002;235:1–52. [Google Scholar]
- 42.Wang S., Li Q.-L., Zhang R.-F., Du J.-Y., Li Y.-X., Ma C.-L. Novel organotin(IV) complexes derived from 4-carboxybenzenesulfonamide: synthesis, structure and in vitro cytostatic activity evaluation. Polyhedron. 2019;158:15–24. [Google Scholar]
- 43.Islam A., Da Silva J.G., Berbet F.M., Da Silva S.M., Rodrigues B.L., Beraldo H., Melo M.N., Frézard F., Demicheli C. Novel triphenylantimony(V) and triphenylbismuth (V) complexes with benzoic acid derivatives: structural characterization, in vitro antileishmanial and antibacterial activities and cytotoxicity against macrophages. Molecules. 2014;19:6009–6030. doi: 10.3390/molecules19056009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Addison A.W., Rao T.N., Reedijk J., van Rijn J., Verschoor G.C. Synthesis, structure, and spectroscopic properties of coppe (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper(II) perchlorate. Dalton Trans. 1984:1349–1356. [Google Scholar]
- 45.Dharmaraj N., Viswanathamurthi P., Natarajan K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transit. Met. Chem. 2001;26:105–109. [Google Scholar]
- 46.Singh K.S., Roy M., Roy S., Ghosh B., Devi N.M., Singh C.B., Mun L.K. Synthesis, characterization and antimicrobial activities of triorganotin(IV) complexes with azo-azomethine carboxylate ligands: crystal structure of a tributyltin (IV) and a trimethyltin(IV) complex. J. Coord. Chem. 2017;70:361–380. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The CCDC for the reported compounds are 1968935. It can be free downloaded from CCDC data base. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.