Abstract
The Spectroscopic profile of Chromone-3-Carboxylic Acid (abbreviated as C3CA) was examined using FT-IR, FT-Raman, UV, 1H and 13C NMR techniques. The geometrical parameters and energies attained from DFT/B3LYP method with 6–311++G (d,p) basis sets calculations. The geometry of the molecule was fully optimized, vibrational spectra were calculated and assigned the fundamental vibrations on the basis of the total energy distribution (TED) of the vibrational modes, calculated with scaled quantum mechanics (SQM) method. The XRD data obtained from the computed geometric parameters shows that there is little deviation in the structure due to the substitution of the COOH group in the molecule. Using NBO study, the delocalization of the electron and the corresponding attraction between the orbitals shows that the lone pair transition has higher stabilization energy when compared with remaining atoms. The 1H and 13C NMR chemical shifts are calculated using GIAO method and the experimental chemical shifts were analysed with theoretical values which reflects better coincidence. The electronic properties, HOMO and LUMO energies, are performed with TD-DFT reproduces good with the experimental findings. Besides, frontier molecular orbitals (FMO), the high reactive nature of the molecule is identified with MEP and global reactivity descriptor analysis are performed. In addition, the molecular docking study was conducted, and results of the docking study identified the sugar phosphatase inhibitor activity of the target molecule (C3CA).
Keywords: Materials science, Materials chemistry, Theoretical chemistry, Chromone-3-carboxylic acid, DFT, Bacillus subtilis, Hirshfeld surface analysis, Charge analysis, Electronic properties
Materials Science, Materials Chemistry, Theoretical Chemistry, Chromone-3-Carboxylic Acid; DFT; Bacillus subtilis; Hirshfeld surface analysis, charge analysis, electronic properties
1. Introduction
Chromone-3-carboxylic acid is a heterocyclic compound (benzopyrone type), an isomer of coumarin with a substituted keto group of pyrone ring. Our human diet has some amount of Chromone and has a wide spectrum of biological activities. This molecule possess anti-oxide rutin activity [1], anticancer activity and used as interlenkin-5- inhibitors, monvamine oxidase inhibiors, neuroleptic agents [2]. Chromone is a natuarally occurring pharmo therapeutic compounds and its derivatives are used in the treatment of asthma. These compounds are used as antioxidant, anti-inflammatory, anti-allergic, anti-bacterial, anti-parasitic, anti-fungal, antimicrobial, anti-diabetic and anti-carcinogenic properties [3]. The bioactive nature of this molecule makes this candidate more effective in the medicinal chemistry. There are more studies in the derivatives of chromone [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14].
By scrutinizing earlier studies, it is obvious that a study with hybrid quantum mechanical and molecular modeling approach on this title compound is not available. This study was to originate new perceptions into the structure and binding properties. To interpret the structural optimization, MEP, charge delocalization (NBO) and chemical shift (NMR), DFT Method was implemented. The derived optimized structure was utilized for docking the chromone 3-carboxylic acid to the biologically relevant metabolic protein.
2. Experimental details
The sample was collected from Sigma-Aldrich chemicals company, USA with 99% purity. The FT-Raman spectrum (400-4000cm−1) of C3CA was recorded using Nd-YAG laser (1064 nm). By KBr pellet technique using IFS 66V spectrophotometer, IR spectrum (400-4000cm−1) of the compound was recorded. The compound with di-methyl sulphoxide (DMSO) solvent was prepared and NMR spectrum was taken between 20-200 ppm with the scanning interval of 20 ppm. With the aid of UV-1700 series instrument, UV-Visible spectrum was recorded between 200-400 nm, with the scanning interval of 0.2 nm.
3. Computational details
3.1. Quantum chemical calculations
The structure optimization, vibrational harmonic frequencies of the title compound are calculated using GAUSSIAN09 [15] package program using B3LYP/6311++G (d,p) basis set. The minimum PES has been obtained and the stability of the optimized geometries were confirmed by getting positive values to all the obtained wavenumbers. The relative contributions of the redundant internal coordinates to each normal vibrational mode of the molecule was analysed by calculating PED using VEDA4 program [16]. With the aid of GAUSSVIEW [17] program with symmetry considerations, vibrational frequency assignments were made with a high degree of accuracy. With the support of UV–Visible study using TD-DFT with same basis set, the major contributions in HOMO–LUMO energies, absorption wavelengths, oscillator strengths etc. based on the optimized structure in the solvent (DMSO) & in gas phase were calculated. Furthermore, MEP, the global reactivity descriptors were analysed. This study was also focused to envisage about Mullikan charges, Hirshfeld analysis, chemical shift (NMR) and charge delocalization of the title compound.
3.2. Molecular docking calculations
In order to attain further understandings on the molecule, molecular docking study was conducted. The ligand was prepared and stored in PDB file format. Then this ligand was imported into the AutoDock work space and the output was saved in PDBQT file format. For Protein preparation, AutoDock Protein preparation wizard was used. The sugar phosphatase inhibitor activity of the molecule was identified, and the suitable protein was selected for the same. The protein taken for the molecular dynamic simulation study is 4UAR (PDB ID) of Bacillus subtilis from Protein Data Bank. The polar hydrogens were added to satisfy the valency, lone pairs were merged to the selected protein, to mask the surface the water molecules were removed and the file was saved in a PDBQT file format. The receptor grids were generated using 90 Å x 90 Å x 90 Å grid size.
4. Result and discussion
4.1. Conformational analysis
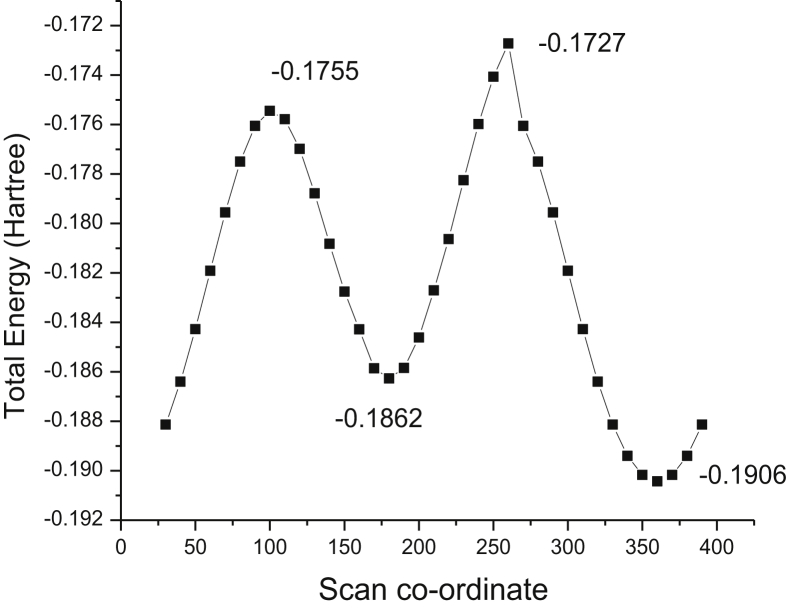
The molecule has a carboxyl and pyrone substituent group attached to a benzene ring. By applying the PES technique, the structural confirmation was, in which the dihedral angle H20–O18–C15–C12 is varied in 36 ways in steps of 100. The scanning graph is shown in Figure 1 which clearly depicts that two minimum energies were observed at 180ᵒ and 360ᵒ with -0.186 and -0.190 hartree respectively, which are structurally identical and acting as the most stable conformer of the compound. In addition, the maximum energy points at 260ᵒ is -0.172 hartree, which are considered as the least conformer.
Figure 1.
The potential energy curve of chromone-3-carboxylic acid.
4.2. Structural analysis
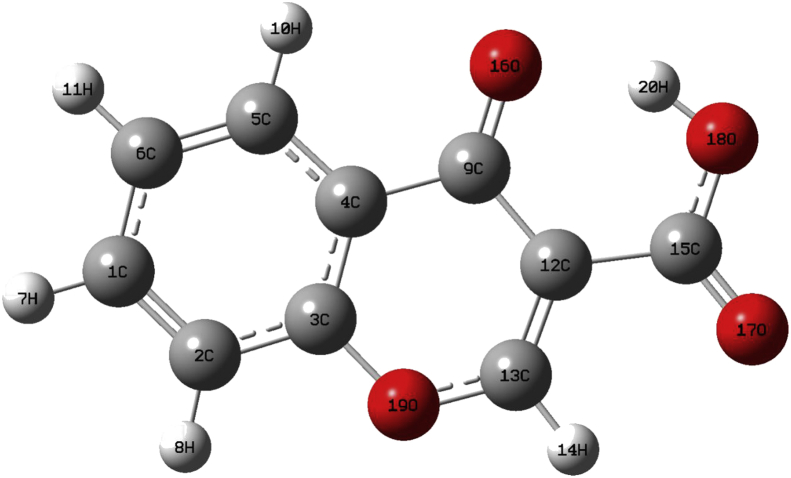
The crystal structure of the compound was reported previously [18] depicts that compound belong to monoclinic, P21/n space with cell parameter a = 18.017 (8) Å, b = 5.549 (3) Å, c = 8.017 (5) Å, β = 92.49 (4) Å and Z = 4. The structure of the C3CA compound and experimentally determined XRD values are shown in Figure 2 & Table 1 respectively. In general, C–C–C angles of ipso atoms, which are known to be sensitive to the electronic properties of the substituent's [19]. The information about supplementary to the CIF pack is taken from Cambridge Crystallographic Database centre, No.CCDC. 1412580.
Figure 2.
The structure of chromone-3-carboxylic acid.
Table 1.
Optimized Geometrical parameter for Chromone-3-Carboxylic acid Computed at B3LPY/6-311+G (d,p).
| Bond Length (Å) | Methods | Exp. | Bond Angle () | Methods | Exp. | |
|---|---|---|---|---|---|---|
| B3LYP | B3LYP | |||||
| 6-311++G (d,p) | 6-311++G (d,p) | |||||
| Benzene ring (C–C) | Benzene ring (C–C–C) | |||||
| C1–C2 | 1.3857 | 1.376 | C1–C2–C3 | 118.54 | 118.7 | |
| C2–C3 | 1.3942 | 1.394 | C2–C3–C4 | 121.90 | 121.8 | |
| C3–C4 | 1.3996 | 1.397 | C3–C4–C5 | 118.40 | 118.7 | |
| C4–C5 | 1.4052 | 1.404 | C4–C5–C6 | 120.32 | 119.7 | |
| C5–C6 | 1.3831 | 1.384 | C1–C6–C5 | 120.07 | 120.2 | |
| C1–C6 | 1.4039 | 1.402 | C2–C1–C6 | 120.74 | 120.9 | |
| Pyrone ring (C–C) | Pyrone ring (C–C–C) | |||||
| C4–C9 | 1.4704 | 1.464 | C3–C4–C9 | 119.88 | 119.6 | |
| C9–C12 | 1.4601 | 1.445 | C5–C4–C9 | 121.70 | 121.7 | |
| C12–C13 | 1.3555 | 1.348 | C4–C9–C12 | 114.87 | 115.2 | |
| Benzene ring (C–H) | C9–C12–C13 | 119.77 | 120.6 | |||
| C1–H7 | 1.0837 | 0.951 | C9–C12–C15 | 122.97 | 115.7 | |
| C2–H8 | 1.0827 | 0.950 | Benzene ring (C–C–H) | |||
| C5–H10 | 1.0832 | 0.950 | C2–C1–H7 | 119.39 | 119.6 | |
| C6–H11 | 1.0832 | 0.950 | C6–C1–H7 | 119.86 | 119.5 | |
| Pyrone ring (C–H) | C1–C2–H8 | 122.03 | 122.7 | |||
| C13–H14 | 1.0822 | 0.950 | C3–C2–H8 | 119.42 | 120.7 | |
| Pyrone ring (C–O) | C6–C5–H10 | 121.73 | 120.2 | |||
| C3–O19 | 1.3756 | 1.379 | C4–C5–H10 | 117.94 | 120.1 | |
| C13–O19 | 1.3358 | 1.344 | C1–C6–H11 | 119.81 | 119.9 | |
| C9–O16 | 1.2374 | 1.248 | C5–C6–H11 | 120.10 | 119.8 | |
| C15–O17 | 1.2070 | 1.213 | Pyrone ring (C–C–H) | |||
| C15–O18 | 1.3338 | 1.323 | C12–C13–H14 | 122.37 | 121.8 | |
| Out of rings | Pyrone ring (C–C–O) | |||||
| C12–C15 | 1.5074 | 1.497 | C4–C9–O16 | 122.30 | 121.9 | |
| O18–H20 | 0.9893 | 0.840 | C12–C9–O16 | 122.81 | 122.9 | |
| C12–C13–O19 | 124.88 | 124.3 | ||||
| C2–C3–O19 | 116.75 | 116.6 | ||||
| C4–C3–O19 | 121.34 | 121.6 | ||||
| Ring (C–O–C) | ||||||
| C3–O19–C13 | 119.23 | 118.8 | ||||
| Ring (C–O–H) | ||||||
| C13–O19–H14 | 112.73 | 117.7 | ||||
| Out of rings | ||||||
| C12–C15–O17 | 121.99 | 132.2 | ||||
| C12–C15–O18 | 115.40 | 110.5 | ||||
| O17–C15–O18 | 122.60 | 121.1 | ||||
| C15–O18–H20 | 109.26 | 109.4 | ||||
Taken from ref. [30].
The C3–C4 bond is common for both benzene ring and substituted pyrone ring. The bond length calculated using computation method for C3–C4 is 1.39Å is almost equal to the experimental value. The theoretically observed other bond lengths C1–C2, C2–C3, C4–C5, C5–C6 and C1–C6 are compared with experimental one is well agreed with one another. In pyrone ring, due to single bond, C4–C9 and C9–C12 bond lengths are 1.4Å while presence of two oxygen atoms O16 and O19 in this ring the bond C12 = C13 is 1.35Å which implies that it is purely double bond.
The bond lengths of C9–O16 and C15–O17 are1.23Å and 1.20Å respectively while that of C15–O18, it is 1.33Å. This difference in bond lengths of C–O group is mainly due to the bonding nature ie., the bonding nature between C15–O18 (single bonded) and C9–O16, C15O17 (double bonded). Due to phase changes, the bond lengths obtained through theoretical method (gaseous phase) is higher than the experimental values (solid phase) The C–H bond lengths obtained from previous literature [20] was between 0.95 and 0.951 ˚A while of our theoretical values ranges between 1.0822 and 1.0837Å suggests that low scattering of hydrogen in X-ray diffraction makes larger deviation of C–H bond lengths which was not included in theoretical calculation. This overestimation is also verified in our calculation as represented in Table 1. Oxygen atoms in both ring has not influenced by the C–H bond lengths.
The C–O bond length values of C3–O19 and C13–O19 present in the pyrone ring are 1.37Åand 1.33Årespectively shows higher values than the other C–O bonds. The bond length between the hydroxyl group (O18–H20) is 0.9893 Å is the lowest value than all other bonds present in the compound. The C12–C15, i.e. Cpyrone–Ccarboxylic acid, bond length calculated by theoretical calculation is 1.5075 Å which is very close to the experimental value (1.497 Å).
The molecule with sp2 hybridization. suggests that the bond angles observed are expected to be in the range of 1200 but here, the bond angles vary from 1170 to 1220 may be due to substitution effects. Also, due to electronic conjugation among the bonds and presence of oxygen atom, there are small variations in the bond angles from the expected value of 120o Additionally, it is observed that the electronic conjugation is lesser in benzene ring while higher in pyrone ring.
4.3. Mullikan and natural atomic charge analysis
Population analysis is constructed on the LCAO and the wave function of the molecule uses the density matrix (P) and the overlap matrix (S) to build the population matrix. MPA explains about the atomic charges, dipole moment, electronic structure and biological activity of the molecule. As the biological activity increases, the charge on the atom also increases [21]. The charges on the C3CA was computed by MPA & NAC and compared in the Table 2.
Table 2.
Mullikan Charges and NAC of Chromone-3-Carboxlic acid.
| Atoms | Mullikan Atomic Charge |
Natural atomic Charge |
|---|---|---|
| B3LYP/6-311+G (d,p) | ||
| C1 | -0.22779 | -0.16963 |
| C2 | -0.16411 | -0.22119 |
| C3 | -1.56454 | 0.34275 |
| C4 | 1.95887 | -0.17557 |
| C5 | -0.11718 | -0.15014 |
| C6 | -0.16941 | -0.1971 |
| H7 | 0.14486 | 0.21465 |
| H8 | 0.14278 | 0.22297 |
| C9 | -0.37049 | 0.50276 |
| H10 | 0.16304 | 0.23653 |
| H11 | 0.14171 | 0.21373 |
| C12 | 0.76583 | -0.26374 |
| C13 | -0.40561 | 0.24131 |
| H14 | 0.19524 | 0.19425 |
| C15 | 0.12549 | 0.77377 |
| O16 | -0.32889 | -0.52357 |
| O17 | -0.31274 | -0.50512 |
| O18 | -0.21584 | -0.69127 |
| O19 | -0.04791 | -0.51071 |
| H20 | 0.28664 | 0.46532 |
MPA predicts that the charge on C1 atom shows the substitution of carboxylic group which will redistribute electronic charge over the chromone ring system. In benzene ring, charges on C4, C7, C11, C12 and H8, H10, H11 are positive while others are negative and in case of pyrone ring, the charges on H14, C15, H20 are positive, C4 is highly positive, C3 is highly negative, C12 is very low positive and C13 is very low negative in the molecule.
NAC shows that charges on C3, C9, C13, C15 are positive while others are negative. All H7, H8, H10, H11, H14, H20 has positive charges which are attached with carbon in the carboxylic group whereas all the oxygen have negative charges.
4.4. NMR analysis
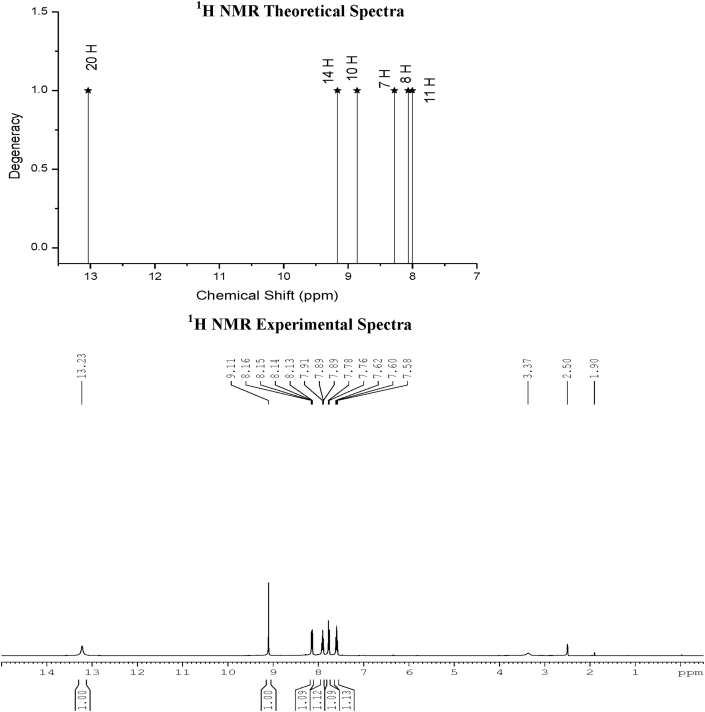
In this study, 1H and 13C chemical shifts of the title molecule in gas and DMSO solvent with DFT theory and the experimental NMR in DMSO Solvent are gathered in Table 3. The experimental 1H and13C NMR spectra of the C3CA are given in Figures 3 and 4 respectively. Primarily, the full geometry optimization of the molecule was performed at the gradient corrected DFT using the hybrid B3LYP method and GIAO [22, 23, 24]. 1H and 13C chemical shift calculations of the compound was made by the same method using 6–311++G (d,p) basis set in gas and DMSO solvent.
Table 3.
Calculated 1H and13 C NMR Chemical shifts (ppm) of Chromone-3-Carboxylic acid at B3LYP/6311++G (d,p) basis set.
| Atom | Gas (ppm) | DMSO (ppm) | Experimental DMSO |
|---|---|---|---|
| C1 | 135 | 137 | 135 |
| C2 | 118 | 120 | 118 |
| C3 | 156 | 157 | 155 |
| C4 | 124 | 123 | 123 |
| C5 | 129 | 127 | 125 |
| C6 | 127 | 128 | 126 |
| C9 | 177 | 178 | 176 |
| C12 | 114 | 113 | 114 |
| C13 | 163 | 166 | 163 |
| C17 | 161 | 164 | 163 |
| H7 | 8.4 | 8.2 | 8.1 |
| H8 | 7.7 | 8.0 | 7.9 |
| H10 | 8.9 | 8.8 | 7.7 |
| H11 | 8.8 | 7.7 | 7.6 |
| H14 | 9.0 | 9.0 | 9.1 |
| H20 | 13 | 13 | 13 |
Figure 3.
1H NMR Theoretical and Experimental Spectra of Chromone-3-carboxylic acid.
Figure 4.
13C NMR Theoretical and Experimental Spectra of Chromone-3-carboxylic acid.
Electron-withdrawing atom or group can decrease the shielding and move the resonance of attached proton towards the higher frequency, whereas electron-donating atom or group increases the shielding and moves the resonance towards the lower frequency [21]. The chemical shifts of aromatic protons of organic molecules are usually observed in the range of 7.00–9.00 ppm.
The signals of 4 aromatic protons (1H) were calculated theoretically as 8.2–8.4, 7.7–8, 8.8–8.9 and 7.7–8.8 ppm for gas and DMSO solvent, respectively. These theoretically obtained values were verified with the reported experimental values in DMSO solvent at 8.1,7.9,7.7 and 7.6 ppm respectively in Table 3.
From the computed and experimental chemical shift values, H7, H8, H10 & H11 attached to the carbon atoms of the benzene ring has smaller values than the pyrone ring proton (H14) and carboxyl proton (H20) signals are due to the electronic charge density around the ring.
In experimental 13C NMR spectrum (DMSO), the value of δ (chemical shift) of carbon atoms is absorbed between 118-176 ppm. The molecule has ten carbons however these carbons are differentiated in three groups (attached with benzene, pyrone, carboxyl) which is consistent with the structure and molecular symmetry. The chemical shift δ of the carbons present in the benzene ring (C1 to C6) are 135, 118, 155, 123, 125 & 126 ppm respectively; in the pyrone ring (C9, C12 and C13) are 176,114,163 ppm while the carbon present in carboxyl group (C15) has δ of 163 ppm. The chemical shift (theoretical) in gas phase and DMSO solvent are presented in Table 3. The value of δ for C9 & C13 attached in the pyrone ring is larger and the value of δ for C12 is very less than others due to the substituent effect of the carboxyl group. The absorption peak value of C9 and C15 are larger (176 & 163 ppm) due to carbon-oxygen double bond.
4.5. Vibrational analysis
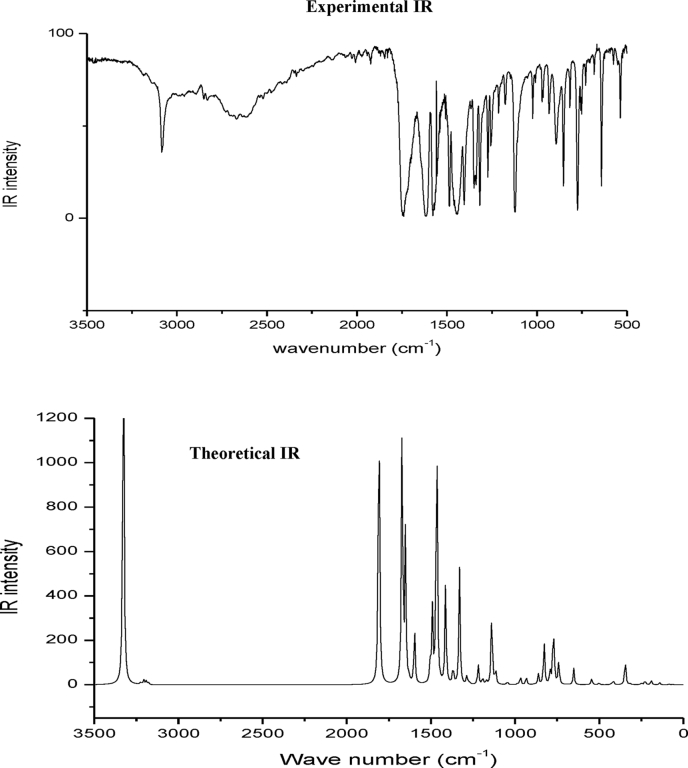
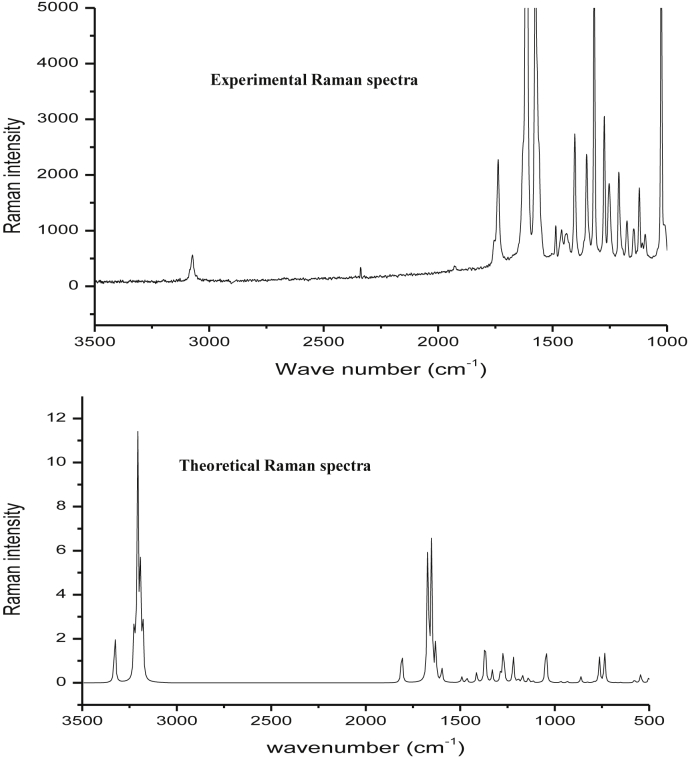
The C3CA molecule consists of 20 atoms with 54 normal modes of vibrations and considered under Cs point group symmetry. The computed and recorded FTIR spectra are as shown in Figures 5 and 6. The FT-IR and FT-Raman experimental frequencies, unscaled and scaled vibrational frequencies and PED of C3CA are calculated using B3LYP method with 6–311 G (d,p) basis set and tabulated in Table 4. The fundamental frequencies are assigned using PED values. The difference of values in theoretical and experimental frequencies are due to the over estimation of force constants in the quantum calculations, combination of electron correlations effects, and basis set deficiencies. To attain the closer difference between experimental and theoretical one, the theoretical values are scaled with the scaling factor of 0.99.
Figure 5.
Experimental and theoretical Infrared spectra of chromone-3-carboxylic acid.
Figure 6.
Experimental and Theoretical Raman spectra of chromone-3-carboxylic acid.
Table 4.
Experimental and Theoretical (B3LYP 6–311++G (d,p)) vibrational frequencies of Chromone-3-carboxylic acid.
| Symmetry species (Cs) | Experimental frequency (cm−1) |
B3LYP 6-311++G (d,p) |
Assignment | PED% | |
|---|---|---|---|---|---|
| FT-IR | FT-RAMAN | Scaled (cm−1) | |||
| A′ | 3183 | 3194 | ν OH | ν OH (99) | |
| A′ | 3089 | 3095 | ν CH | ν CH (99) | |
| A′ | 3073vw | 3078 | ν CH | ν CH (99) | |
| A′ | 3071m | 3074 | ν CH | ν CH (97) | |
| A′ | 3062m | 3063 | ν CH | ν CH (98) | |
| A′ | 3052 m | 3051 | ν CH | ν CH (96) | |
| A′ | 1737s | 1734w | 1736 | ν C=O | ν OC (77) |
| A′ | 1615s | 1608vs | 1604 | ν C=O | ν OC (51) |
| A′ | 1580w | 1584 | ν C=C | ν CC (22) | |
| A′ | 1562s | 1563 | ν C=C | ν CC (23) | |
| A′ | 1544w | 1533 | ν C=C | ν CC (26) | |
| A′ | 1438w | 1443 | β OH | ν CC (11) HOC(18) + δ OH (40)+ HCC(14) | |
| A′ | 1432s | 1431 | ν C=C | í CC (10) | |
| A′ | 1402w | 1406 | ν C–O | δ HOC(74) | |
| A′ | 1360w | 1355 | ν C–O | ν OC (10) | |
| A′ | 1320w | 1313 | ν C–O | ν CC (17) + δ HCO (10) OC(74) | |
| A′ | 1263m | 1260w | 1276 | ν C–C | δ HCO (38) |
| A′ | 1251m | 1248w | 1234 | ν C–C | δ HCC(17) + δ HCO (10) |
| A′ | 1225w | 1220 | ν C–C | ν OC (20)+ ν CC (10) | |
| A′ | 1183w | 1177w | 1170 | ν C–C | ν OC (12) HCC(34) |
| A′ | 1130w | 1144 | ν C–C | ν CH (25) + ν CC (11) + δ CCC(10) | |
| A′ | 1110s | 1123 | ν C–C | ν OC (11) + δ HCC (21) | |
| A′ | 1095vw | 1092 | δ CH | ν OC (23) | |
| A′ | 1089w | 1070 | δ CH | δ CCH (50) + δ CCC (13) | |
| A′ | 1017w | 1012w | 1004 | δ CH | ν CC (45)+ â HCC (40) |
| A′ | 953w | 969 | δ CH | δ CCCH (11) | |
| A′ | 940m | 946 | δ CH | δ HCCH (43) + δ CCCH (37) | |
| A′ | 926vw | 929 | δ C=O | δ HCOC(79) | |
| A′ | 898m | 895 | δ C=O | ν CC (13) + δ CCO (23) | |
| A′ | 847s | 849w | 849 | δ OH | δ CCCH (45) |
| A″ | 813s | 811w | 826 | δ C–O | δ CCC (40) + δ COC (14) |
| A″ | 799w | 793 | δ C–C | δ HOCC (83) | |
| A″ | 794w | 760 | δ CH | δ CCCH (17) | |
| A″ | 751s | 740w | 741 | δ CH | δ CCCH (12) |
| A″ | 728w | 732 | δ CH | δ OCH (14) | |
| A″ | 697w | 712 | δ CH | δ CCCH(15) | |
| A″ | 689w | 705 | δ CH | δ OCH (38) | |
| A″ | 643vw | 642vw | 649 | δ C=O | δ CCCC (10) |
| A″ | 620s | 624 | δ C=O | δ CCO (19) + δ CCC (20) | |
| A′ | 548m | 553 | δ C–O | δ OCO (21) | |
| A′ | 538w | 527 | δ C–C | δ OCCC (40) | |
| A′ | 525w | 522 | δ COH | δ CCC (34) + δ OC (13) + δ CCC (31) | |
| A′ | 480w | 480 | δ CCC | δ CCC(38) + δ CCO (14) | |
| A′ | 445w | 443 | δ CCC | δ CCC(14) + δ CCC (11) | |
| A′ | 426m | 408w | 411 | δ CCC | δ CC(15)+ δ CCO (43) |
| A′ | 386m | 399 | δ CCC | δ CCCC(27)+ δ CCOC (17) | |
| A′ | 332s | 331 | δ CCO | δ CCO (13) | |
| A″ | 306m | 304 | δ COC | δ CC(19) + δ CCC (11) | |
| A″ | 230w | 238 | δ CCC | δ CCCC(23) | |
| A″ | 220w | 217 | δ CCO | δ CCOC (21) | |
| A″ | 184w | 182 | δ CCC | δ CCC (10) | |
| A″ | 137w | 134 | δ CCO | δ CCCC(49) | |
| A″ | 78s | 81 | δ CCO | δ CCCO(72) | |
| A″ | 66s | 56 | δ COC | δ CCCO (24) | |
ν–stretching; β–in–plane bending; δ–deformation; ρ–rocking; γ–out of plane bending; ω–wagging and τ–torsion. IR and Raman intensities are normalized to 100.
4.5.1. O–H vibrations
The OH group stretching vibrations in the carboxylic acid occurs between 3300 -2500 cm−1 and O–H bending from 1440-1395 and 950-910 cm−1. Also, the chromone ring is attached with a single O–H group makes the stretching vibration of OH around 3300 cm−1 [25]. In this study, the O–H stretching vibration is assigned at 3183cm−1,in-plane and out-of-plane bending vibrations at 1438 cm−1 and 849 cm−1 (FT-Raman) respectively shows that the decrease in the wavenumbers is due to the substituent effect of chromone ring.
4.5.2. C–H vibrations
The hetero aromatic structure shows the presence of C–H stretching vibrations in the region 3000–3100 cm−1. The strong vibration at 3089 cm−1 (FTIR), weak vibration at 3073 cm−1 (FT-R), medium vibration at 3071, 3062 and 3052 cm−1 in FTIR are due to C–H stretching modes. In this work, C–H in-plane bending vibrations are assigned at 1095 (FT-R), 1089, 1017 (FTIR) and 953 cm−1 (FTIR) and out of plane bending vibrations are assigned at 794 (FT-R), 751 (FTIR), 728 (FT-R), 697 (FTIR), 689 cm−1 (FT-R).
4.5.3. Ring vibrations
The C=C stretching vibrations are assigned in the range 1650-1500 cm−1 and C–C stretching vibrations are assigned in the range 1500-1400 cm−1 in previous literature [26]. In this work, the C=C stretching vibrations are assigned at 1580 cm−1 (FT-R), 1562 cm−1 (FTIR), 1544 cm−1 (FT-R) and 1432 cm−1 (FTIR). Similarly, C–C stretching vibrations in the regions are assigned at 1263, 1251, 1183, 1110 cm−1 in FT-IR and 1260, 1248, 1225, 1117, 1130 cm−1 in Raman spectra respectively. The theoretical C–C stretching wavenumbers are observed at 1276, 1234, 1220, 1170, 1144, 1123, cm−1. The torsion and CCC bending modes are mixed with other modes.
4.5.4. CO vibrations
As per the previous literatures, the C=O stretch of carboxylic acids appeared between 1740-1660 cm-1 which is similar as C=O stretch in ketones. Also, the C=O asymmetric and symmetric stretching should be expected at 1832 cm−1 and 1761 cm−1 respectively. In this present work, the C=O asymmetric stretching at 1737 cm−1 (FTIR) with strong intensity and 1734 cm−1(FT-R) with weak intensity. Similarly, the strong intensity peak at 1615 cm−1 (FTIR) and very strong peak at 1608 cm−1 (FT-R) is assigned to the symmetric C=O stretching vibration. The variation in expected frequencies shows the effect of substituents. Theoretically, the C=O stretching vibrations are observed at 1667 and 1691 cm−1 respectively. The C–O stretching vibrations are assigned to the weak FT-Raman peaks at 1402, 1360 and 1320 cm−1. The in-plane bending vibrations of C=O are assigned at 926 cm−1 (FT-R) and 898 cm−1 (FTIR) frequencies. The C=O out of plane bending vibrations are assigned to the strong band at 620 (FTIR), 624 cm−1 (FT-Raman) and medium intensity peak at 548 cm−1 (FTIR).
4.6. Hirshfeld and finger print analysis
The hirshfeld surface on the title molecule is analysed with crystal explorer 3.1 software [27] to draw the 3D image of hirshfeld surface and is used to study the intermolecular interactions in detail. The two factors, ‘de’, the distance between any surface point nearest to interior atom, and ‘di’, the distance between the surface point nearest to the exterior atom are noted with vanderwaals radii of the atom. The mapping of hirshfeld shape index (-1.0000 to 1.0000), curvedness (-4.000 to 0.4000), surface over dnorm (-1.2738 to 1.1038), di (0.6990–2.4462), de (0.2903–2.2232) are shown in Figure 7. The observed fingerprints are shown in Figure 8, plotted using d norm descriptor which indicates the contributions of inter contacts to the Hirshfeld surfaces, C–H…H–C (42.1%), O–C…C–O (12.1%), C–C…C–C (7.2%), H–O...O–H (36.5%), and O–O...O–O (2.2%). The inter contacts in the hydrogen bonds are represented by the red spots over the surface.
Figure 7.
Hirshfeld surface for d-norms, de, di, shape index and curvedness of chromone-3-carboxylic acid.
Figure 8.
Finger print of total Hirshfeld, O–C⋯C–O, H–O….O–H, C–C….C–C, C–H….H–C, H–H….H–H, O–O….O–O.
From the shape index diagram, the red triangles in the concave region (above the plane of the molecule) indicating that the atoms of the π-stacked molecule above them, and the blue triangles represent by convex regions indicating the ring atoms of the molecule inside the surfaces. The red triangles on the shape index mapping are referring to the C2–H8···π interaction with the contribution of 42.4%. The curvedness is a measure of the shape of the surface area of the molecule. The flat areas of the surface correspond to low values of curvedness, while sharp curvature areas correspond to high values of curvedness indicating the interactions between neighboring molecules. The large flat region which delineated by a blue outline refer to the π···π stacking interactions. The curvedness of the compound reveals that π···π stacking interaction is absent.
4.7. NBO analysis
To study the inter and intra molecular interactions, charge transfer from electron donor to acceptor, the NBO analyses were made. The fock matrix was elucidating in the donor-acceptor interactions in the NBO analysis [20].
The high stabilization energy of the transitions gives a measure of the probabilities of the transitions. In this molecule, the top ten highly probable transitions can be listed as follows in the descending order, based on the stablisation energy: O17 to C15–O18 (n -σ ∗, 30.33Kcal/mol), O18 to C15–O17 (n -π ∗, 25.15Kcal/mol), O19 to C12–O13 (n -π ∗, 20.15Kcal/mol), O16 to C9–C12 (n -σ∗, 19.58Kcal/mol), O19 to C3–C4 (n -π ∗, 18.52Kcal/mol), O17 to C12–C15 (n -σ∗, 17.23Kcal/mol, C3–C4 to C9–O16 (π -π∗, 17.16Kcal/mol), C12–C13 to C9–O16 (π -π∗, 15.94Kcal/mol), C12–C13 to C15–O17 (π -π∗, 15.37Kcal/mol) and O17 to C12–C15 (π -π∗, 15.27Kcal/mol). The highest probable transition is O17 to C15–O18 (n -σ ∗, 30.33Kcal/mol). Furthermore, most of the above transitions will not appear in UV-Visible spectra due to the forbidden nature of the transitions. Only a very few transitions will be allowed by the selection rules, which can be predicted by HOMO- LUMO analysis; the peaks appeared both in theoretical and experimental spectra are identified with the help of the oscillator strengths of the bands. Table 5 gives the details about the types of bond, acceptors, donors, occupancy levels, energy (E2), energy difference with polarized energy.
Table 5.
Natural Bonding Orbital energy profile of Chromone-3-Carboxylic Acid.
| Donor | Type of bond | Occupancy | Acceptor | Type of bond | Occupancy | Energy E (2) kcal/mol | Energy difference E(j)-E(i) a.u. | Polarized energy F (i,j) a.u. |
|---|---|---|---|---|---|---|---|---|
| C1–C2 | σ | 1.98152 | C3–O19 | σ∗ | 0.03344 | 2.89 | 0.98 | 0.048 |
| C1–C2 | π | 1.82167 | C3–C4 | π∗ | 0.2694 | 11.25 | 0.3 | 0.053 |
| C1–C2 | π | 1.82167 | C5–C6 | π∗ | 0.14554 | 9.42 | 0.32 | 0.049 |
| C1–C6 | σ | 1.97879 | C2–H8 | σ∗ | 0.01116 | 3.15 | 1.06 | 0.052 |
| C1–C6 | σ | 1.97879 | C5–H10 | σ∗ | 0.01078 | 3.03 | 1.08 | 0.051 |
| C1–H7 | σ | 1.97941 | C2–C3 | σ∗ | 0.02878 | 5.4 | 0.91 | 0.063 |
| C2–C3 | σ | 1.9742 | C1–H7 | σ∗ | 0.01022 | 2.57 | 1.09 | 0.047 |
| C2–C3 | σ | 1.9742 | C3–C4 | σ∗ | 0.01116 | 2.74 | 1.25 | 0.052 |
| C2–C3 | σ | 1.9742 | C4–C9 | σ∗ | 0.06074 | 3.85 | 1.04 | 0.057 |
| C2–H8 | σ | 1.97925 | C1–C6 | σ∗ | 0.02117 | 5.05 | 0.93 | 0.061 |
| C3–C4 | π | 1.75875 | C1–C2 | π∗ | 0.16418 | 9.01 | 0.32 | 0.049 |
| C3–C4 | π | 1.75875 | C5–C6 | π∗ | 0.14554 | 9.42 | 0.33 | 0.051 |
| C3–C4 | π | 1.75875 | C9–O16 | π∗ | 0.20158 | 17.16 | 0.3 | 0.064 |
| C4–C5 | σ | 1.96596 | C3–C4 | σ∗ | 0.01116 | 3.04 | 1.23 | 0.055 |
| C4–C5 | σ | 1.96596 | C3–O19 | σ∗ | 0.03344 | 5.84 | 0.85 | 0.063 |
| C4–C5 | σ | 1.96596 | C6–H11 | σ∗ | 0.01109 | 2.88 | 1.08 | 0.05 |
| C4–C9 | σ | 1.97599 | C2–C3 | σ∗ | 0.02878 | 3.92 | 1.02 | 0.057 |
| C5–C6 | π | 1.81447 | C1–C2 | π∗ | 0.16418 | 11.61 | 0.3 | 0.053 |
| C5–C 6 | π | 1.81447 | C3–C4 | π∗ | 0.2694 | 11.15 | 0.29 | 0.052 |
| C5–H10 | σ | 1.97834 | C1–C6 | σ∗ | 0.02117 | 5.24 | 0.92 | 0.062 |
| C5–H10 | σ | 1.97834 | C3–C4 | σ∗ | 0.01116 | 2.54 | 1.14 | 0.048 |
| C6–H11 | σ | 1.98034 | C4–C5 | σ∗ | 0.02396 | 5.23 | 0.92 | 0.062 |
| C9–C12 | σ | 1.97383 | C13–H14 | σ∗ | 0.01668 | 2.75 | 1.04 | 0.048 |
| C9–O16 | π | 1.96403 | C12–C13 | π∗ | 0.1534 | 5.75 | 0.35 | 0.041 |
| C12–C13 | π | 1.84517 | C9–O16 | π∗ | 0.20158 | 15.94 | 0.32 | 0.065 |
| C12–C13 | π | 1.84517 | C12–C13 | π∗ | 0.1534 | 2.55 | 0.31 | 0.025 |
| C12–C13 | π | 1.84517 | C15–O17 | π∗ | 0.19384 | 15.37 | 0.31 | 0.062 |
| C12–C15 | σ | 1.97604 | C13–O19 | σ∗ | 0.02683 | 4.63 | 0.89 | 0.057 |
| C13–H14 | σ | 1.98137 | C9–C12 | σ∗ | 0.06758 | 4.82 | 0.99 | 0.062 |
| C15–O17 | π | 1.97381 | C12–C13 | π∗ | 0.1534 | 5.56 | 0.36 | 0.041 |
| O18–H20 | σ | 1.98175 | C15–O17 | σ∗ | 0.01624 | 3.04 | 1.28 | 0.056 |
| O16 | n | 1.88826 | C4–C9 | σ∗ | 0.06074 | 17.23 | 0.65 | 0.095 |
| O16 | n | 1.88826 | C9–C12 | σ∗ | 0.06758 | 19.58 | 0.64 | 0.101 |
| O17 | n | 1.84395 | C12–C15 | σ∗ | 0.06674 | 15.27 | 0.6 | 0.088 |
| O17 | n | 1.84395 | C15–O18 | σ∗ | 0.10554 | 30.33 | 0.49 | 0.111 |
| O18 | n | 1.9753 | C12–C15 | σ∗ | 0.06674 | 4.89 | 0.99 | 0.063 |
| O18 | n | 1.87016 | C15–O17 | π∗ | 0.19384 | 25.15 | 0.34 | 0.084 |
| O19 | n | 1.97109 | C3–C4 | σ∗ | 0.01116 | 4.33 | 1.21 | 0.065 |
| O19 | n | 1.97109 | C12–C13 | σ∗ | 0.01679 | 3.61 | 1.18 | 0.058 |
| O19 | n | 1.78958 | C3–C4 | π∗ | 0.2694 | 18.52 | 0.39 | 0.077 |
| O19 | n | 1.78958 | C12–C13 | π∗ | 0.1534 | 20.15 | 0.36 | 0.078 |
4.8. Electronic properties
4.8.1. Electronic absorption spectra
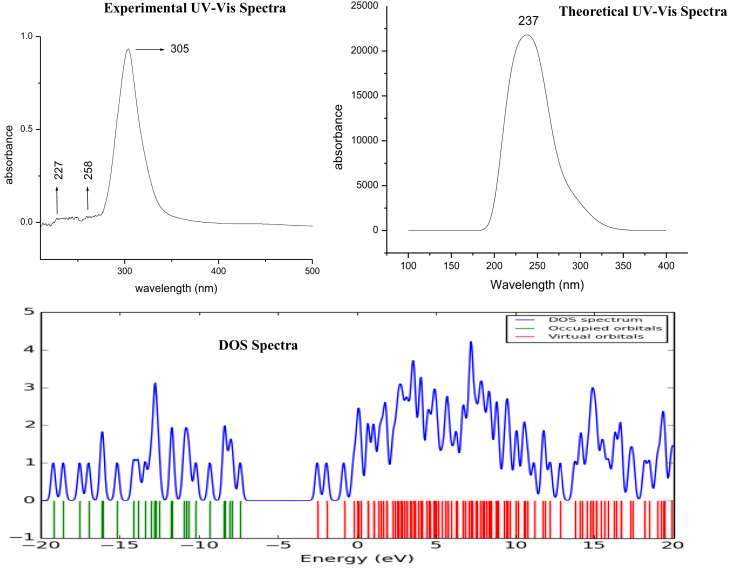
UV-Visible spectra of C3CA have been analysed with theoretical calculations, major contributions were designated with Swizard program [28] and tabulated in Table 6. The UV- Experimental and theoretical spectra are presented in Figure 9. According to the Table.6 the top ten most probable transitions at gas phase have absorption wavelengths 308, 285, 281, 263, 260, 245, 241, 239, 223, 220 nm and their energy gaps are 4.0218, 4.3370, 4.3981, 4.6991, 4.7650, 5.0422, 5.1295, 5.1740, 5.5485, 5.6253 eV respectivelyy, and corresponding oscillator strengths are 0.0001, 0.0000, 0.0627, 0.0000, 0.0019, 0.0447, 0.0001, 0.2923, 0.0435, 0.0810. The oscillator strength 0.0000 shows singlet transition also the gaussian software reflects as 0.0000 when the oscillator strength value is lower than 0.0001. The observation of oscillator strength and absorption co-efficient values indicate that only the third transition will have the appreciable intensity of absorption, which means the peak at 281 nm wavelength will have the maximum intensity, as its HOMO-LUMO contribution is 89%.
Table 6.
Theoretical electronic absorption spectra of chromone-3-carboxylic acid (absorption wavelength λ (nm), excitation energies E (ev) and oscillator strengths (f) using TD-DFT/B3LYP/6–311++G (d,p) method.
| λ (nm) Theoretical Gas |
Experimental | E (eV) | (f) | Major contribution |
|---|---|---|---|---|
| 308 | 4.0218 | 0.0001 | H-1→LUMO (60%) | |
| 285 | 4.3370 | 0.0000 | H-2→LUMO (55%) | |
| 281 | 4.3981 | 0.0627 | HOMO→LUMO (89%) | |
| 263 | 4.6991 | 0.0000 | H-1→L+1 (80%) | |
| 260 | 4.7650 | 0.0019 | HOMO→L+1 (50%) | |
| 245 | 5.0422 | 0.0447 | H-4→LUMO (60%) | |
| 241 | 5.1295 | 0.0001 | H-2→L+1 (97%) | |
| 239 | 5.1740 | 0.2923 | HOMO→L+1 (41%) | |
| 223 | 5.5485 | 0.0435 | H-5→LUMO (74%) | |
| 220 | 5.6253 | 0.0810 | H-3→L+1 (30%) | |
| DMSO | ||||
| 291 | 4.2510 | 0.0001 | H-2→LUMO (91%) | |
| 288 | 305 | 4.2775 | 0.0786 | HOMO→LUMO (91%) |
| 267 | 4.6258 | 0.0068 | H-1→LUMO (46%) | |
| 253 | 4.8826 | 0.0000 | H-4→LUMO (45%) | |
| 252 | 4.9055 | 0.3693 | H-1→LUMO (44%) | |
| 241 | 5.1287 | 0.0000 | H-4→LUMO (39%) | |
| 232 | 5.3224 | 0.2091 | H-3→LUMO (76%) | |
| 231 | 5.3658 | 0.0001 | H-4→L+1 (80%) | |
| 226 | 5.4972 | 0.1555 | H-3→L+1 (13%) | |
| 214 | 5.7793 | 0.2034 | H-3→L+1 (63%) | |
Figure 9.
Experimental, Theoretical UV-Vis Spectra & DOS spectra of chromone-3-carboxylic acid.
Similarly, for DMSO phase in which the experimental spectrum was also recorded, the respective absorption wavelengths are 291, 288, 267, 253, 252, 241, 232, 231, 226, 214 nm with energy gaps 4.25, 4.27, 4.62, 4.88, 4.90, 5.12, 5.32, 5.36, 5.49, 5.77 ev respectively along the oscillator strengths of 0.0001, 0.0786, 0.0068, 0.0000, 0.3693, 0.0000, 0.2091, 0.0001, 0.1555, 0.2034 respectively. In this case, the second transition has the highest HOMO-LUMO contribution of 91%. The theoretical wavelength for this absorption is 288 nm, which appeared at 305 nm in experimental UV spectrum. The deviation from the experimental value may be due to the solvent effect. This transition (H→L) which takes place in both phases is due to n - π ∗ transitions; O18 to C15–O17 (n -π ∗, 25.15Kcal/mol) and O19 to C12–O13 (n -π ∗, 20.15Kcal/mol). The pictorial representation of DOS of C3CA molecule is plotted in Figure 8 using the GaussSum3 program [29].
4.8.2. Frontier molecular orbital analysis
The diagram of HOMO and LUMO for the title molecule is shown in Figure 10 and the positive negative phases are represented in red and green colour where green indicates the strongest attraction & red indicates the strongest repulsion. From the Figure 10, the HOMO is localized over the C=C bond in the benzene ring but the LUMO is located over the oxygen atoms of the pyrone ring. Hence the HOMO → LUMO electron density transfer occurs from pyrone ring to carboxylic acid group. The transition from the ground state to first excited state occurs from HOMO to LUMO. HOMO has the energy of 5.6844eV while LUMO has 2.735 eV and the energy gap between HOMO to LUMO molecular orbital is 2.9494eV.
Figure 10.
Frontier molecular orbitals of chromone-3-caboxylic acid.
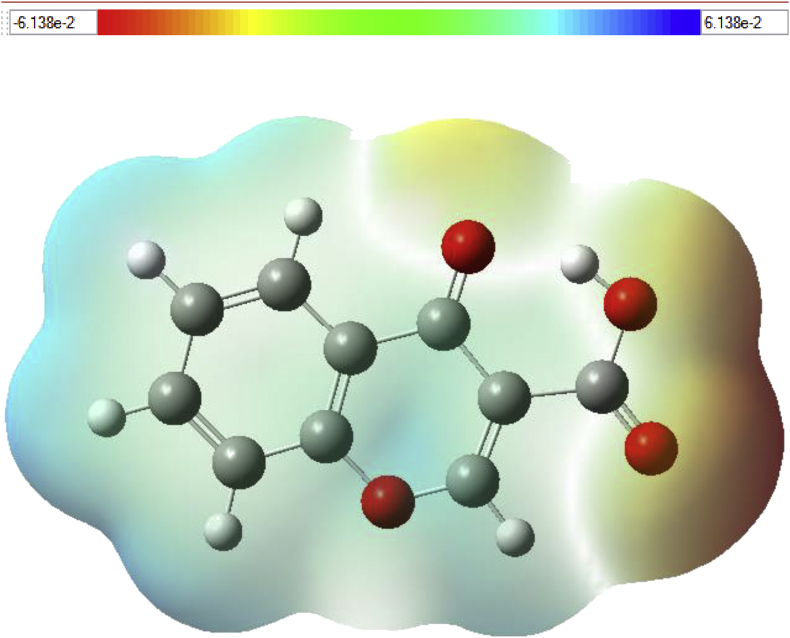
4.8.3. Molecular electrostatic potential (MEP)
The MEP map for C3CA molecule is as shown in Figure 11 and it is evident that the strongest affinity for a proton was observed at the carboxylic acid site with the maximum negative region (electrophilic) shown in red color a while the maximum positive region (nucleophilic) referred in blue color around the chromone ring indicating the strongest affinity for electron. Also, the O19 atom possess neutral potential while O16, O17 and O18 are highly repulsive potential. Moreover, H7, H8, H10 and H11 are strongly attractive in nature. The positive and negative potential of the molecule ranges from -6.138e-2 au. to 6.138e-2 au. It is also clear that the blue color are delocalized over the entire chromone ring which offer multiple binding sites through electron acceptance and donation.
Figure 11.
Molecular electrostatic potential map of chromone-3-carboxylic acid.
4.8.4. Global reactivity descriptors
Table 7 shows the calculated values of the global reactivity descriptors for the title molecule. The calculation of DFT based reactivity descriptors namely, global hardness (η), global softness (S), electronegativity (χ), electrophilicity index (ω) are important to describe the reactivity and site selectivity of various bio-molecules. The electrophilicity is used to predict the biological activities, toxicity and various properties of the molecule. In particular, the energy of the LUMO+1 orbital, electrophilicity and van der Waals surface area are related to biological activities of the molecule.
Table 7.
Homo, Lumo, Kubo gap, global electro negativity, global hardness and softness, global electrophilicity index of Chromone-3-Carboxylic acid.
| Parameters | Gas |
|---|---|
| EHOMO (ev) | 5.6844 |
| ELUMO (ev) | 2.7350 |
| ΔEHOMO-LUMO gap (ev) | 2.9494 |
| Elecronegativity (÷) (ev) | -4.2090 |
| Global hardness (ç) (ev) | -1.4747 |
| Global softness (S) (ev) | 5.8988 |
| Electrophilicity index (ω) (ev) | 6.3200 |
| Dipole Moment (μ) (debye) | 7.7040 |
Electrophilicity index (ω) is considered as a measure of electrophilic power of a molecular system towards a nucleophile. If higher the electrophilicity index, higher will be the reactivity as an electrophile while if it is lower, more reactivity as nucleophile. In the present study, we have calculated the electrophilicity index as 6.32 ev which is greater demonstrates that the molecule has higher reactivity as an electrophile.
According to MHP, at constant external potential the stability of a molecule increases with hardness and with the increase in stability the reactivity decreases. The value of chemical hardness calculated in this study is -1.4747eV (gas phase). Here we have calculated the chemical hardness as -1.4747eV (gas phase) while the global softness is 5.8988 ev shows that the molecule has soft in nature and the small HOMO- LUMO energy gap (2.9494 ev) implies that the molecule is more reactive.
4.9. Docking study
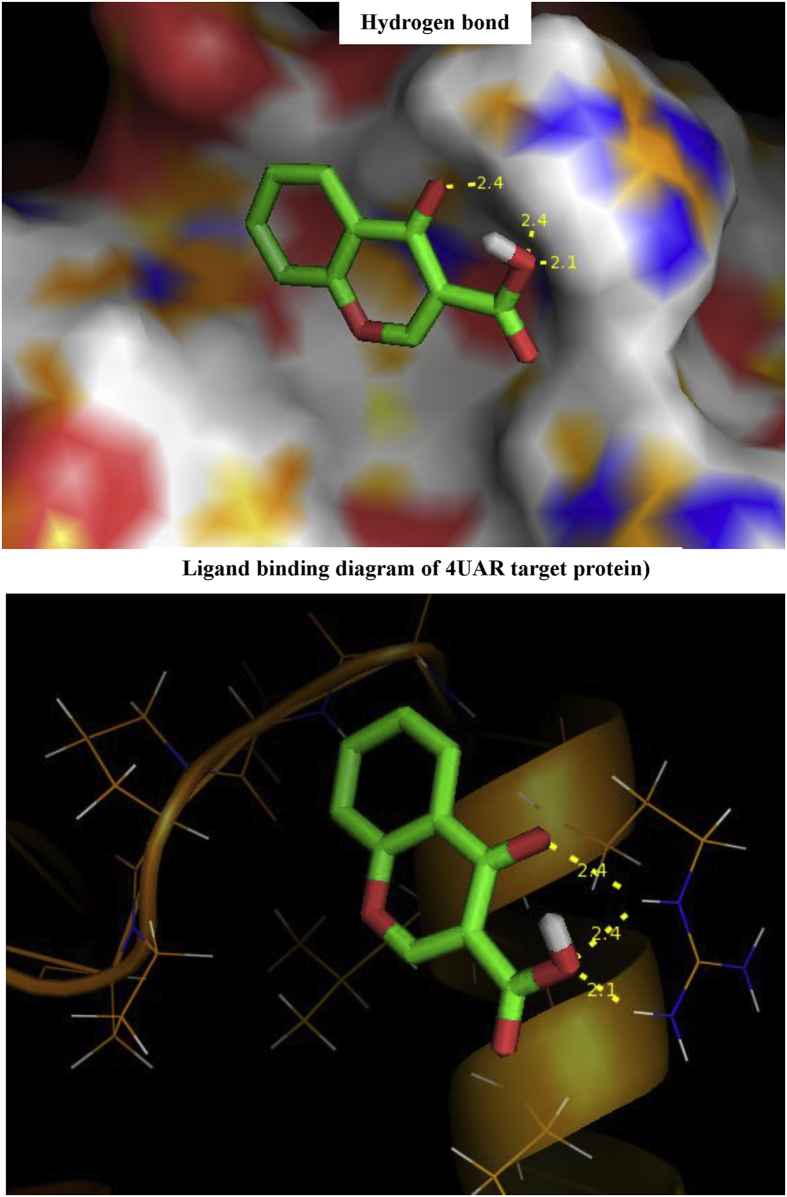
In docking, the host is the receptor (protein) and the binder is the title compound (ligand). Docking calculations were performed on Auto Dock software [30] and visualized through Discovery Studio Visualizer software 4.0 [30]. The optimized structure of C3CA was docked with the protein 4UAR of Bacillus subtilis. The 3D crystal structure of protein -ligand interaction was obtained from PDB (PDB ID: 4UAR). The active site of the protein ligand interaction is shown Figure 12 with grid size of 90 Å x 90 Å x 90 Å.
Figure 12.
Hydrogen bond, ligand binding diagram of 4UAR target protein with ligand.
The docking positions with lowest docking energy (binding affinity) is studied and the bond lengths at these positions were calculated by RMSD method and the values are presented in Table 8. RMSD values above to 2 Å are considered reliable for hydrogen bond formation in docking protocol. Different sets of hydrogen bonding interactions with polar side chain residues of AAR 162, and non-polar residue of LEU159 are observed at distances around 2Å ie., 2.4 Å and 2.1 Å respectively. The ligand binding sites for various modes of interactions with the protein is shown in Figure 12.
Table 8.
Interaction between Anti-bacterial Sugar-phosphatase inhibitor with target protein 4UAR.
| Protein (PDB ID) | No.of hydrogen bond | Bonded Residues | Bond Distance Å |
|---|---|---|---|
| 4UAR | 3 | AAR 162 | 2.4 |
| AAR 162 | 2.4 | ||
| LEU159 | 2.1 |
5. Conclusion
The presence of carboxylic group decreases the electron density in the chromone ring system which consists of benzene and pyrone rings. The conformational analysis was performed by Potential Energy Surface Scan (PES) to predict the stable conformer. The minimum energy of the compound was found to be -0.190 hartree at 360o respectively. From the MPA it is confirmed that the charge of carbon atoms in the benzene ring were redistributed due to the substitutional groups. The charges predicted by MPA were found supported by the chemical shift analysis from the NMR study. From NBO analysis, the most probable transitions in the molecule are identified. The vibrational wavenumbers computed theoretically were found agreeing well with the experimental values. UV-Visbile analysis of the compound indicates that among the ten probable electronic transitions, only the second and third transitions in the list will have the appreciable intensity of absorption. From HOMO & LUMO analysis, global reactivity descriptors, it is confirmed that the molecule is considered as soft (as the energy gap is less), has higher reactivity as an electrophilic (higher electrophilicity index), more reactive (hardness is less). The MEP analysis implied that the skeleton of the benzene ring and edges of the molecule shows more zero potential regions. The molecule is also analysed, and the molecular docking was made with the protein of 4UAR of Bacillus subtilis. The antioxidant and bioactive nature of this molecule which neutralize the active nature of oxygen and reducing free radical process makes this molecule as a active candidate for anti-inflammatory, antifungal, antimicrobial, antiviral, antitumour and anticancer drugs.
Declarations
Author contribution statement
K. Jayasheela: Performed the experiments.
S. Periandy: Contributed reagents, materials, analysis tools or data.
P.B. Nagabalasubramanian: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We remain grateful to Kanchi Mamunivar Govt. Institute for Post Graduate Studies and Research, Lawspet, Puducherry and the Department of Physics, St. Joseph's College of Arts and Science (Autonomous), Cuddalore for providing the Quantum Computational Research Lab for this study.
References
- 1.Phosrithong N., Samee W., Nunthanavanit P., Ungwitayatorn J. In vitro antioxidant activity study of novel chromone derivatives. Chem. Biol. Drug Des. 2012;79:981–989. doi: 10.1111/j.1747-0285.2012.01368.x. [DOI] [PubMed] [Google Scholar]
- 2.Mikhail yu, Kornev Vyacheslav. Synthesis and chemical properties of chromone-3-carboxylic acid. Chem. Heterocycl. Compd. 2016;52:71–83. [Google Scholar]
- 3.Teixeira J.G., Dias C.B., Teixeira D.M. Electrochemical characterization and quantification of the strong antioxidant and antitumor agent pomiferin. Electroanalysis. 2009;21:2345. [Google Scholar]
- 4.Sabel I., Fernandes P.G. Electrochemical oxidation mechanisms of the antioxidants daidzein and 7-hydroxy-4-chromone. Electroanalysis. 2012;24(3):618–626. [Google Scholar]
- 5.Liang J., Tian Y.X., Fu L.M., Wang T.H. Daidzein as an antioxidant of lipid: Effects of the microenvironment in relation to chemical structure. Agr. Food Chem. 2008;56:10376. doi: 10.1021/jf801907m. [DOI] [PubMed] [Google Scholar]
- 6.Aliabadi A., Shamsa F., Ostad S. Synthesis and biological evaluation of 2-phenylthiazole-4-carboxamide derivatives as anticancer agents. Eur. J. Med. Chem. 2010;45:5384. doi: 10.1016/j.ejmech.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 7.Sebastian S. Molecular structure, Normal Coordinate Analysis, harmonic vibrational frequencies, Natural Bond Orbital, TD-DFT calculations and biological activity analysis of antioxidant drug 7-hydroxycoumarin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;101:370–381. doi: 10.1016/j.saa.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Dias M.M. Dietary chromones as antioxidant agents—the structural variable. Food Func. 2011;2:595. doi: 10.1039/c1fo10098j. [DOI] [PubMed] [Google Scholar]
- 9.Magdy Ibrahim A. Ring transformation of chromone-3-carboxylic acid under nucleophilic conditions. J. Arkivoc. 2008;17:192–204. [Google Scholar]
- 10.Agostinha M., Matos R., Sousa S., Morais F. Thermochemistry of chromone- and coumarin-3-carboxylic acid. J. Therm. Anal. Calorim. 2010;100:519–526. [Google Scholar]
- 11.Sahan R. Modulating the reactivity of chromone and its derivatives through encapsulation in a self-assembled phenylethynylene bis-urea host. Salp. J. Photochem. Photobiol. A: Chem. 2016;315:14–24. [Google Scholar]
- 12.Shahriarkhadem Chromone and flavonoid alkaloids: Occurrence and bioactivity. J. Molec. 2012;17:191–206. doi: 10.3390/molecules17010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magdy A. Synthesis and chemical reactivity of 2-methylchromones. Ibrahim J. Arkivoc. 2010:98–135. [Google Scholar]
- 14.Jayasheela K., Periandy S., Xavier S., Niveditha K. Docking and spectral investigations (FT-IR, FT-Raman, NMR, UV-Vis) on 7-hydroxyl-4-chromone using quantum computational (DFT) analysis. Int. J. Renew. Energy Technol. 2017 [Google Scholar]
- 15.Frisch M.J. Gaussian, Inc.; Wallingford, CT: 2009. GAUSSIAN 09, Revision A.1. [Google Scholar]
- 16.Jamroz M.H. 2004– 2010. Vibrational Energy Distribution Analysis VEDA 4, Warsaw. [DOI] [PubMed] [Google Scholar]
- 17.Frisch A., Nielson A.B., Holder A.J. Gaussian Inc.; Pittsburgh, PA: 2000. GAUSSVIEW User Manual. [Google Scholar]
- 18.Ishikawa Y. Topic crystal structure of 3-(hydroxymethyl)chromone. J. Acta Cryst. E. 2015;496:580–591. [Google Scholar]
- 19.Nagabalasubramanian P.B., Karbacak M., Periandy S. Molecular structure, polarizability, characterization hyperpolarizability analysis and spectroscopic of 1-(chloromethyl)-2-methylnaphthalene (FT-IR with experimental and FT-Raman) techniques and quantum chemical calculations. Spectrochim. Acta Part A. 2012;85:43–52. doi: 10.1016/j.saa.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Mariappan G., Sundaraganesan N. FT-IR, FT-Raman, NMR spectra, density functional computations of the vibrational assignments (for monomer and dimer) and molecular geometry of anticancer drug 7-amino-2-methylchromone. J. Mol. Struct. 2014;1063:192–202. [Google Scholar]
- 21.Nagabalasubramanian P.B., Periandy S., Karabacak Mehmet, Govindarajan M. Molecular structure, vibrational, electronic and thermal properties of 4-vinylcyclohexene by quantum chemical calculations. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;145:340–352. doi: 10.1016/j.saa.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Ditchfield R. Molecular orbital theory of magnetic shielding and magnetic susceptibility. J. Chem. Phys. 1972;56:5688–5691. [Google Scholar]
- 23.Wolinski K., Hinton J.F., Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990;112:8251–8260. [Google Scholar]
- 24.Kalinowski H.O., Berger S., Braun S. John Wiley &Sons; Chicheser: 1988. Carbon-13 NMR Spectroscopy. [Google Scholar]
- 25.Wade L.G., editor. Journal of Advanced Organic Chemistry. fourth ed. Wiley; New York: 1992. p. 723. [Google Scholar]
- 26.Roeges N.P.G. Wiley; New York: 1994. A Gide to the Completer Interpretation of Infrared Specra of Organic Structures. [Google Scholar]
- 27.Garrett M.M., David S.G., Rober s.H., Ruth H., William E.H., Richard K.B., Arthur J.O. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. [Google Scholar]
- 28.Gorelsky S.I. University of Ottawa; Ottawa, Canada: 2010. SWizard Program Revision 4.5.http://www.sg.chem.net [Google Scholar]
- 29.O’Boyle N.M., Tenderholt A.L., Langner K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008;29:839–845. doi: 10.1002/jcc.20823. [DOI] [PubMed] [Google Scholar]
- 30.Jayasheela K., Periandy S. Probing vibrational activities, electronic properties, molecular docking and Hirshfeld surfaces analysis of 4-chlorophenyl ({[(1E)-3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)methanone: A promising anti-Candida agent. J. Mol. Struct. 2018;1159:83–95. [Google Scholar]