Abstract
The therapeutic management of patients with severe steroid-refractory ulcerative colitis still represents a critical clinical challenge. In this setting, cyclosporin is an effective and rapidly acting induction treatment that is applied in combination with maintenance therapeutic agents like thiopurines or vedolizumab. Here, we present the case of a 33-year-old ulcerative colitis patient with severe steroid-refractory ulcerative colitis who refused surgical intervention and previously demonstrated no long-term benefit to anti-TNF antibody, vedolizumab, cyclosporin, thiopurines or tofacitinib treatment. Intravenous cyclosporin therapy was re-initiated in the patient and, after signs of clinical response, therapy with ustekinumab was additionally applied. After 11 weeks of well tolerated cyclosporin and ustekinumab combination therapy, cyclosporin was discontinued upon clinical and endoscopic remission. Subsequently, ustekinumab treatment has been effective in maintaining remission during the follow-up period of 195 days.
Keywords: cyclosporin, ulcerative colitis, ustekinumab, trough levels
Introduction
Medical management of severe steroid-refractory ulcerative colitis represents a formidable clinical challenge as possible therapeutic options are rather limited. They consist mainly of the anti-TNF antibody infliximab, as well as the substance class of calcineurin inhibitors (cyclosporin, tacrolimus), which have both been tested in randomised clinical trials in acute severe ulcerative colitis (ASUC) patients.1,2 Recent small case series have also indicated possible therapeutic effectiveness of standard or high-intensity tofacitinib induction therapy3,4; however, these observations must be verified in corresponding trials. Although cyclosporin and infliximab have proven to be similarly efficacious in randomised trials,5,6 non-randomized studies suggest that infliximab is associated with better treatment response and lower risk of colectomy at 12 months.7 However, ASUC patients with previous failure to infliximab are becoming more prevalent, necessitating the need for effective second-line rescue therapy with cyclosporin.8 Treatment with cyclosporin has proven to be highly efficacious at inducing remission, but its effectiveness in maintenance treatment of ulcerative colitis is limited and its use can be associated with occurrence of severe adverse events, like opportunistic infections, nephrotoxicity and hypertension.9 Consequently, there is the need for sequential combination with a maintenance therapeutic agent, such as thiopurines or vedolizumab.10–13 However, patients who have previously failed thiopurines or vedolizumab treatment are currently not deemed to be appropriate for calcineurin therapy due to a lack of effective maintenance therapy.
Here, we report the first combination therapy approach of cyclosporin and ustekinumab in a patient with severe steroid-refractory ulcerative colitis, who previously failed anti-TNF, thiopurine and vedolizumab treatment.
Case report
A 33-year-old Caucasian male with ASUC presented in our department in October 2019. The patient reported abdominal pain, recurrent fever and a stool-frequency ranging from 14 to 16 per day, with presence of blood in most of the stools. The partial Mayo score at the time of presentation was 8. The CRP-level was elevated with 64 mg/l and faecal calprotectin level was reported at 627 μg/l prior to admission.
The patient was first diagnosed with left-sided ulcerative colitis at the age of 25, which later progressed to the manifestation of pancolitis. Successive therapies with adalimumab, infliximab, vedolizumab and cyclosporin combined with azathioprine and mercaptopurine, as well as tofacitinib did not lead to lasting clinical improvement or had to be discontinued due to side-effects. Prior to admission to our department, the patient had also not responded to high-dose intravenous prednisolone (100 mg/day) treatment. Recommended restorative proctocolectomy was declined by the patient.
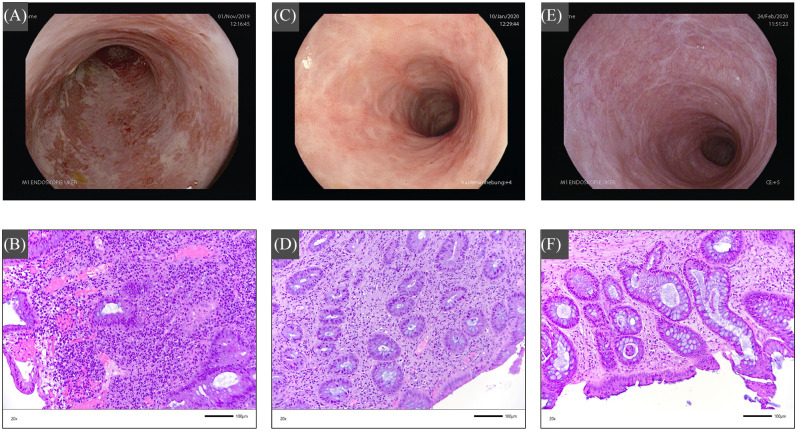
Upon admission, sigmoidoscopy showed signs of severe endoscopic inflammation (endoscopic Mayo score: 3) with spontaneous bleeding and extended superficial mucosal ulcerations (Figure 1A) and histology confirmed severe inflammation (Geboes score: 5) (Figure 1B). Cytomegalovirus (CMV)-colitis was excluded based on negative CMV-DNA in the blood and tissue of the patient, and other infectious causes could also be ruled out.
Figure 1.
Endoscopy (upper images) and corresponding histology findings (lower image) in the distal sigmoid area during the course of the cyclosporin/ustekinumab therapy. (A) Before initiation of cyclosporin therapy, there are multiple superficial mucosal ulcers and spontaneous bleeding as signs of severe endoscopic disease. (B) Histology findings indicate signs of severe acute and chronic inflammation with architectural disorders, surface erosions and ulcerations. (C) At 8 weeks after initiating combination therapy with cyclosporin and ustekinumab, there is mucosal erythema and decreased vascular pattern as signs of mild endoscopic inflammation. (D) Histology demonstrates changes compatible with moderate inflammation. The surface appears intact, and there is moderate chronic and less acute cell infiltration with neutrophils and some eosinophils, granulocytic cells as well as plasmocytic infiltration and less mucin depletion. Furthermore, mild architectural disorder with interstitial, sometimes scar-like, fibrosis. (E) Under ongoing ustekinumab maintenance monotherapy, there is endoscopic remission with normal mucosal appearance. (F) Histology indicates mild mucosal inflammation with an intact surface, moderate chronic and just few signs of acute inflammation with regressive infiltration of neutrophils and just few eosinophilic granulocytes, as well as few plasmocytic cells and no mucin depletion. Mild architectural disorder with interstitial, sometimes scar-like, fibrosis. Histological images (20×) were taken using a Leica DM 4000 B microscope (Leica Microsystems, Wetzlar, Germany) with Jenoptik Progres Gryphax SUBRA camera (Jenoptik, Jena, Germany).
Due to the steroid-refractory course of the disease, intravenous cyclosporin therapy was started in the patient with 2 mg/kg per day (day 0). The patient had initially responded well to previous cyclosporin therapy 2 years ago; however, this had to be stopped after 3 months due to cephalgia and vertigo. The additionally initiated maintenance therapy with azathioprine had to be stopped 1 month later, as the patient developed severe nausea. Occurrence of nausea also led to the discontinuation of subsequently initiated mercaptopurine treatment.
During the current presentation, the patient demonstrated clinical response to intravenous cyclosporin treatment. As the patient was intolerant to thiopurines and previously failed vedolizumab treatment, 390 mg intravenous ustekinumab were additionally applied on day 6 of cyclosporin treatment. At the time of discharge, the CRP-level was 26 mg/l and the cyclosporin trough level was 251 μg/l. We aimed for serum trough levels of 250–300 ng/ml during ongoing oral cyclosporin therapy.
At day 62, the patient presented himself at our outpatient clinic with clinical remission (partial Mayo score: 0) under ongoing oral cyclosporin and ustekinumab therapy. Endoscopy demonstrated signs of only mild mucosal disease (endoscopic Mayo score: 1) with mucosal erythema and decreased vascular pattern (Figure 1C), while histology also confirmed mild inflammation (Geboes score: 3) (Figure 1D). Faecal calprotectin and serum CRP-levels were within normal range. The ustekinumab drug level was 6.47 μg/ml (measured with Promonitor-UTK Kit, Progenika, Derio, Spain). Maintenance therapy with subcutaneous ustekinumab 90 mg was administered. The oral cyclosporin component of the combination therapy was discontinued at day 83.
After day 139, the patient presented himself in clinical remission (partial Mayo score: 0). Sigmoidoscopy demonstrated endoscopic remission (endoscopic Mayo score: 0) without signs of active inflammation (Figure 1E) and histology just showed signs of mild inflammatory activity (Geboes score: 2) (Figure 1F). The faecal calprotectin level was 58 μg/ml and the CRP-level was within normal range. The ustekinumab drug level at this time point was 3.52 μg/ml. Maintenance therapy with subcutaneous ustekinumab 90 mg was continued. The patient presented himself in sustained clinical remission (partial Mayo score: 0) at day 195 for continuation of ustekinumab therapy. Faecal calprotectin and CRP-levels remained within normal range. During the whole treatment period, no therapy-associated side effects occurred, and cyclosporin as well as ustekinumab were well tolerated.
Discussion
The role of cyclosporin in remission induction therapy in patients with steroid-refractory ulcerative colitis is well established.14 Since its initial use for this indication in the early 1990s,1 its potency to induce remission has been proven in several studies.15,16 Furthermore, it has been shown to be non-inferior to infliximab rescue therapy under those circumstances.5,17 While thiopurines have long been used as combination partners for maintenance therapy,9,10,18 vedolizumab has recently been combined with cyclosporin with encouraging results.11,19
As our patient opposed surgical intervention, was resistant to previous infliximab therapy, and had formerly responded well to cyclosporin induction therapy, we decided to proceed with cyclosporin re-induction, which was again successful. The choice of a necessary agent for remission maintenance therapy was difficult as established combination therapies (azathioprine, mercaptopurine) had previously already failed to control the patient’s disease. Furthermore, the other potential combination partner vedolizumab had already proven ineffective as monotherapy.
Ustekinumab, an IL-12/IL-23 antagonist, has recently been approved as an effective treatment option for both induction and maintenance therapy in patients with ulcerative colitis.20 While there is even more long-term experience regarding efficacy and safety in the treatment of Crohn’s disease,21 an expanding amount of data suggests that ustekinumab is not only an effective but also very safe treatment for patients with ulcerative colitis.22,23 For this reason, we decided to use a combination treatment of cyclosporin and ustekinumab, which proved to be both an effective and well-tolerated therapy. As safety is the most important concern in combination therapy approaches, the patient was closely monitored for adverse events, in particular infectious complications. Overall, no adverse events were observed during the time of combination treatment. While there are isolated descriptions of the combined use of cyclosporin and ustekinumab in psoriasis arthritis,24 this is, to the best of our knowledge, the first reported case of this combination therapy being employed in the treatment of ulcerative colitis. Our experience over more than 27 weeks after initiation of this therapy are encouraging regarding the efficacy and safety of this combination therapy and warrant further studies.
Footnotes
Author contributions: I.G. and R.A. designed the study, analysed the data, and drafted the manuscript. C.G. provided pathological expertise and analysed the histology samples. S.H., A. N., C.G., A.H., T.R., and M.F.N. provided important intellectual content and critically revised the manuscript. L.O. and D.N. performed the ustekinumab concentration measurements, provided important intellectual content, and critically revised the manuscript.
Conflict of interest statement: R.A. and M.F.N. received honoraria for lectures and consulting fees from Janssen-Cilag
Ethics approval: An ethics approval was not required for this case report at our institution.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from DFG project BE3686/2, research unit FOR2438, SFB1181 Project C02 (RA), SFB796, SPP1656, and DFG-SFB/TRR241 Project No. C02 (RA). The German Research Council DFG funds the Heisenberg Professorship of R.A. The Interdisciplinary Centre for Clinical Research (IZKF) Erlangen supported I.G. by participation in the Clinician-Scientist Program.
Guarantor: Raja Atreya
Informed consent: Informed patient consent for publication of this case report has been obtained in written form.
ORCID iD: Ingo Ganzleben  https://orcid.org/0000-0003-0571-6641
https://orcid.org/0000-0003-0571-6641
Contributor Information
Ingo Ganzleben, Department of Medicine 1, University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Carol Geppert, Department of Pathology, University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany.
Lourdes Osaba, Progenika Biopharma, A Grifols Company, Derio, Spain.
Simon Hirschmann, Department of Medicine 1, University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Andreas Nägel, Department of Medicine 1, University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Christian Glück, Department of Medicine 1, University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Arthur Hoffman, Department of Internal Medicine III, Clinic Aschaffenburg-Alzenau, Aschaffenburg, Germany.
Timo Rath, Department of Medicine 1, University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Daniel Nagore, Progenika Biopharma, A Grifols Company, Derio, Spain.
Markus F. Neurath, Department of Medicine 1, University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Raja Atreya, Department of Medicine 1, University Hospital, Friedrich-Alexander-University Erlangen-Nürnberg, Ulmenweg 18, Erlangen, 91054, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
References
- 1. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994; 330: 1841–1845. [DOI] [PubMed] [Google Scholar]
- 2. Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005; 128: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 3. Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis 2020; 14: 1026–1028. [DOI] [PubMed] [Google Scholar]
- 4. Berinstein JA, Steiner CA, Regal RE, et al. Efficacy of induction therapy with high-intensity tofacitinib in 4 patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 988-90 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012; 380: 1909–1915. [DOI] [PubMed] [Google Scholar]
- 6. Laharie D, Bourreille A, Branche J, et al. Long-term outcome of patients with steroid-refractory acute severe uc treated with ciclosporin or infliximab. Gut 2018; 67: 237–243. [DOI] [PubMed] [Google Scholar]
- 7. Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: Infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol 2016; 111: 477–491. [DOI] [PubMed] [Google Scholar]
- 8. Weisshof R, Ollech JE, El Jurdi K, et al. Ciclosporin therapy after infliximab failure in hospitalized patients with acute severe colitis is effective and safe. J Crohns Colitis 2019; 13: 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999; 94: 1587–1592. [DOI] [PubMed] [Google Scholar]
- 10. Actis GC, Fadda M, David E, et al. Colectomy rate in steroid-refractory colitis initially responsive to cyclosporin: a long-term retrospective cohort study. BMC Gastroenterol 2007; 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensen B, Gibson PR, Micic D, et al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol 2019;17:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pellet G, Stefanescu C, Carbonnel F, et al. Efficacy and safety of induction therapy with calcineurin inhibitors in combination with vedolizumab in patients with refractory ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 494–501. [DOI] [PubMed] [Google Scholar]
- 13. Ollech JE, Dwadasi S, Rai V, et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by vedolizumab maintenance in 71 patients with severe steroid-refractory ulcerative colitis. Aliment Pharmacol Ther 2020; 51: 637–643. [DOI] [PubMed] [Google Scholar]
- 14. Pham CQ, Efros CB, Berardi RR. Cyclosporine for severe ulcerative colitis. Ann Pharmacother 2006; 40: 96–101. [DOI] [PubMed] [Google Scholar]
- 15. D’Haens G, Lemmens L, Geboes K, et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 2001; 120: 1323–1329. [DOI] [PubMed] [Google Scholar]
- 16. Van Assche G, D’Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003; 125: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 17. Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (construct): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol 2016; 1: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyake N, Ando T, Ishiguro K, et al. Azathioprine is essential following cyclosporine for patients with steroid-refractory ulcerative colitis. World J Gastroenterol 2015; 21: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szanto K, Molnar T, Farkas K. New promising combo therapy in inflammatory bowel diseases refractory to anti-tnf agents: cyclosporine plus vedolizumab. J Crohns Colitis 2018; 12: 629. [DOI] [PubMed] [Google Scholar]
- 20. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019; 381: 1201–1214. [DOI] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for crohn’s disease through the second year of therapy. Aliment Pharmacol Ther 2018; 48: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adedokun OJ, Xu Z, Marano C, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis: ustekinumab PK and exposure-response in UC. Clin Gastroenterol Hepatol. Epub ahead of print 6 December 2019. DOI: /10.1016/j.cgh.2019.11.059. [DOI] [PubMed] [Google Scholar]
- 23. Amiot A, Filippi J, Abitbol V, et al. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a getaid multicentre real-world cohort study. Aliment Pharmacol Ther 2020; 51: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 24. Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol 2013; 12: 1098–1102. [PubMed] [Google Scholar]