Abstract
Background
Clinical observations support the hypothesis that stressful events increase relapse occurrence in multiple sclerosis patients, while stress-reduction strategies can modulate this effect. However, a direct cause-effect relationship between stress level and relapse cannot be firmly established from these data.
Objectives
The purpose of this work was to address whether modulation of stress could interfere with symptom relapse in an animal model of multiple sclerosis with relapsing-remitting course.
Methods
Mice bred in standard or enriched environment were subjected to repeated acute stress during the remission phase of relapsing-remitting PLP-induced experimental autoimmune encephalomyelitis.
Results
We report that repeated acute stress induced a twofold increase in relapse incidence in experimental autoimmune encephalomyelitis. On the other hand, environmental enrichment reduced relapse incidence and severity, and reversed the effects of repeated acute stress.
Conclusion
These data provide the platform for further studies on the biological processes that link stress and multiple sclerosis relapses in a suitable animal model.
Keywords: Psychological stress, multiple sclerosis, relapse, water avoidance stress, environmental enrichment
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that leads to various neurological manifestations, including sensitive and motor symptoms. In the most frequent form, the relapsing-remitting subtype, these symptoms occur in an unpredictable manner during periods of relapses, followed by periods of remissions when signs of disease activity are reduced or absent. There is considerable interest in identifying the factors that lead to the occurrence of relapse.
Clinical observations have suggested a link between acute stress and occurrence of relapses in MS patients. Considering the lack of prospective studies using objective measures of stress, there is still no consensus to conclude on the effect of stress on MS onset and relapses.1 However, several studies report that acute stressful life events (marital separation, difficulties at work, changes in place of residence, death of a relative, etc.)2 increase the probability for MS patients to experience a relapse.3 A prospective study showed that three or more stressful life events in a four week period led to a fivefold increase of MS relapse rate, and that the presence of at least one stressful life events is sufficient to increase by threefold the rate of relapse during the following four weeks.4 Conversely, stress reduction strategies have shown positive effects in MS patients.5
Considering these clinical observations, several studies have attempted to reproduce the effects of stress in animal models of MS. In most cases, stress was shown to reduce, rather than increase, the severity of experimental autoimmune encephalomyelitis (EAE).6 However, most of the studies have used chronic stress (more than one hour per day for more than five days),6 which has long been known to inhibit immune response, rather than acute stress events. One study reported an advance in the onset of EAE after repeated acute stress.7 More recently, mild chronic stress was shown to exacerbate and accelerate the clinical symptoms of rat EAE.8 However, in all of these earlier studies, the stress paradigm was applied in the early phase, prior to symptom onset, and not during the remission phase. Thus, these studies did not allow addressing the effect of stress on relapses in relapsing-remitting context.
Overall, the question remains of whether acute stress, when occurring during remission periods, can influence the incidence and severity of relapses. In addition, the effect of stress reduction on relapses was never tested in relapsing-remitting animal models of MS. To answer these questions, we set up a paradigm of repeated acute stress applied during the remission phase of relapsing-remitting EAE. We observed that stress exposure precipitated relapse, so that in stressed animals, the incidence of relapse was doubled, and the delay before the occurrence of relapse was significantly reduced. Conversely, environmental enrichment, previously shown to reduce stress in rodents, reduced relapse incidence and severity, and reversed the effects of stress.
Methods
Animals
Experiments were performed on female SJL/J mice (Janvier, Le Genest-Saint-Isle, France) maintained under specific pathogen-free conditions at the Centre Universitaire de Ressources Biologiques (Basse-Normandie, France). This study and the procedures thereof were approved by the French ministry of education and research (Project licence APAFIS#2887-2015112017418114v2; Center agreement #D14118001) in accordance with the French (Decree 87/848) and European (Directive 86/609) guidelines. Animals were monitored once a day for signs of pain, posture, reactivity, activity, signs of distress, food/drink consumption and follow-up of body weight. Humane euthanasia was planned to be applied to any animal showing at least one of the following signs: prostration, body weight loss > 20%, lack of reaction, prolonged inactivity or other signs of distress, stop of food and/or drink consumption.
Experimental autoimmune encephalomyelitis (EAE)
Relapsing remitting EAE (PLP-induced EAE) was induced in 8-week-old female SJL/J mice via subcutaneous immunization with 200 µg recombinant myelin proteolipid protein (PLP139–151, Eurogentec) in an emulsion mix (volume ratio 1:1) with Complete Freund's Adjuvant (CFA; Difco Laboratories) containing 800 µg of heat-killed Mycobacterium tuberculosis H37Ra (MBT; Difco). The emulsion was administered to regions above the shoulders and the flanks (total of 4 sites; 50 μl at each injection site). All animals were injected intraperitoneally with 200 ng pertussis toxin derived from Bordetella pertussis (Sigma-Aldrich) in 200 µL saline at the time of, and after 48 hours following immunization. EAE induction was performed with the application of local analgesia (lidocaine patch) to prevent discomfort and pain. The animals were euthanized 80 days after EAE induction under deep anesthesia (5% isoflurane, O2/N2O 1/1) by exsanguination via intracardiac infusion of saline.
Clinical score
Mice were examined daily for clinical signs of EAE and were scored as followed: 0, no disease; 1, limp tail; 2, hindlimb weakness; 3, complete hindlimb paralysis; 4, hindlimb paralysis plus forelimb paralysis; and 5, moribund or dead. All clinical score were assessed daily by an examiner blinded to the EAE group. A relapse was defined as a sustained increase (minimum duration of 2 days) of at least 0.5 in clinical score.
Water avoidance stress (WAS)
WAS (one hour per day during four days) was performed by placing asymptomatic EAE mice on a platform (4cm diameter) positioned at the center of a plastic container filled with water at room temperature up to 1 cm below the top of the platform. EAE mice were randomly assigned to the two experimental groups. Non-WAS mice were handled identically, but placed in an empty and clean cage.
Standard breeding conditions
Mice in standard (STD) conditions were placed in standard cages [32 × 16 × 14 cm (L × W × H)], which allowed social interactions (5 mice per cage). Nesting material was placed in the cage and renewed if needed. The animals had ad libitum access to food and water.
Environmental enrichment (EE)
Enriched mice were housed in Marlau™ cages [Viewpoint; 58 × 40 × 32 cm (L × W × H)], which allowed social interactions (15 mice per cage). These cages are composed of two floors. The ground floor has two compartments: one containing food, the other one containing water bottles, three running wheels and an elevated nest. The upper floor contains a maze changed three times a week. Because of one-way doors between the two ground compartments, mice had to climb to the upper floor using a ladder, pass through the maze and go down to the food compartment using a slide tunnel to reach food. Enriched mice were housed in these Marlau™ cages from the age of six weeks to the end of the experiment. Enrichment was thus applied before EAE induction and during all the phases of EAE, including presymptomatic phase, first peak, remission and relapses. There are no documented differences on stress scores, stress measurements or stress biomarkers across the two particular breeding conditions used in the present work. Nevertheless, environmental enrichment has been described before to reduce stress in laboratory rodents.9
Statistical analysis
Results are presented as the mean ± SEM. Normality tests were performed on all samples (D'Agostino-Pearson omnibus test and Shapiro-Wilk test). When normality could not be assumed, we used non-parametric tests (Mann-Whitney's U-test), which are the most stringent in these conditions. For the comparison of the mean clinical scores, we used a two-way repeated measures ANOVA followed by a Bonferroni’s multiple comparison post hoc test. For the comparison of the number of feces in STD, STD+WAS, EE and EE+WAS groups, we used a Kruskall-Wallis test followed by a Dunn’s multiple comparison as a post hoc test. Onset and relapse incidence curves were analysed with Gehan-Breslow-Wilcoxon test. Data were analysed using GraphPad Prism 7.0. Two groups were considered to be significantly different when P< 0.05.
Results
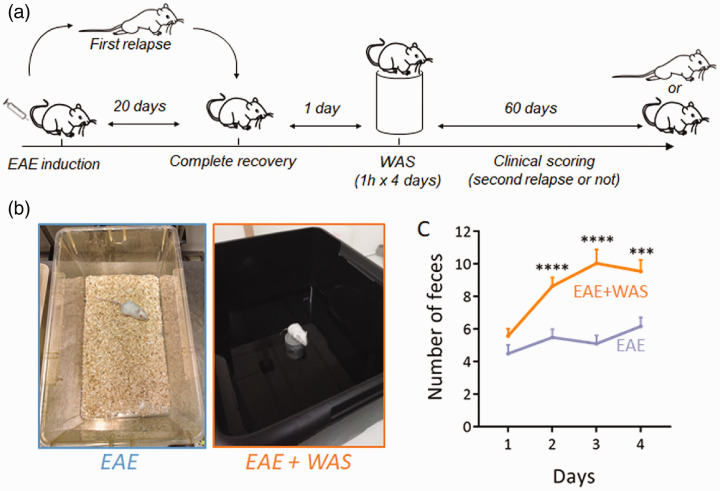
Repeated acute stress has been defined as a short exposure to stressing stimulus (<1h) repeated once daily during less than 5 days (as opposed to repeated chronic stress: >1h during more than 5 days).6 Here, to induce repeated acute stress, we applied a paradigm in which mice were exposed to water avoidance stress (WAS) once a day during 4 days during the remission period of EAE (Figure 1(a) and (b)). The stressful nature of this paradigm was assessed by measuring fecal pellet output during the exposure to stress (Figure 1(c)), as a measure of stress-induced colonic motility.10 This procedure is fully non-invasive and was preferred to other methods such as measurement of stress hormones in the blood-stream that require invasive procedures and thus constitute a source of stress for the animals.
Figure 1.
Experimental design for water avoidance stress. (a) Water avoidance stress (WAS, one hour per day for four days) was applied during the remission phase of PLP-induced relapsing-remitting EAE mice and the clinical scoring was assessed during 60 days. (b) Photography of the experimental system. Mice were placed for 1 hour on a platform positioned at the center of a plastic container filled with water at room temperature up to 1 cm below the platform level. Mice were randomly assigned to the two experimental groups. Non-WAS mice were handled identically, but placed in a standard cage. (c) Measure of fecal pellet outputs in the two experimental groups as an index of stress-induced colonic motility (n = 26 and n = 28 for EAE and EAE+WAS groups respectively). ***P<0.001; ****P<0.0001, Mann Whitney’s U-test.
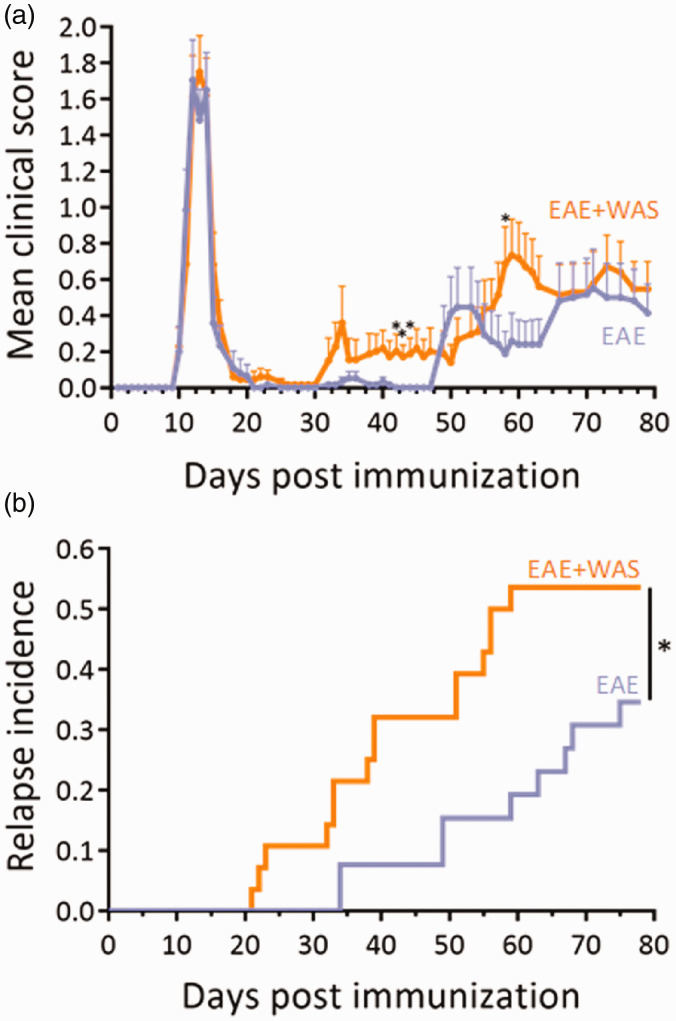
The exposure to stress induced an increase in clinical score which reached significance at days 42, 43, 44, and 58 (Figure 2(a)). These differences in mean clinical score were explained by the fact that WAS advanced the appearance of the relapse (mean day of relapse onset: 40.53 ± 3.43 vs. 55.33 ± 4.92; P = 0.0189; mean duration of remission: 23.67 ± 3.66 vs. 38.0 ± 4.91; P = 0.0273; Table 1), so that the incidence of relapse was increased by 1.58-fold at day 75 (Figure 2(b); Supplementary Figure 1A). Noteworthy, the severity of EAE was not affected by stress (mean peak score: 2.77 ± 0.29 vs. 2.50 ± 0.47. Table 1).
Figure 2.
Acute stress precipitates relapse in EAE animals. (a) Clinical score was assessed daily by an examiner blinded to the treatment (n = 26 and n = 28 for EAE and EAE+WAS groups respectively; *P<0.05, two-way repeated measures ANOVA + Bonferroni’s multiple comparison). (b) Clinical evaluation expressed as the long-term relapse incidence in EAE and EAE+WAS groups (*P<0.05, Gehan-Breslow-Wilcoxon test).
Table 1.
Values for mean day of onset or relapses and mean peak clinical score in the EAE and EAE+WAS groups.
| EAE | N | EAE + WAS | N | P-value | |
|---|---|---|---|---|---|
| Mean day of onset | 11.50 ± 0.15 | 30/35 | 11.57 ± 0.21 | 30/35 | 0.7510 |
| Peak score | 2.52 ± 0.19 | 2.52 ± 0.19 | 0.7036 | ||
| Mean duration of remission | 38.00 ± 4.91 | 23.67 ± 3.66 | 0.0273* | ||
| Mean day of first relapse onset | 55.33 ± 4.92 | 9/26 | 40.53 ± 3.43 | 15/28 | 0.0189* |
| Peak score | 1.72 ± 0.41 | 2.20 ± 1.40 | 0.4066 | ||
| Mean day of second relapse onset | 62.80 ± 6.00 | 5/26 | 52.40 ± 4.36 | 10/28 | 0.1885 |
| Peak score | 3.00 ± 0.55 | 2.05 ± 0.28 | 0.1092 | ||
| Mean day of third relapse onset | 66.00 ± 0 | 1/26 | 63.50 ± 2.92 | 6/28 | N.D. |
| Peak score | 4 ± 0 | 2.42 ± 0.42 | N.D. | ||
| Peak score (all relapse) | 2.50 ± 0.47 | 9/26 | 2.77 ± 0.29 | 15/28 | 0.6130 |
N corresponds to the number of animals with first surge or relapse divided by the total number of animals within the group (see Supplementary Figure 1A for a detailed description). The data are given for the animals that develop a first peak. P values are given for t-test or, when normality could not be assumed, Mann-Whitney’s U-test. *p < 0.05.
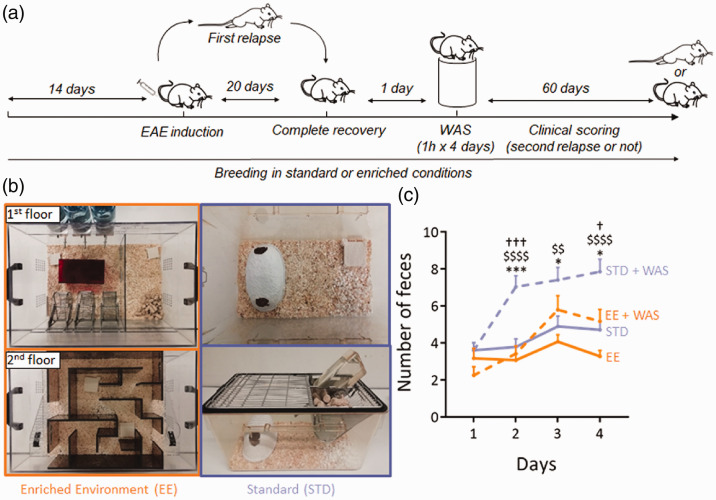
Environmental enrichment has been described as an efficient paradigm to reduce stress in laboratory rodents.9 Here, we bred mice in standard or enriched environment prior to EAE induction and exposure to repeated acute stress (Figure 3(a) and (b)). This paradigm of standard enrichment reversed the increase of fecal pellet output (used as index of stress-induced colonic motility) induced by WAS (Figure 3(c)).
Figure 3.
Experimental design for environment enrichment. (a) Environmental enrichment (EE) was applied from 14 days pre-EAE induction to the end of the protocol. Standard (STD) animals were kept in standard breeding conditions. WAS was applied to half of the animals of each group during the remission phase of EAE. In total, 4 experimental groups were studied: STD, STD+WAS, EE, EE+WAS. (b) Photography of the STD and EE breeding cages (see methods for details). (c) Measure of fecal pellet outputs in the four experimental groups as an index of stress-induced colonic motility (n = 16, n = 21, n = 12 and, n = 12 for STD, STD+WAS, EE, and EE+WAS groups respectively; *P<0.05; ***P<0.001, STD vs. STD+WAS; P<0.0001, EE vs. STD+WAS; †††P<0.001, EE+WAS vs. STD+WAS; Kruskall-Wallis test + Dunn’s multiple comparison).
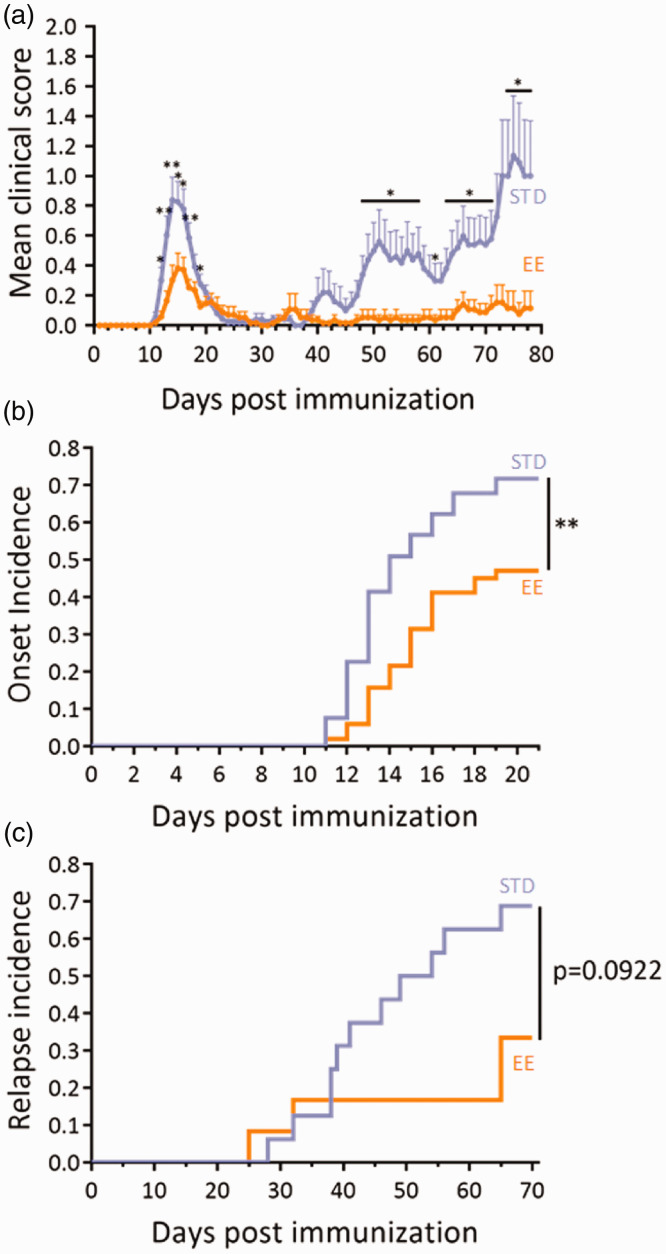
Strikingly, animals bred in enriched environment showed a reduction in the first symptomatic peak of EAE (Days 12-17 post immunization, Figure 4(a)) and a drastic reduction of clinical score during the relapse phase (days 48-71 and 73-78, Figure 4(a)). These differences in mean clinical score along the course of EAE were the result of several parameters: First, the incidence of the disease and the incidence of relapse were reduced in enriched animals (Figure 4(b) and (c)), so that the incidence of disease was decreased by 1.52-fold at day 25 (Figure 4(b), Supplementary Figure 1B) and the incidence of relapse was decreased by 2.06-fold at day 70 (Figure 4(c), Supplementary Figure 1C). Second, the onset of the disease tended to occur later in enriched animals (mean day of relapse onset: 14.67 ± 0.41 vs. 13.74 ± 0.35 vs.; P = 0.0581; Table 2).
Figure 4.
Environmental enrichment reduced EAE severity. (a) Clinical score assessed daily by an examiner blinded to the treatment in the standard (STD) and enriched environment (EE) groups (n = 53 and n = 51 for STD and EE groups respectively; *P<0.05; **P<0.01, two-way repeated measures ANOVA + Bonferroni’s multiple comparison). (b) Clinical evaluation expressed as the EAE onset incidence in EE and STD groups (**P<0.01, Gehan-Breslow-Wilcoxon test). (c) Clinical evaluation expressed as the long-term EAE relapse incidence in EE and STD groups (n = 16 and n = 12 for STD and EE groups respectively; P = 0.0922, Gehan-Breslow-Wilcoxon test).
Table 2.
Values for mean day of onset, mean day of relapse and mean peak clinical score in the STD or EE groups.
| STD EE | N | P-value | N | ||
|---|---|---|---|---|---|
| Mean day of onset | 13.74 ± 0.35 | 38/53 | 14.67 ± 0.41 | 24/51 | 0.0581 |
| Peak score | 1.64 ± 0.17 | 1.27 ± 0.16 | 0.2070 | ||
| Mean day of first relapse onset | 44.18 ± 3.33 | 11/16 | 46.75 ± 10.63 | 4/12 | 0.7597 |
| Peak score | 1.64 ± 0.44 | 1.63 ± 0.47 | 0.5963 | ||
| Mean day of second relapse onset | 55.29 ± 4.11 | 7/16 | 47.00 ± 0 | 1/12 | N.D. |
| Peak score | 2.43 ± 0.46 | 1.50 ± 0 | N.D. | ||
| Mean day of third relapse onset | 65.00 ± 2.42 | 4/16 | 69.00 ± 0 | 1/12 | N.D. |
| Peak score | 2.63 ± 0.55 | 1.50 ± 0 | N.D. | ||
| Peak score (all relapse) | 2.77 ± 0.37 | 11/16 | 1.63 ± 0.47 | 4/12 | 0.1143 |
N corresponds to the number of animals with first surge or relapse divided by the total number of animals within the group (see Supplementary Figure 1B for a detailed description). The data are given for the animals that develop a first peak. P values are given for t-test or, when normality could not be assumed, Mann-Whitney’s U-test.
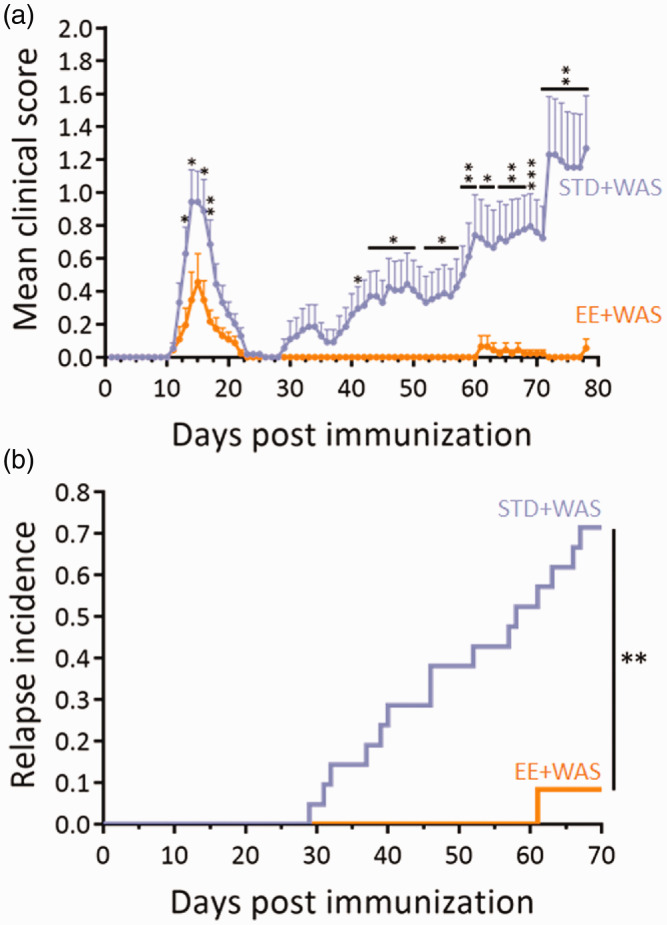
Considering that repeated acute stress increased relapse rate and that environmental enrichment reduced the severity of EAE, our next question was to ask as to whether environmental enrichment may reduce the effects of stress. Mice bred in standard or enriched environment were subjected to WAS during the remission period (Figure 3(a)). In agreement with above results, mice bred in enriched environment showed a reduction in clinical score during the first peak of disease (days 13–14 and 16–17, Figure 5(a)). The mean clinical score gradually increased after the remission period in standard animals, but not in enriched animals (Figure 5(a)), reaching statistical difference at day 41, between days 43 and 50 and from day 52 to the end of the experiment (day 78). This difference was the reflect of a dramatic difference in the incidence of relapse (71.4% vs. 8.3%, P = 0.0011; Figure 5(b), Supplementary Figure 1B). Only one animal (from n = 12) suffered a relapse in the enriched group (Supplementary Figure 1B), and that relapse occurred much later than what observed in the standard group (61 days vs. a mean of 43.73 ± 3.15 in standard animals; Table 3). The peak score of this relapse was also reduced as compared to the mean of the standard group (1.5 ± 0 vs. 2.00 ± 0.32; Table 3). In addition, although 60% of the animals with a first relapse (9 animals out of 15) also suffered a second relapse later in the standard group (58.43 ± 4.28 days post immunization; Table 3), no second relapse was observed in the enriched group.
Figure 5.
Environmental enrichment reversed the effects of stress on EAE severity. (a) Clinical score assessed daily by an examiner blinded to the treatment in the STD+WAS and EE+WAS groups (n = 21 and n = 12 for STD+WAS and EE+WAS groups respectively; *P<0.05; **P<0.01; ***P<0.001, two-way repeated measures ANOVA +Bonferroni’s multiple comparison). (b) Clinical evaluation expressed as the long-term EAE relapse incidence in STD+WAS and EE+WAS groups (n = 21 and n = 12 for STD+WAS and EE+WAS groups respectively; **P<0.01, Gehan-Breslow-Wilcoxon test).
Table 3.
Values for mean day of relapse onset and mean peak clinical score in the STD+WAS and EE+WAS groups.
| STD+WAS EE+WAS | P-value | N | N | ||
|---|---|---|---|---|---|
| Mean day of first relapse onset | 43.73 ± 3.15 | 15/21 | 61.00 ± 0 | 1/12 | N.D. |
| Peak score | 2.00 ± 0.32 | 1.50 ± 0 | N.D. | ||
| Mean day of second relapse onset | 58.43 ± 4.28 | 9/21 | N.D. | 0/12 | N.D. |
| Peak score | 2.36 ± 0.31 | N.D. | N.D. | ||
| Mean day of third relapse onset | 56.00 ± 2.00 | 2/21 | N.D. | 0/12 | N.D. |
| Peak score | 2.50 ± 1.00 | N.D. | N.D. | ||
| Mean day of fourth relapse onset | 76.00 ± 0 | 1/21 | N.D. | 0/12 | N.D. |
| Peak score | 1.00 ± 0 | N.D. | N.D. | ||
| Peak score (all relapse) | 2.50 ± 0.30 | 15/21 | 1.50 ± 0 | 1/12 | N.D. |
N corresponds to the number of animals with first surge or relapse divided by the total number of animals within the group (see Supplementary Figure 1B for a detailed description). The data are given for the animals that develop a first peak.
Discussion
The present study overall shows that repeated acute stress precipitates relapses in RR-EAE and that environmental enrichment, previously reported to reduce stress in laboratory rodents,9 prevents the occurrence of relapses and alleviates the effect of repeated acute stress on RR-EAE severity.
Controversial results have been reported in the past concerning the effects of stress on EAE.11 A general consensus has emerged to state that chronic stress ameliorates, while acute stress worsens, disease course. The outcome of these studies was generally the evolution of clinical score in a chronic, monophasic model, rather than the effect on relapses in a relapsing-remitting model, as described here. The data presented here show that repeated acute stress during the remission phase of RR-EAE is sufficient to precipitate relapses in absence of other inducers. These data complement previous reports of an exacerbation of disease by chronic mild stress.8 Our data match clinical observation in MS patients, previously summarized in a meta-analysis12 and describing that stressful life events exacerbate MS. The paradigm developed in the present study may therefore be used to investigate the mechanisms responsible for stress-induced occurrence of relapses. In complement, other paradigms of repeated acute stress, such as restraint stress or exposure to predator odors may be used in future studies.
The two major stress response systems, the hypothalamic-adrenal-pituitary axis and the sympathetic nervous system, have been suggested to mediate the effects of stress on relapses.13 In addition to these pathways, stress also increases intestinal barrier permeability.14 Animal studies give a possible link between gut inflammation and MS: intestinal barrier dysfunction occurs at the onset of monophasic EAE15 and a pro-inflammatory response in the gut has been shown to trigger EAE.16,17 Conversely, the sequestration of Th17 cells in the gut confers resistance to EAE.18 Together, these studies suggest a sequence of events involving the gut in the pathogenesis of EAE: (i) initial expansion and activation of T-cell in gut-associated lymphoid tissues, leading to (ii) recruitment of auto-antibody-producing B cells and (iii) migration to brain draining cervical nodes to finally trigger autoimmune encephalomyelitis. Increased permeability of the intestinal barrier is a key feature of gut inflammation19 and could thus initiate this cascade of events, which may potentiated by stress. In addition, stress may also induce neurovascular pathology, leading to BBB permeation20,21 which could provide an additional explanation for the effect of stress in EAE. The paradigm described in the present work could be useful to test these mechanistic hypotheses in further studies.
While the effects of stress on RR-EAE disease described here corroborate previous reports in experimental models8 and coincide with clinical data,12 more intriguing and novel are our data concerning the effects of environmental enrichment. Indeed, we show that animals bred in enriched environment show a reduced severity and incidence of the first RR-EAE peak, and a drastic amelioration of the second phase of the disease. In addition, environmental enrichment alleviated the effects of repeated acute stress, applied during the remission phase, on the incidence of relapse. These data suggest that animals bred in an enriched environment have lost their sensitivity to repeated acute stress on RR-EAE symptoms. The fact that environmental enrichment reduces the severity of RR-EAE from the first peak suggests that this breeding regimen exert a “conditioning” effect on animals towards a lesser sensitivity to EAE. Interestingly, although repeated acute stress prompts standard animals towards a higher rate of relapse, this stress paradigm does not compensate for the beneficial “conditioning” effect brought by environmental enrichment. Future experiments in which enrichment would be applied only during remission is a very exciting perspective that would test the possibility of a therapeutic effect of enrichment in EAE.
The mechanism by which environmental enrichment alleviates the effects of stress is also an intriguing question. Previous report suggested that environmental enrichment increases cerebral activity in the prefrontal cortex and that this increased activity participates in setting up stress resiliency.22 This suggests that environmental enrichment could drive a manner of cerebral training in rodents that would lead to the reported beneficial effects. However, one cannot exclude that this regimen may reverse the effects of stress by other-though not necessarily exclusive- ways. Several positive effects of environmental enrichment, in addition to cerebral training, may account for its beneficial effects, including increase in physical activity, or changes in metabolic parameters.23
In conclusion, this study shows that repeated acute stress increases relapse rate and incidence in an experimental paradigm relevant to relapsing-remitting MS, the most frequent form of this disease. These data in animal models have to be seen in the light of recent successful attempts to use stress management strategies as an adjunct to the available MS treatments.24,25 The use of preclinical animal models may provide rationales and mechanistic explanations for the use of these strategies in MS management.
Availability of Data and Materials
The datasets used and analysed during the current study are available from the corresponding author.
Conflict of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_2055217320959806 for Environmental enrichment alleviates the deleterious effects of stress in experimental autoimmune encephalomyelitis by Antoine Philippe Fournier, Erwan Baudron, Isabelle Wagnon, Philippe Aubert, Denis Vivien, Michel Neunlist, Isabelle Bardou and Fabian Docagne in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Contributor Information
Antoine Philippe Fournier, Normandie Univ, Unicaen, Inserm, Physiopathology and Imaging of Neurological Disorders, Cyceron Centre, Institut Blood and Brain@Caen-Normandie, Caen, France; Université de Montréal, Faculté de Médecine, Département de Neuroscience, Montréal, Canada.
Philippe Aubert, Université de Nantes, Inserm, The Enteric Nervous System in Gut and Brain Disorders, Nantes, France; Institut des Maladies de l‘Appareil Digestif, Nantes, France.
Denis Vivien, Normandie Univ, Unicaen, Inserm, Physiopathology and Imaging of Neurological Disorders, Cyceron Centre, Institut Blood and Brain@Caen-Normandie, Caen, France; Department of clinical research, CHU Côte de Nacre, Caen, France.
Michel Neunlist, Université de Nantes, Inserm, The Enteric Nervous System in Gut and Brain Disorders, Nantes, France; Institut des Maladies de l'Appareil Digestif, Nantes, France.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the ARSEP foundation and the Fondation pour la recherche médicale (FRM). APF and IW received a fellowships from the Conseil Régional de Normandie.
Ethics Approval
This study and the procedures thereof were approved by the French ministry of education and research (Project reference APAFIS#2887-2015112017418114v2; Center agreement #D14118001) in accordance with the French (Decree 87/848) and European (Directive 86/609) guidelines.
ORCID iD
Fabian Docagne https://orcid.org/0000-0003-1745-0625
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Briones-Buixassa L, et al. Stress and multiple sclerosis: a systematic review considering potential moderating and mediating factors and methods of assessing stress. Health Psychol Open 2015; 2: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res 1967; 11: 213–218. [DOI] [PubMed] [Google Scholar]
- 3.Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev 2013; 12: 947–953. [DOI] [PubMed] [Google Scholar]
- 4.Mitsonis CI, et al. The impact of stressful life events on risk of relapse in women with multiple sclerosis: a prospective study. Eur Psychiatry J Assoc Eur Psychiatr 2008; 23: 497–504. [DOI] [PubMed] [Google Scholar]
- 5.Lovera J, Reza T. Stress in multiple sclerosis: review of new developments and future directions. Curr Neurol Neurosci Rep 2013; 13: 398. [DOI] [PubMed] [Google Scholar]
- 6.Heesen C, Gold SM, Huitinga I, et al. Stress and hypothalamic-pituitary-adrenal axis function in experimental autoimmune encephalomyelitis and multiple sclerosis – a review. Psychoneuroendocrinology 2007; 32: 604–618. [DOI] [PubMed] [Google Scholar]
- 7.Chandler N, Jacobson S, Esposito P, et al. Acute stress shortens the time to onset of experimental allergic encephalomyelitis in SJL/J mice. Brain Behav Immun 2002; 16: 757–763. [DOI] [PubMed] [Google Scholar]
- 8.Gerrard B, et al. Chronic mild stress exacerbates severity of experimental autoimmune encephalomyelitis in association with altered non-coding RNA and metabolic biomarkers. Neuroscience 2017; 359: 299–307. [DOI] [PubMed] [Google Scholar]
- 9.Gurfein BT, et al. The calm mouse: an animal model of stress reduction. Mol Med Camb Mass 2012; 18: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology 1993; 104: 716–723. [DOI] [PubMed] [Google Scholar]
- 11.Heesen C, et al. Stress regulation in multiple sclerosis: current issues and concepts. Mult Scler Houndmills Basingstoke Engl 2007; 13: 143–148. [DOI] [PubMed] [Google Scholar]
- 12.Mohr DC, Hart SL, Julian L, et al. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. Br Med J 2004; 328: 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold SM, et al. The role of stress-response systems for the pathogenesis and progression of MS. Trends Immunol 2005; 26: 644–652. [DOI] [PubMed] [Google Scholar]
- 14.Vanhaecke T, et al. L. fermentum CECT 5716 prevents stress-induced intestinal barrier dysfunction in newborn rats. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc 2017; 29: e13069. [DOI] [PubMed] [Google Scholar]
- 15.Nouri M, Bredberg A, Weström B, et al. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One 2014; 9: e106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berer K, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011; 479: 538–541. [DOI] [PubMed] [Google Scholar]
- 17.Lee YK, Menezes JS, Umesaki Y, et al. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2011; 108: 4615–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berer K, Boziki M, Krishnamoorthy G. Selective accumulation of pro-inflammatory T cells in the intestine contributes to the resistance to autoimmune demyelinating disease. PLoS One 2014; 9: e87876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neunlist M, et al. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 2013; 10: 90–100. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann ML, et al. Decoding microglia responses to psychosocial stress reveals blood-brain barrier breakdown that may drive stress susceptibility. Sci Rep 2018; 8: 11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard C, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci 2017; 20: 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci Off J Soc Neurosci 2011; 31: 6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toth LA, Kregel K, Leon L, et al. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med 2011; 61: 314–321. [PMC free article] [PubMed] [Google Scholar]
- 24.Levin AB, Hadgkiss EJ, Weiland TJ, et al. Meditation as an adjunct to the management of multiple sclerosis. Neurol Res Int 2014; 2014: 704691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohr DC, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology 2012; 79: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_2055217320959806 for Environmental enrichment alleviates the deleterious effects of stress in experimental autoimmune encephalomyelitis by Antoine Philippe Fournier, Erwan Baudron, Isabelle Wagnon, Philippe Aubert, Denis Vivien, Michel Neunlist, Isabelle Bardou and Fabian Docagne in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author.