Abstract
Restoring bone defects are the major challenge facing clinical trial therapy, particularly skull related problems. Morin, a naturally occurring compound, has pro-osteogenesis. This research focuses on assessing the role of morin for its pro-osteogenesis activities. We utilized in vivo and in vitro models to investigate the molecular-level mechanisms of morin’s osteoblastic biological activity. The effectiveness of morin on pro-osteogenesis (100 mg/kg/day) was assessed by monitoring modifications in the bone histomorphometry score, the development of immature osteoblasts from mesenchymal stems cells and improvements in the expression of pro-osteogenic cytokines in skull defected (SD) mice. Quantitative—PCR, Western blot analysis, and immunofluorescence were studied to investigate the signaling pathways. Morin has a substantial in vivo pro-osteogenesis effect which can facilitate the development of osteoblasts, the production of osteoblast related marker genes, and in vitro protein markers for osteoblasts. From a molecular biology standpoint, morin contributes to the development of osteoblasts and stimulation of the Wnt pathway with the activation and translocation of β-catenin nuclei. Our findings from the study revealed that morin may be a beneficial substitute for helping regenerate bone defects.
Keywords: morin, bone defect, osteogenesis, osteoblast, western blot
Introduction
Bone defect repair is the biggest challenge faced in clinical trial therapy, particularly skull related problems.1 Bone being quite different from the rest of the tissues, it has an inherent capacity to approach injury regeneration because of its hard nature. In most cases of bone injury, a proper treatment can enhance healing without permanent bone damage.2 There are numerous medical indications that necessitate several curative methods for the development and reinforcement of bone rejuvenation.3 Autologous bone grafting is the most commonly employed advanced method, although the procedure or approach is analogous to several drawbacks, in particular the morbidity of the donor site, the restriction of the grafting material and also the quality of the bone in patients with osteoporosis.4–6 This is the main reason why we need safe, reliable, realistic and even cost-effective approaches to treat bone regeneration therapies in patients with bone defects.
Further to several studies, mesenchymal stem cells (MSCs) were shown to depict several promising characteristics, because of which they can be used in regenerative medicine as a tool. The potential to offer a better therapeutic capacity in degenerative, metabolic and even effective therapy is the treatment of several other forms of diseases that were the merits for utilizing MSC’s as a tool.7 Several bioactive compounds were shown to occur in fruits and vegetables and have the capacity to modulate auto-renewal and distinguish the potential of matured stem cells as well as affect a wide array of intracellular signal transduction pathways.8–10
With the introduction of bioactive ingredients, bone regeneration was surprisingly boosted. In addition to bone grafts such bioactive compounds include growth factors, nanoparticles, phytoconstituents, and even anabolic agents are investigated thoroughly to promote bone development. Flavonoids’ activity was widely reported, referring to the anti-cancer, antioxidant, anti- inflammatory, anti-viral and anti-thrombogenic actions. There was also a decline in osteoclast activity, an improvement in osteoblast actions and a reduction of the depletion of trabecular bone thickness.11–14 In specific, other flavonoids, quercetin, and silibinin induce differentiation of osteoblasts and even promote angiogenesis. It was revealed in vitro that quercetin derivatives and other flavonoids effectively inhibit osteoclast proliferation and alkaline phosphatase activity and stimulate osteoblast proliferation.15,16
In yet another study, Avdeeva et al.17 showed that flavonoids are a possible therapy for integrated osteomyelitis treatment by promoting myelopoiesis and bone tissue regeneration with a decline in the severity of inflammatory processes. Their research outcomes revealed that signs of regenerative processes (endoosteum and periosteum activation, the development of granulation tissue) were evident in the femur, but more prominent in the epiphysis. Upon treatment with flavonol glycosides from S.controversa, most bone plates had a normal appearance and a uniform mineralization.17
Morin’s antioxidant property18 and its involvement in lipid peroxidation inhibition,19 ability to activate or inactivate the enzymes and transporter proteins,20 anti-proliferative action,21 promoting apoptosis induction of cancer cell death,22 anti-inflammatory action,23 and anti-cancer activity24 were observed. It is reported that morin protects diabetic osteopenia by reducing trabecular bone mineral density (BMD) evaluated by micro-CT scan and other bone related marker proteins such as osteocalcin, bone related alkaline phosphatase (ALP), telopeptides of collagen type I (CTX). These modifications were restored to normal after 5 weeks of Morin treatment,25 by resisting bone loss. Further, the anti-proliferative activity of flavanoids was investigated on liver and colorectal cancer cells,26,27 followed by the cytotoxic potential of morin flavonoids on MCF-7 and MDA-MB-231 breast cancer cell lines,28,29 indicating that morin possess both anti-cancer and stem cell inducing activities.30 Recently a study reported the role of morin and its zinc complexes on osteoblast differentiation with molecular level investigations on human osteoblast-like cells (MG63) and mouse mesenchymal stem cells (MSCs) (C3H10T1/2) with enhanced expression levels of Runx2, Type 1 collagen, osteocalcin (OCN) and osteonectin (ON), thus showing the potential osteogenic ability of morin.31 Nonetheless, the specific molecular mechanisms have not been reported so far. Therefore, we utilized in vivo and in vitro models in the current research to investigate the specific molecular mechanisms of morin’s osteoblastic bioactivity through Wnt signaling pathways.
Materials and methods
Chemicals, reagents and media
Morin (purity ⩾ 95%, HPLC) was purchased from Sigma-Aldrich, China. Dulbecco’s and alpha modified Eagles media (DMEM; α-MEM); fetal bovin serum (FBS) from Thermo Fisher Scientific, China; L-Glutamine–Pencillin-Streptomycin were obtained from Himedia labs, China. MTT (3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazoliumbromide) was procured from Sigma Aldrich (China). Primary antibodies namely β-actin, Dvl3; Dvl2, Naked 2; Runx2; p-LRP6; LRP6 and β-catenin and secondary antibodies (Horseradish Peroxidase-conjugated; HRP-conjugated) for mice and rabbit IgGs respectively were obtained from Cell Signaling Technology (China).
Separation and culture of mesenchymal stem cells (MSCs) for the mouse bone marrow
The study was carried out using wild type mice. Along with all the muscles and cartilages, the arm and leg bones were removed from the skeleton. The α-MEM (Alpha modified Eagle’s medium) and 1% FBS were used to remove the bone marrow with a 10 ml syringe (27-gage needle) and then to sieve the cells on a 70 mm screen. The cells (2.5 × 107 cells/ml) were incubated at 37°C for 5 h in the presence of CO2. Altering the medium (α-MEM + 2 mM L-Glutamine + 100 mg/ml pencillin/streptomycin + 10% FBS) eliminated the non-adherent cells. Furthermore, the medium was changed after 8 h. PBS was used to wash cells after 72 h, and added a new medium. For every 4–5 days the medium was changed. After 3–4 weeks, the MSC colonies were isolated from cultures employing Trypsin/EDTA (Ethylenediaminetetraacetic acid). Cell surface markers were used to attain the MSC characteristics. The cells 2 × 104 cells/well were placed in 24-well plates containing DMEM (Dulbecco’s modified Eagles medium) to ensure a noticeable differentiation, which was supplemented by an osteogenic inducer. The osteogenic inducer used was Vitamin C (60 μM), β-glycerophosphate disodium (20 mM) and Dexamethasone (100 nM) respectively. Morin was used for conducting drug assays at various concentrations. It was added in the culture medium at varying concentrations, thus maintaining the volume constant. The cells were then incubated at 37°C after the assay was performed. The medium was changed daily for the next 4–10 days.
MTT assay
Mesenchymal stem cells were seeded on 96-well plates at 2 × 104 cells per well. Different concentrations of morin (25, 50 and 75 μg/mL) were applied to the plates following overnight incubation. Following 24 and 48 h incubation, cell growth was determined through the MTT assay using a plate reader. The proliferation rate was computed as follows
Rate of proliferation rate (%) = Absorbance 486 (sample) / Absorbance 486 (control) × 100%
Alizarin staining red procedure
The most important biomarker for osteoblast differentiation is the deposition of extracellular matrix mineralization nodules. This technique of alizarin red staining is directly proportional to the mineralization nodules and also to the maturity of the differentiated osteoblasts. The next step for the later 10 days was to treat with an osteogenic medium using different concentrations of morin (0.1, 0.3, 0.5, 0.7 and 1.0 μg/mL). PBS (phosphate buffer saline) was used for washing the cells with 4% paraformaldehyde for 20 min. The cells were stained at 37°C for 20 min using the staining kit (Alizarin Red). The extracellular images of the mineral deposited nodules were taken with the aid of microscope (inverted) attached to a camera (digital). The Alizarin Red Dye is used for calcium concentration measurement in MSCs with 500 μl of 15% cetylpyridium chloride with 10 mM sodium phosphate solution (10 min at 40°C). These MSCs were computed on microplate reader at 526 nm.
Process of staining alkaline phosphatase
Among numerous biomarkers for osteoblast differentiation, alkaline phosphatase (ALP) is the most commonly used biomarker due to its ability to promote osteogenesis activity in the early stages. The higher the ALP activity, the greater the ALP staining that implies more active osterogenesis. The cells are placed in the medium with varying amounts of MORIN (0.1, 0.3, 0.5, 0.7 and 1.0 μl/ml) for 7 days. The cells were gently washed for 20 min (PBS + 4% paraformaldehyde) despite having placed in the osteogenic medium, and stained using alkaline phosphate staining kit at 37°C for 90 min. The excess dye materials were eliminated by washing with PBS three times. The alkaline phosphatase nodule images were captured with the aid of microscope (inverted) attached to a camera (digital). With the osteogenic inducer, MSCs were activated. ALP activity levels in the cells were assessed using the kit for ALP activity. Later, the cells were co-cultured in different concentrations with morin for the next 8 days.
Western blot analysis
Using a buffer solution containing 45 mM Tris hydrochloride (pH 7.3), 170 mM NaCl, 0.5% Nonidet P-40, 0.15% SDS, and 2% sodium deoxycholate) mixed with 45 μg/mL PMSF (phenylmethylsulfonyl fluoride), whole cell protein substances were isolated from cultured MSC cells or their derived osteoblasts. In the SDS buffer, 50 μg of total cell protein components were added and allowed to run through the SDS PAGE gels (10%) and the obtained proteins were blotted onto membranes (nitrocellulose). TBS-Tween (Tris–buffer saline; 0.01 M Tris, 0.2 M NaCl, pH 7.2 and 0.3% Tween-20) with 7.5% (w/v) skimmed milk was used to block the membranes for 1 h. These membranes were added to primary antibodies, previously diluted using 2% skim milk, and incubated for 3 h at 4°C. These membranes were subsequently washed 3–4 times using TBS-Tween medium, to which secondary conjugated antibodies (HRP) were added and allowed to incubate for 3 h at room temperature. Antibody activation was ascertained following exposure to an ECL (enhanced luminol-based chemiluminescent) substrate in an Imaging System (Bio rad, USA). Each specific genes relative expression was calculated using Image J, and normalized to β-actin.
RNA Isolation and Quantitative—PCR detection
The total RNA was separated from the cultured cell using RNeasy kit, according to the manufacturer’s instructions. This RNA (2 µg) was used with the oligo primers and reverse transcriptase to produce the cDNA. For PCR reactions, one microliter (μL) of cDNA, suitable primers and 40 cycles of annealing temperature at 94°C for 40 s were used, followed by 70°C for 40 s. Quantitative-PCR was done in combination with SYBR Green Master Mix (China) and a PCR Detection System for normalization using β-actin.
Immunofluorescence confocal microscopy
The cultivation of the cells was prepared on the glass slides and fixed using 4% paraformaldehyde for 15 min. Then it was permeabilized in PBS containing 0.1% triton X-100 for 5 mins. Non-specific binding of an antibody was prevented by incubating for 30 min in PBS containing 3% BSA (bovine serum albumin). The cycle was carried out by diluting the primary antibodies with PBS containing 0.3% BSA and incubated for 2 h. Such cells were carefully washed, and secondary antibodies conjugated with Alexa Fluor-488 were allowed to bind those antibodies. A dye was used to stain the nuclei of the cells, and the samples were mounted to a ProlongTM diamond anti-fade medium. The microscope was used to capture the images that were used with the aid of Image—Pro plus 5.0 software for analysis.
In vivo experiments
Animals
Eight weeks old mice (specific pathogen free, SPF grade, Kunming (KM) mice) were procured from Animal Experimental Centre of Ningxia Medical University, Yinchuan, Ningxia, China. Isoflurane was administered an anesthetic to the mice. The crest of the cranium was trimmed and a vertical opening was made to expose the cranium. The bone tissue was exposed through retraction of surrounding tissues. In the parietal area of the skull, a craniotomy defect was developed. All the mice were kept in a cage, at a temperature of 25°C in a dark cycle of 12/12. Animal Ethics Committee approval was obtained, and the study was conducted in accordance to their guidelines.
All the mice were divided into eight different groups with five animals in each group randomly. Control-F group (received vehicle), MORIN - F- 50 mg/kg, MORIN - F- 100 mg/kg, MORIN - F- 150 mg/kg [four female (F) groups respectively]; Control-M group (received vehicle), MORIN-M-50 mg/kg, MORIN-M-100 mg/kg and MORIN-150 mg/kg [four male (M) groups respectively]. Morin was dissolved in a corn oil and administered regularly through intragastric administration. The dose administered was modified according to the body weight which was recorded on a weekly basis. Morin administration began 7 days after the incision and was performed until euthanasia for the next 28 days.
Sample size calculation and justification
The sample size was determined using power; standard deviation for evaluating sample variability; type-1 error at P ⩽ 0.05; two tailed tests involving study power at 80%. The sample size was estimated using G power program.
Organ index determination
Blood samples were drawn from mice that were fasted overnight until euthanasia. The serum osteoclacin (OCN) levels were assessed using ELISA (enzyme-linked immunosorbent assay) from the samples stored at ‒80°C. The organs from thoracic and abdominal regions such as heart, liver, and spleen were carefully collected. The connective tissue and the residual fat on the surface were removed. They were rinsed after separating the organs, and then weighed to calculate the index of the organs.
Microcomputed tomography assessment of mice’s bones
The entire skull was carefully separated without any relevant soft tissues and positioned for 24 h in 4% paraformaldehyde preceded by washing with a solution of sucrose (10%). After 12 h, micro-CT (micro-computed tomography) images were obtained at a resolution of 9000 mm for an exposure time of 900 ms per 360 rotational mode, 60 kV voltage, and 220 amperes tube current. Three-dimensional (3D) reconstructions were generated from two-dimensional (2D) images using multimodal 3D visualization software supplied by micro-CT devices. According to the ASBMR (American Society for Bone and Mineral Research) guidelines, parameters were determined as follows: BV/TV (bone volume/total volume) in the area of skull defect (1 mm distal to proximal epiphysis).
Statistical analysis
Data was expressed as mean ± SEM values to record the outcomes of the study. ANOVA test was used to perform analyzes of all results. Values of these tests were significant with P < 0.05.
Results
Morin promotes effective treatment for bone defects
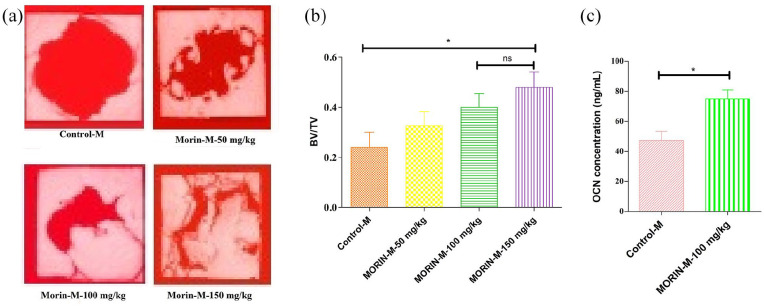
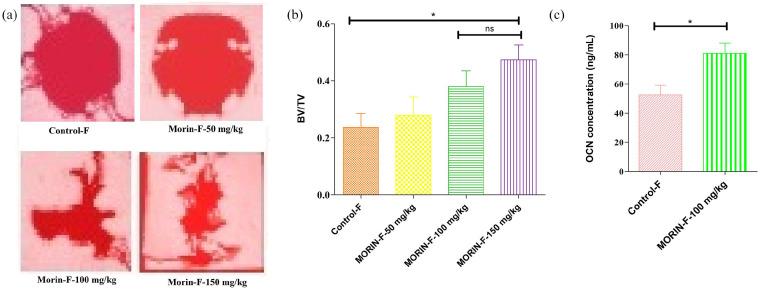
In order to accurately evaluate the morin activity for skull defects, in vivo experiments were performed in which all 44-week-old mice were grouped into eight groups that were administered different concentrations of morin (0, 50, 100, and 150 mg/kg/d) respectively. The micro-CT results for Figures 1(a) and 2(a) revealed a significant spherical defect that was effectively repaired after 28 days of treatment with different concentrations of morin in both male and female groups showing regeneration and the formation of new bone at the defect regions. With increased morin concentration the therapeutic effect was also increased. Male and female groups provided approximately the same features of the dose-effect of morin. There were no significant differences in bone regeneration and its formation with decreased bone region defects following morin treatment in both male and female groups, thereby demonstrating no gender-dependent efficacy of morin. The activity of morin, where 100 mg and 150 mg/kg/d groups were not significant (P > 0.05), was explained quantitatively in Figures 1(b) and 2(b) showing bone volume/total volume (BV/TV), indicating that specified volume of defect produced (total volume) was occupied by the formation of mineralized bone (bone volume). This illustrates that dose of 100 mg/kg/d was sufficient for effective treatment of skull defect regions. Both male and female BV/TV values were the same that showed increased characteristics of bone repair and bone regeneration. In Figures 1(c) and 2(c), the ELISA test revealed morin’s capability to improve the concentration of OCN (osteoclasin), which inturn proves its ability to treat skull defects by enhancing osteogenesis. The toxic dose of morin could also be assessed through this study of organ indices of animals and summarized in Table 1. There was no significant difference in body weight and indices of the individual organs (Table 1) were noticed. However, 150 mg/kg/d could augment the spleen indices, but 100 mg/kg/d might not, implying that the morin activity was in the range from 100 mg to 150 mg/kg/d as a non-toxic dose. Consequently the effective morin dose was 100 mg/kg/d.
Figure 1.
The beneficial effects of morin on imperfection of skull. (a) Representative micro-CT images of regions of skull defects in male mice at various doses showing the imperfections of bone region defects of the skull in male mice were greatly repaired and regenerated a new bone formation, with decreasing spaces in a dose dependent manner for 28 days. The concentrations of morin at 100 and 150 mg/kg showed significant osteogenic capacity with bone regeneration as compared to control. (b) Quantitative analysis of ratio of bone volume to total volume (BV/TV) showing histomorphometry assessments in male mice showing that skull defects were greatly repaired with a significant differences between them in bone regeneration bone region defects. However, no significant difference was noticed between morin at 100 and 150 mg/kg respectively in histomorphometric analysis. (c) Quantitative analysis of osteocalcin (OCN) serum levels in male group of mice at 100 mg/kg of morin indication that concentration of osteogenesis marker (OCN) was increased in skull defects after treatment with morin thus stimulating osteogenesis.
Data are represented as the mean ± SD.
*P < 0.05.
Figure 2.
The beneficial effects of morin on imperfection of skull. (a) Representative micro-CT images of regions of skull defects in female mice at various doses showing the imperfections of bone region defects of the skull in female mice were greatly repaired and regenerated a new bone formation, with decreasing spaces in a dose dependent manner for 28 days. The concentrations of morin at 100 and 150 mg/kg showed significant osteogenic capacity with bone regeneration as compared to control. (b) Quantitative analysis of ratio of bone volume to total volume (BV/TV) showing histomorphometry assessments in female mice showing that skull defects were greatly repaired with a significant differences between them in bone regeneration bone region defects. However, no significant difference was noticed between morin at 100 and 150 mg/kg respectively in histomorphometric analysis. (c) Quantitative analysis of osteocalcin (OCN) serum levels in female group of mice at 100 mg/kg of morin indication that concentration of osteogenesis marker (OCN) was increased in skull defects after treatment with morin thus stimulating osteogenesis.
Data are represented as the mean ± SD.
*P < 0.05.
Table 1.
Morin’s therapeutic effect on skull defects and shows all experimental animals.
| Model | Morin-50 mg/kg | Morin-100 mg/kg | Morin-150 mg/kg | |
|---|---|---|---|---|
| Body weight | ||||
| Male | 52.57 ± 0.28 | 56.47 ± 0.55 | 56 ± 0.25 | 52.6 ± 0.36 |
| Female | 39.83 ± 1.08 | 38.03 ± 1.12 | 38.4 ± 0.66 | 36.63 ± 0.76 |
| Liver indices | ||||
| Male | 4.51 ± 0.2 | 4.54 ± 0.17 | 4.59 ± 0.15 | 4.4 ± 0.08 |
| Female | 4.44 ± 0.25 | 4.36 ± 0.25 | 4.43 ± 0.09 | 4.36 ± 0.05 |
| Renal indices | ||||
| Male | 1.2 ± 0.1 | 1.27 ± 0.12 | 1.2 ± 0.07 | 1.23 ± 0.11 |
| Female | 1.86 ± 0.03 | 1.89 ± 0.03 | 1.78 ± 0.05 | 1.91 ± 0.03 |
| Lung indices | ||||
| Male | 0.63 ± 0.03 | 0.58 ± 0.04 | 0.54 ± 0.03 | 0.54 ± 0.02 |
| Female | 0.65 ± 0.02 | 0.64 ± 0.02 | 0.68 ± 0.03 | 0.62 ± 0.02 |
| Heart indices | ||||
| Male | 0.67 ± 0.01 | 0.66 ± 0.02 | 0.64 ± 0.02 | 0.69 ± 0.01 |
| Female | 0.65 ± 0.02 | 0.61 ± 0.02 | 0.6 ± 0.02 | 0.61 ± 0.01 |
| Spleen indices | ||||
| Male | 0.34 ± 0.01 | 0.35 ± 0.02 | 0.34 ± 0.01 | 0.55 ± 0.02*** |
| Female | 0.35 ± 0.02 | 0.36 ± 0.02 | 0.39 ± 0.01 | 0.74 ± 0.02*** |
All data is displayed as mean ± SD.
P < 0.001, indicated significant when compared between the values of model against morin treatment at 150 mg/kg/day.
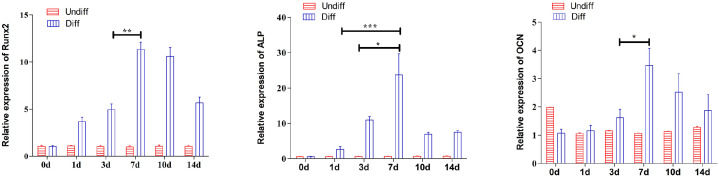
MSCs showed remarkable improvement in the expression of osteogenic genes such as OCN, ALP, and Runx2 (runt-related transcription factor 2), when cells were grown in an osteogenic medium for a 14-day period (Figure 3). The results in Figure 3 demonstrated the potential of mesenchymal stem cells to distinguish into osteoblasts under osterogenic cultural environment employed in our study. The expression of osteogenic related genes were significantly (P < 0.05) increased on 7th day under the influence of standard osteogenic inducing medium as compared against 1st and 3rd day respectively.
Figure 3.
Quantitative PCR evaluation of expression of osteogenic marker gene as Runx2 (Runt-related transcription factor 2), ALP (alkaline phosphatase) and OCN (osteocalcin) in osteogenically induced mesenchymal stem cells and non-induced control indicating that differentiation of MSCs in osteogenic medium showed increased expression of osteogenic related genes remarkably on 7th day.
All data appears as mean ± SD.
*P < 0.05; **P < 0.01; ***P < 0.001.
Evaluating morin for a safe dose through MTT assay
Morin’s cytotoxic effects on mesenchymal stem cells in the concentration range of 25–75 μg/mL were tested at 24 and 48 h. The test indicated that there was no decrease in the rate of proliferation of MSCs compared with control group without morin. The findings are shown in Figure 4 and suggested that morin was non-toxic on mesenchymal stem cells at a concentration range of 25–75 μg/mL.
Figure 4.
Cell viability performed by MTT assay for mesenchymal stem cells treated with morin at various concentration (25, 50, and 75 μg/mL) in osteogenic media for 24 and 48 h respectively indicating that no significant change in the mesenchymal stem cells proliferation was notices at the tested concentration of morin when compared with untreated controls cells.
All data appears as mean ± SD.
Morin promotes osteogenic demarcation of mesenchymal stem cells
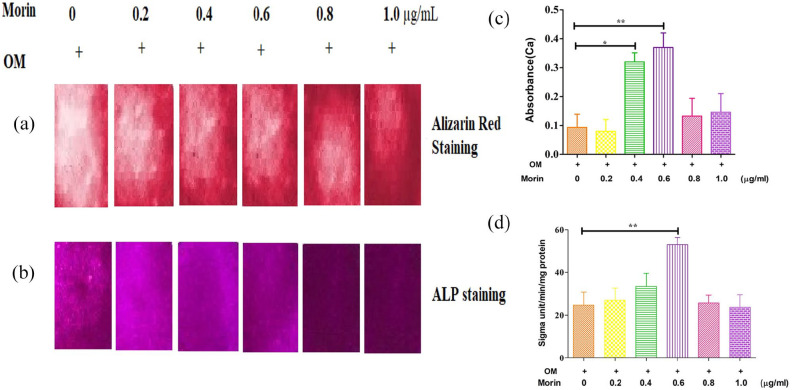
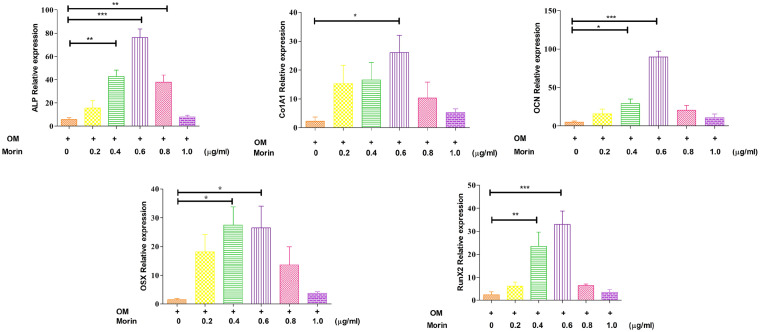
We observed whether co-culturing with morin might result MSCs to differentiate osteogenically. To assess whether or not morin can influence the actual differentiation, an analysis was performed, in which six experimental groups were taken, one of which was a control group and others were the morin groups, set at concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 μg/ml respectively. The control was co-cultured with osteogenic media and morin groups were co-treated with osteogenic media along with varying morin concentrations. On comparing with the control group, the ALP staining and activity was assessed to show a significant increase in morin administering groups (Figure 5(b) and (d)). Calcium deposition and Alizarin red staining was improved as a consequence of the morin co-culture (Figure 5(a) and (c)). The Quantitative—PCR analysis of ALP, ColA1 (alpha-1 type I collagen), OCN, OSX (osteoblast-specific transcription factor) and Runx2 further supported those results. In the morin administering groups, the expressions of ALP, Runx2, OSX, OCN and ColA1 were significantly increased (P < 0.05; Figure 6). The overall result of these analyzes showed that morin is a compound that is naturally effective in controlling MSC differentiation in osteogenesis.
Figure 5.
Morin promoted the development of osteoblasts. (a) All experimental groups were treated with various concentrations of morin (0.1–1.0 μg/Ml) and compared against control group. All the test groups were grown in the presence of osteogenesis inducing medium. After the osteogenic induction on all test groups, alizarin red staining (a) and ALP staining (b) were conducted on the day 14 and 7 respectively. (b) In all test groups, qualification of the cellular calcium level (c) and ALP activity (d) was determined on the da 14 and 7 respectively indicating that calcium deposition was increased quantified from alizarin red staining when co-cultured with various concentration of morin. OM indicates osteogenic media.
All data appears as mean ± SD.
*P < 0.05; **P < 0.01.
Figure 6.
Quantitative—PCR analysis of ALP, ColA1, OCN, OSX, and Runx2 mRNA expression levels in experimental groups treated with various morin concentrations during osteogenic induction indicating that morin was able to regulate the differentiation of mesenchymal stem cells with increase in the expression of osteogenic related marker genes. The result were represented as mean ± SD.
*P < 0.05; **P < 0.01; ***P < 0.001.
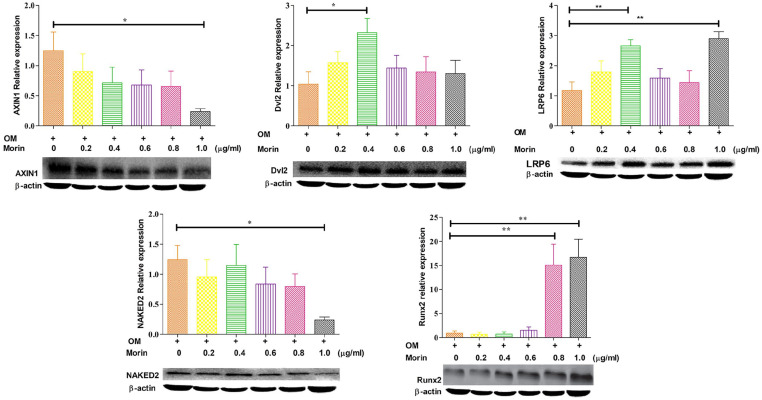
Morin motivates in vitro stimulation of the Wnt (Wingless-related integration site) pathway to boost osteogenesis
To determine the morin’s mechansim of action for promoting differentiation through osteogenesis, we investigated how morin affects the Wnt signaling pathway. Four days after the stimulation with morin in the presence of osteogenic inducer showed a decline in the expression of Axin1 and NAKED2 (Figure 7) was decreased with various morin concentrations. At the same time, the expression of LRP6 and Dvl2 (Figure 7) was increased with morin concentration. It is well known that Runx2 protein expression is important for transcriptional regulation of osteoblasts development involving Wnt signaling pathways. The results of morin treatment on MSCs showed an elevated expression of Runx2 protein after stimulation for 4 days followed by the increased expression of downstream transcriptional targets of ALP, ColA1 OSX, and OCN (Figure 6). These assessments indicated that morin could activate osteogenesis in MSCs by boosting the expression of the Runx2 protein.
Figure 7.
Morin displays it’s activity through Wnt signaling pathway. Western blot analysis involving immunoblotting with antibodies specific to the components of Wng signaling pathway was performed on the extracts obtained from mesenchymal stem cells treated with various concentration of morin (0.2–1.0 vg/mL) with an osteogenic inducing medium for the indicated times. The levels of protein expressions were normalized to β-actin expressions and used as a internal reference (control) for comparison. OM indicates osteogenic inducing media.
The results are shown as mean ± SD.
*P < 0.05; **P < 0.01.
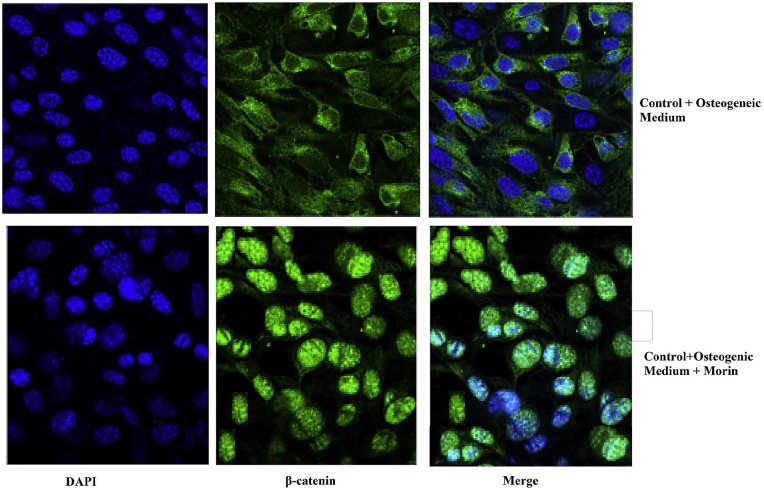
Morin facilitates expression and translocation of β-Catenin
To activate the target gene, β-catenin is required to translocate from cytoplasm to nuclei. In this study, immunofluorescence microscopy was used to demonstrate the β-catenin translocation to induce the development of adult osteoblasts during Wnt signaling pathway. After treatment with morin, the most significant factor for signaling the Wnt pathway, the β-catenin translocation was substantially increased (Figure 8). These findings showed that morin promoted osteogenesis by activating Wnt signaling pathways to increase translocation of β-catenin (the most important factor required for the Wnt signaling pathway) from the cytoplasm into the nuclei
Figure 8.
Morin was able to facilitate the expression of—catenin and nuclear localization. MSCs grown on glass cover slips treated with osteogenic inducer and morin (1 μg/mL) were fixed and stained with DAPI (nuclei) and—β catenin for 7 days and observed in confocal microscopy under immunofluorescence. It is obvious form the illustration that morin was able to promote expression of—β catenin and its translocation from the cytoplasm into the nucleus in order to transactivate the target genes responsible for the activation of Wnt signaling pathways, which further induces the formation of mature osteoblasts, thus indicating that morin was able to promote osteogenesis. It can be noticed from the illustration that—catenin is being displaced from the cytoplasm in the presence of morin, thus translocating into the nucleus to induce the expression and activation of Runx2, the primary transcriptional regulator of osterogenesis.
Discussion
Bone regeneration progresses by inducing osteogenic differentiation of the progenitor populations (mesenchymal stem cells) locally residing in the bone marrow after a bone defect. Enhancing the osteoblastic cells at the site of bone loss accelerates bone regeneration. The mesenchymal stem cells stimulated embryonic osteogenic cells, as per previous bone reconstruction investigations, help in the development of a new bone.32 To be precise, when specific bioactive molecules were added, they could boost and stimulate faster bone development, and even to a larger extent.33 When these bioactive molecules were combined with mesenchymal stem cells, they contribute to improve cell migration along with cell proliferation as well as differentiation that helps the cells to form the bone’s functional building units.11 In this study, for the first time, the activity of morin, as an active natural compound showed pro-osteogenesis action in vivo, without the aid of any of the stem cells or scaffold transplantation to heal the cranium defects and also promoting the Wnt signaling pathway that led to the development of expression and improvement of the activity by translocating the β-catenin actively. Despite having explicitly used morin to activate MSC cells, the outcomes were satisfactory and clearly suggested that morin possesses significant ability to treat bone defects.
To validate morin’s substantial activity, we considered and experimented with a skull defected mice. BV/TV analysis was considered to be the most commonly employed analysis tool conducted on bone defects using micro CT testing. Since the BV/TV value revealed increasing regions of bone defects which indicated that the bone defects were treated and repaired substantially. With the help of BV/TV analysis, the therapeutic action of morin was proven to be significant in both males and females (Figures 1 and 2), indicating the total volume (TV) was occupied by newly formed mineralized bone (BV). This study also evaluated the OCN (a marker protein for osteoblast development) serum concentration. The concentration increased rapidly in mice administered with morin than in the control group (Figures 1(c) and 2(c)) which implies that the osteogenic process had begun to activate the formation of new bone. The potential of morin being active in the pro-osteogenesis process was confirmed by the results of significantly enhanced gene expression levels (OCN, OSX, ALP, and ColA1) in the osteogenesis phase (Figure 3)11 in the presence of osteogenic inducing medium. These findings were also confirmed by Vimalraj et al.31 demonstrating that morin and morin-zinc complexes at 60 µM remarkably stimulated ALP activity under osteogenis supplementation on MG-63 cells. In accordance to our findings, they also showed that morin and its zinc complexes at 60 µM noticeably increased the mineralized matrix by performing alizarin red staining on MG-63 cells to analyze calcium deposition.31
The MTT assay findings shown in Figure 4 demonstrated that morin was non-toxic on mesenchymal stem cells at a tested concentration range of 25–75 μg/mL, which are in agreement with the study by Vimalraj et al.31 where they indicated that morin and morin-zinc complexes were safe without showing any detrimental effects at a tested concentrations of 20, 40, and 60 μM on mouse mesenchymal stem cells (C3H20T1/2) and myoblast (H9C2) cells for an incubation period of 48 hr. Further, authors also tested the effects of morin and morin-zinc complexes on chick embryo model, demonstrating that various concentration of morin and morin-zinc complexes at a tested concentrations between 40 and 100 μM showed no toxic effects and deformities after comprehensive evaluation of all physical parameters such as twisted neck, hemorhage (rupture blood vessels), microphthalmia (abnormal eyes), polydactyly (extra finger), micromelia (smallness of one limb), excencephaly (brain outside skull) omphalocele (organ develop outside).31
Cytosolic factors regulation activates Wnt signaling cascade that triggers MSCs to mediate the expression of osteoblast-related genes to foster osteoblast formation. Occupying Wnt ligands binding site by Wnt ligands stimulates Dvl, which in turn contributes to the inhibition of the β-catenin-Axin-morin complex. In the short period, Wnt ligand phosphorylation inhibits β-catenin degradation, followed by translocation into the nucleus, which induces the transcription and stimulation of Runx2, the primary osteogenesis transcriptional regulator.34 These findings were also illustrated by the study where it was shown that ALP, an early osteoblast differentiation marker is mainly regulated by Runx2, bone specific transcriptional factor.31
Wnt ligand required to stimulate early in the sequential process of osteoblast formation to activate the Wnt Signaling Axis.12 This formation process was often influenced by two factors – direction of differentiation, and the force of differentiation. Previously it was established that morin alone could not control the direction of differentiation of the MSCs.13 That was the reason why MSCs were also given with an osteogenic inducer, which helps morin to promote the osteogenic process14 with an osteogenic inducer present in the cell media when compared to control cells (cells grown with osteogenic medium only). In Wnt signaling pathway, NAKED2 might suppress the Dvl activity to reinforce the stability of β-catenin-Axin-morin complex, thus accelerating β-catenin to obstruct Wnt signals. It is well known that LRP6 is a significant factor for Wnt ligand receptor, whose increased expression could reinforce the Wng signals. Our study results demonstrated that morin was able to act through Wnt signaling pathway by reducing NAKED2 expression and increasing LRP6 expression in the presence of osteogenic inducing medium. Such reported improvements were the primary source of evidence in suggesting that Wnt signaling would potentially be improved by morin.18 The secondary evidence of morin activity was Dvl2’s increased expression that could activate the Wnt signaling. The morin’s enhanced expression of Dvl2 was attributed to inhibition of β-catenin degradation by after the complex was phosphorylated by GSK-3β.19 Morin co-culture significantly increased Axin1 gene expression as the third evidence provided in this study to reinforce Wnt signaling.20
Our results also showed that Morin was promoting the expression of Runx2, an important transcription factor involved in the Wnt signaling pathway for osteoblast differentiation.35,36 The expression of Runx2 elucidates the up-regulation of gene expressions (OSX, OCN, ColA1 and ALP), since they are explicitly involved in downstream transcription targets of Runx2.37 Our findings were further supported by the study results, where it was shown that Runx2, OCN, ON (osteonectin) and type 1 collagen was significantly increased with Morin and Morin zinc complexes compared to control.31 According to our findings, in previous studies, it is reported that Runx2 is also master transcription factor in the MAPK pathway was fundamental for both skeletogenesis and bone homeostasis in mice. It was recognized as the most crucial signaling in bone biology and modulates most of the extracellular signals.38 Mechanistic study shows that the TAK1-MKK3/6-p38 MAPK axis phosphorylates Runx2 and facilitates its interaction with the co-activator CREB-binding protein to combine master transcription factors in the osteoblast genetic program’s.39 Many reports have nevertheless followed our results demonstrating that BMP-2, ALP, Runx-2, OCN, type I collagen, OPN (osteopontin), TGF-β1 and AR (androgen receptor) as potent biomarkers responsible for regulation of osteoblast differentiation providing evidences of formation of extracellular matrix, upregulation of osteoblast proliferation, alkaline phosphatase (ALP) activity and deposition of minerals (calcium and phosphate) and collagen respectively.40–43
Our findings reported that the maximum effective in vitro and in vivo morin concentration was 0.6 μg/mL and 100 mg/kg/d respectively, which was consistent with previous findings that indicated a significant increase in morin concentration increases osteogenic activity.31 Our findings showed a substantial decrease in osteogenic activity if morin was co-cultured as shown through ALP and red alizarin staining, accompanied by osteogenic gene expression (Figure 3).
Limitations of the study
Our findings are required more in vivo studies to explore the clinical potential of morin in bone defects.
Other specific mechanistically studies need to be evaluated to present an evidence for morin to explore its therapeutic benefits in bone defects.
More studies of tissue engineering scaffolds and other delivery mechanisms for bone regeneration are therefore needed to justify the future applications of morin.
Conclusion
Bone defects and their repair mechanism are often a serious concern, paving ways for new agents to be discovered that enable in bone regeneration. Our findings showed that morin was able to exhibit osteoblast differentiation. Hence, morin therapy was found to be effective and innovative in treatment modalities to bone defects. Our research shows that morin possesses pro-osteogenic properties by activating osteogenic inducer to trigger Wnt signaling and thereby leading to up-regulated Runx2 expression. The results of our research showed that morin exhibited therapeutic impact by promoting the formation of osteoblasts through its pro-osteogenesis properties, and may therefore be a promising substance in the treatment of bone defects.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained by the ANIMAL ETHICS COMMITTEE (NMU/YIN/20/SHAQUI/056-1) of corresponding author’s Department of Orthopaedic Trauma, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China, 750004.
Animal welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation of the country.
ORCID iD: Shaodong Qiu  https://orcid.org/0000-0003-2564-2723
https://orcid.org/0000-0003-2564-2723
References
- 1. Wan W, Shi P. (2010) Scaffold modeling application in the repair of skull defects. Artificial Organs 34(4): 339–342. [DOI] [PubMed] [Google Scholar]
- 2. Verrier S, Alini M, Alsberg E, et al. (2016) Tissue engineering and regenerative approaches to improving the healing of large bone defects. European Cells and Materials 32: 87–110. [DOI] [PubMed] [Google Scholar]
- 3. Ghadakzadeh S, Mekhail M, Aoude A, et al. (2016) Small players ruling the hard game: siRNA in bone regeneration. Journal of Bone and Mineral Research 31(3): 475–487. [DOI] [PubMed] [Google Scholar]
- 4. Cabraja M, Kroppenstedt S. (2012) Bone grafting and substitutes in spine surgery. Journal of Neurosurgical Sciences 56(2): 87–95. [PubMed] [Google Scholar]
- 5. Garcia-Gareta E, Coathup MJ, Blunn GW. (2015) Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 81: 112–121. [DOI] [PubMed] [Google Scholar]
- 6. Miller CP, Chiodo CP. (2016) Autologous bone graft in foot and ankle surgery. Foot and Ankle Clinics 21(4): 825–837. [DOI] [PubMed] [Google Scholar]
- 7. Galipeau J, Sensébé L. (2018) Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 22(6): 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewandowski J, Kolanowski TJ, Kurpisz M. (2017) Techniques for the induction of human pluripotent stem cell differentiation towards cardiomyocytes. Journal of Tissue Engineering and Regenerative Medicine 11(5): 1658–1674. [DOI] [PubMed] [Google Scholar]
- 9. Ventura C, Cavallini C, Bianchi F, et al. (2008) Stem cells and cardiovascular repair: A role for natural and synthetic molecules harboring differentiating and paracrine logics. Cardiovascular & Hematological Agents in Medicinal Chemistry 6(1): 60–68. [DOI] [PubMed] [Google Scholar]
- 10. Kornicka K, Kocherova I, Marycz K. (2017) The effects of chosen plant extracts and compounds on mesenchymal stem cells—A bridge between molecular nutrition and regenerative medicine-concise review. Phytotherapy Research 31(7): 947–958. [DOI] [PubMed] [Google Scholar]
- 11. Vimalraj S, Rajalakshmi S, Preeth DR, et al. (2018) Mixed-ligand copper (II) complex of quercetin regulate osteogenesis and angiogenesis. Materials Science and Engineering: C 83: 187–194. [DOI] [PubMed] [Google Scholar]
- 12. Vimalraj S, Rajalakshmi S, Saravanan S, et al. (2018) Synthesis and characterization of zinc-silibinin complexes: A potential bioactive compound with angiogenic, and antibacterial activity for bone tissue engineering. Colloids and Surfaces B: Biointerfaces 167: 134–143. [DOI] [PubMed] [Google Scholar]
- 13. Vimalraj S, Bhuvaneswari S, Lakshmikirupa S, et al. (2018) Nitric oxide signaling regulates tumor-induced intussusceptive-like angiogenesis. Microvascular Research 119: 47–59. [DOI] [PubMed] [Google Scholar]
- 14. Rajalakshmi S, Vimalraj S, Saravanan S, et al. (2018) Synthesis and characterization of silibinin/phenanthroline/neocuproine copper (II) complexes for augmenting bone tissue regeneration: An in vitro analysis. JBIC Journal of Biological Inorganic Chemistry 23(5): 753–762. [DOI] [PubMed] [Google Scholar]
- 15. Shou D, Zhang Y, Shen L, et al. (2017) Flavonoids of Herba epimedii enhances bone repair in a rabbit model of chronic osteomyelitis during post-infection treatment and stimulates osteoblast proliferation in vitro. Phytotherapy Research 31(2): 330–339. [DOI] [PubMed] [Google Scholar]
- 16. Kim CS, Ha HK, Song KY. (2004) Therapeutic agent or osteoporosis comprising an active ingredient of quercetin derivatives. US Patent 0162247. [Google Scholar]
- 17. Avdeeva E, Shults E, Skorokhodova M, et al. (2018) Flavonol glycosides from Saussurea controversa and their efficiency in experimental osteomyelitis. Planta Medica International Open 5: e24–e29. [Google Scholar]
- 18. Duthie GG, Duthie SJ, Kyle JAM. (2000) Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutrition Research Reviews 13: 79–106. [DOI] [PubMed] [Google Scholar]
- 19. Treml J, Smejkal K. (2016) Flavonoids as potent scavengers of hydroxyl radicals. Comprehensive Reviews in Food Science and Food Safety 15(3): 111–119. [DOI] [PubMed] [Google Scholar]
- 20. Venturelli S, Burkard M, Biendl M, et al. (2016) Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 32(11): 1171–1178. [DOI] [PubMed] [Google Scholar]
- 21. Zhamanbayeva GT, Aralbayeva AN, Murzakhmetova MK, et al. (2016) Cooperative antiproliferative and differentiation-enhancing activity of medicinal plant extracts in acute myeloid leukemia cells. Biomedicine & Pharmacotherapy 82: 80–89. [DOI] [PubMed] [Google Scholar]
- 22. Caselli A, Cirri P, Santi A, et al. (2016) Morin: A promising natural drug. Current Medicinal Chemistry 23(8): 774–791. [DOI] [PubMed] [Google Scholar]
- 23. Nishizuka Y. (1988) The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334: 661–665. [DOI] [PubMed] [Google Scholar]
- 24. Davis WL, Matthew SB. (2000) Antioxidants and cancer III: Quercetin. Alternative Medicine Review 5: 196–208. [PubMed] [Google Scholar]
- 25. Abuohashish HM, Al-Rejaie SS, Al-Hosaini KA, et al. (2013) Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetology & Metabolic Syndrome 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hussain J, Ali L, Khan A, et al. (2014) Isolation and bioactivities of the flavonoids morin and morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—A wild Arabian medicinal plant. Molecules 19(11): 17763–17772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rehman NU, Mabood F, Khan AL, et al. (2019) Evaluation of biological potential and physicochemical properties of Acridocarpus orientalis (Malpighiaceae). The Pakistan Journal of Botany 51(3): 1099–1106. [Google Scholar]
- 28. Jin H, Lee WS, Eun SY, et al. (2014) Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. International Journal of Oncology 45(4): 1629–1637. [DOI] [PubMed] [Google Scholar]
- 29. Okubo T, Kano I. (2003) Studies on estrogenic activities of food additives with human breast cancer MCF-7 cells and mechanism of estrogenicity by BHA and OPP. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan 123(6): 443–452. [DOI] [PubMed] [Google Scholar]
- 30. Jamshidi-adegani F, Vakilian S, Rehman NU, et al. (2020) Secondary metabolites from acridocarpus orientalis inhibits 4T1 cells and promotes mesenchymal stem cells (MSCs) proliferation. Molecular Biology Reports 47: 5421–5430. [DOI] [PubMed] [Google Scholar]
- 31. Vimalraj S, Subramaniyam R, Sekaran S, et al. (2019) Zinc chelated morin promotes osteoblast differentiation over its uncomplexed counterpart. Process Biochemistry 82: 167–172. [Google Scholar]
- 32. Nash LA, Sullivan PJ, Peters SJ, et al. (2015) Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Molecular Nutrition & Food Research 59: 443–453. [DOI] [PubMed] [Google Scholar]
- 33. Mamun MA, Hosen MJ, Islam K, et al. (2015) Tridax procumbens flavonoids promote osteoblast differentiation and bone formation. Biological Research 18(48): 65. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Lerner UH, Ohlsson C. (2015) The WNT system: Background and its role in bone. Journal of Internal Medicine 277(6): 630–649. [DOI] [PubMed] [Google Scholar]
- 35. Cai T, Sun D, Duan Y, et al. (2016) WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Experimental Cell Research 345(2): 206–217. [DOI] [PubMed] [Google Scholar]
- 36. Vega OA, Lucero CMJ, Araya HF, et al. (2017) Wnt/β-catenin signaling activates expression of the bone-related transcription factor RUNX2 in select human osteosarcoma cell types. Journal of Cellular Biochemistry 118(11): 3662–3674. [DOI] [PubMed] [Google Scholar]
- 37. Otto F, Lubbert M, Stock M. (2003) Upstream and downstream targets of RUNX proteins. Journal of Cellular Biochemistry 89(1): 9–18. [DOI] [PubMed] [Google Scholar]
- 38. Greenblatt MB, Shim JH, Zou W, et al. (2010) The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. The Journal of Clinical Investigation 120: 2457–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwon HS, Johnson TV, Tomarev SI. (2013) Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling. Journal of Biological Chemistry 288: 16882–16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hnin ET, Zahid H, Isa NM, et al. (2018) Exploring molecular mechanism of bone-forming capacity of Eurycoma longifolia: Evidence of enhanced expression of bone-related biomarkers. Journal of Ayurveda and Integrative Medicine 9(4): 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hnin ET, Zahid H, Isa NM, et al. (2019) Eurycoma longifolia, a promising suppressor of RANKL-induced differentiation and activation of osteoclasts: An in vitro mechanistic evaluation. Journal of Ayurveda and Integrative Medicine 10(2): 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bukhari SNA, Hussain F, Thu HE, et al. (2019) Synergistic effects of combined therapy of curcumin and Fructus Ligustri Lucidi for treatment of osteoporosis: Cellular and molecular evidence of enhanced bone formation. Journal of Integrative Medicine 17(1): 38–45. [DOI] [PubMed] [Google Scholar]
- 43. Shao M, Hussain Z, Thu HE, et al. (2017) Emerging trends in therapeutic algorithm of chronic wound healers: Recent advances in drug delivery systems, concepts-to-clinical application and future prospects. Critical Reviews™ in Therapeutic Drug Carrier Systems 34(5): 387–452. [DOI] [PubMed] [Google Scholar]