Abstract
Objective
To investigate the related risk factors and predictive nomogram of postoperative hypoxaemia in elderly patients with femoral neck fractures.
Methods
This study included patients aged ≥65 years who underwent surgical treatment of acute femoral neck fractures. Univariate and multivariate logistic analyses were performed to determine the incidence of and risk factors for postoperative hypoxaemia. A predictive nomogram was constructed based on the multivariable model. Using the bootstrap method, discrimination was determined by the C-index and calibration plot.
Results
The logistic regression analysis showed that the anaesthesia type, surgical procedure, American Society of Anesthesiologists (ASA) classification, preoperative hypoxaemia occurrence, and age were independent predictors of development of postoperative hypoxaemia. The predictive formula for hypoxaemia was established as follows: hypoxaemia=−0.8668×spinal anaesthesia (whether)+0.1162×nerve anaesthesia (whether)+1.9555×plate/screw fixation (whether)+1.4950×hip replacement (whether)+0.4883×ASA classification+1.7153×preoperative oxygenation index+0.1608×age. With the bootstrap method, the prediction curve fit well with the ideal curve, suggesting that the prediction curve constructed in this study has good predictive ability.
Conclusions
Anaesthesia type, surgical procedure, ASA classification, preoperative hypoxaemia occurrence, and age were risk factors for postoperative hypoxaemia in elderly patients with femoral neck fractures. The predictive nomogram was designed for preoperative assessment of the risk of postoperative hypoxaemia by calculating the risk score.
Keywords: Femoral neck fracture, hypoxaemia, risk factors, perioperative period, predictive nomogram, elderly patient
Introduction
In China, femoral neck fractures account for 3.6% of all fractures throughout the whole body and 48% to 54% of hip fractures. Elderly individuals are the most commonly affected population, and most femoral neck fractures in these patients are caused by low-energy injuries such as falls.1 With the ageing of the world’s population, it has been estimated that the incidence of femoral neck fractures increases by 25% annually, and the number of hip fractures is expected to reach three times the current rate in 50 years.1 With the continuous development of medical science, anaesthetic techniques, and perioperative monitoring technology, the conditions under which elderly patients with poor pulmonary function undergo hip surgery are improving. Surgery is the most common treatment for most femoral neck fractures.2 Elderly patients with femoral neck fractures often develop acute lung injury (ALI) during the perioperative period, and hypoxaemia is the most common symptom in patients with ALI. However, little is known regarding the occurrence of hypoxaemia following femoral neck fracture surgery.
Elderly patients have a high risk of developing hypoxaemia because of their respiratory system deterioration and decreased respiratory function.3 Elderly patients with preoperative respiratory disease complications have a high incidence of hypoxaemia because of poor tolerance of surgical trauma, slow clearance of the anaesthetic, and the physiological influences of surgery and anaesthesia.4 Unfortunately, hypoxaemia is often undetected, misdiagnosed, or undertreated. The mechanisms underlying preoperative hypoxaemia and the causes of lung injury in patients who have undergone surgical treatment of femoral neck fractures remain elusive. No studies have examined the relationship between postoperative hypoxaemia and femoral neck fracture.
In the present study, univariate and multivariate logistic analyses were performed to determine the incidence of and risk factors for hypoxaemia. A predictive nomogram was constructed based on the multivariable model. Using the bootstrap method, the discriminatory ability of the nomogram was determined by the C-index and calibration plot. Our aim was to identify the predictors of hypoxaemia in an effort to improve the early treatment of hypoxaemia.
Patients and methods
Patient population
This study involved consecutive patients who underwent surgical treatment for femoral neck fractures at the Shanghai Changhai Hospital trauma centre from January 2012 to December 2017. The exclusion criteria were pathological fractures, age of <65 years, and multiple fractures. Hypoxaemia was defined by an arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio of ≤300. PaO2 was obtained from an arterial blood gas analysis. FiO2 was equal to 21 + (4 × oxygen flow).5 The patients were divided into two groups according to whether they did or did not develop hypoxaemia postoperatively.
The patients were characterised at baseline according to sex; age; cause of injury; pre-injury walking ability; smoking status; prevalence of hypertension, coronary disease, and diabetes; current drug use; anaesthesia type; American Society of Anesthesiologists (ASA) classification; surgical procedure; preoperative hypoxaemia occurrence; body mass index; preoperative test indexes (white blood cell count, alanine aminotransferase level, aspartate aminotransferase level, albumin level, platelet count, ejection fraction, and serum creatinine level); time to surgery; and operation time (Table 1).
Table 1.
Baseline characteristics.
| Variable | Without hypoxaemia(n = 390) | With hypoxaemia(n = 169) | Statistic | P value |
|---|---|---|---|---|
| Sex | ||||
| Female | 287 (73.59) | 119 (70.41) | χ2 = 0.64 | 0.4255 |
| Male | 103 (26.41) | 50 (29.59) | ||
| Cause of injury | ||||
| Fall | 370 (95.36) | 161 (95.27) | χ2 = 0.03 | 0.8616 |
| Spontaneous | 4 (1.03) | 1 (0.59) | ||
| Unknown | 14 (3.61) | 7 (4.14) | ||
| Missing | 2 | |||
| Pre-injury walking ability | ||||
| No walking disability | 117 (30.00) | 45 (26.63) | χ2 = 0.76 | 0.3830 |
| Moderate walking disability | 266 (68.21) | 119 (70.41) | ||
| Unable to walk | 4 (1.03) | 4 (2.37) | ||
| Smoking | ||||
| Never smoked | 339 (86.92) | 142 (84.02) | 1.0000 | |
| Current smoking habit | 19 (4.87) | 9 (5.33) | ||
| Former smoking habit | 31 (7.95) | 18 (10.65) | ||
| Hypertension | ||||
| No | 228 (58.46) | 98 (57.99) | χ2 = 0.01 | 0.9170 |
| Yes | 162 (41.54) | 71 (42.01) | ||
| Coronary disease | ||||
| No | 372 (95.38) | 159 (94.08) | χ2 = 0.42 | 0.5170 |
| Yes | 18 (4.62) | 10 (5.92) | ||
| Diabetes | ||||
| No | 329 (84.36) | 149 (88.17) | χ2 = 1.38 | 0.2403 |
| Yes | 61 (15.64) | 20 (11.83) | ||
| Current drug use | ||||
| None | 173 (44.82) | 68 (40.72) | 1.0000 | |
| NSAIDs | 3 (0.78) | 2 (1.20) | ||
| General cardiac drugs | 19 (4.92) | 5 (2.99) | ||
| Pulmonary drugs | 1 (0.26) | 1 (0.60) | ||
| Anti-hypertension drugs | 158 (40.93) | 74 (44.31) | ||
| Osteoporosis drugs | 1 (0.26) | 1 (0.60) | ||
| Not included above | 31 (8.03) | 16 (9.58) | ||
| Anaesthesia mode | ||||
| General | 6 (1.81) | 5 (3.47) | χ2 = 3.02 | 0.0822 |
| Spinal | 263 (79.22) | 98 (68.06) | ||
| Nerve block | 63 (18.98) | 41 (28.47) | ||
| ASA classification | ||||
| 1 | 13 (3.36) | 1 (0.60) | χ2 = 13.78 | 0.0002 |
| 2 | 283 (73.13) | 102 (61.08) | ||
| 3 | 86 (22.22) | 61 (36.53) | ||
| 4 | 5 (1.29) | 3 (1.80) | ||
| Surgical procedure | ||||
| Hollow screw fixation | 68 (17.66) | 5 (2.98) | χ2 = 21.08 | <0.0001 |
| Plate/screw fixation | 8 (2.08) | 4 (2.38) | ||
| Hip replacement | 309 (80.26) | 159 (94.64) | ||
| Missing | 5 | 1 | ||
| Preoperative hypoxaemia | ||||
| No | 262 (67.18) | 71 (42.01) | χ2 = 31.01 | <0.0001 |
| Yes | 128 (32.82) | 98 (57.99) | ||
| Age, years | 76.05 ± 6.86 | 82.99 ± 6.09 | Z = 10.39 | <0.0001 |
| BMI, kg/m2 | 26.57 ± 53.15 | 25.11 ± 28.71 | Z = 0.76 | 0.4447 |
| Preoperative WBC count, ×109/L | 8.31 ± 2.46 | 8.30 ± 2.57 | Z = 0.18 | 0.8598 |
| Preoperative ALT level, U/L | 22.77 ± 40.59 | 20.52 ± 19.79 | Z = 2.06 | 0.0398 |
| Preoperative AST level, U/L | 26.89 ± 65.81 | 24.64 ± 13.34 | Z = 0.16 | 0.8763 |
| Preoperative ALB level, g/L | 36.70 ± 5.52 | 34.71 ± 4.60 | Z = 5.45 | <0.0001 |
| Preoperative PT, s | 13.43 ± 1.04 | 14.21 ± 9.80 | Z = 1.29 | 0.1975 |
| Preoperative EF, % | 62.29 ± 4.34 | 62.43 ± 3.82 | Z = 0.17 | 0.8676 |
| Preoperative serum creatinine level, µmol/L | 75.58 ± 48.88 | 82.43 ± 74.79 | Z = 1.32 | 0.1882 |
| Time to surgery, days | 4.0 (3.0–5.0) | 4.0 (3.0–6.0) | Z = 1.91 | 0.0563 |
| Operation time, minutes | 88.37 ± 50.48 | 88.57 ± 36.69 | Z = 0.48 | 0.6304 |
| Length of hospital stay, days | 10.18 ± 3.79 | 11.92 ± 4.38 | Z = 4.78 | <0.0001 |
Data are presented as n (%), mean ± standard deviation, or median (range).
NSAIDs, nonsteroidal anti-inflammatory drugs; ASA, American Society of Anesthesiologists; BMI, body mass index; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; PT, platelet count; EF, ejection fraction.
All participants provided written informed consent. This study was approved by the Changhai Hospital Clinical Research Ethics Committee.
Statistical analysis
Continuous data are expressed as mean ± standard deviation. Normally distributed variables were analysed using Student’s t-test, and non-normally distributed variables were analysed using the Wilcoxon rank sum test. Qualitative data were analysed using the chi-square test or Fisher’s exact test. A P value of <0.05 was considered statistically significant. Logistic stepwise regression was used to analyse single factors with a P value of <0.1 to predict the independent risk factors. The odds ratios and 95% confidence intervals were also calculated. A predictive nomogram was constructed based on the multivariable model. Using the bootstrap method, discrimination was determined by the C-index and calibration plot.
Results
Patients’ baseline data and comparison of clinical features between patients with and without postoperative hypoxaemia
In total, 559 patients met the inclusion criteria for this study (153 men and 406 women; age range, 65–104 years; average age, 79 years). The incidence of postoperative hypoxaemia was 30.23% (169/559). The single-factor analysis showed that among the perioperative characteristics, the anaesthesia type, surgical procedure, ASA classification, preoperative hypoxaemia occurrence, alanine aminotransferase level, albumin level, and age were significantly associated with postoperative hypoxaemia (p < 0.05) (Table 1).
The logistic regression analysis showed that the anaesthesia type, surgical procedure, ASA classification, preoperative hypoxaemia occurrence, and age were independent predictors of the development of postoperative hypoxaemia (Table 2). According to the results of the multifactor analysis, the predictive formula of hypoxaemia was established as follows: hypoxaemia = −0.8668 × spinal anaesthesia (whether) + 0.1162 × nerve anaesthesia (whether) + 1.9555 × plate/screw fixation (whether) + 1.4950 × hip replacement (whether) + 0.4883 × ASA classification + 1.7153 × preoperative oxygenation index + 0.1608 × age.
Table 2.
Multivariable logistic regression of predictors of postoperative hypoxaemia.
| Variable | Coefficient | 95% CI | P value |
|---|---|---|---|
| Anaesthesia mode: spinal vs. general | −0.8668 | 0.42 (0.239–0.738) | 0.0026 |
| Anaesthesia mode: nerve block vs. general | 0.1162 | 1.123 (0.24–5.25) | 0.8826 |
| Surgical procedure: plate/screw fixation vs. hollow screw fixation | 1.9555 | 7.067 (0.916–54.546) | 0.0607 |
| Surgical procedure: hip replacement vs. hollow screw fixation | 1.4950 | 4.459 (1.542–12.896) | 0.0058 |
| ASA classification | 0.4883 | 1.63 (1.022–2.599) | 0.0404 |
| Preoperative hypoxaemia | 1.7153 | 5.558 (3.272–9.443) | <0.0001 |
| Age, years | 0.1608 | 1.174 (1.127–1.224) | <0.0001 |
CI, confidence interval; ASA, American Society of Anesthesiologists.
Note: Multivariate regression included variables from the univariate analysis with statistical significance (P < 0.1). Stepwise regression was used to screen variables. The inclusion criterion was 0.05, and the exclusion criterion was 0.1.
Development of nomogram
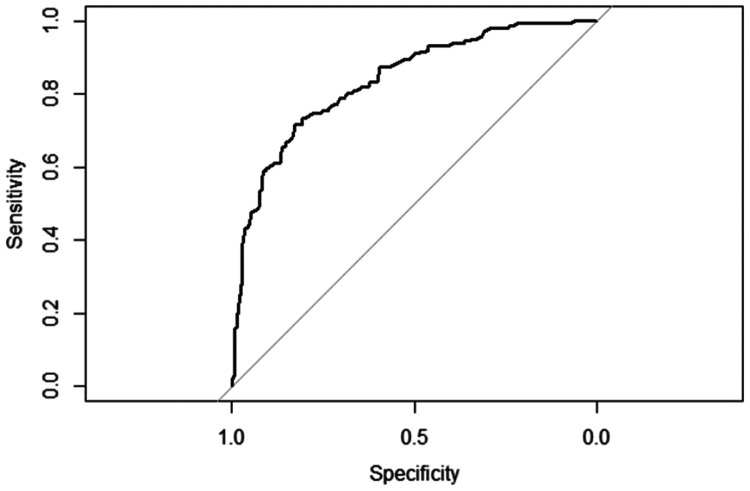
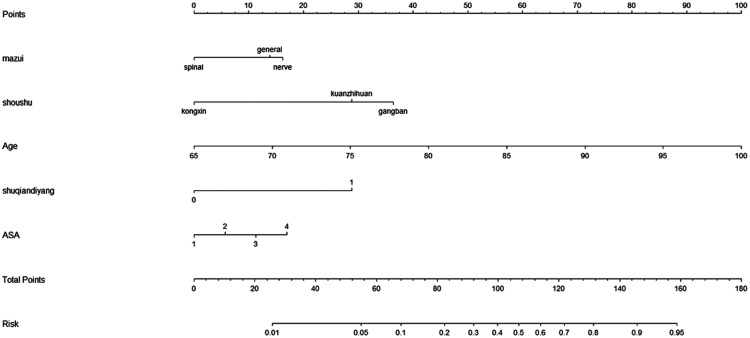
To provide physicians with a quantitative method for predicting the risk of postoperative hypoxaemia, we constructed a nomogram based on the multivariable logistic regression results. The receiver operating characteristic curve of the prediction formula is presented in Figure 1. The area under the curve was 0.8348 (0.7948–0.8748), indicating that the predictive ability was strong. The predictive nomogram was constructed based on the multivariable model (Figure 2). To use the nomogram, a vertical line is drawn up to the top point row to assign points for each variable. The total number of points is then calculated, and a vertical line is drawn downward from the total point row to obtain the probability of postoperative hypoxaemia.
Figure 1.
Receiver operating characteristic curve of the prediction formula. The area under the curve was 0.8348 (0.7948–0.8748), indicating strong predictive ability.
Figure 2.
Predictive nomogram for postoperative hypoxaemia. ASA, American Society of Anesthesiologists.
Clinical utility of nomogram for predicting postoperative hypoxaemia
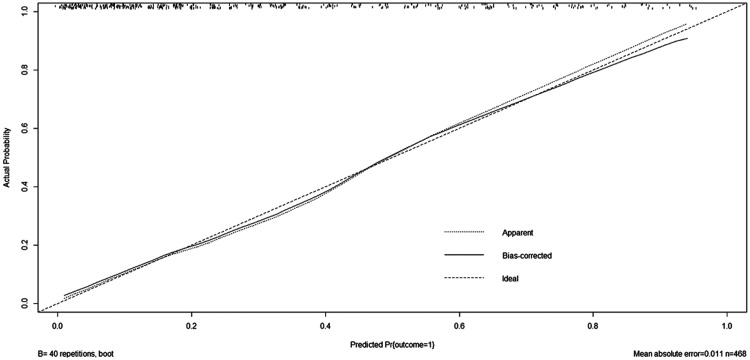
Using the bootstrap method, a calibration plot was constructed to compare the predicted outcome of hypoxaemia with the actual outcome of hypoxaemia (Figure 3). The close fit between the predictive curve and the ideal curve indicates that the predictive curve constructed in this study had good predictive ability, and there was no obvious change in the overfit correction of the predictive curve. The area under the curve after calculation and correction was 0.8223, indicating that the predictive model constructed in this study was not overfit; however, it still requires external validation in future studies.
Figure 3.
Calibration plot. Using the bootstrap method, a calibration plot was drawn to compare the predicted outcome of hypoxaemia and the actual outcome of hypoxaemia.
Relationship between postoperative hypoxaemia and postoperative adverse events
Patients with postoperative hypoxaemia had a longer length of stay than those without postoperative hypoxaemia (Table 1). A high proportion of those with postoperative hypoxaemia entered the intensive care unit (Table 3). Patients with postoperative hypoxaemia showed a higher rate of pneumonia, delirium, arrhythmia, multiple organ dysfunction syndrome, and even death during the perioperative period than patients without postoperative hypoxaemia (Table 3).
Table 3.
Postoperative adverse events.
| Variable | With hypoxaemia(n = 169) | Without hypoxaemia(n = 390) | Statistic | P value |
|---|---|---|---|---|
| Pneumonia | 15 (8.88) | 14 (3.59) | χ2 = 5.67 | 0.017 |
| Delirium | 39 (23.08) | 43 (11.03) | χ2 = 13.68 | <0.001 |
| Arrhythmia | 29 (17.16) | 40 (10.26) | χ2 = 5.19 | 0.023 |
| MODS | 5 (2.96) | 1 (0.26) | χ2 = 5.67 | 0.016 |
| Enter ICU | 31 (18.34) | 45 (11.54) | χ2 = 4.65 | 0.031 |
| Death | 3 (1.78) | 0 (0.00) | χ2 = 4.03 | 0.045 |
Data are presented as n (%).
All of the above adverse events occurred postoperatively.
MODS, multiple organ dysfunction syndrome; ICU, intensive care unit.
Discussion
Femoral neck fracture is a common cause of morbidity and mortality in the elderly population,6 and it is the most common reason for admission to the acute orthopaedic ward.7 Conservative treatment is rarely applied because of poor outcomes.8 Thus, most femoral neck fractures are treated surgically. Elderly patients with femoral neck fractures often develop ALI, and hypoxaemia is the most common symptom in patients with ALI.9 According to the diagnostic criteria for acute respiratory distress syndrome established by the American-European Consensus Conference, hypoxaemia is defined as a PaO2/FiO2 ratio of ≤300. In our study, hypoxaemia was not associated with heart failure.
Compared with deep vein thrombosis, which has been regarded as the most threatening fatal complication, postoperative hypoxaemia has received little attention in the orthopaedic literature. However, postoperative hypoxaemia causes delayed recovery, prolonged hospitalisation, and wasted medical resources. Patients with hypoxaemia have a significantly longer overall hospital stay than those without hypoxaemia. Hypoxaemia affects multiple organs and tissues in the body. When cerebral anoxia occurs, brain cells can undergo morphologic anoxic damage. Arrhythmia is readily induced by myocardial hypoxia.10 Postoperative hypoxaemia is also considered to be the cause of myocardial ischaemia, arrhythmia, and neurological dysfunction.11
Clinical research on hypoxaemia in patients with femoral neck fractures has rarely been performed because hypoxaemia is generally transient and has multiple underlying causes. The prevention and treatment of postoperative hypoxaemia is an important factor for a successful operation.12 Therefore, postoperative hypoxaemia in elderly patients who have undergone hip arthroplasty must be corrected in a timely manner. Hypoxaemia can result in adverse events such as myocardial ischaemia, mental disorders, and cognitive impairment.11
In elderly patients with femoral neck fractures, the development of hypoxaemia can be influenced by multiple factors. However, the specific risk factors remain controversial. No studies have focused on the relationship between postoperative hypoxaemia and femoral neck fracture. The present study was performed to determine the incidence of and risk factors for postoperative hypoxaemia and to construct a predictive nomogram. The incidence of hypoxaemia in our study was 30.23%. The results of our research indicate that the anaesthesia type, surgical procedure, ASA classification, preoperative hypoxaemia, and age are strongly and independently associated with postoperative hypoxaemia. A preoperative PaO2/FiO2 ratio of <300 mmHg is an independent risk factor for postoperative hypoxaemia. Preoperative chronic respiratory disease is the most important factor affecting postoperative pulmonary complications. Respiratory organ degenerative lesions occur in elderly patients and are often accompanied by pulmonary diseases prior to surgery.13,14
Pulmonary function tests, clinical examination (to check for palpitations, chest tightness, shortness of breath, and similar abnormalities), physical examination (auscultation of respiratory sounds in both lungs), chest X-ray examination (to check for increased lung texture, enlargement, pulmonary patchiness, or strip shadows), blood gas analysis, and other relevant examinations should be performed in elderly patients with femoral neck fractures.15 Because of the high risk associated with anaesthesia in elderly patients with different degrees of organ dysfunction, especially cardiopulmonary insufficiency, preoperative evaluation is extremely important. The ASA classification is frequently used to assess a patient’s anaesthesia risk in the clinical setting.16 The ASA classification can be used to estimate elderly patients’ tolerance of anaesthesia before surgery, enabling better control of respiratory and circulatory function during the operation and more accurate prediction of cardiopulmonary events after the operation.17 Epidural anaesthesia can inhibit the perioperative stress response, and the incidence of hypoxaemia after an operation is lower when epidural anaesthesia is used in conjunction with general anaesthesia than when general anaesthesia is used alone.18
Postoperative hypoxaemia can induce complications such as myocardial ischaemia, arrhythmia, mental disorders, acute respiratory distress syndrome, and multiple organ failure. Therefore, early diagnosis of and intervention for hypoxaemia are very important. When the patient returns to the ward after the operation, an electrocardiographic monitor should be immediately connected, and the SpO2 should be observed. Changes in the heart rate and rhythm and in the respiratory sounds in both lungs should also be observed.21 Elderly patients often experience psychological tension caused by the serious deterioration of their physical function as well as increased pain and fear after an operation.19 They are often unwilling to cooperate with medical staff to assist in expectoration and oxygen inhalation. Medical staff need to be patient in communicating with elderly patients, informing them of the importance of active expectoration and encouraging and demonstrating expectoration. Proper sedation and analgesia can relieve pain and smooth muscle spasms and improve oxygen consumption. Continuous low-flow oxygen inhalation is necessary for patients at high risk of hypoxaemia. Inhaled dexamethasone and ambroxol hydrochloride (Mucosolvan; Boehringer Ingelheim, Ingelheim am Rhein, Germany) should be used to humidify the airways to alleviate laryngeal oedema and bronchial spasms. Patients with severe respiratory obstruction should undergo tracheotomy as early as possible. Additionally, proper nutritional support is crucial.20
The mechanism underlying the development of preoperative hypoxaemia remains unclear. To the best of our knowledge, there is no published study reporting the association between femoral neck fracture and postoperative hypoxaemia. Generally, long-term smoking and a smoking history can damage alveolar epithelial cells and tracheal and bronchial epithelial cells, resulting in pulmonary function damage.21 However, we found no significant difference in the effects of smoking between the two groups in our study. As a retrospective study, however, the patients’ smoking history might have been biased. The smoking rate among adult women in China is very low. Our study included 406 (72.6%) women, which might explain the lack of a significant difference in smoking history between patients with and without postoperative hypoxaemia.
The main limitation of this study is its retrospective design. In addition, the diagnosis of hypoxaemia was based on an objective testing method; no subjective observations were obtained from nursing staff or the attending doctors, and no expert opinions were obtained.
Conclusion
The anaesthesia type, surgical procedure, ASA classification, preoperative hypoxaemia occurrence, and age were independent predictors of the development of postoperative hypoxaemia. The risk of postoperative hypoxaemia increased as the risk score on the predictive nomogram increased. Further research is required to obtain more evidence regarding the prevention and treatment of hypoxaemia in elderly patients with femoral neck fractures.
Acknowledgements
The authors thank the Department of Orthopedics and the Records Room of Changhai Hospital Affiliated with Second Military Medical University.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Xu DF, Bi FG, Ma CY, et al. A systematic review of undisplaced femoral neck fracture treatments for patients over 65 years of age, with a focus on union rates and avascular necrosis. J Orthop Surg Res 2017; 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu XB, Wang JQ, Sun X, et al. Guidance for the treatment of femoral neck fracture with precise minimally invasive internal fixation based on the orthopaedic surgery robot positioning system. Orthop Surg 2019; 11: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 2010; 23: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samejima J, Tajiri M, Ogura T, et al. Thoracoscopic lung biopsy in 285 patients with diffuse pulmonary disease. Asian Cardiovasc Thorac Ann 2015; 23: 191–197. [DOI] [PubMed] [Google Scholar]
- 5.Takayuki N, Kohei K, Hiroshi et al. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today 2006; 36: 680–685. [DOI] [PubMed] [Google Scholar]
- 6.Tandjung R, Rosemann T. Post hip fracture care. Praxis 2011; 100: 917–921. [DOI] [PubMed] [Google Scholar]
- 7.Norring-Agerskov D, Madsen CM, Bathum L, et al. History of cardiovascular disease and cardiovascular biomarkers are associated with 30-day mortality in patients with hip fracture. Osteoporos Int 2019; 30: 1767–1778. [DOI] [PubMed] [Google Scholar]
- 8.Chang CH, Tsai SW, Wu PK. Suboptimal outcomes after internal fixation for displaced intracapsular femoral neck fractures in 50- to 60-year-old patients. Hip Int 2020; 30: 474–480. [DOI] [PubMed] [Google Scholar]
- 9.Dostálová V, Dostál P. Acute respiratory distress syndrome. Vnitr Lek 2019; 65: 193–203. [PubMed] [Google Scholar]
- 10.Kang M, Kempker JA. Definitions, epidemiology, clinical risk factors, and health disparities in acute respiratory distress syndrome. Semin Respir Crit Care Med 2019; 40: 3–11. [DOI] [PubMed] [Google Scholar]
- 11.Gouda MM, Shaikh SB, Bhandary YP, et al. Inflammatory and fibrinolytic system in acute respiratory distress syndrome. Lung 2018; 196: 609–616. [DOI] [PubMed] [Google Scholar]
- 12.Choi H, Cho JH, Kim HK, et al. Prevalence and clinical course of postoperative acute lung injury after esophagectomy for esophageal cancer. J Thorac Dis 2019; 11: 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill SE, Yamashita CM, Veldhuizen RA, et al. Lung remodeling associated with recovery from acute lung injury. Cell Tissue Res 2017; 367: 495–509. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Zhang L. Research progress in perioperative ventilator-induced lung injury. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019; 44: 346–353. [DOI] [PubMed] [Google Scholar]
- 15.Chen CY, Liao KM. The impact of atrial fibrillation in patients with COPD during hospitalization. Int J Chron Obstruct Pulmon Dis 2018; 13: 2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedenstierna G, Tokics L, Scaramuzzo G, et al. Oxygenation impairment during anesthesia: influence of age and body weight. Anesthesiology 2019; 131: 46–57. [DOI] [PubMed] [Google Scholar]
- 17.Hansson S, Nemes S, Kärrholm J, et al. Reduced risk of reoperation after treatment of femoral neck fractures with total hip arthroplasty. Acta Orthop 2017; 88: 500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharrock NE, Finerty E. Hip replacement, hip seeding, and epidural anaesthesia. Lancet 2005; 365: 1011–1012. [DOI] [PubMed] [Google Scholar]
- 19.Shoemaker WC, Appel PL, Kram HB, et al. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest 1992; 102: 208–215. [DOI] [PubMed] [Google Scholar]
- 20.Kaku S, Nguyen CD, Htet NN, et al. Acute respiratory distress syndrome: etiology, pathogenesis, and summary on management. J Intensive Care Med 2020; 35: 723–737. [DOI] [PubMed] [Google Scholar]
- 21.Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA 2019; 321: 786–797. [DOI] [PubMed] [Google Scholar]