Abstract
Unfortunately, malaria still remains a major problem in tropical areas, and it takes thousands of lives each year and causes millions of infected cases. Besides, on December 2019, a new virus known as coronavirus appeared, that its rapid prevalence caused the World Health Organization (WHO) to consider it a pandemic. As a potential drug for controlling or treating these two undesired diseases at the cellular level, chloroquine and its derivatives are being investigated, although they possess side effects, which must be reduced for effective and safe treatments. With respect to the importance of this medicine, the current research aimed to calculate the solubility of chloroquine in supercritical carbon dioxide, and evaluated effect of pressure and temperature on the solubility. The pressure varied between 120 and 400 bar, and temperatures between 308 and 338 K were set for the measurements. The experimental results revealed that the solubility of chloroquine lies between 1.64 × 10−5 to 8.92 × 10−4 (mole fraction) with different functionality to temperature and pressure. Although the solubility was indicated to be strong function of pressure and temperature, the effect of temperature was more profound and complicated. A crossover pressure point was found in the solubility measurements, which indicated similar behaviour to an inflection point. For the pressures higher than the crossover point, the temperature indicated direct effect on the solubility of chloroquine. On the other hand, for pressures less than the crossover point, temperature enhancement led to a reduction in the solubility of chloroquine. Moreover, the obtained solubility results were correlated via semi-empirical density-based thermodynamic correlations. Five correlations were studied including: Kumar & Johnston, Mendez-Santiago-Teja, Chrastil, Bartle et al., and Garlapati & Madras. The best performance was obtained for Mendez-Santiago-Teja's correlation in terms of average absolute relative deviation percent (12.0%), while the other examined models showed almost the same performance for prediction of chloroquine solubility.
Keywords: Pharmaceuticals, Chloroquine, Thermodynamics, Solubility, Crossover pressure
1. Introduction
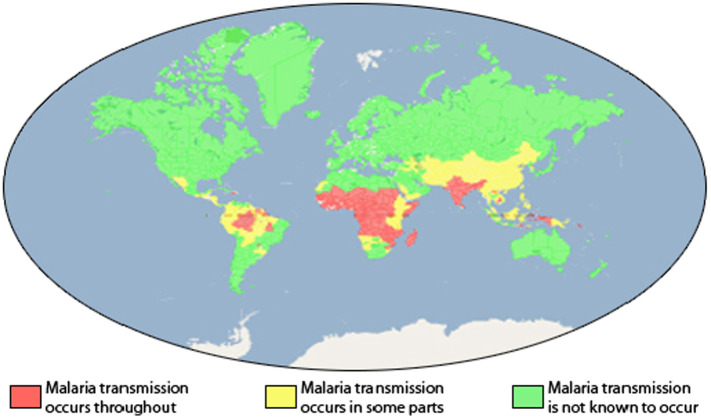
Globally, malaria leads to nearly 1–3 million casualties annually, whereas around half the world's people live in regions that are at the risk of malaria transmission. Unfortunately, most of these deaths related to children aged 5 years or younger, placing malaria as the world's fourth leading cause of mortality rate in children younger than five years [1]. Fig. 1 shows distribution of malaria infections worldwide.
Fig. 1.
Malaria distribution worldwide [1].
Beside malaria which is a worldwide severe concern, recently spread virus called COVID-19 is the other disease, putting the world in a great shock after its first appearance in Wuhan, China in December 2019. Unfortunately, the rapid spread of this new coronavirus called intense acute respiratory syndrome coronavirus (SARS-CoV-2) forced the World Health Organization (WHO) to promulgate it as a pandemic in March 2020 leading to more shocking results in the world [2]. With respects to the severe conditions these two diseases introduced in the globe, the researchers are seeking for efficient and safe treatments and medications for controlling them in cellular level in daily attempts. One of the widely examined drugs is called Chloroquine which is a 4-aminoquinoline compound discovered in Germany (1934) during a program which was concentrated on the treatment of malaria [[3], [4], [5], [6]]. The efficiency of this drug was acceptable such that it was rapidly selected as a treatment for all malaria throughout the world. An enormous amount of this drug (almost 100 million malaria treatment doses) was yearly dispended, and China announced production of more than 400 tons [3]. This is why chloroquine can claim as the 12 drugs that people have been most exposed. However, similar to the other medications, chloroquine, and hydroxychloroquine have some adverse effects such as indigestion, nausea, sporadically vomiting, ocular disorder, and headache [[7], [8], [9]].
Unfortunately, it is reported that not only the lethality of chloroquine in overdose is more than other drugs, but also chloroquine introduces abnormal pharmacokinetic characteristics with huge certain amounts of distribution and very slow deletion from body (terminal deletion half-lives > 1 month) [6,10]. Considering the concerns associated with chloroquine administration, it is of vital importance to produce a dosage form of this drug in a way that reduces its health risks. Different approaches have been suggested to enhance drug bioavailability such that low dosage can be administered. Co-crystallization [11], salt formation [12], amorphous solid dispersion (ASD) [13], and nanonization [14] have been reported in literature among which nanonization can be utilized to prepare drugs at nano size in combination with supercritical based processes. Indeed, at nano scale the drug particles indicate higher solubility due to high surface area and energy. Therefore, low dosage of drugs can be used at nano scale, which in turn decreases the drug's side effects.
A novel solution for preparation of nano-sized medicine is to produce via supercritical carbon dioxide (SC-CO2)-based particle formation technologies which can also alter the morphology of the particles in a desired manner [[14], [15], [16], [17]]. In this technology, usually a gas at supercritical condition is used as dense solvent for preparation of drug nanoparticles. CO2 has been extensively used in supercritical-based technologies due to its mild supercritical pressure and temperature, non-explosive property, cheap gas, and non-toxic solvent for application in pharmaceuticals [18]. One of the required parameters of drug to be processed through supercritical technology is its solubility in the solvent. Indeed, drug solubility in supercritical solvent determines whether the supercritical process is viable for the particular drug or not [19,20]. Moreover, the solubility needs to be determined at wide range of temperatures and pressures in order to size and design the process at industrial scale [21]. Different studies have been carried out to measure the solubility of active pharmaceutical ingredients (APIs) in supercritical CO2 as solvent via different techniques [14,22]. Therefore, for a API candidate to be processed using supercritical-based technology the solubility must be measured at different conditions, e.g. temperature (T) and pressure (P).
Given that measuring solubility of whole API candidates in supercritical solvents in wide ranges of T & P is expensive and time consuming, it is required to develop theoretical framework to predict solubilities. In terms of API solubility in supercritical solvents, thermodynamic models can be utilized among which semi-empirical correlations have been employed and proposed for API solubility in supercritical carbon dioxide due to their simplicity and predictive characteristics [15,[23], [24], [25], [26], [27]]. The solubility of two APIs, namely Lansoprazole and Esomeprazole in supercritical CO2 between 120 and 270 bar, and 308–338 K was reported by Sodeifian et al. [28,29]. It was reported that Lansoprazole solubility was in the range of 1.15 × 10−5 to 7.36 × 10−4, while for Esomeprazole the solubility was between 1.11 × 10−5 to 9.10 × 10−4 in mole fraction unit. They also reported solubility modeling for the case of Lansoprazole applying six semi-empirical models along with two distinct EoSs (Equation of State) e.g. Peng–Robinson (PR) and SAFT-VR. It was revealed that there is no significant superiority between the employed semi-empirical correlations and EoSs in terms of API solubility predictions. A comprehensive review regarding the possible approaches for API solubility modeling in supercritical CO2 which can be used as a guideline for the researchers to pick the best choice according to their systems has been published by Sodeifian et al. [30].
Given that there is no reported solubility for chloroquine under various pressures and temperatures in supercritical carbon dioxide, the current study is focused on the evaluating solubility of this drug. In this way, in the first stage, the solubility values were measured in various P & T, and then the measured solubility data were correlated utilizing five density-based correlations, i.e. Mendez-Santiago-Teja (MST) [31], Bartle et al. [32], Chrastil [25], Kumar-Johnston (KJ) [26], and Garlapati and Madras [33].
2. Experiments
2.1. Materials and method
Chloroquine (C18H26ClN3), with molecular weight of 319.87 g·mol−1 was provided from Matrix Scientific, USA with purity > 95%. Chloroquine was further purified by treatment with CO2 (purity > 99.8%) with the pressure of 500 bar and temperature of 338 K for 3 h to ensure that there is not any impurities in the API used for the solubility experiments. The molecular structure of chloroquine is shown in Fig. 2 .
Fig. 2.
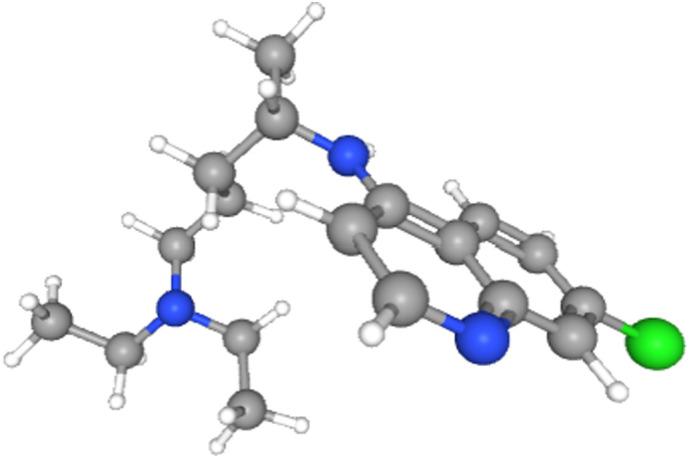
Chemical structure of chloroquine [5].
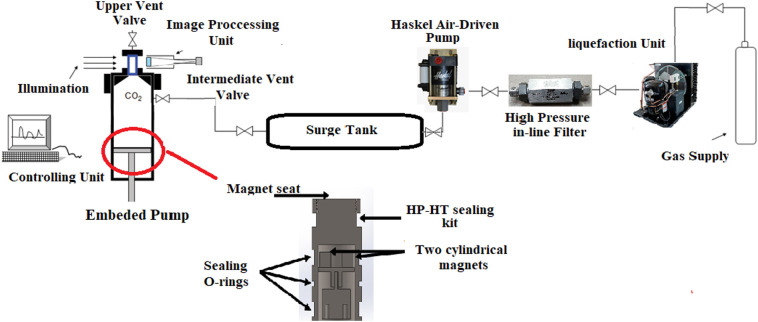
A homemade PVT cell was utilized throughout the solubility measurement experiments in this work. The cell was designed for the operating pressure and temperature of up to 600 bar and 426 K, respectively (Designed and constructed by Fanavari Atiyeh Pouyandegan Exir company, Arak, Iran). The process schematics is represented in Fig. 3 . The sample was placed in the PVT cell which has a capacity of 0.4 L with an embedded motorized pump. The measurement system constitutes of two compartments including CO2 liquefication and PVT cell. In the first compartment of experimental setup, CO2 gas is liquefied by reducing temperature down to 253 K, and rising the pressure up to 70 bar. Then the condensed CO2 passes through a filter (5 micron pore size) to remove impurities and suspended solids. The purified condensed CO2 enters the PVT cell after passing through a surge tank (1 L) to dampen the pressure fluctuations in the PVT cell. Pressure inside PVT cell is controlled using a pressure transmitter (Keller, Switzerland). 5 g of compacted chloroquine was placed in the PVT cell, and gently mixed for 3 h, and finally the solubility of API was measured using gravimetric method. All measurements were carried out in triplicate. The detailed description of the solubility measurements have been reported in our previous publications [15,16,20].
Fig. 3.
The schematics of used machine for the solubility measurements [16].
Reprinted from [16], Copyright (2020), with permission from Elsevier.
3. Results and discussions
3.1. Influence of temperature and pressure on chloroquine solubility
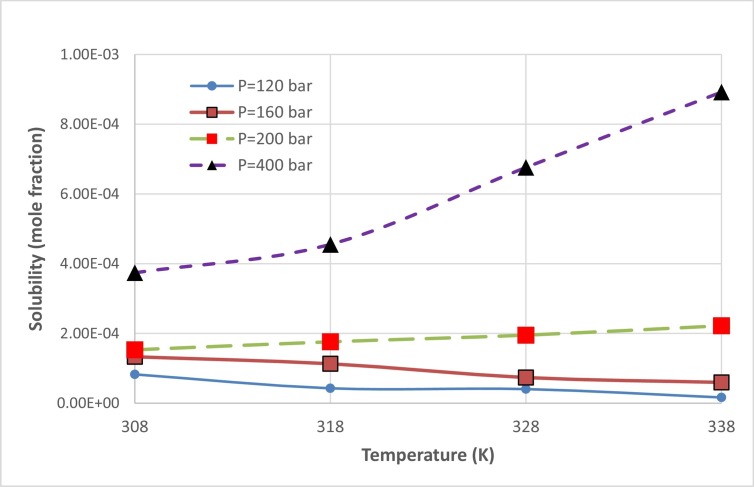
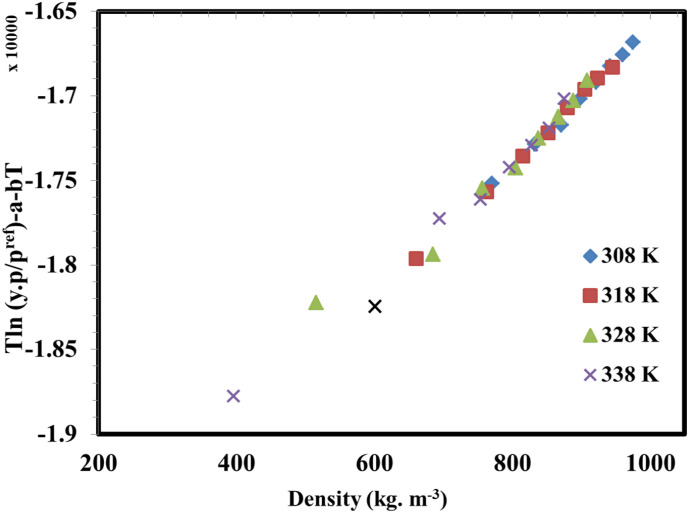
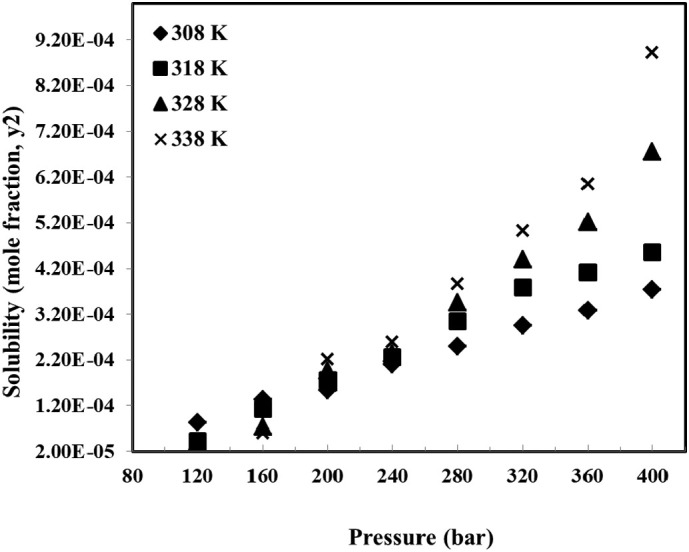
The effect of temperature and pressure on chloroquine solubility is shown in Fig. 4 and Table 1 . In the measurements, 8 pressure levels were considered between 120 and 400 bar, and 4 levels were considered for temperature between 308 and 338 K. The results indicate that chloroquine solubility varies between 1.64 × 10−5 and 8.92 × 10−4 (mole fraction), for the entire range of pressure and temperature (see Table 1). The results revealed that the measured data is reproducible with a maximum relative standard deviation of 8%, and average relative standard deviation of 5%. The deviations could be attributed to the fluctuations in measuring and controlling temperature and pressure in the PVT cell, due to operation at high pressures.
Fig. 4.
Solubility of chloroquine as function of T & P.
Table 1.
Chloroquine solubility at different temperatures and pressures.a
| P/bar | T/K |
|||||||
|---|---|---|---|---|---|---|---|---|
| 308 |
318 |
328 |
338 |
|||||
| y | SD | y | SD | y | SD | y | SD | |
| 120 | 8.26 × 10−5 | 6.72 × 10−6 | 4.26 × 10−5 | 3.09 × 10−6 | 4.04 × 10−5 | 3.06 × 10−6 | 1.64 × 10−5 | 1.06 × 10−6 |
| 160 | 1.33 × 10−4 | 1.06 × 10−5 | 1.13 × 10−4 | 3.93 × 10−6 | 7.35 × 10−5 | 3.40 × 10−6 | 5.96 × 10−5 | 2.90 × 10−6 |
| 200 | 1.53 × 10−4 | 5.17 × 10−6 | 1.76 × 10−4 | 4.73 × 10−6 | 1.95 × 10−4 | 1.27 × 10−5 | 2.22 × 10−4 | 1.30 × 10−5 |
| 240 | 2.11 × 10−4 | 9.32 × 10−6 | 2.26 × 10−4 | 1.34 × 10−5 | 2.33 × 10−4 | 1.44 × 10−5 | 2.59 × 10−4 | 2.20 × 10−5 |
| 280 | 2.50 × 10−4 | 1.03 × 10−5 | 3.05 × 10−4 | 4.35 × 10−6 | 3.45 × 10−4 | 1.24 × 10−5 | 3.87 × 10−4 | 2.73 × 10−5 |
| 320 | 2.95 × 10−4 | 1.92 × 10−5 | 3.78 × 10−4 | 1.72 × 10−5 | 4.40 × 10−4 | 1.84 × 10−5 | 5.02 × 10−4 | 3.57 × 10−5 |
| 360 | 3.28 × 10−4 | 1.04 × 10−5 | 4.12 × 10−4 | 1.54 × 10−5 | 5.21 × 10−4 | 1.35 × 10−5 | 6.04 × 10−4 | 4.59 × 10−5 |
| 400 | 3.74 × 10−4 | 2.65 × 10−5 | 4.55 × 10−4 | 2.13 × 10−5 | 6.76 × 10−4 | 4.62 × 10−5 | 8.92 × 10−4 | 2.27 × 10−5 |
Standard uncertainty, u, are u (T) = 0.1 K and u (P) = 0.35 bar.
The solubility data as function of temperature (T) and pressure (P) depicted in Fig. 4 reveals that increasing pressure led to chloroquine solubility enhancement for all temperatures. This behaviour can be attributed to the density enhancement of solvent at high pressures which increases the solvating power of supercritical solvent. For the solubility measurements at the temperature of 338 K, it is observed that solubility increases from 1.64 × 10−5 to 8.92 × 10−4 which is the highest solubility value, when the pressure rises from 120 to 400 bar. The effect of temperature on solubility at 4 different pressures is illustrated in Fig. 5 . It is observed that the solubility of chloroquine is decreased with enhancement of temperature for the pressures of 120 and 160 bar. However, a reverse trend is observed for the higher pressures, i.e. 200 and 400 bar. It is indicated that at pressures greater than 160 bar, chloroquine solubility is increased with temperature. The reason for this shifting behaviour in the chloroquine solubility could be due to the dual influence of temperature regarding the crossover pressure phenomenon. There is a point in which the temperature effect on solubility shows reducing trend, for the pressures less than this point, while for the values greater than crossover pressure point the temperature effect is increasing. For the case of chloroquine solubility in supercritical carbon dioxide, the crossover point lies between 160 and 200 bar. From the thermodynamic point of view, temperature has two different influences on solubility, i.e. density change as well as modification of sublimation pressure which act inversely. For the pressures below the crossover pressure, decreasing the density because of increasing the temperature is predominant which resulted in decreasing the solubility, while for the pressures higher than crossover point sublimation pressure modification can compensate for the reductive effect of density, leading to an enhancement of chloroquine solubility.
Fig. 5.
Effect of temperature on solubility of chloroquine.
3.2. Thermodynamic modeling
In order to predict the solubility data as function of pressure and temperature, five different semi-empirical correlations were employed. The models consist of unknown parameters which need to be determined by curve fitting techniques. The used correlations include: Mendez-Santiago-Teja (MST) [31], Bartle et al. [32], Chrastil [25], Kumar-Johnston (KJ) [26], and Garlapati and Madras [33]. The mathematical formula of each correlation can be found elsewhere [15,16]. The fitting parameters of these correlations were determined via multiple linear regression approach, and the corresponding AARD % of each correlation is listed in Table 2 . The results revealed that among the employed correlations, MST led to the most accurate predictions (AARD % of 12.0%). However, other models show almost similar accuracy in terms of AARD, and no significant privilege was observed among the used correlations.
Table 2.
Fitting parameters of the semi-empirical correlations.
| Model | AARD %a |
|---|---|
| Bartle et al. | 13.0 |
| Mendez-Santiago-Teja | 12.0 |
| Kumar and Johnstone | 12.3 |
| Chrastil | 13.3 |
| Garlapati and Madras | 13.6 |
AARD % = 100 × ∑ ((ycalc − yexp.) / yexp.).
Having determined the unknown parameters of the correlations, we can estimate the thermodynamic properties of chloroquine such as heat of sublimation and total heat as follows [25,32]:
| (1) |
| (2) |
In Eqs. (1), (2), R denotes the gas universal constant, b denotes the fitting parameter in Bartle et al.'s model, and a refers to the fitting parameter in Chrastil model. Using Eqs. (1), (2), heat of sublimation was calculated to be 59.4 kJ/mol, and the total heat equals 39.1 kJ/mol. Moreover, the solvation enthalpy (Δ sol H) was estimated about −20.3 kJ/mol using Hess's law.
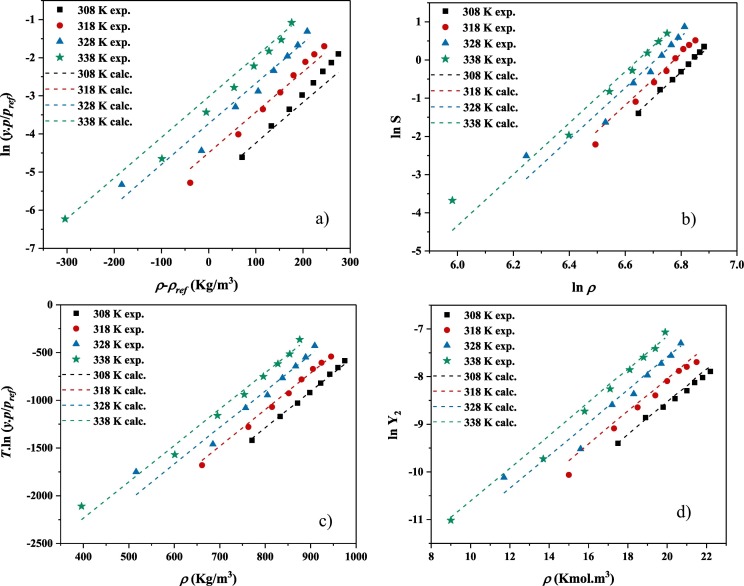
The comparisons between the measured and calculated solubility of chloroquine are represented in Fig. 6 for four correlations. It is clearly observed that the used thermodynamic models are well capable to correlate the measured solubility data at different pressures and temperatures, and can be utilized as predictive models for chloroquine solubility in supercritical carbon dioxide as solvent. Furthermore, performing self-consistency test for MST correlation as a representative of the other models revealed a successful self-consistency test indicating the extrapolative ability of the models. In detail, the self-consistency test revealed that not only the examined models possess correlative capability for the examined temperatures and pressures, but also they can be used to extrapolate the solubility of chloroquine in P and T out of the measured values (see Fig. 7 ).
Fig. 6.
Chloroquine solubility results based on a) Bartle et al. model, b) Chrastil, c) MST and d) KJ model.
Fig. 7.
Self-consistency results using MST correlation.
4. Conclusions
Solubility of chloroquine as an important API at different P & T in supercritical carbon dioxide using gravimetric method was obtained. A PVT cell connected to a liquefier unit was used to measure the solubility at different conditions. The pressure and temperature varied between 120 and 400 bar and 308 and 338 K, respectively. The measured solubility data were in the range of 1.64 × 10−5 and 8.92 × 10−5 based on mole fraction with a maximum relative standard deviation of about 8%. The evaluated solubility results showed a direct relationship between pressure and solubility with significant impact for temperatures of 328 and 338 K compared with 308 and 318 K. Besides, the measurements illustrated a complicated trend between temperature and solubility due to existence of crossover pressure. Indeed, the evaluated solubility results revealed a crossover pressure of about 200 bar where the effect of temperature changes on the solubility. For the pressures less than crossover point, increasing the temperature resulted in decreasing the solubility of chloroquine, on the other hand, for the pressures greater than this point led to an increase in the solubility of chloroquine due to the greater influence of sublimation pressure rather density reduction. Finally, the evaluated solubility results were correlated utilizing five semi-empirical three-parameter density-based correlations: Bartle et al., Chrastil, MST, KJ and Garlapati & Madras models. The results demonstrated that all of the investigated models have the same accuracy and no one indicated a significant superiority over the other correlations. Moreover, the self-consistency test was conducted for MST model, and revealed that not only it is possible to correlate the solubility of chloroquine in the examined conditions, but also it is viable to extrapolate the solubility of chloroquine in the ranges out of the examined temperatures and pressures which makes them an excellent choice for solubility modeling approach in supercritical state.
CRediT authorship contribution statement
Mahboubeh Pishnamazi: Conceptualization, Modeling, Data analysis. Saber Hosseini: Project administration, Writing-review, Validation. Samyar Zabihi: Writing-draft, Validation, Analysis. Fatemeh Borousan: Writing-draft, Conceptualization. Ali Zeinolabedini Hezave: Performing experiments, Experimental design. Azam Marjani: Funding, Data analysis, Revision. Saeed Shirazian: Supervision, Modeling, Analysis, Writing-review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Arak Science and Technology Park (Iran), Fanavari Atiyeh Pouyandegan Exir and Fanavari Arena Exir Sabz companies that supported this investigation by providing the required types of equipment, materials and budget. This work was supported by the Government of the Russian Federation (Act 211, contract 02.A03.21.0011) and by the Ministry of Science and Higher Education of Russia (grant FENU-2020-0019).
References
- 1.2020. https://www.cdc.gov/malaria/malaria_worldwide/impact.html
- 2.World Health Organization Coronavirus disease 2019 (COVID-19) situation report - 75. https://www.who.int/docs/default-source/coronaviruse/situationreports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2 Available at.
- 3.Tonnesmann E., Kandolf R., Lewalter T. Chloroquine cardiomyopathy - a review of the literature. Immunopharmacol. Immunotoxicol. 2013;35(3):434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Zvi I., et al. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://pubchem.ncbi.nlm.nih.gov/compound/Chloroquine (accessed 2020)
- 6.White N.J., et al. COVID-19 prevention and treatment: a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips-Howard P.A., ter Kuile F.O. CNS adverse events associated with antimalarial agents. Drug Saf. 1995;12(6):370–383. doi: 10.2165/00002018-199512060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Huzly D., et al. Malaria chemoprophylaxis in German tourists: a prospective study on compliance and adverse reactions. J. Travel Med. 1996;3(3):148–155. doi: 10.1111/j.1708-8305.1996.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli C., Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin. Drug Saf. 2017;16(3):411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 10.Ball D.E., Tagwireyi D., Nhachi C.F.B. Chloroquine poisoning in Zimbabwe: a toxicoepidemiological study. J. Appl. Toxicol. 2002;22(5):311–315. doi: 10.1002/jat.864. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh R., et al. Pharmaceutical cocrystal drug products: an outlook on product development. Trends Pharmacol. Sci. 2018;39(12):1033–1048. doi: 10.1016/j.tips.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Suresh A., et al. Improving solubility and intrinsic dissolution rate of ofloxacin API through salt formation via mechanochemical synthesis with diphenic acid. J. Mol. Struct. 2020;1221 [Google Scholar]
- 13.Liu G., et al. Development of nimesulide amorphous solid dispersions via supercritical anti-solvent process for dissolution enhancement. Eur. J. Pharm. Sci. 2020;152 doi: 10.1016/j.ejps.2020.105457. [DOI] [PubMed] [Google Scholar]
- 14.Chen B.-Q., et al. Continuous nanonization of lonidamine by modified-rapid expansion of supercritical solution process. J. Supercrit. Fluids. 2018;133: p:486–493. [Google Scholar]
- 15.Pishnamazi M., et al. Thermodynamic modelling and experimental validation of pharmaceutical solubility in supercritical solvent. J. Mol. Liq. 2020;319 [Google Scholar]
- 16.Pishnamazi M., et al. Measuring solubility of a chemotherapy-anti cancer drug (busulfan) in supercritical carbon dioxide. J. Mol. Liq. 2020;317 [Google Scholar]
- 17.Zabihi S., et al. Measuring salsalate solubility in supercritical carbon dioxide: experimental and thermodynamic modelling. J. Chem. Thermodyn. 2021;152 [Google Scholar]
- 18.Dehghani F., Foster N.R. Dense gas anti-solvent processes for pharmaceutical formulation. Curr. Opin. Solid State Mater. Sci. 2003;7(4–5):363–369. [Google Scholar]
- 19.Johannsen M., Brunner G. Solubilities of the fat-soluble vitamins A, D, E, and K in supercritical carbon dioxide. J. Chem. Eng. Data. 1997;42(1):106–111. [Google Scholar]
- 20.Zabihi S., et al. Experimental solubility measurements of fenoprofen in supercritical carbon dioxide. J. Chem. Eng. Data. 2020;65(4):1425–1434. [Google Scholar]
- 21.Hezave A.Z., et al. Analyzing the solubility of fluoxetine hydrochloride in supercritical carbon dioxide. J. Supercrit. Fluids. 2013;73:57–62. [Google Scholar]
- 22.Pitchaiah K.C., et al. Solubility of trioctylmethylammonium chloride in supercritical carbon dioxide and the influence of co-solvents on the solubility behavior. J. Supercrit. Fluids. 2018;138:102–114. [Google Scholar]
- 23.Yamini Y., Hassan J., Haghgo S. Solubilities of some nitrogen-containing drugs in supercritical carbon dioxide. J. Chem. Eng. Data. 2001;46(2):451–455. [Google Scholar]
- 24.Garnier S., et al. Modelling solubility of solids in supercritical fluids using fusion properties. Fluid Phase Equilib. 1999;158:491–500. [Google Scholar]
- 25.Chrastil J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982;86(15):3016–3021. [Google Scholar]
- 26.Kumar S.K., Johnston K.P. Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids. 1988;1(1):15–22. [Google Scholar]
- 27.Bian X.Q., Du Z.M., Tang Y. An improved density-based model for the solubility of some compounds in supercritical carbon dioxide. Thermochim. Acta. 2011;519(1–2):16–21. [Google Scholar]
- 28.Sodeifian G., Detakhsheshpour R., Sajadian S.A. Experimental study and thermodynamic modeling of esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids. 2019;154 [Google Scholar]
- 29.Sodeifian G., Sajadian S.A., Derakhsheshpour R. Experimental measurement and thermodynamic modeling of Lansoprazole solubility in supercritical carbon dioxide: application of SAFT-VR EoS. Fluid Phase Equilib. 2020;507 [Google Scholar]
- 30.Sodeifian G., et al. A comprehensive comparison among four different approaches for predicting the solubility of pharmaceutical solid compounds in supercritical carbon dioxide. Korean J. Chem. Eng. 2018;35(10):2097–2116. [Google Scholar]
- 31.Mendez-Santiago J., Teja A.S. The solubility of solids in supercritical fluids. Fluid Phase Equilib. 1999;158:501–510. [Google Scholar]
- 32.Bartle K.D., et al. Solubilities of solids and liquids of low volatility in supercritical carbon-dioxide. J. Phys. Chem. Ref. Data. 1991;20(4):713–756. [Google Scholar]
- 33.Garlapati C., Madras G. New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochim. Acta. 2010;500(1–2):123–127. [Google Scholar]