Abstract
High-grade serous ovarian carcinoma (HGSOC) is one of the most lethal gynecological malignancies; however, the precise molecular mechanisms have not been fully characterized. Fibulin-5 (FBLN-5) is an extracellular matrix (ECM) glycoprotein, and plays a crucial role in maintaining the stability of ECM structures, regulating cell proliferation and tumorigenesis. In the present study, the expression of FBLN-5, as determined by western blot analysis and immunohistochemistry, was significantly increased in normal fallopian tube (FT) samples compared with that in HGSOC samples, and decreased FBLN5 expression was associated with unfavorable prognosis of HGSOC. Functional characterization revealed that FBLN5 overexpression significantly inhibited migration, invasion and proliferation abilities of ovarian cancer cells in vitro. Furthermore, micro (mi)RNA-27a-3p (miR-27a-3p) was revealed to be increased in HGSOC, and dual-luciferase reporter assay indicated that miR-27a-3p was functioned as a negative regulator of FBLN5 by directly binding with its 3′-untranslated region. Collectively, FBLN5 expression was associated with prognosis, proliferation, and metastasis in HGSOC. We hypothesized that FBLN5 was targeted by miR-27a-3p and may serve as a biomarker and provide a new therapeutic approach for the treatment of HGSOC.
Keywords: microRNA-27a-3p, fibulin-5, high-grade serous ovarian carcinoma, prognosis, proliferation, metastasis
Introduction
Ovarian cancer (OC) is one of the leading causes of cancer-associated deaths. There was an estimated 52,100 newly-diagnosed cases and 22,500 fatalities from OC in China in 2015 and 21,750 newly-diagnosed cases and 13,940 fatalities in the USA in 2020 (1,2). High-grade serous ovarian carcinomas (HGSOC) account for 70–80% of all OC cases and the mortality rate is markedly high (3). Despite the important advances in diagnosis and research, the role and precise mechanisms of HGSOC have not been fully elucidated.
The fibulin protein family widely exists in the extracellular matrix (ECM), and plays a crucial role in the formation and stabilization of the basement membrane, and loose connective tissue and elastic fibers (4,5). In addition to the role in tissue framework, fibulin proteins have also been revealed to be involved in tumorigenesis and progression of cancer (6). Depending on the cell type and cellular context, different fibulins possess both antitumor and pro-tumor properties (7). Fibulin-5 (FBLN5), a 66-kDa secreted glycoprotein, plays significant roles in cell adhesion and motility, and cell-to-cell and cell-to-matrix communication (8,9). In addition, FBLN5 has been revealed to regulate cell growth, cell migration, tissue repair and tumorigenesis (10). Numerous studies have indicated the prognostic potential of FBLN5, as a tumor suppressor in a diverse range of cancers, such as breast cancer, lung cancer and hepatocellular carcinoma (11–14). However, the molecular mechanisms and prognostic significance of FBLN5 in HGSOC have not been characterized.
Micro(mi)RNAs are endogenous, short (21 to 25 nucleotides) non-coding RNAs that repress gene expression at the post-transcriptional level (15). miRNAs target the mRNAs by complementary binding to the homology sequence in the 3′-untranslated region (3′-UTR) (16), and have an overarching regulatory role during carcinogenesis and tumor development (17). Specifically, micro (mi)RNA-27a-3p (miR-27a-3p) was revealed to be a significant positive regulator of tumorigenesis and progression in different types of cancer, including hepatocellular (18), nasopharyngeal (19), and oral squamous carcinoma (20).
In the present study, the expression level of FBLN5 and the molecular mechanisms in patients with HGSOC were elucidated. FBLN5 was identified and proved to negatively regulate the malignant behavior of ovarian cancer both in vitro and in vivo, implying that FBLN5 and its upstream regulator miR-27a-3p has the potential to be a novel therapeutic target of ovarian cancer.
Materials and methods
Tissue samples
In the present study, a total of 216 samples [57 normal fallopian tubes (FT) and 159 HGSOC tissues] were collected at Qilu Hospital of Shandong University (Shandong, China) from May 2006 to July 2013. The ages of all included patients ranges from 35 to 78 years. The normal FT samples were obtained from patients who had a benign gynecological tumor, while the HGSOC samples were collected from patients undergoing surgical resection without previous chemotherapy. Written informed consent was provided by each patient prior to surgery. In addition, the guidelines developed by the Ethics Committee of Shandong University Qilu Hospital (KYLL-2018-229) were also adhered to.
Cells culture
The SKOV3 and 293T cell lines were purchased from the American Type Culture Collection and the Chinese Academy of Sciences, respectively. The HEY and A2780 cell lines were a kind gift from the laboratory of Dr. Wei (Department of Gynecology and Obstetrics, Northwestern University, Feinberg School of Medicine). The cells were cultured in the appropriate medium (SKOV3 and A2780 cells, RPMI-1640 medium and HEY and 293T cells, DMEM) (all from Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), and all the cells were maintained in an incubator under standard growth conditions.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from cells and samples was extracted using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). The mRNA and miRNA were reverse-transcribed using the Prime Script RT Reagent kit and One Step Prime Script miRNA cDNA Synthesis kit, respectively, (both from Takara Bio, Inc.) following the manufacturer's guidelines and recommended thermocycling conditions. The cDNA was amplified using the SYBR Green (Takara Bio, Inc.) and Real-Time PCR (qPCR) System (QuantStudio3; Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: 95°C for 30 sec, 40 cycles at 95°C for 5 sec and 60°C for 30 sec, 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. U6 and ACTB were used as the internal controls for normalization and comparison. The 2−ΔΔCq method (21) was used to calculate the expression level of the specific genes. The primers are presented in Table SIA.
Western blot analysis
The total protein from the cells and tissues was isolated in ice-cold lysis solution containing RIPA buffer, phenylmethylsulfonyl fluoride and sodium fluoride (100:1:1) (all from Beyotime Bio, Inc.), and then centrifuged for 15 min at 12,000 × g at 4°C. The supernatants were used to calculate the protein concentration using a bicinchoninic acid Assay (Merck KGaA). The proteins (50 µg) from each sample were separated using electrophoresis (separation gel, 10–12%; stacking gel, 5%). Subsequently, the proteins were transferred onto a PVDF membrane (0.22 µm), blocked (5% skim milk for 1 h at room temperature), and incubated with the primary antibodies overnight at 4°C. Then the membrane was incubated with the horseradish peroxidase-conjugated secondary antibodies for 1–2 h. Finally, the protein signals were visualized using an enhanced chemiluminescence detection kit (PerkinElmer, Inc.), and the densitometry of the protein bands were analyzed using the Image J v1.8.0 software (National Institutes of Health). GAPDH was used as the endogenous control. The primary antibodies in this study included: Anti-FBLN5 (dilution 1:1,000, cat. no. ab66339; Abcam), anti-GAPDH (dilution 1:1,000, cat. no. 2118; Cell Signaling Technology), anti-N-cadherin (dilution 1:1,000, cat. no. 13116; Cell Signaling Technology), anti-Snail (dilution 1:1,000, cat. no. 3879; Cell Signaling Technology), anti-E-cadherin (dilution 1:1,000, cat. no. 3195; Cell Signaling Technology), anti-p21 (dilution 1:1,000, cat. no. 2947; Cell Signaling Technology), anti-p27 (dilution 1:1,000, cat. no. 3686; Cell Signaling Technology). The secondary antibodies were purchased from KPL: Anti-mouse IgG (cat. no. 5220-0341, dilution 1:6,000) and anti-rabbit IgG (cat. no. 5220-0336, dilution 1:4,000).
Immunohistochemical (IHC) staining
A tissue microarray (TMA) of HGSOC tissues (4-µm thick, made by our laboratory) were incubated at 60°C for 35 min. The TMAs were immediately deparaffinized, rehydrated with xylene and a graded ethanol series. Subsequently, antigen retrieval was performed using citric acid buffer and microwave irradiation. The non-specific antigens were blocked using a normal goat serum and the slides were then probed with rabbit anti-FBLN5 antibody (1:300; cat. no. ab202977; Abcam) overnight at 4°C. The signal was detected using 3,3′-diaminobenzidine chromogenic reagent kit (ZSGB Bio, Inc.) and hematoxylin-counterstained (Solarbio Bio, Inc.) according to the manufacturer's instructions. The final score of FBLN5 staining was determined on the basis of extent and intensity.
Plasmid construction and transfection
The coding DNA sequences of FBLN5 were obtained from Shanghai GeneChem Co., Ltd., and then ligated into a pLenti-C-Myc- DDK-IRES-Puro (PCMV) plasmid (OriGene Technologies, Inc.). Lentivirus expressing FBLN5 were obtained using the 293T cell line packaged with the pMD2.G (Addgene, Inc.) and psPAX2 (Addgene, Inc.) vectors. The FBLN5-expressing stable cells were produced using the infected lentivirus for 24 h and selected for a week in medium containing antibiotics. Small interfering (si)RNA targeting FBLN5 was synthesized from Shanghai Biosune Biotechnology Co., Ltd., and were similar to those stated in previous studies (4,22). The sequences of the siRNA and miRNA are presented in Table SIB and C.
Cell proliferation assays
The MTT (Sigma-Aldrich; Merck KGaA) assay was used to measure cell growth. A total of (0.8–1)x103 cells/well were plated in 96-well plates in sextuplicate, for 1–5 days. At the same time every day, 20 µl (5 mg/ml) MTT reagent was added to each well, and continued to culture for 4 h at 37°C. Subsequently, the supernatant was removed and DMSO (Sigma-Aldrich; Merck KGaA) was then added to each well. The optical density was detected at 490 nm using a microplate reader (Thermo Fisher Scientific, Inc.). All experiments were performed in triplicate.
Clonogenic assays
To evaluate the colony formation ability, single cells were seeded in a 6-well plate (1,000 cells/well) and cultured for two weeks, under standard culture conditions. Thereafter, 100% methanol was used to fix the colonies for 15 min, and stained with 0.1% crystal violet for 15 min at room temperature. Colonies of more than 50 cells were counted. All experiments were performed in triplicate.
Invasion and migration assays
For both the assays, the cells were seeded in a Transwell chamber (8-µm), but only the membrane for the invasion assays was coated with Matrigel (both from BD Biosciences). A total of 200 µl suspension (1×105 cells) was added to the upper chamber, and 700 µl medium supplemented with 20% FBS was added in the lower chamber. Following routine incubation at 37°C for the appropriate time-points (6–24h), the cells in the upper chamber were removed using a cotton swab. Subsequently, the cells that reached the lower surface were fixed and stained with 100% methanol and 0.1% crystal violet for 15 min at room temperature, respectively.
Tumor formation assays in nude mice
Female nude mice (BALB/c; 4–5 weeks old; average weight, 15 g) were obtained from the NBRI of Nanjing University (Jiangsu, China), and maintained in specific-pathogen-free (SPF) conditions with 25°C temperature, 50% humidity, 12-h light/dark cycle, and had free access to water and food. Nude mice health and behavior were monitored every day. Then, a 200-µl cell suspension (5×106 cells) was injected subcutaneously into either side of the armpit, in each nude mouse. After 2–3 weeks, all the mice were euthanized by intraperitoneal injection of pentobarbital sodium (200 mg/kg), which was confirmed by respiratory and cardiac arrest, and then the tumors were excised and weighed. The diameter of the longest of these tumors was <2 cm, and all animal experiments adhered to the guidelines and policy of the Shandong University Animal Care and Use Committee.
Bioinformatics analyses
The GEPIA database (http://gepia.cancer-pku.cn/) was used to evaluate the mRNA expression levels of FBLN5 in serous ovarian cancer and normal tissues (23). The TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org) were employed to identify predicted miRNA sequences that may regulate FBLN5 (24,25).
Statistical analysis
The data analysis was performed using SPSS v18.0 software (SPSS, Inc.). The unpaired Student's t-test and χ2 test were used to evaluate the association and the significant difference between groups. The curve of overall survival (OS) was assessed using the Kaplan-Meier method and the log-rank test. The experimental data are presented as the mean ± standard error of the mean and P<0.05 was considered to indicate a statistically significant difference.
Results
FBLN5 expression is significantly downregulated in the HGSOC tissue
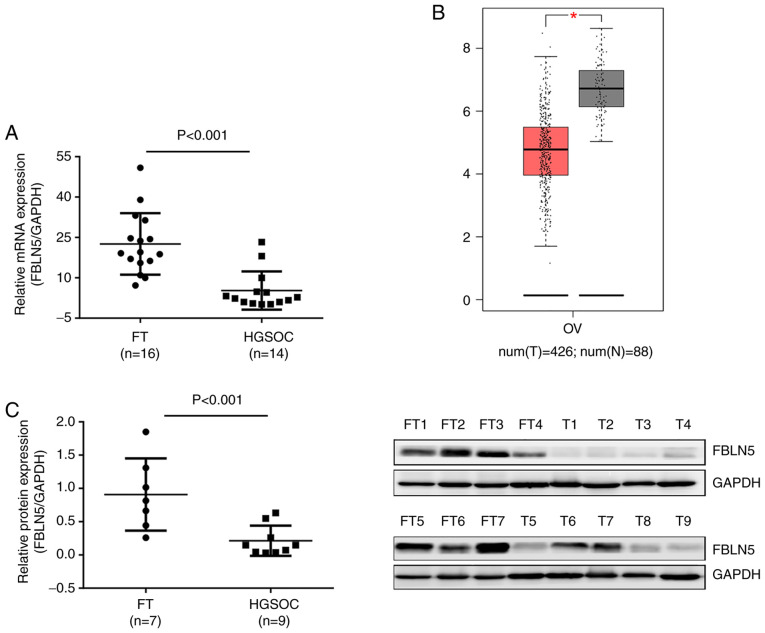
The mRNA expression level of FBLN5 in HGSOC and FT tissues (HGSOC, n=14; FT, n=16), was initially investigated and the expression levels of FBLN5 were upregulated in FT tissues compared with that in HGSOC tissues (P<0.001; Fig. 1A). The results from the GEPIA database also revealed that FBLN5 was overexpressed in normal control tissues compared with that in OC samples (Fig. 1B). Then, the protein levels of FBLN5 in FT (n=7) and HGSOC (n=9) tissue samples were investigated using western blot analysis and were revealed to be significantly different (P<0.001; Fig. 1C).
Figure 1.
FBLN5 is downregulated in HGSOC tissues. (A) Reverse transcription-quantitative PCR analysis of the FBLN5 mRNA expression level in HGSOC tissues (n=14) and normal FT tissues (n=16). (B) FBLN5 mRNA expression level in ovarian cancer and normal tissues from the GEPIA database. (C) FBLN5 protein expression level of FBLN5 in HGSOC (n=9) and normal FT (n=7) tissues using western blot analysis. *P<0.05. FBLN5, fibulin-5; HGSOC, high-grade serous ovarian cancer; T, tumor; FT, fallopian tube.
Low expression level of FBLN5 is associated with unfavorable prognosis
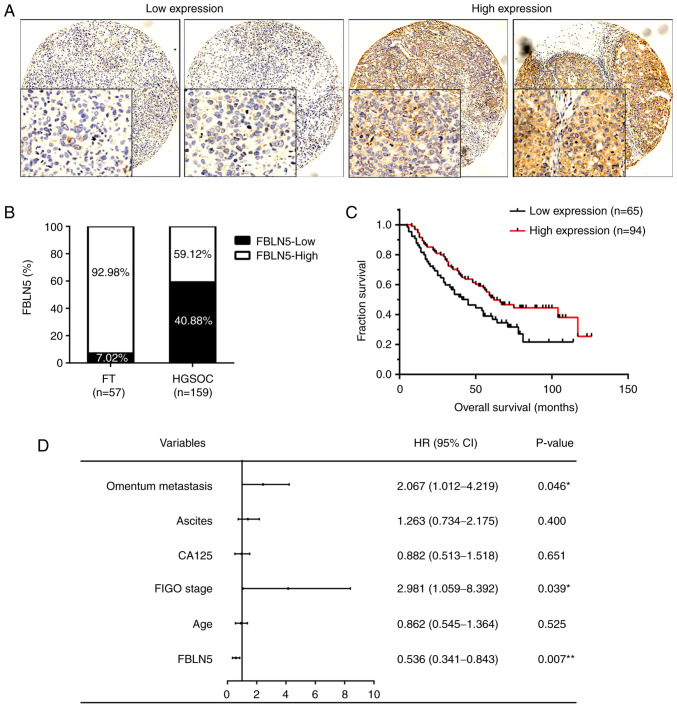
To further investigate the role of FBLN5 in HGSOC, IHC was performed to analyze the protein expression level in a TMA (FT, n=57; HGSOC, n=159). With respect to the staining degree, the HGSOC tissues were divided into either high (n=94) and low FBLN5 expression groups (n=65) (Fig. 2A). There was markedly higher expression levels of FBLN5 in the FT tissues (92.98%; 53/57) compared with that in the HGSOC tissues (59.12%; 94/159; Fig. 2B). In addition, the OS of the patients in the FBLN5-high expression group was significantly longer compared with that in patients in the FBLN5-low expression group (P<0.05; Fig. 2C). Using multivariate analysis of the clinicopathological parameters, the data presented in Fig. 2D revealed that OS was significantly associated with FBLN5 expression (HR, 0.536; P=0.007), FIGO stage (HR, 2.981; P=0.039) and omentum metastasis (HR, 2.067; P=0.046). In addition, analysis of the clinicopathological features indicated that FBLN5 expression was associated with age (P=0.024), omentum metastasis (P=0.040), recurrence (P=0.008), and OS (P=0.016; Table I).
Figure 2.
Association of FBLN5 expression with clinical information in patients with HGSOC. (A) Representative immunohistochemical staining of FBLN5 in HGSOC. (B) The high and low expression level of FBLN5 in 57 FT and 159 HGSOC tissues. (C) Overall survival rates in patients with HGSOC, with either high or low expression levels of FBLN5. (D) The multivariate analysis for OS. *P<0.05, **P<0.01. FBLN5, fibulin-5; HGSOC, high-grade serous ovarian cancer; FT, fallopian tube.
Table I.
Association between FBLN5 expression and clinicopathological features.
| FBLN5 expression | ||||
|---|---|---|---|---|
| Groups | No. | Low level | High level | P-value |
| Age (years) | 0.024 | |||
| <55 | 74 | 23 | 51 | |
| ≥55 | 85 | 42 | 43 | |
| FIGO stage | 0.244 | |||
| I+II | 35 | 11 | 24 | |
| III+IV | 120 | 52 | 68 | |
| CA125 (U/ml) | 0.868 | |||
| <600 | 64 | 27 | 37 | |
| ≥600 | 87 | 35 | 52 | |
| Ascites (ml) | 0.502 | |||
| <1,000 | 66 | 24 | 42 | |
| ≥1,000 | 84 | 36 | 48 | |
| Omentum metastasis | 0.040 | |||
| Negative | 54 | 16 | 38 | |
| Positive | 101 | 48 | 53 | |
| Lymph node metastasis | 0.566 | |||
| Negative | 41 | 11 | 30 | |
| Positive | 22 | 8 | 14 | |
| Recurrence | 0.008 | |||
| No | 28 | 5 | 23 | |
| Yes | 97 | 45 | 52 | |
| OS (years) | 0.016 | |||
| <48 | 79 | 40 | 39 | |
| ≥48 | 80 | 25 | 55 | |
FBLN5, fibulin-5; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125; OS, overall survival.
FBLN5 inhibits OS cell proliferation
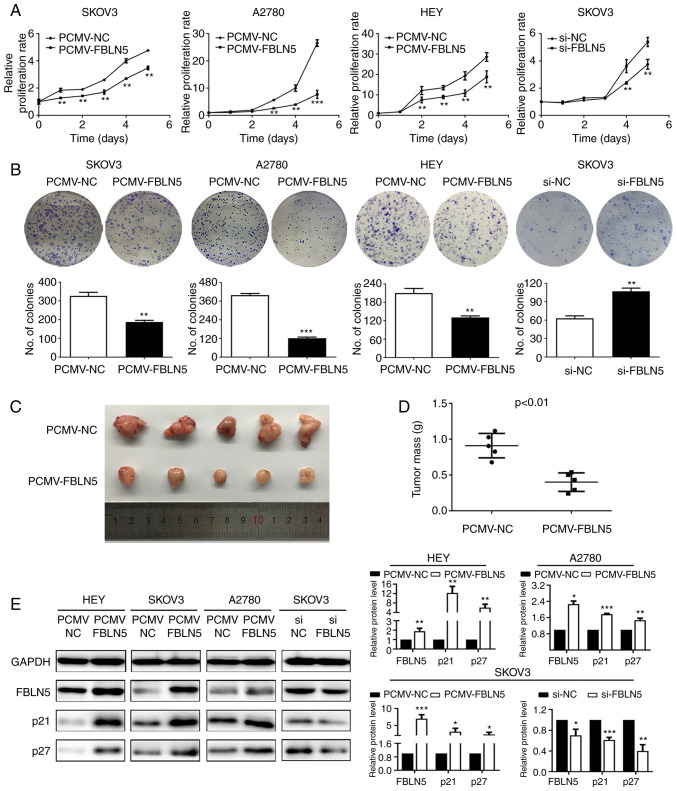
To evaluate the functional role of FBLN5, three cell lines, including SKOV3, A2780 and HEY were transfected stably with FBLN5 overexpression vector, while the SKOV3 cell line was transfected transiently with FBLN5 siRNA. The growth curve analysis demonstrated that upregulation of FBLN5 notably inhibited cell growth compared with that in the control group (Fig. 3A). Furthermore, the clonogenic assays revealed that overexpression of FBLN5 significantly inhibited the clonogenicity efficiency compared with that in the empty vector control group (Fig. 3B). In addition, the HEY cells transfected with PCMV-FBLN5 and PCMV-NC, were subcutaneously injected into the nude mice. As revealed in Fig. 3C and D, FBLN5 overexpression could significantly suppress the growth of OC cell lines in the nude mice. IHC staining of the xenograft tissue was utilized to detect the protein expression level of FBLN5. As demonstrated in Fig. S1, the expression level of FBLN5 in the nucleus was higher in the PCMV-FBLN5 group compared with that in the PCMV-NC group. Lastly, FBLN5 overexpression significantly increased p21 and p27 protein expression levels (Fig. 3E). These data indicated that FBLN5 could suppress the proliferation of OC cells.
Figure 3.
FBLN5 inhibits ovarian cancer cell proliferation in vitro and in vivo. The (A) growth curve and (B) colony formation assay were used to assess the effect of FBLN5 on the proliferation of ovarian cancer cells. (C) The HEY cell line with or without FBLN5 overexpression was injected subcutaneously into nude mice (n=5). (D) The tumor weights in each group were assessed. Data are presented as the mean ± standard error of the mean. n=5. (E) p21 and p27 expression was measured using western blot analysis in ovarian cancer cell lines with FBLN5 overexpression or knockdown. Data are presented as the mean ± standard error of the mean. n=3. *P<0.05, **P<0.01, ***P<0.001. FBLN5, fibulin-5; si, small interfering; NC, negative control.
FBLN5 inhibits OC cell invasion and migration by suppressing epithelial-mesenchymal transition (EMT)
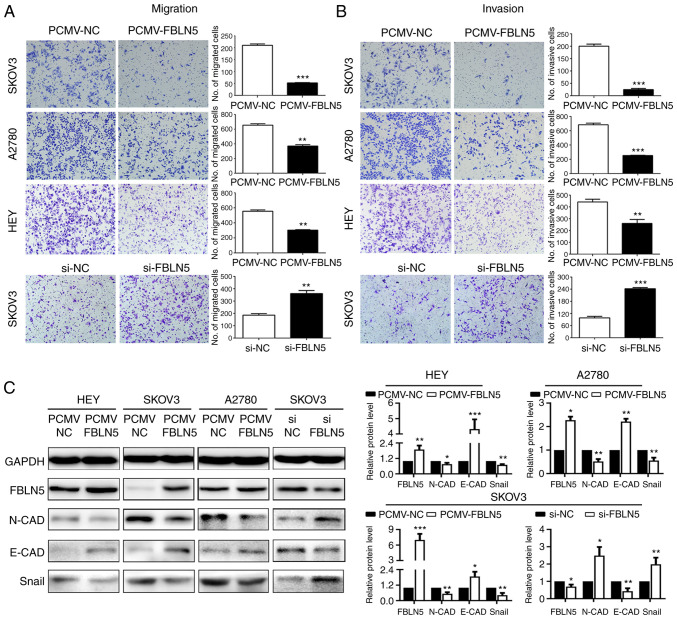
To determine the impact of altered FBLN5 expression on the metastasis of the OC cell lines, the invasion and migration abilities were detected using Transwell and Matrigel assays. As indicated in Fig. 4A and B, FBLN5 downregulation significantly promoted cell invasion and migration abilities. In agreement, overexpression of FBLN5 significantly attenuated the abilities of migration and invasion of the SKOV3, HEY and A2780 cell lines. Then, the molecular mechanism was investigated by analyzing EMT markers. The results revealed that FBLN5 overexpression significantly upregulated an epithelial marker (E-cadherin) and reduced the levels of 2 mesenchymal markers (N-cadherin and Snail) compared with that in the empty vector group, transfected in the HEY, A2780 and SKOV3 cell lines. In addition, FBLN5 knockdown downregulated the epithelial marker (E-cadherin) and increased the expression of 2 mesenchymal markers (N-cadherin, Snail) compared with that in the control group in the SKOV3-transfected cells (Fig. 4C). These data demonstrated that FBLN5 suppressed metastasis of OC cells by inhibiting EMT in vitro.
Figure 4.
FBLN5 inhibits the migration and invasion of ovarian cancer cells in vitro. Transwell assays were used to assess the effect of FBLN5 overexpression or knockdown on the (A) migration and (B) invasion of ovarian cancer cells. (C) Western blot analysis of the EMT markers, N-cadherin, E-cadherin and Snail. Data are presented as the mean ± standard error of the mean. n=3. *P<0.05, **P<0.01, ***P<0.001. FBLN5, fibulin-5; EMT, epithelial-mesenchymal transition; si, small interfering; NC, negative control; N-CAD, N-cadherin; E-CAD, E-cadherin.
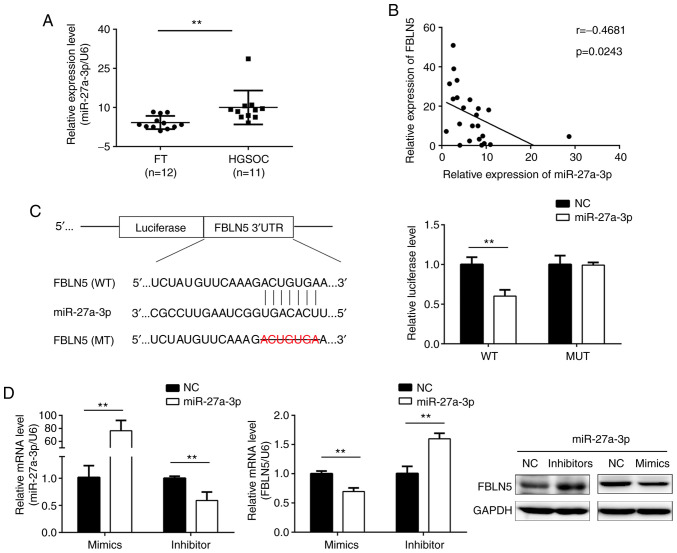
miR-27a-3p is a direct regulator of FBLN5
Finally, the molecular mechanisms underlying the downregulation of FBLN5 expression in OC was investigated. The potential miRNA that targets the mRNA of FBLN5 was predicted using the miRanda and TargetScan online databases, and miR-27a-3p was selected for further examination. In addition, prior research revealed that miR-27a-3p was upregulated in OC (26,27). As demonstrated in Fig. 5A, the expression level of miR-27a-3p was upregulated in HGSOC compared with that in FT tissues. Further analysis revealed that the expression level of FBLN5 was negatively correlated with miR-27a-3p expression level in HGSOC (Fig. 5B). To further verify the association of direct interaction between miR-27a-3p and FBLN5, the dual-luciferase reporter assay was subsequently performed. The data indicated that upregulation of miR-27a-3p markedly inhibited the luciferase activities in cells co-transfected with wild-type FBLN5 sequences and miR-27a-3p mimics (Fig. 5C). The SKOV3 cell line was transfected with miR-27a-3p inhibitors or mimics. Western blot and RT-qPCR assays demonstrated that miR-27a-3p mimics significantly reduced the protein and mRNA expression levels of FBLN5, respectively, which was reversed by miR-27a-3p inhibitors (Fig. 5D).
Figure 5.
FBLN5 is the target gene of miR-27a-3p. (A) The mRNA level of miR-27a-3p was higher in HGSOC tissues compared with that in FT tissues, using RT-qPCR. (B) Correlation analysis of FBLN5 and miR-27a-3p expression levels. (C) Prediction of the binding sites between miR-27a-3p and 3′-UTR of FBLN5. The binding sequence of miR-27a-3p with the FBLN5 3′UTR sequence was deleted in the mutated type. Dual-luciferase reporter assays were used to analyzed the luciferase activity of the WT or MT FBLN5 3′UTR. (D) The mRNA expression level of miR-27a-3p was detected using RT-qPCR following SKOV3 cell transfection with miR-27a-3p mimics or inhibitor. RT-qPCR and western blot analysis revealed an inverse association between FBLN5 and miR-27a-3p expression. Upregulation of miR-27a-3p inhibited FBLN5 expression level and downregulated miR-27a-3p expression level could promote the expression level of FBLN5. Data are presented as the mean ± standard error of the mean. **P<0.01. FBLN5, fibulin-5; miR-27-3p, microRNA-27-3-p; HGSOC, high-grade serous ovarian cancer; FT, fallopian tube; RT-qPCR, reverse transcription-quantitative PCR; UTR, untranslated region; WT, wild-type; MT, mutant; NC, negative control.
Discussion
Ovarian carcinoma is the fifth leading cause of cancer-related mortality in females (28,29), and HGSOC is the most malignant type of OC. Most patients with OC are diagnosed at an advanced stage, which leads to poor prognosis (30,31). FBLN5 has been verified to be an anti-oncogene in different types of malignancy (11–14). In the present study, it was revealed that a low expression level of FBLN5 was associated with unfavorable prognosis of HGSOC, providing a potential biomarker for the prediction of diagnosis and prognosis. However, the detailed mechanism of FBLN5 in HGSOC progression remains unknown.
FBLN5 has an anti-proliferative effect in several types of human malignancy (11–13), and FBLN5 overexpression was revealed to suppress DNA synthesis and cyclin A expression in mink lung epithelial cells (32). p21 and p27 are two important cell cycle-related genes, which regulate cell cycle progression during G1/S and G0/S phases, respectively (33). In the present study, cell proliferation, colony formation and tumor formation assays were performed. Notably, the results revealed that FBLN5 overexpression inhibited OC cell growth in vitro and in vivo, and markedly increased the protein expression level of p21 and p27. Based on these data, we hypothesized that FBLN5 inhibited the proliferation of OC cells by regulating the cell cycle-related proteins. Limitedly, we did not explore the potential relations between FBLN5 and the other essential cell cycle regulators in the present study, such as AURKA, AURKB and FOXM1, which would be carried out relevantly in our future research.
Migration and invasion are important causes of death in patients with cancer. As a vital regulator of metastasis, EMT has been associated with tumor progression, and promotes mobility and resistance to apoptosis in various types of cancer (34). Emerging evidence has revealed that tumor-associated matrix metallopeptidases (MMPs) stimulate EMT (4). Tu et al (13) and Lee et al (35), reported that FBLN5 was involved in EMT and affected the invasion and migration by tumor-associated MMPs in breast cancer and hepatocellular carcinoma cells. EMT is a cellular process and is often defined as the loss of epithelial characteristics and the increase in mesenchymal features (36,37). Consistent with this hypothesis, the results in the present study revealed that FBLN5 overexpression increased the epithelial marker, E-cadherin and decreased the mesenchymal markers, N-cadherin and Snail. Therefore, we hypothesized that FBLN5 inhibited the migration and invasion of OC cells by inhibiting the EMT pathway.
Currently, numerous studies have demonstrated that miRNAs are important regulators in a diverse range of human malignancies (18–20). Furthermore, it has been reported that the function and aberrant expression of miRNAs play a crucial role in several biological processes (38,39). For example, miR-27a-3p promoted cell proliferation in glioma cells by the cooperative regulation of MXI1 (40). Li et al (26) and Wang et al (27), reported that the miR-27a-3p expression level was increased in OC, and primarily affected the growth and metastasis of cancer cells. Consistent with these previous studies, the results from the present study indicated that miR-27a-3p was upregulated in the tissue from patients with HGSOC. In addition, it was verified that FBLN5 was the target of miR-27a-3p, and the miR-27a-3p expression level was negatively correlated with FBLN5 expression level. Therefore, the increased miR-27a-3p expression level could explain why FBLN5 was downregulated in HGSOC.
In summary, the present study indicated that FBLN5 was targeted by miR-27a-3p and suppressed cell proliferation, invasion and metastasis in OC cells. Furthermore, FBLN5 downregulation was associated with unfavorable prognosis. These findings revealed that increasing FBLN5 expression could be a potential new strategy for the treatment of HGSOC.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81572554 and 81874107), and the Science Foundation of Qilu Hospital of Shandong University (grant no. 2019QLQN03).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
RL and HW designed and conceived the experiments, and wrote the original draft of the manuscript. HJ, QW and ZD collected and analyzed the data. HM, CY and SY provided technical support and revised the manuscript. NY assisted with acquisition and analysis of clinical information. BK directed the project and interpreted the results. All authors have read and approved the manuscript.
Ethics approval and consent to participate
This study was approved by the Shandong University Qilu Hospital Ethics Committee and Shandong University Animal Care and Use Committee (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 3.Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ, Bast RC, Jr, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668–679. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heo JH, Song JY, Jeong JY, Kim G, Kim TH, Kang H, Kwon AY, An HJ. Fibulin-5 is a tumour suppressor inhibiting cell migration and invasion in ovarian cancer. J Clin Pathol. 2016;69:109–116. doi: 10.1136/jclinpath-2015-203129. [DOI] [PubMed] [Google Scholar]
- 5.Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: A versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4:479–489. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher WM, Currid CA, Whelan LC. Fibulins and cancer: Friend or foe? Trends Mol Med. 2005;11:336–340. doi: 10.1016/j.molmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Obaya AJ, Rua S, Moncada-Pazos A, Cal S. The dual role of fibulins in tumorigenesis. Cancer Lett. 2012;325:132–138. doi: 10.1016/j.canlet.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa H, Schluterman MK, Brekken RA. Fibulin-5, an integrin-binding matricellular protein: Its function in development and disease. J Cell Commun Signal. 2009;3:337–347. doi: 10.1007/s12079-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vega S, Iwamoto T, Yamada Y. Fibulins: Multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. 2009;66:1890–1902. doi: 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albig AR, Schiemann WP. Fibulin-5 function during tumorigenesis. Future Oncol. 2005;1:23–35. doi: 10.1517/14796694.1.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Song X, Yue W, Chen D, Yu J, Yao Z, Zhang L. Fibulin-5 inhibits Wnt/β-catenin signaling in lung cancer. Oncotarget. 2015;6:15022. doi: 10.18632/oncotarget.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamedi Y, Fontanil T, Solares L, Garcia-Suárez O, García- Piqueras J, Vega JA, Cal S, Obaya AJ. Fibulin-5 downregulates Ki-67 and inhibits proliferation and invasion of breast cancer cells. Int J Oncol. 2016;48:1447–1456. doi: 10.3892/ijo.2016.3394. [DOI] [PubMed] [Google Scholar]
- 13.Tu K, Dou C, Zheng X, Li C, Yang W, Yao Y, Liu Q. Fibulin-5 inhibits hepatocellular carcinoma cell migration and invasion by down-regulating matrix metalloproteinase-7 expression. BMC Cancer. 2014;14:938. doi: 10.1186/1471-2407-14-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue W, Sun Q, Landreneau R, Wu C, Siegfried JM, Yu J, Zhang L. Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer Res. 2009;69:6339–6346. doi: 10.1158/0008-5472.CAN-09-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 16.Ranganathan K, Sivasankar V. MicroRNAs-biology and clinical applications. J Oral Maxillofac Pathol. 2014;18:229–234. doi: 10.4103/0973-029X.140762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76:3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo D, Li Y, Chen Y, Zhang D, Wang X, Lu G, Ren M, Lu X, He S. DANCR promotes HCC progression and regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1 pathway. Cell Prolif. 2019;52:e12628. doi: 10.1111/cpr.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Luo Z. Dysregulated miR-27a-3p promotes nasopharyngeal carcinoma cell proliferation and migration by targeting Mapk10. Oncol Rep. 2017;37:2679–2687. doi: 10.3892/or.2017.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng G, Xun W, Wei K, Yang Y, Shen H. MicroRNA-27a-3p regulates epithelial to mesenchymal transition via targeting YAP1 in oral squamous cell carcinoma cells. Oncol Rep. 2016;36:1475–1482. doi: 10.3892/or.2016.4916. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi Y, Tsuruga E, Nakashima K, Sawa Y, Ishikawa H. Fibulin-4 and-5, but not fibulin-2, are associated with tropoelastin deposition in elastin-producing cell culture. Acta Histochem Cytochem. 2010;43:131–138. doi: 10.1267/ahc.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45((W1)):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008;36((Database Issue)):D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li E, Han K, Zhou X. microRNA-27a-3p down-regulation inhibits malignant biological behaviors of ovarian cancer by targeting BTG1. Open Med (Wars) 2019;14:577–585. doi: 10.1515/med-2019-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Ji G, Wu Q, Feng S, Zhao Y, Cao Z, Tao C. Integrated microarray meta-analysis identifies miRNA-27a as an oncogene in ovarian cancer by inhibiting FOXO1. Life Sci. 2018;210:263–270. doi: 10.1016/j.lfs.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA, Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 29.Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, Berek JS, Osann K. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108:521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 30.Kurman RJ. Origin and molecular pathogenesis of ovarian high- grade serous carcinoma. Ann Oncol. 2013;24(Suppl 10):x16–x21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 31.Mezzanzanica D. Ovarian cancer: A molecularly insidious disease. Chin J Cancer. 2015;34:1–3. doi: 10.5732/cjc.014.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiemann WP, Blobe GC, Kalume DE, Pandey A, Lodish HF. Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by transforming growth factor-beta and affects protein kinase cascades. J Biol Chem. 2002;277:27367–27377. doi: 10.1074/jbc.M200148200. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Guan H, Guo Y, Liang W, Liu L, He X, Ke W, Cao X, Xiao H, Li Y. CITED1 promotes proliferation of papillary thyroid cancer cells via the regulation of p21 and p27. Cell Biosci. 2018;8:57. doi: 10.1186/s13578-018-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 35.Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis. 2008;29:2243–2251. doi: 10.1093/carcin/bgn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Chen H, Gao W, Wang S, Wu K, Lu C, Luo X, Li L, Yu C. Girdin interaction with vimentin induces EMT and promotes the growth and metastasis of pancreatic ductal adenocarcinoma. Oncol Rep. 2020;44:637–649. doi: 10.3892/or.2020.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong GO, Jeon H-S, Han HS, Son JW, Lee YH, Hong DG, Lee YS, Cho YL. Differential microRNA expression profiles in primary and recurrent epithelial ovarian cancer. Anticancer Res. 2015;35:2611–2617. [PubMed] [Google Scholar]
- 39.Llauradó M, Majem B, Altadill T, Lanau L, Castellví J, Sánchez-Iglesias JL, Cabrera S, De la Torre J, Díaz-Feijoo B, Pérez-Benavente A, et al. MicroRNAs as prognostic markers in ovarian cancer. Mol Cell Endocrinol. 2014;390:73–84. doi: 10.1016/j.mce.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Liu M, Peng X, Zhou P, Zhou J, Xu K, Xu H, Jiang S. miR-24-3p and miR-27a-3p promote cell proliferation in glioma cells via cooperative regulation of MXI1. Int J Oncol. 2013;42:757–766. doi: 10.3892/ijo.2012.1742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.