Abstract
Dietary restriction of phenylalanine combined with a protein substitute prevents intellectual disability in patients with phenylketonuria (PKU). However, current protein substitutes are associated with low adherence owing to unpalatability and burdensome administration regimens. This prospective, observational acceptability study in children with PKU assessed the use of a prolonged-release protein substitute designed with an ethyl cellulose and arginate coating masking the bitter taste, smell and reducing the osmolarity of free amino acids. The study product was mixed with the subject’s food or drink and replaced ≥1 dose per day of the subject’s usual protein substitute for 7 days. Seven of 13 subjects were able to take their prescribed dose over the 7 day period. Most subjects mixed the test protein substitute with food or fruit juice. Reduced blood phenylalanine levels (n = 5) and improved phenylalanine/tyrosine ratio (n = 4) were recorded from baseline to Day 7, respectively. Four subjects reported fewer gastrointestinal symptoms compared to baseline. There were no cases of diarrhoea, constipation, bloating, nausea or vomiting. No adverse reactions were reported. In conclusion, the novel prolonged-release protein substitute was taken in a different way to a typical protein substitute and enabled satisfactory blood phenylalanine control. The study product was well tolerated; subjects experienced fewer gastrointestinal symptoms than with their previous treatment. Although the results of this pilot study provide reassuring data, longer-term studies evaluating adherence and blood phenylalanine control are necessary.
Keywords: phenylketonuria, diet therapy, phenylalanine, protein substitute, gastrointestinal symptoms, prolonged release
1. Introduction
Phenylketonuria (PKU) is a rare metabolic disorder caused by a deficiency of phenylalanine hydroxylase (PAH), the enzyme that catalyses the hydroxylation of phenylalanine to tyrosine, and which leads to irreversible intellectual impairment in untreated children [1]. While there is no cure for PKU, the dietary restriction of phenylalanine has been highly successful in preventing intellectual disability and achieving near-normal intellect. However, current dietary treatments are associated with some major issues, such as low adherence attributed to unpalatable and burdensome dietary supplements and subtle but chronic neuropsychological impairments despite early intervention, particularly in adulthood, including mood and psychiatric issues [2,3,4,5].
Lifelong dietary management of PKU involves severe restriction of phenylalanine plus supplementation with protein substitutes, usually consisting of phenylalanine-free amino acids [6]. Protein substitutes provide essential and nonessential amino acids and commonly include micronutrients that would otherwise be lacking in a low-phenylalanine diet [6,7]. Since the 1960s, when the first manufactured protein substitutes were introduced, improvements have been made to their nutritional composition, presentation, taste and acceptability [7,8]. Currently, protein substitutes are available in a variety of forms, including powders, gels, liquids and tablets [9], and are traditionally administered as high-volume hyperosmolar drinks (if diluted with too little water, they may cause abdominal pain, diarrhoea or constipation [10]).
Generally, many patients have a poor acceptance of protein substitutes and parents struggle to ensure that their children take them as prescribed [11,12]. Poor adherence to a low-phenylalanine diet and protein substitute increases with age and is associated with worsening of blood phenylalanine control [11,13,14]. Furthermore, children may take up to an hour to take their full dose of protein substitute, with some failing to take the prescribed quantity [15]. Although reasons for poor adherence to protein substitutes are multiple, the bitter taste and aftertaste of synthetic amino acids are frequently reported as important factors [9,16]. Manufacturers have tried to improve the taste by lowering the quantities of unpalatable sulphurous and dicarboxylic amino acids and adding flavourings and sweeteners, but the results have been suboptimal [17], with minimal impact on aftertaste [18]. Commonly, children complain of breath odour following consumption of protein substitutes [19]. Although reports of adding protein substitute to food are few [20], this is perceived to have low acceptance [21].
Synthetic amino acids bypass degradation by proteases in the digestive process, resulting in blood levels that are higher, peak faster and decrease more quickly than when compared with natural protein. Therefore, it is recommended to take synthetic protein substitutes in small, frequent dosages in equally distributed amounts [8]. If protein substitutes are taken less frequently, there may be wide variations in blood phenylalanine levels over 24 h, which is associated with reduced blood phenylalanine control in PKU [8,22]. Protein substitutes with the ability to delay absorption of phenylalanine and tyrosine, mimicking physiological absorption kinetics, are expected to improve the rate of protein accretion, minimizing fluctuations in quantitative plasma amino-acid levels [8]. However, despite previous efforts, there has been little success in developing a slow-release protein substitute that mirrors the physiological absorption kinetics of intact natural proteins.
There is a need for more palatable and physiological protein substitutes that are both effective at reducing 24 h variability in phenylalanine levels and are accepted by patients. Here, we report outcomes from an observational study assessing the introduction of a prolonged-release protein substitute to the diets of children with PKU [16,23].
2. Materials and Methods
2.1. Study Design
This was a prospective, observational acceptability study performed in children with PKU aged 3–16 years who attended a single clinic at Birmingham Women’s and Children’s Hospital, Birmingham, United Kingdom. Caregivers of eligible children were identified and sent a study information sheet. Research dietitians discussed the study details with interested parents and patients on their request. This study was conducted according to the requirements stated by the UK Advisory Committee for Borderline Substances (ACBS). This is a committee that recommends dietary products to be reimbursed by the National Health Service (NHS). The study product met the criteria of a Type 2 product: “a formulation which was broadly similar in composition to existing products already on the market.” According to ACBS guidelines, “All acceptability studies must be for at least 1 week and at least 15 patients must complete the study. Where nutritional products are intended for use in very rare conditions, such as inherited metabolic disorders, fewer patients are acceptable”. Thirteen patients were enrolled for 7 days of treatment and were able to complete the study, but only 54% (7 of 13) were able to take 100% of the prescribed product and were included in the final analyses. These studies conformed to the principles of good clinical practice.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria included: male or female; aged 3–16 years; proven diagnosis of PKU requiring a protein substitute; already taking a protein substitute; and willing to take the study product for 7 days.
Exclusion criteria included: presence of serious concurrent illness; chief investigator’s uncertainty about the willingness or ability of the patient to comply with protocol requirements; participation in any other study involving investigational or marketed products within two weeks prior to study entry; and children who received antibiotics over the two weeks prior to the study.
2.3. Study Product
The study product, PKU GOLIKE PLUS 3–16, (APR Applied Pharma Research, Switzerland) is a protein substitute for oral use in the form of off-white/light yellow granules, consisting of a prolonged-release amino-acid mixture with vitamins and minerals and other nutrients (i.e., carnitine, taurine, choline and inositol). The study product was developed with a coating able to overcome practical issues associated with free amino acids, such as bitter taste, smell, aftertaste and osmolarity. The coating consisted of ethyl cellulose plus alginates encasing granules of amino acids (without phenylalanine). The study product could be mixed with food or fruit juice or taken as granules. It was gluten and lactose-free and contained no added fat, with a nutritional profile suitable for patients aged 3–16 years (Table 1).
Table 1.
Nutritional declaration for the study product (PKU GOLIKE PLUS 3–16) 1.
| Component | Per 100 g | Per Sachet of 24 g |
|---|---|---|
| Energy | 280 kcal/1187 kJ | 67 kcal/286 kJ |
| Fat | 0 g | 0 g |
| of which saturated | 0 g | 0 g |
| Carbohydrate | 4.3 g | 1.0 g |
| of which sugars | 0 g | 0 g |
| Fibre | 7.1 g | 1.7 g |
| Protein equivalent 1 | 62.2 g | 15 g |
| Salt | 0.06 g | 0.015 g |
| Amino Acids | ||
| L-serine | 2.5 g | 0.6 g |
| L-threonine | 3.8 g | 0.9 g |
| L-leucine | 8.6 g | 2.1 g |
| Glycine | 3.8 g | 0.9 g |
| L-alanine | 2.3 g | 0.5 g |
| L-arginine | 3.0 g | 0.7 g |
| L-cysteine | 1.5 g | 0.4 g |
| L-glutamine | 15.0 g | 3.6 g |
| L-histidine | 2.1 g | 0.5 g |
| L-aspartic acid | 4.5 g | 1.1 g |
| L-proline | 4.5 g | 1.1 g |
| L-isoleucine | 4.1 g | 1.0 g |
| L-lysine | 5.3 g | 1.3 g |
| L-tryptophan | 1.5 g | 0.4 g |
| L-valine | 3.8 g | 0.9 g |
| L-methionine | 1.0 g | 0.3 g |
| L-tyrosine | 7.5 g | 1.8 g |
| Vitamins | ||
| Vitamin A (RE) | 1295 mcg | 311 mcg |
| Vitamin D | 25 mcg | 6.0 mcg |
| Vitamin E (αTE) | 13 mg | 3.2 mg |
| Vitamin K | 100 mcg | 24 mcg |
| Vitamin C | 135 mg | 32.31 mg |
| Thiamine | 2.0 mg | 0.5 mg |
| Riboflavin | 1.9 mg | 0.5 mg |
| Niacin | 27 mg | 6.4 mg |
| Vitamin B6 | 2.6 mg | 0.6 mg |
| Folic acid | 267 mcg | 64.1 mcg |
| Vitamin B12 | 4.2 mcg | 1.0 mcg |
| Biotin | 54 mcg | 13 mcg |
| Pantothenic acid | 11 mg | 2.6 mg |
| Minerals | ||
| Potassium | 1250 mg | 300 mg |
| Calcium | 1339 mg | 321 mg |
| Magnesium | 304 mg | 72.9 mg |
| Phosphorus | 1060 mg | 254 mg |
| Chloride | 0.75 mg | 0.18 mg |
| Sodium | 25 mg | 5.9 mg |
| Iron | 23 mg | 5.6 mg |
| Zinc | 14 mg | 3.4 mg |
| Copper | 1.4 mg | 0.3 mg |
| Manganese | 2.5 mg | 0.6 mg |
| Selenium | 58 mcg | 14 mcg |
| Chromium | 46 mcg | 11 mcg |
| Molybdenum | 88 mcg | 21 mcg |
| Iodine | 225 mcg | 54.0 mcg |
| Other Nutrients | ||
| Carnitine | 0.08 g | 0.02 g |
| Taurine | 0.21 g | 0.05 g |
| Choline | 321 mg | 77.1 mg |
| Inositol | 214 mg | 51.4 mg |
1 1 g of protein equivalent = 1.2 g of amino acids. The protein content is provided by the amino acids. Ingredients: L-glutamine, L-leucine, L-tyrosine, L-lysine acetate, glazing agent: ethyl cellulose; calcium hydrogen phosphate dihydrate, maltodextrin, L-aspartic acid, L-proline, L-isoleucine, L-threonine, glycine, L-valine, potassium bicarbonate, L-arginine, L-serine, L-alanine, L-histidine, L-cysteine, L-tryptophan, L-methionine, choline bitartrate, magnesium oxide, iron, maize starch, ferric pyrophosphate, glazing agent: sunflower lecithin, stabiliser: sodium alginate; inositol, taurine, L-ascorbic acid, L-carnitine, zinc sulphate, nicotinamide, DL-alpha tocopheryl acetate, chromium chloride hexahydrate, sodium molybdate, manganese gluconate, calcium-d-pantothenate, cupric gluconate, retinyl palmitate, pyridoxine hydrochloride, thiamine hydrochloride, riboflavin, cholecalciferol, folic acid, potassium iodide, phytomenadione, sodium selenite, D-biotin, cyanocobalamin.
The study product was introduced into the standard therapeutic diets of enrolled children for 7 days by replacing at least one dose per day of the patient’s usual protein substitute, according to ACBS requirements. Considering the short study duration, all the protein substitute requirements were not replaced as patients had little time to adapt to a different type of protein substitute given in another format.
Patients followed their usual low-phenylalanine diets during the study. Any foods containing protein up to 0.5 g/100 g and fruits and vegetables containing phenylalanine up to 75 mg/100 g were given without measurement. We aimed to maintain the same total protein equivalent intake for each patient whilst taking the prolonged-release protein substitute.
2.4. Preparation of Study Product
The research dietitians explained the study product’s characteristics and its theoretical advantages to the caregivers and patients. Verbal and written information was given about how to administer the study product, i.e., granules could be taken either in liquids or semisolid foods with a creamy consistency (fruit smoothies, low-protein vegetable soup, fruit or vegetable purees, low-protein puddings or desserts). Each caregiver and patient were also given a practical demonstration on how to mix the study product. The type of food or drink that the study product was mixed with was selected by the patient.
2.5. Assessments
Subject demographics were recorded at study baseline. Prior to treatment initiation, information was recorded regarding the current protein substitute (dose, type, palatability and presence of any gastrointestinal side effects).
During treatment, parents/caregivers and subjects completed daily questionnaires to record treatment preparation/administration and any problems, including adverse events or gastrointestinal side effects. An additional questionnaire was completed at the end of treatment.
All patients had known adherence with their usual protein substitute prior to entering this study as well as routine blood spot phenylalanine monitoring, with three retrospective blood spots available in addition to blood tests taken during the study.
A fasting blood spot for phenylalanine was taken at home by parents/caregivers both before and at the end of the treatment period. Children aged ≤12 years aimed to maintain blood phenylalanine between 120–360 µmol/L; children aged >12 years aimed to maintain levels between 120–600 µmol/L. Early-morning fasted blood spots were collected on filter cards (Perkin Elmer 226, Greenville, SC, USA, UK Standard NBS). Blood specimens were sent via first-class post to the laboratory at Birmingham Children’s Hospital. All cards had a standard thickness and the blood phenylalanine concentrations were calculated on a 3.2 mm punch by Waters Xevo TQD tandem mass spectrometer (Elstree, Herts, UK).
2.6. Ethical Permission
The Solihull Research Ethics Committee granted a favourable ethical opinion (REC reference: 19/WM/0151, IRAS project ID: 256519). Written consent was obtained for all subjects from at least one caregiver with parental responsibility and written assent obtained from the subject if appropriate for their age and level of understanding.
2.7. Statistical Analysis
Descriptive statistics were used to examine demographics and disorder characteristics, protein substitute use, adverse events and plasma phenylalanine and tyrosine levels.
3. Results
3.1. Subjects
A total of 13 subjects were enrolled into the acceptability study (12 with classical PKU; 1 with moderate PKU); mean age was 11.6 years (range 7 to 16 years). All subjects were diagnosed with PKU via newborn screening and started a low-phenylalanine diet from the time of screening. None were treated with sapropterin as an adjunctive therapy. At the start of the study, the subjects routinely took either one or two different types/brands of protein substitute daily (eight subjects took a single protein substitute daily; five subjects took two different types). Seven of the subjects routinely took a protein substitute derived from casein glycomacropeptide (CGMP), and three subjects took this as their sole source of protein substitute, with four subjects taking CGMP with an amino acid substitute. In total, five subjects usually took both a liquid and a powdered protein substitute, five took a liquid substitute only, and three took a powdered substitute only. The median (range) dose of protein equivalent from protein substitute was 1.4 (1.0–3.1) g/kg/day.
3.2. Substudy Cohort Results
The substudy cohort consisted of subjects who were able to take the entire prescribed dose of the study product (n = 7; mean age 10.9 years) (Table 2). The mean percentage of the prescribed dose consumed over 7 days for these patients was 98% (range 92–100%). Subjects that consumed less than 90% of the prescribed dose during the 7 days of treatment are not included in the substudy cohort analyses. Subjects were prescribed either 15 g/day (n = 6) or 20 g/day (n = 1) protein equivalent of the study product to replace one dose of the total protein substitute intake. The study product provided 20–27.3% of the subject’s usual protein equivalent intake per day; the remaining protein equivalent intake was from each subjects’ usual protein substitute. Three subjects (Subjects 1, 2 and 6) were 5 g of protein equivalent short of their pre-study dose, due to differences in sizes of protein substitute sachets/pouches.
Table 2.
Baseline demographics and treatment in the substudy cohort.
| Baseline Demographics | |||||||
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Age (years) | 11 | 11 | 12 | 9 | 7 | 11 | 15 |
| Weight (kg) | 60.3 | 53.3 | 45.9 | 25.8 | 26.6 | 45.6 | 55.8 |
| Height (cm) | 152.3 | 147.5 | 155.8 | 124.5 | 119.7 | 154.8 | 174.4 |
| PKU classification | Classical | Classical | Moderate | Classical | Classical | Classical | Classical |
| Blood Phe on diagnosis (µmol/L) | 1700 | 1680 | 900 | 1390 | 1590 | 2520 | 2690 |
| Gender | Female | Female | Male | Male | Male | Male | Male |
| Ethnicity | Pakistani | Pakistani | White European | White British | Mixed race | White British | White British |
| Diet and Protein-Substitute Profile | |||||||
| Natural protein allowance (g/day) | 4.0 | 4.0 | 7.0 | 3.0 | 6.5 | 7.5 | 18.0 |
| Protein equivalent from usual protein substitutes g/day (g/kg/day) | 60.0 (1.0) | 60.0 (1.1) | 60.0 (1.3) | 80.0 (3.1) | 60.0 (2.3) | 80.0 (1.7) | 60.0 (1.2) |
| Number of different protein substitutes/day | 2 | 2 | 2 | 1 | 2 | 2 | 1 |
| Number of doses/day | 3 | 3 | 4 | 4 | 4 | 4 | 3 |
| Study Product Treatment Schedule and Preparation | |||||||
| Daily dose (g) | 24.0 | 24.0 | 24.0 | 32.0 | 24.0 | 24.0 | 24.0 |
| Protein equivalent from study product (g) | 15.0 | 15.0 | 15.0 | 20.0 | 15.0 | 15.0 | 15.0 |
| Total daily protein equivalent from all protein substitutes (g) 1 | 55.0 | 55.0 | 55.0 | 80.0 | 60.0 | 75.0 | 55.0 |
|
Protein equivalent from study product
(% of daily intake) |
27.3 | 27.3 | 27.3 | 25.0 | 25.0 | 20.0 | 27.3 |
| Method of administration | In fruit juice | In fruit juice | Food and drinks | In fruit juice | In fruit juice and food | Fruit smoothie | Smoothie |
| Timing of administration of study product | Evening | Evening | Evening | Evening | Morning midday and bedtime | Morning or evening | Morning or evening |
|
Comments on
study product |
Left some bits behind on cup | Last bit was hard to take | No comments | No comments | No comments | Required blender to mix | No comments |
1 Subject 1, Subject 2 and Subject 6 were 5 g of protein short of their pre-study dose due to differences in sizes of protein substitute sachets/pouches when incorporating the study product with existing protein-substitute diet plan. Abbreviations: Phe: phenylalanine; PKU: phenylketonuria.
3.3. Administration of Study Product
The study product was mixed with a variety of different drinks and foods, but the preferred method was to prepare it as a fruit smoothie or mixed with fruit juice (Table 2). All subjects or their parents found the study product easy to prepare. Seven of 13 children commented that the product had little taste or that it tasted “okay”. The reason why children (n = 6 of 13) were unable to take the entire prescribed dose of the study product was primarily because of texture (described as bitty and gritty) and one child described the amount of powder as “a little overpowering” when added to food.
3.4. Adherence with Study Product
All but one of the subjects included in the substudy cohort took 100% of the prescribed study product on at least one day during the study period; five subjects took 100% of the dose every day. The lowest percentage daily intake of the study product was 75%, which occurred once throughout the study (Day 1, Subject 2).
3.5. Blood Phenylalanine and Tyrosine Control
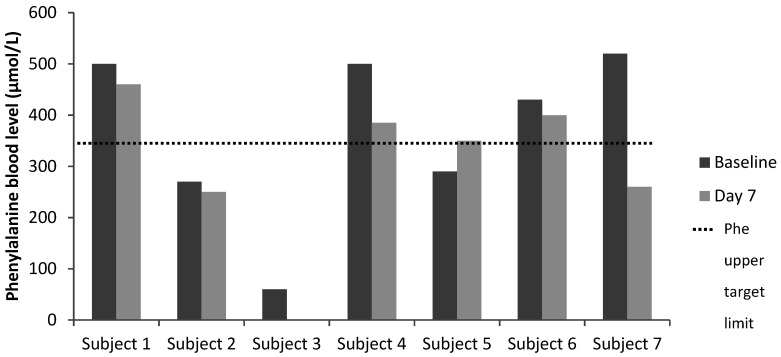
Blood phenylalanine and tyrosine control was satisfactory over the study period, with lower blood phenylalanine levels recorded in five of the seven subjects (Subject 1: −40%, Subject 2: −7%, Subject 3: NA, Subject 4: −33%, Subject 6: −7%, Subject 7: −50%) (Table 3 and Figure 1). One subject had blood sample labelling issues, so blood phenylalanine was not reported whilst on the study product (Subject 3). The blood phenylalanine level increased in one child (Subject 5: +21%) but remained within the recommended target range. Tyrosine baseline and Day 7 data were available for five subjects: tyrosine levels increased in three subjects and were lower in two subjects. The phenylalanine/tyrosine ratio improved in four of five subjects.
Table 3.
Phenylalanine and tyrosine blood levels of the substudy patient cohort during the study.
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Target Phe levels (µmol/L) | 120–360 | 120–360 | 120–360 | 120–360 | 120–360 | 120–360 | 120–600 |
| Phenylalanine Levels (µmol/L) | |||||||
| Baseline | 500 | 270 | 60 | 500 | 290 | 430 | 520 |
| Day 7 | 460 | 250 | NA 1 | 385 | 350 | 400 | 260 |
| Tyrosine Levels (µmol/L) | |||||||
| Baseline | 110 | 120 | 50 | 30 | 60 | 50 | 70 |
| Day 7 | 150 | 160 | NA 1 | NA 1 | 40 | 60 | 50 |
| Phenylalanine/Tyrosine Ratio | |||||||
| Baseline | 4.5 | 2.3 | 1.2 | 16.7 | 4.8 | 8.6 | 7.4 |
| Day 7 | 3.1 | 1.6 | NC 1 | NC 1 | 8.8 | 6.7 | 5.2 |
1 Sample labelling issue. NA: not available; NC: not calculable.
Figure 1.
Phenylalanine blood levels of the substudy patient cohort over the study period. Phe target range was 120–360 µmol/L for patients ≤12 years and 120–600 µmol/L for patients >12 years. (Note: Sample labelling issue for Subject 3 on Day 7, so no result recorded). Abbreviation: Phe: phenylalanine.
3.6. Gastrointestinal Symptoms
The study product was well tolerated by all seven subjects for the entire study period. Four subjects reported fewer gastrointestinal symptoms, with less burping, flatulence and regurgitation whilst taking the study product. In one subject, ‘severe’ burping, flatulence and regurgitation was recorded at baseline and reduced to ‘mild’ with the study product. There were no cases of mild, moderate or severe diarrhoea, constipation, bloating/distension, nausea or vomiting during the study. There was one case of moderate abdominal discomfort and pain (attributed to menstruation).
3.7. Adverse Events
No adverse reactions were reported.
4. Discussion
In this study, we assessed the introduction of a novel protein substitute with a coating that supports a more physiological release of amino acids than existing substitutes and also masks their bitter taste, aftertaste and smell [16]. The study design followed the guidelines of the UK ACBS with the objective of evaluating the acceptability of the new protein substitute with a limited number of subjects and within a short evaluation time. Despite a short time period for adaptation to the study product, subjects did not report that taste and smell were an issue. During the study, most of the subjects either showed improved blood phenylalanine and tyrosine control or at least maintained them within their target range. This is an encouraging preliminary outcome.
Many patients with PKU are food neophobic [24,25]: and are very suspicious of anything different. Consequently, this short evaluation study was particularly challenging. The study product was dissimilar from current protein substitutes, which patients were well used to taking. Each child understood the potential advantages of taking a prolonged-release protein in terms of impact on blood phenylalanine control and product taste and was very open to trying this new product. However, none had previously experienced taking a protein substitute added to their usual food or drink, so this was an unfamiliar concept to them. The abrupt introduction of a new substitute was overwhelming to some children. Understandably, it is likely to take time to adapt to change, and a slower, more gradual introduction may have been more acceptable. Thereby, a program of slow and systematic introduction, with consistent support given by the PKU team, is essential. Additionally, accompanying educational messages may significantly influence motivation and persistence when introducing any new protein substitute [26]. Generally, patients with PKU and their caregivers welcome new treatments, changes in treatment strategies, new foods, and different presentations of protein substitutes if it improves acceptability and tolerance of treatment [27].
Most of the subjects added the study product to fruit smoothies or juice. This form of administration was quick and similar to their current method of taking protein substitute. This study group has always treated protein substitute differently to food, consumed it immediately before or after meals. If the study evaluation had been extended, they might have eventually accepted mixing the new protein substitute with food or drinks. Due to the study product’s coating, the taste and smell were masked, enabling the protein substitute to be taken as an ‘add-on’ to meals and snacks without affecting the taste of the original food. This type of protein substitute may be particularly useful for patients returning to a low-phenylalanine diet, who have unpleasant memories associated with the smell and taste of protein substitute. It may also be helpful during pregnancy for women struggling with nausea and vomiting associated with the taste and smell of protein substitutes [28]. The excipients used for the coating of the study product are considered safe for pregnancy and lactation [29].
The study product was well tolerated over the treatment period. Protein substitutes are known to have a high osmolarity and therefore may cause gastrointestinal upsets, including abdominal pain, diarrhoea and constipation [12,15,30]. In this cohort, there were no cases of diarrhoea, constipation, bloating/distension, nausea or vomiting during the study. Four subjects reported improvements in the severity of burping, flatulence and regurgitation that they had experienced while receiving their previous treatment regimen. Importantly, there were no adverse reactions reported during the study period. The use of an ethyl cellulose and alginate coating on the study product resulted in granules of amino acids that were stable in the stomach with a gradual disintegration during small-intestine transit [29,31]. This may explain the favourable gastrointestinal tolerability in this study. Both excipients (ethyl cellulose and alginate) are widely used in pharmaceutical technology and recognised as safe for use in medical foods [16]. It would be important to observe whether benefits to gastrointestinal tolerance could be observed longer-term, particularly if the study product provided a higher proportion of protein-substitute intake.
In recent years, several new protein-substitute compositions and formulations have been developed with the aim of improving adherence, which remains suboptimal especially in adolescents and adults with PKU [32]. However, all contain free L-amino acids with absorption kinetics that are more rapid than intact protein sources, causing a lower biological and functional efficacy [33,34,35]. The introduction of a prolonged-release protein substitute with the ability to prolong absorption of synthetic amino acids is expected to minimise blood phenylalanine level fluctuations and improve phenylalanine control and other metabolic markers in PKU [8,23], but longer-term studies are required to examine the impact on blood phenylalanine control and 24 h blood phenylalanine variability.
There were limitations to this study evaluation. It represented a small patient population with a limited treatment period. Only around 20 to 30% of the usual protein substitute requirements were substituted with the study product due to the short time of adaptation. There were limited blood phenylalanine control data. Additional, more extensive studies performed in a larger population and over a longer timeframe will provide more evidence regarding the adherence and tolerability of this protein substitute, together with impact on metabolic control in patients with PKU.
5. Conclusions
In children with PKU, partial replacement of standard protein-substitute with a prolonged-release protein substitute was achievable. The study product was mixed with food or fruit juice. Subjects maintained satisfactory blood phenylalanine control and were able to take their protein substitute in a different way to their usual practice. The prolonged-release protein substitute was well tolerated, with subjects experiencing fewer gastrointestinal symptoms than with their previous treatment regimen.
Acknowledgments
The authors thank Sam Coates for their assistance in preparing the manuscript for publication and Patrizia Marzorati at APR Applied Pharma Research S.A. for her review and comments.
Author Contributions
Conceptualization, A.M.; methodology, A.M., A.D., S.E.; software, A.M.; validation, A.M.; formal analysis, A.M., C.A., A.D., and A.P.; investigation, A.M.; resources, A.M.; data curation, A.M.; writing—original draft preparation, A.M., C.A., A.D., A.P., and S.E.; writing—review and editing, A.M., C.A., A.D., A.P., and S.E.; visualization, A.M.; supervision, A.M.; project administration, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by APR Applied Pharma Research SA.
Conflicts of Interest
A.M. is an advisory board member for ELEMENT Danone-Nutricia, Arla, and Applied Pharma Research, and has received research funding and honoraria from Nutricia, and Vitaflo International. S.E. has received research funding from Nutricia, and financial support and honoraria from Nutricia and Vitaflo International to attend/speak at study days and conferences. C.A., and A.D. declare no conflict of interest. A.P. has received an educational grant from Cambrooke Therapeutics, research funding from Vitaflo, Nutricia, and Biomarin. Biomarin, Mevalia, Vitaflo and Nutricia have provided research funding to attend scientific meetings.
References
- 1.Van Wegberg A.M.J., MacDonald A., Ahring K., Belanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Gizewska M., et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood A., Grange D.K., Christ S.E., Steiner R., White D.A. Variability in phenylalanine control predicts IQ and executive abilities in children with phenylketonuria. Mol. Genet. Metab. 2014;111:445–451. doi: 10.1016/j.ymgme.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeRoche K., Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: Intelligence and executive function. Dev. Neuropsychol. 2008;33:474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- 4.Simon E., Schwarz M., Roos J., Dragano N., Geraedts M., Siegrist J., Kamp G., Wendel U. Evaluation of quality of life and description of the sociodemographic state in adolescent and young adult patients with phenylketonuria (PKU) Health Qual. Life Outcomes. 2008;6:25. doi: 10.1186/1477-7525-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Hafid N., Christodoulou J. Phenylketonuria: A review of current and future treatments. Transl. Pediatr. 2015;4:304–317. doi: 10.3978/j.issn.2224-4336.2015.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Spronsen F.J., van Wegberg A.M., Ahring K., Belanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Gizewska M., et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017;5:743–756. doi: 10.1016/S2213-8587(16)30320-5. [DOI] [PubMed] [Google Scholar]
- 7.Macleod E.L., Ney D.M. Nutritional Management of Phenylketonuria. Ann. Nestle. 2010;68:58–69. doi: 10.1159/000312813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald A., Singh R.H., Rocha J.C., van Spronsen F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019;32:70–78. doi: 10.1017/S0954422418000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MaCdonald A., van Rijn M., Feillet F., Lund A.M., Bernstein L., Bosch A.M., Gizewska M., van Spronsen F.J. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann. Nutr. Metab. 2012;61:289–295. doi: 10.1159/000342256. [DOI] [PubMed] [Google Scholar]
- 10.Shaw V., editor. Clinical Paediatric Dietetics. 4th ed. Wiley-Blackwell; Oxford, UK: 2014. p. 864. [Google Scholar]
- 11.MacDonald A. Diet and compliance in phenylketonuria. Eur. J. Pediatr. 2000;159(Suppl. S2):S136–S141. doi: 10.1007/PL00014375. [DOI] [PubMed] [Google Scholar]
- 12.Ford S., O’Driscoll M., MacDonald A. Living with Phenylketonuria: Lessons from the PKU community. Mol. Genet. Metab. Rep. 2018;17:57–63. doi: 10.1016/j.ymgmr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter J.H., White F.J. Blood phenylalanine control in adolescents with phenylketonuria. Int. J. Adolesc. Med. Health. 2004;16:41–45. doi: 10.1515/IJAMH.2004.16.1.41. [DOI] [PubMed] [Google Scholar]
- 14.Green B., Browne R., Firman S., Hill M., Rahman Y., Kaalund Hansen K., Adam S., Skeath R., Hallam P., Herlihy I., et al. Nutritional and metabolic characteristics of UK adult phenylketonuria patients with varying dietary adherence. Nutrients. 2019;11:2459. doi: 10.3390/nu11102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald A., Harris G., Rylance G., Asplin D., Booth I.w. Abnormal feeding behaviours in phenylketonuria. J. Hum. Nutr. Diet. 1997;10:163–170. doi: 10.1046/j.1365-277X.1997.00050.x. [DOI] [Google Scholar]
- 16.Giarratana N., Gallina G., Panzeri V., Frangi A., Canobbio A., Reiner G. A new Phe-free protein substitute engineered to allow a physiological absorption of free amino acids for phenylketonuria. J. Inborn Errors Metab. 2018;6:1–9. doi: 10.1177/2326409818783780. [DOI] [Google Scholar]
- 17.Pena M.J., de Almeida M.F., van Dam E., Ahring K., Belanger-Quintana A., Dokoupil K., Gokmen-Ozel H., Lammardo A.M., MacDonald A., Robert M., et al. Protein substitutes for phenylketonuria in Europe: Access and nutritional composition. Eur. J. Clin. Nutr. 2016;70:785–789. doi: 10.1038/ejcn.2016.54. [DOI] [PubMed] [Google Scholar]
- 18.Paul D.B., Brosco J.P. The PKU Paradox. A Short History of a Genetic Disease. Johns Hopkins University Press; Baltimore, MD, USA: 2013. [Google Scholar]
- 19.Tiele A., Daly A., Hattersley J., Pinto A., Evans S., Ashmore C., MacDonald A., Covington J.A. Investigation of paediatric PKU breath malodour, comparing glycomacropeptide with phenylalanine free L-amino acid supplements. J. Breath Res. 2019;14:016001. doi: 10.1088/1752-7163/ab4097. [DOI] [PubMed] [Google Scholar]
- 20.Lim K., van Calcar S.C., Nelson K.L., Gleason S.T., Ney D.M. Acceptable low-phenylalanine foods and beverages can be made with glycomacropeptide from cheese whey for individuals with PKU. Mol. Genet. Metab. 2007;92:176–178. doi: 10.1016/j.ymgme.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald A., Gokmen-Ozel H., van Rijn M., Burgard P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. 2010;33:665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- 22.Duran G.P., Rohr F.J., Slonim A., Guttler F., Levy H.L. Necessity of complete intake of phenylalanine-free amino acid mixture for metabolic control of phenylketonuria. J. Am. Diet. Assoc. 1999;99:1559–1563. doi: 10.1016/S0002-8223(99)00382-X. [DOI] [PubMed] [Google Scholar]
- 23.Scheinin M., Barassi A., Junnila J., Lovro Z., Reiner G., Sarkkinen E., MacDonald A. Amino acid plasma profiles from a prolonged-release protein substitute for phenylketonuria: A randomized, single-dose, four-way crossover trial in healthy volunteers. Nutrients. 2020;12:1653. doi: 10.3390/nu12061653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans S., Daly A., Chahal S., MacDonald J., MacDonald A. Food acceptance and neophobia in children with phenylketonuria: A prospective controlled study. J. Hum. Nutr. Diet. 2016;29:427–433. doi: 10.1111/jhn.12346. [DOI] [PubMed] [Google Scholar]
- 25.Evans S., Daly A., Chahal S., Ashmore C., MacDonald J., MacDonald A. The influence of parental food preference and neophobia on children with phenylketonuria (PKU) Mol. Genet. Metab. Rep. 2018;14:10–14. doi: 10.1016/j.ymgmr.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernstein L.E., Helm J.R., Rocha J.C., Almeida M.F., Feillet F., Link R.M., Gizewska M. Nutrition education tools used in phenylketonuria: Clinician, parent and patient perspectives from three international surveys. J. Hum. Nutr. Diet. 2014;27(Suppl. S2):4–11. doi: 10.1111/jhn.12065. [DOI] [PubMed] [Google Scholar]
- 27.Giovannini M., Verduci E., Salvatici E., Paci S., Riva E. Phenylketonuria: Nutritional advances and challenges. Nutr. Metab. 2012;9:7. doi: 10.1186/1743-7075-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford S., O’Driscoll M., MacDonald A. Reproductive experience of women living with phenylketonuria. Mol. Genet. Metab. Rep. 2018;17:64–68. doi: 10.1016/j.ymgmr.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasilewska K., Winnicka K. Ethylcellulose—A pharmaceutical excipient with multidirectional application in drug dosage forms development. Materials (Basel) 2019;12:3386. doi: 10.3390/ma12203386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PBMK A. Nutritional Management of Patients with Inherited Disorders of Aromatic Amino Acid Metabolism. Jones and Bartlett; Boston, MA, USA: 2010. [Google Scholar]
- 31.Arica B., Calis S., Atilla P., Durlu N.T., Cakar N., Kas H.S., Hincal A.A. In vitro and in vivo studies of ibuprofen-loaded biodegradable alginate beads. J. Microencapsul. 2005;22:153–165. doi: 10.1080/02652040400026319. [DOI] [PubMed] [Google Scholar]
- 32.Rocha J.C., MacDonald A. Dietary intervention in the management of phenylketonuria: Current perspectives. Pediatr. Health Med. Ther. 2016;7:155–163. doi: 10.2147/PHMT.S49329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacroix M., Bos C., Leonil J., Airinei G., Luengo C., Dare S., Benamouzig R., Fouillet H., Fauquant J., Tome D., et al. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am. J. Clin. Nutr. 2006;84:1070–1079. doi: 10.1093/ajcn/84.5.1070. [DOI] [PubMed] [Google Scholar]
- 34.Bujko J., Schreurs V.V., Nolles J.A., Verreijen A.M., Koopmanschap R.E., Verstegen M.W. Application of a [13CO2] breath test to study short-term amino acid catabolism during the postprandial phase of a meal. Br. J. Nutr. 2007;97:891–897. doi: 10.1017/S0007114507433049. [DOI] [PubMed] [Google Scholar]
- 35.Metges C.C., Barth C.A. Metabolic consequences of a high dietary-protein intake in adulthood: Assessment of the available evidence. J. Nutr. 2000;130:886–889. doi: 10.1093/jn/130.4.886. [DOI] [PubMed] [Google Scholar]